Abstract
Background:
Major surgical procedures, such as gastrectomy, result in extensive postoperative pain, which can lead to increased morbidity, discomfort and dissatisfaction among the patients.
Objectives:
The aim of this study was to evaluate the effect of adding diclofenac suppositories or intravenous paracetamol, on morphine consumption and on the quality of postgastrectomy pain control.
Patients and Methods:
This randomized double blinded clinical trial was carried out in 90 patients with gastric cancer, who were candidates for gastrectomy, which were divided into three similar groups. The patients were transferred to an intensive care unit after the operation and received patient-controlled analgesia (PCA) with morphine, morphine PCA plus intravenous paracetamol 1 g, every 6 hours, and morphine PCA plus diclofenac suppositories, 100 mg every 8 hours. The patients were evaluated for up to 24 hours after the operation for the severity of pain, alertness, and opioid complications.
Results:
There was no significant difference in pain scores among the three groups (P values, after extubation, at 2, 4, 6, 12, 18 and 24 hours were 0.72, 0.19, 0.21, 0.66, 0.54, 0.56, and 0.25, respectively), although morphine consumption was greater in the morphine group, compared with the other two groups (21.4 ± 7.7 mg in morphine group vs. 14.3 ± 5.8 mg in morphine-paracetamol group and 14.3 ± 3.9 in morphine-diclofenac group; P = 0.001). In morphine group, during the first 24 hours, the patients had lower levels of consciousness (P values, after extubation, at 2, 4, 6, 12, 18 and 24 hour were 0.6, 0.95, 0.28, 0.005, 0.027, 0.022 and 0.004 respectively), even though the incidence of complications was similar among the three groups.
Conclusions:
In this study, intravenous paracetamol or diclofenac suppositories, administered for postgastrectomy pain control, decreased morphine consumption by almost 32% and also improved alertness. Nevertheless, the amount of opioids did not affect the incidence of complications.
Keywords: Acetaminophen, Diclofenac, Morphine, Gastrectomy, Pain Management
1. Background
Acute postoperative pain is a complex physiological reaction, due to damage and inflammation, which increases morbidity, mortality rate, costs, and also reduces patient satisfaction (1, 2). Postoperative pain leads to the pulmonary, cardiac and metabolic complications, impaired ulcer healing, and restlessness. Therefore, pain control is important for prevention and treatment of complications (3-5). Postoperative pain intensity varies depending on the type and place of surgery and is higher in major surgeries, such as laparotomy (6).
Different methods are used for postoperative pain control. Therapeutic regimens depend on type of surgery, age, physical and mental states of patients (7). In major surgeries, such as gastrectomy, combined and multi-drug methods are recommended (8, 9). The opioids, non-steroid anti-inflammatory drugs (NSAIDs), and paracetamol are commonly used for pain control (10, 11). Performing nerve blocks or intraspinal injection of opiates can be useful in thoracotomy, hepatic resection, and esophagectomy (12-14). Combination of these methods improves quality of pain control (due to interaction with different mechanisms) and also reduces opioid and NSAIDs dosage and complications (respiratory depression, dependency, renal failure, gastrointestinal bleeding, etc.). In 1995, The American Society of Anesthesiologists presented a guideline for acute postoperative pains, with emphasis on the standardization of care and use of epidural analgesia, patient controlled analgesia (PCA), and concurrent analgesic methods (15).
Although different studies have been conducted on pain control, after a search in PubMed, Medline, SCOPUS and Cochrane database, no studies have been identified regarding the postgastrectomy pain.
2. Objectives
The aim of our study was the evaluation of adding intravenous paracetamol or diclofenac suppository on the dose of morphine, using the patient controlled analgesia method and also, the improvement of quality of postgastrectomy pain control and consciousness.
