Abstract
Objective
The purpose of this work was to compare measured and estimated volumetry prior to liver resection.
Methods
Data for consecutive patients submitted to major liver resection for colorectal liver metastases at two centres during 2004–2012 were reviewed. All patients underwent volumetric analysis to define the measured total liver volume (mTLV) and measured future liver remnant ratio (mRFLR). The estimated total liver volume (eTLV) standardized to body surface area and estimated future liver remnant ratio (eRFLR) were calculated. Descriptive statistics were generated and compared. A difference between mRFLR and eRFLR of ±5% was considered clinically relevant.
Results
Data for a total of 116 patients were included. All patients underwent major resection and 51% underwent portal vein embolization. The mean difference between mTLV and eTLV was 157 ml (P < 0.0001), whereas the mean difference between mRFLR and eRFLR was −1.7% (P = 0.013). By linear regression, eTLV was only moderately predictive of mTLV (R2 = 0.35). The distribution of differences between mRFLR and eRFLR demonstrated that the formula over- or underestimated mRFLR by ≥5% in 31.9% of patients.
Conclusions
Measured and estimated volumetry yielded differences in the FLR of ≥5% in almost one-third of patients, potentially affecting clinical decision making. Estimated volumetry should be used cautiously and cannot be recommended for general use.
Introduction
Major hepatectomy is commonly used in the treatment of primary and secondary liver malignancies. Following major hepatectomy, liver insufficiency or ‘small-for-size syndrome’ is associated with significant morbidity and mortality.1
Liver insufficiency is a clinical syndrome whereby the remnant liver fails to sustain adequate organ function, leading to hyperbilirubinaemia, coagulopathy, ascites, encephalopathy and hypoalbuminaemia. It may lead to further renal and/or respiratory failure, infectious complications, and ultimately to postoperative death.1,2 Despite this general understanding of the syndrome, it remains ill defined, as evidenced by the varying interchangeable terminology utilized in the literature. It has been referred to as liver ‘insufficiency’, ‘failure’ and ‘dysfunction’, as well as ‘small-for-size syndrome’. At least four groups have attempted to define this syndrome based on various clinical parameters, in two instances utilizing postoperative death from liver failure as an objective outcome.3–6
Despite varying definitions, there is consensus in the literature regarding the importance of maintaining adequate remnant volume following liver resection. Although other factors are thought to influence post-resection liver function, such as the health of the remaining parenchyma, age, diabetes, chemotherapy-associated injury, operative blood loss and cholestasis,2 most authors agree that a minimal future liver remnant (FLR) exists for safe resection. In a young and otherwise healthy patient with normal underlying liver parenchyma, commonly reported FLR cut-off values typically represent 20–30% of the patient's total liver volume (TLV).4,7–9
Several techniques exist to measure the FLR. Most centres measure liver volumes directly on cross-sectional imaging and compute the remnant to TLV ratio using only functional non-tumoral liver as representative of TLV. Alternatively, another technique has been described by Vauthey's group and is often used in practice.10 It consists of estimating the TLV based on a patient's body surface area (BSA), measuring the future liver volume on cross-sectional imaging, and then calculating the percentage of the FLR that can now be considered to be standardized to the patient's BSA. Given that these two techniques are inherently different, the objective of this work was to determine the accuracy and variability of each volumetric method in the context of major hepatic resection.
Materials and methods
Patients
A retrospective review of the medical records of consecutive patients submitted to major liver resection at two major tertiary hepatobiliary units in Canada and the Netherlands, respectively, during 2004–2012 was carried out. Approval for this study was sought and obtained from the Ethics Committee of the Centre de Recherche du Centre Hospitalier de l'Université de Montréal (CR-CHUM 12.221). In the Netherlands, research ethics approval was waived for this type of study by the Academic Medical Centre Ethics Board.
Patient selection criteria were defined a priori, before any data acquisition or analysis. Inclusion criteria required that: (i) the patient had undergone major liver resection (three or more segments)6 for metastatic colorectal cancer, and (ii) the patient had undergone volumetric analysis to determine the volume of his or her FLR. Exclusion criteria ruled out data for: (i) patients for whom volumetric data measured prior to any liver surgery or intervention [e.g. staged resection or portal vein embolization (PVE)] were not available; (ii) patients for whom data on height and weight were not available, and (iii) patients with chronic liver disease. These criteria were chosen to define a homogeneous study population in which volumetric analysis would not be affected by hepatic remodelling from prior liver interventions, chronic liver disease, or biliary tract dilation. All patients were thus pre-PVE or pre-staged resection, if necessary, and were thus expected to yield a comparison of volumetric assessment techniques that was as objective as possible. At the Canadian centre, all measurements were recorded within a prospective database for clinical utilization. At the Dutch centre, measurements included both prospectively recorded volumes and some retrospective volumes generated from the original pre-intervention imaging. At both centres, volumetry was utilized commonly for major hepatectomy at the surgeon's discretion.