3. Patients and Methods
After the approval by The Research Chancellor of the Medical University and Ethics Committee No: 900184 on 2012.2.2, registered in Iranian Registry of Clinical Trials Center No: IRCT201112078322N1 and with the written letter of consent from the patients, this double blind randomized clinical trial was conducted on the candidate patients of gastrectomy. Ninety gastric cancer patients, who were candidate for gastrectomy, aged 40 - 70 years old were selected. The patients were evaluated for perceptibility and working with PCA pump and trained by a skilled person, before starting. Exclusion criteria included cardiac, hepatic, and renal failure, insulin-dependent diabetes, severe asthma, morphine, NSAIDs, or acetaminophen sensitivity, weight of more than 110 kg or below 50 kg, long-term intake of narcotic drugs or analgesics before surgery, and previous use of aspirin or clopidogrel. If gastrectomy was not possible, due to the disease progress, the patients would be excluded from the study.
For the patients, two 18 G venous catheters were embedded. Anesthesia induction was done by 2 mg midazolam, 4 µg/kg fentanyl, 2 mg/kg propofol, and 1.5 mg/kg cisatracurium. The patients were intubated and ventilated mechanically, after 2 - 3 minutes. To maintain anesthesia, 50 - 200 µg/kg/minute propofol, 0.2 - 0.5 µg/kg/minute remifentanil, and nitrous oxide (N2O/O2) 50% were used. For all the patients, 0.1 mg/kg morphine sulfate (Darupakhsh Pharmaceutical Co. Tehran, Iran) was slowly injected intravenously, during the last 30 minutes of the surgery. After completion of the surgery and respiration recovery, the patients were extubated and transferred to the recovery and underwent monitoring and oxygen therapy with the fraction of inspired oxygen (FiO2) = 40 - 50%, by a face mask. For all the patients, a PCA pump (1 mg morphine with minimum interval of 5 minutes) was used. Then, they were transferred to the intensive care unit (ICU), after achieving recovery discharge criteria, including relative consciousness, stable hemodynamic, and good respiration.
After 2 hours in the ICU, the patients were divided into three similar groups by computer randomization table (random block software) and closed envelope. The patients, information-recording anesthesia nurse, and the nurse prescribing medicine had no information about the kind of prescribed medication, either suppositories or intravenous. These three groups underwent analgesic treatment, as follows:
M (morphine) group: This group of patients received 100 mL saline, every 6 hours and placebo suppository, for blindness (such as soap, in the same package that was prepared by the staff pharmacist), every 8 hours, in addition to morphine PCA pump.
MP (morphine + paracetamol) group: This group received 1 g intravenous paracetamol (Anad Pharmaceuticals, Anand, Gujarat, India) with 100 mL saline, every 6 hours, and placebo suppository, every 8 hours, in addition to morphine PCA pump.
MD (morphine + diclofenac Na) group: This group received 100 mg diclofenac suppository (Aburaihan Pharmaceutical Company, Tehran, Iran), every 8 hours, and 100 mL saline, every 6 hours, in addition to morphine PCA pump.
The first dose of morphine was prescribed by a nurse, in confused patients, with symptoms such as tachycardia, hypertension, flashing, or sweating. When the patient was completely conscious and well-responding, he/she used the device by him/herself. Consciousness level, pain intensity, and peripheral oxygen saturation (SpO2) were evaluated in the recovery, after 2, 4, 6, 12, 18, and 24 hours. Nausea, vomiting with four point scale (0 = none, 1 = just nausea, 2 = single vomiting on 24 hours, 3 = multiple vomiting, on 24 hours) and itching of the patients were also evaluated during 24 hours.
Consciousness level of the patients was measured using simple numerical rating scale (ACDU scale) as follows: 1- Alert (full awake), 2- Confused (sometimes wakes up and then sleeps), 3- Drowsy (almost always sleeps), and 4- Unresponsive (hardly wakes up). The pain intensity was also evaluated, based on simple four-point categorical verbal rating scale (VRS) with patients expression as: 0- painless (numeric rating scale (NRS) = 0), 1- mild pain (NRS = 1 - 3), 2- medium pain (tolerable pain or NRS = 4 - 6), and 3- severe pain (intolerable pain or NRS = 7 - 10).
3.1. Statistical Studies
The sample size was calculated based on the study by Fayaz et al. (16) and variance of morphine intake was calculated in three groups, with SigmaPlot v12.5 software. Considering average estimation error of 0.85, first type error of 0.05 (α = 0.05) and confidence coefficient of 80% (β = 0.2), a sample size of 88 patients resulted and 90 people were selected for facilitating the study. The results were evaluated and analyzed in SPSS v.13 software (SPSS Inc. Chicago, IL, USA). Parametric data, such as age, surgery duration, recovery, changes in arterial oxygen pressure, and morphine intake were compared by one-way and repeated measures analysis of variance (ANOVA) and also Dunnet test. Nonparametric data, such as consciousness, pain intensity, nausea and itching, with non-normal distribution, were analyzed by Chi-square test. The relationship between consciousness level and morphine intake was also evaluated, using logical regression method. A P < 0.05 was considered significant.
4. Results
This study was conducted on 90 patients, aged 40 - 70 years old, with gastric cancer in three similar groups, with flow diagram. Demographic and clinical information are shown in Table 1. There was no statistically significant difference between the three groups, for age, gender, weight, surgery and recovery duration (from tracheal extubation to transfer to the ICU).
Table 1. Demographic Parameters in the Three Groups a.
| Parameters | Morphine | Morphine-Paracetamol | Morphine-Diclofenac | P Value |
|---|---|---|---|---|
| Gender | 0.14 | |||
| Male | 19 | 21 | 23 | |
| Female | 11 | 9 | 7 | |
| Age, y | 65.3 ± 11.3 | 66.8 ± 10.1 | 63.8 ± 12.6 | 0.6 |
| Weight, Kg | 62.8 ± 17.8 | 63.4 ± 10.9 | 65.9 ± 9.4 | 0.21 |
| Duration of surgery, min | 273.3 ± 51.9 | 251 ± 76.2 | 269.5 ± 68.4 | 0.44 |
a Values are presented as Mean ± SD.
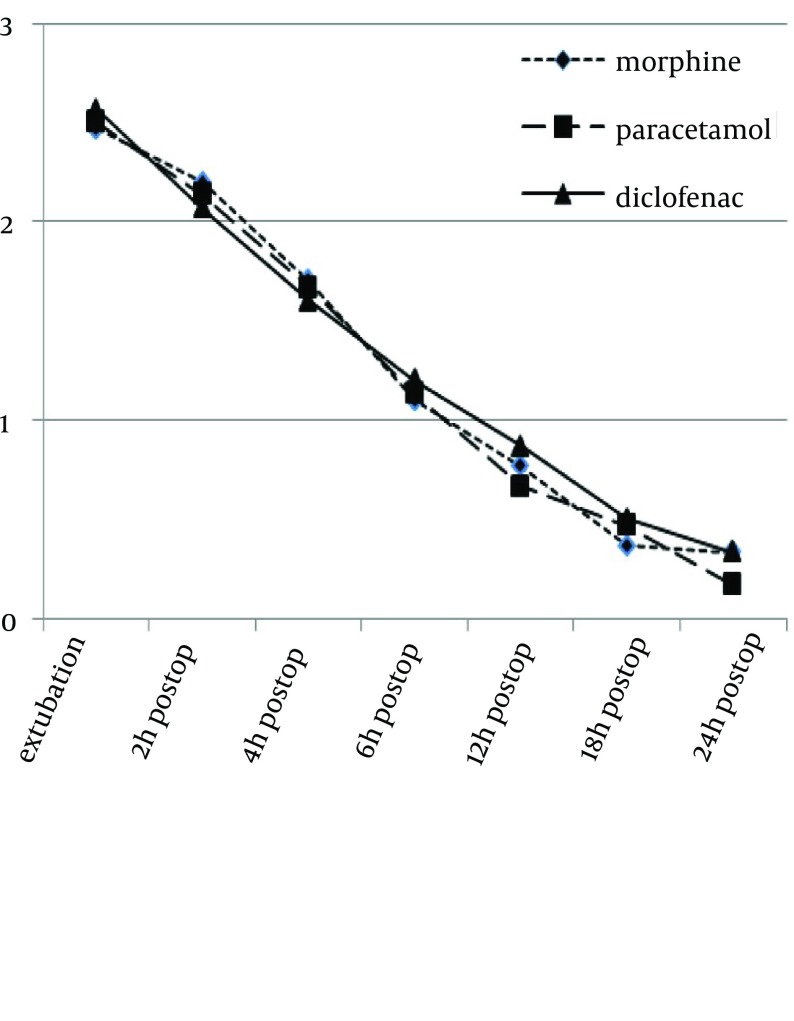
Consciousness level of the patients was not significantly and clearly different in the three groups, in the first 4 hours after the surgery, in the ICU. However, it was significantly different after 4 hours among three groups (P values, after extubation, at 2, 4, 6, 12, 18 and 24 hours, were 0.6, 0.95, 0.28, 0.005, 0.027, 0.022 and 0.004, respectively). Figure 1 shows the difference between morphine group and the paracetamol and diclofenac groups, in terms of consciousness level.
Figure 1. Consciousness State in the Three Groups.
1- Awake, 2- Sometimes wake up and then sleep, 3- Almost always sleeps, 4- Hardly wakes up. The P values, after extubation, at 2, 4, 6, 12, 18 and 24 hours were 0.6, 0.95, 0.28, 0.005, 0.027, 0.022 and 0.004, respectively.
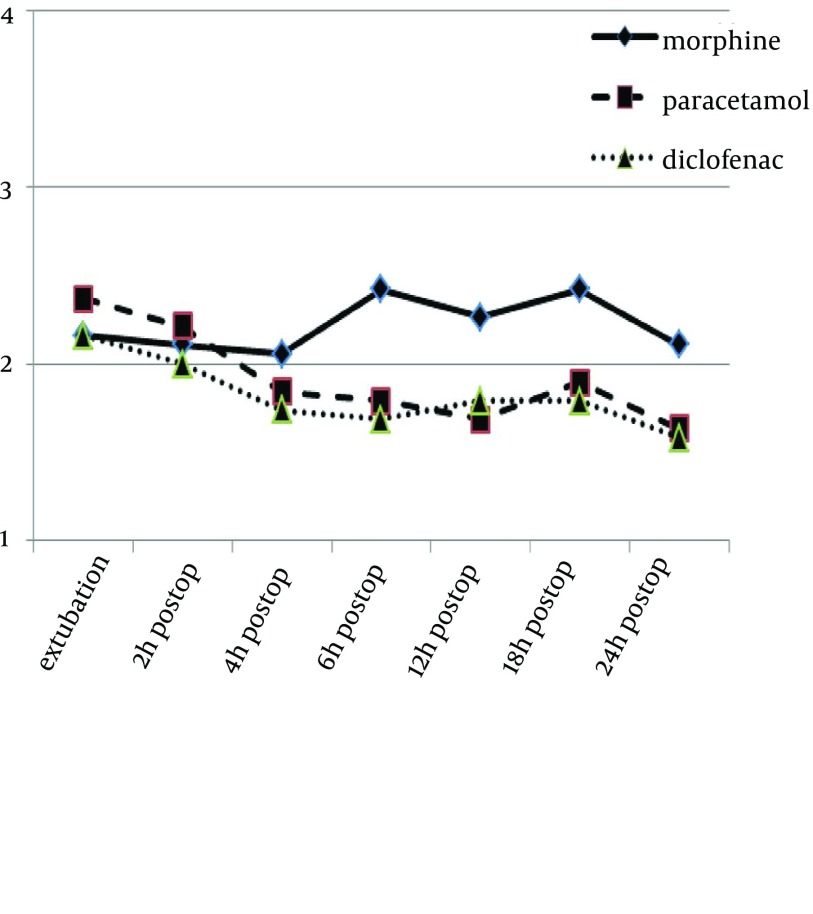
Pain rating scale, during 24 hours, in the three groups, is demonstrated in Figure 2. There was no significant difference between the groups, in terms of pain intensity (P values, after extubation, at 2, 4, 6, 12, 18 and 24 hours, were 0.72, 0.19, 0.21, 0.66, 0.54, 0.56 and 0.25, respectively). Nevertheless, pain reduction during 24 hours was significant in all the three groups (P = 0.00).
Figure 2. Pain Scores of the Three Groups During 24 Hours.
0- Painless, 1- Mild pain (tolerable pain), 2- Medium pain (intolerable pain), and 3- Severe pain (noxious pain). P values, after extubation, at 2, 4, 6, 12, 18 and 24 hours were 0.72, 0.19, 0.21, 0.66, 0.54, 0.56 and 0.25, respectively.
Morphine intake in M group was 32% higher than in the MP (P = 0.00) and MD (P = 0.00) groups (Table 2), although the morphine intake between MD and MP groups was not significantly different (P = 0.75). The relationship between consciousness and morphine intake was significant after 4 hours (P value was 0.49, 0.99, 0.043, 0.006, 0.048, 0.001, and 0.00, after extubation, the 2nd, 4th, 6th, 12th, 18th, and 24th hour, respectively). Complications of morphine intake, such as arterial desaturation, nausea, vomiting, and itching, are shown in Table 2 there was no significant difference between the three groups.
Table 2. Morphine Intake and Opioid Complications in the Three Groups a,b.
| Parameters | Morphine | Morphine-Paracetamol | Morphine-Diclofenac | P Value |
|---|---|---|---|---|
| Total morphine consumption | 21.4 ± 7.7 | 14.3 ± 5.8 | 14.3 ± 3.9 | 0.001 |
| Nausea and vomiting c | 33.3 | 33.3 | 20 | 0.42 |
| Pruritus c | 20 | 13.3 | 16.7 | 0.79 |
| SpO 2 c | 96.5 ± 1.3 | 95.8 ± 1.9 | 96.1 ± 1.8 | 0.24 |
a Abbreviations: SpO2, Peripheral Oxygen Saturation.
b Values are presented as Mean ± SD.
c Values unit is %.
5. Discussion
Patients experience severe postoperative pain, in the first 24 hours. Pain intensity depends on surgery type and therapeutic protocol. In our previous study (in laparotomy), maximum tolerated pain during the first hours was 8.4 ± 1.2 and mean pain of the patients has been reported as 5.8 ± 1.9, in the first 24 hours, without appropriate protocol (17). In this study, by adding paracetamol and diclofenac to PCA morphine method, the effect of these drugs on pain control was comparable.
Pain intensity was identical in the three groups, during 24 hours. However, morphine intake in MP and MD groups was less than in the M group. After the first 4 hours, consciousness level of the patients in the M group was lower than that in MP and MD groups, which could be the result of higher intake of morphine, in this group. Despite reduction of morphine intake, there was no significant difference among the groups for opioid complications, such as nausea, vomiting, itching, and oxygen desaturation, due to same pain intensity in the three groups and supplementary oxygen.
Laparotomy is accompanied by high postoperative pain, which is due to wide incision and more manipulations (9). Different methods have been used for controlling postoperative pain. Nevertheless, use of oral and intravenous compounds is more applicable. Using opiates by PCA method, along with paracetamol, diclofenac, or both, is a suitable method for pain control. The combination therapy is more effective and reduces pain intensity and need for additional analgesics (18). For example, the combination of these two drugs after caesarean section reduces the need for morphine intake by 38% (19).
In another study for pain control, among the patients undergoing coronary artery bypass grafting, morphine + diclofenac, morphine + diclofenac + paracetamol, and morphine alone groups were compared. In this study, it was found that morphine intake in the control group was significantly higher than that in the group receiving diclofenac and diclofenac + paracetamol during 24 hours. The period until extubation was longer, oxygenation level was lower, and nausea and vomiting rates were higher in the control group, compared with the other two groups (16).
Indeed, same results cannot be always found. For example, in a study which was conducted on children after appendectomy, pain intensity and morphine intake were significantly reduced after suppository diclofenac. However, it was not effective in reducing pain (20).
Results of the present study were similar to most of the previous works, confirming improvement of pain quality and reduction of morphine intake by concurrent use of diclofenac or paracetamol and morphine.
In several studies, there is controversy over the use of acetaminophen for severe postoperative pain control. In a systematic review on the results of seven studies, including 265 patients, adding acetaminophen did not reduce complications of morphine or increase patient satisfaction; however, adding acetaminophen to morphine pump reduced the need for morphine by 20% during the first 24 hours after the surgery (21). Intake of some compounds such as diclofenac is sometimes accompanied by several complications, such as increased bleeding (10).
In the present investigation, adding diclofenac suppository or paracetamol infusion had an identical effect on pain intensity control and intake of opiates and reduced average morphine intake within the first 24 hours after the surgery, in both groups, by 32%, which could justify a better consciousness of the patients in these two groups. Lack of difference in consciousness level in the first 4 hours may be due to the residual effect of anesthesia drugs and using morphine in the last hours of surgery.
Our study has several limitations, as follows: Pain scores are measured at fixed time, resulting that the length of analgesic effect of drugs could not be investigated and also, the primary outcome of this study was rescue analgesic requirement, which may not truly reflect the efficacy of these analgesics.
Adding intravenous paracetamol 1 g q6h, or suppository diclofenac 100 mg q8h to the pain control protocol with PCA morphine after gastrectomy reduced morphine intake by 32% at the same rate, which led to better consciousness of the patients within the first 24 hours after the surgery. However, it had no effects on other complications of morphine, such as nausea, vomiting, and itching (1).
Acknowledgments
This study was supported by The Deputy Research of Mashhad University of Medical Sciences, Mashhad, Iran. Authors are thankful to The Cardiac Anesthesia Research Center who helped us in data analysis and editing this report.
Footnotes
Authors’ Contributions:Alireza Bameshki: study concept and design. Hengameh Rezaei Boroujerdi: acquisition of data. Shima Sheybani: analysis and interpretation of data. Arash Peivandi Yazdi: drafting of the manuscript. Mehryar Taghavi Gilani: statistical analysis.
Funding/Support:Deputy for research of Mashhad University of medical sciences.
References
- 1.Turk DC, Okifuji A. Assessment of patients' reporting of pain: an integrated perspective. Lancet. 1999;353(9166):1784–8. doi: 10.1016/S0140-6736(99)01309-4. [DOI] [PubMed] [Google Scholar]
- 2.Watcha MF, Issioui T, Klein KW, White PF. Costs and effectiveness of rofecoxib, celecoxib, and acetaminophen for preventing pain after ambulatory otolaryngologic surgery. Anesth Analg. 2003;96(4):987–94. doi: 10.1213/01.ANE.0000053255.93270.31. [DOI] [PubMed] [Google Scholar]
- 3.Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology. 1995;83(5):1090–4. doi: 10.1097/00000542-199511000-00023. [DOI] [PubMed] [Google Scholar]
- 4.National Guideline C. Post-operative pain management. Guidelines on pain management. Rockville MD: Agency for Healthcare Research and Quality (AHRQ). 2014. [Google Scholar]
- 5.Justins DM. Postoperative pain: a continuing challenge. Ann R Coll Surg Engl. 1992;74(2):78–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Couceiro TC, Valenca MM, Lima LC, de Menezes TC, Raposo MC. Prevalence and influence of gender, age, and type of surgery on postoperative pain. Rev Bras Anestesiol. 2009;59(3):314–20. doi: 10.1590/s0034-70942009000300006. [DOI] [PubMed] [Google Scholar]
- 7.Recart A, Duchene D, White PF, Thomas T, Johnson DB, Cadeddu JA. Efficacy and safety of fast-track recovery strategy for patients undergoing laparoscopic nephrectomy. J Endourol. 2005;19(10):1165–9. doi: 10.1089/end.2005.19.1165. [DOI] [PubMed] [Google Scholar]
- 8.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22(5):588–93. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 9.Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83(1):11–25. [PMC free article] [PubMed] [Google Scholar]
- 10.Legeby M, Sandelin K, Wickman M, Olofsson C. Analgesic efficacy of diclofenac in combination with morphine and paracetamol after mastectomy and immediate breast reconstruction. Acta Anaesthesiol Scand. 2005;49(9):1360–6. doi: 10.1111/j.1399-6576.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 11.Cormie PJ, Nairn M, Welsh J, Guideline Development G. Control of pain in adults with cancer: summary of SIGN guidelines. BMJ. 2008;337:a2154. doi: 10.1136/bmj.a2154. [DOI] [PubMed] [Google Scholar]
- 12.Hurford WE, Dutton RP, Alfille PH, Clement D, Wilson RS. Comparison of thoracic and lumbar epidural infusions of bupivacaine and fentanyl for post-thoracotomy analgesia. J Cardiothorac Vasc Anesth. 1993;7(5):521–5. doi: 10.1016/1053-0770(93)90306-6. [DOI] [PubMed] [Google Scholar]
- 13.Rahimzadeh P, Safari S, Faiz SH, Alavian SM. Anesthesia for patients with liver disease. Hepat Mon. 2014;14(7):e29688. doi: 10.5812/hepatmon.19881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bameshki AR, Zanjankhah MR, Gilani MT, Khashayar P. Intrathecal sufentanil for intraoperative and postesophagectomy pain relief. South Med J. 2010;103(3):197–201. doi: 10.1097/SMJ.0b013e3181cecc81. [DOI] [PubMed] [Google Scholar]
- 15.Practice guidelines for acute pain management in the perioperative setting. A report by the American Society of Anesthesiologists Task Force on Pain Management, Acute Pain Section. Anesthesiology. 1995;82(4):1071–81. [PubMed] [Google Scholar]
- 16.Fayaz MK, Abel RJ, Pugh SC, Hall JE, Djaiani G, Mecklenburgh JS. Opioid-sparing effects of diclofenac and paracetamol lead to improved outcomes after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18(6):742–7. doi: 10.1053/j.jvca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Bameshki A, Jahanbakhsh S, Jangjoo A, Zandi H, Fathi M. Evaluation of acute postoperative pain and patient satisfaction in laparotomy, cholecystectomy and herniorrhaphy. Anesthesiol and Pain. 2013;3(4):196–201. [Google Scholar]
- 18.Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–9. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- 19.Munishankar B, Fettes P, Moore C, McLeod GA. A double-blind randomised controlled trial of paracetamol, diclofenac or the combination for pain relief after caesarean section. Int J Obstet Anesth. 2008;17(1):9–14. doi: 10.1016/j.ijoa.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Morton NS, O'Brien K. Analgesic efficacy of paracetamol and diclofenac in children receiving PCA morphine. Br J Anaesth. 1999;82(5):715–7. doi: 10.1093/bja/82.5.715. [DOI] [PubMed] [Google Scholar]
- 21.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94(4):505–13. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]