Data on patient and tumour characteristics included details of age, gender, weight, height, number of liver lesions, and pathological changes within the peritumoral liver parenchyma. Treatment characteristics recorded included details of neoadjuvant chemotherapy, type of major liver resection, and requirement for PVE.
Liver volume measurements
At the Canadian centre, all volumetric analyses were performed by one trained radiology technician (AB) during the entire duration of the study. Senior liver surgeons verified all measurements and utilized the data for clinical practice (FV-M, RL, MD). A dedicated GE Advantage Workstation 4.2 (GE Healthcare, Inc., Waukesha, WI, USA) was used for this work. For each patient, relevant volumes were measured using portal phase computed tomography (CT) or magnetic resonance imaging (MRI) of the liver. When both CT and MRI scans were available, the CT scan was used preferentially. Measured total liver volume (mTLV) was obtained by delineating the liver contour manually on every cut with slice thickness of 5 mm or every one or two cuts with slice thickness of ≤3 mm. Volume was calculated by the software based on the total surface area measured on each imaging cut and the distance between slices. Total tumour volume (TV) and FLR volume were measured in a similar fashion. The caudate lobe was always included in volume measurements. Couinaud segmental anatomy was defined in the usual fashion on the basis of portal vein and hepatic vein anatomy.11 Intrahepatic portal pedicles and hepatic veins were included within the tracings. The gallbladder, extrahepatic portal pedicles, extrahepatic hepatic veins and inferior vena cava were excluded from volume measurements according to the accepted method of segmenting the liver.10
At the Dutch centre, all volumetric analyses were performed by one surgical trainee experienced in performing volumetric measurements (KPC) and verified by an experienced radiologist (KPvL). Integrated software (Mx-View 3.52; Philips Medical Systems BV, Best, the Netherlands) was used to calculate all liver volumes. All relevant volumes were measured using portal phase CT scans with 5-mm slice thickness. Total volume, FLR volume and mTLV were measured using the same technique as at the Canadian centre.
Volumetric analysis
For each patient, relevant liver volumes were measured ‘manually’ using the technique outlined above (mTLV, TV and FLR volume). From these data, the measured FLR ratio (mRFLR), expressed as the predicted percentage of liver remaining after resection, was calculated as: mRFLR = (FLR volume/mTLV − TV) × 100.
In parallel, each patient's estimated FLR ratio (eRFLR) was also calculated using the technique described by Vauthey et al.10 Using this method, the patient's BSA is calculated12 and then used to estimate the TLV (eTLV) as follows: eTLV = −794.41 + 1267.28 × BSA. From these data, the eRFLR can be calculated as: eRFLR = (FLR volume/eTLV).1 Using this technique, only the FLR volume is measured on cross-sectional imaging. It should also be noted that TV is not incorporated in this formula.
Statistical analysis
Summary statistics were generated for each study centre and for the entire cohort. Continuous variables were reported as means and standard deviations (SDs) or medians as appropriate. Dichotomous and categorical variables were reported as proportions. Data from the two study centres were compared using unpaired t-tests or Wilcoxon tests for continuous variables, as appropriate, and chi-squared or Fisher's tests for dichotomous variables. Different techniques for liver volumetric measurements were compared for the same cohorts of patients using paired t-tests. Measured and estimated TLV and FLR ratios were also compared using Spearman correlations. Finally, simple linear regression was carried out to determine the goodness of fit in models with measured values as independent variables and estimated values as dependent variables. R2 values were derived, indicating the proportion of the response-variable variation that is explained by the linear model.
In order to determine the clinical relevance of computed differences between volumetric measurement techniques, a minimal difference of ±5% in the predicted FLR ratios was defined. Because estimated volumetry was originally derived from manual measurements,10 the latter was considered to represent the reference standard for the purpose of this study. Both measured and estimated volumetric data were thus generated, and the proportion of patients in whom mRFLR and eRFLR differed by ≥5% was calculated. For this comparison, binomial proportions and Ward 95% confidence intervals (CIs) were generated.
All statistical analyses were carried out using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Patients
A total of 116 patients were included in this study, of whom 73 were treated at the Canadian centre and 43 at the Dutch centre (Table1). The study cohort was homogeneous, except for significant differences in median height (170 cm versus 175 cm; P = 0.0058) and body mass index (BMI) (26.4 kg/m2 versus 24.5 kg/m2; P = 0.029), reported pathological changes in the peritumoral liver parenchyma (22.7% versus 51.2%; P = 0.0022), and utilization of PVE (61.6% versus 32.6%; P = 0.0025).
Table 1.
Patient characteristics in 116 patients submitted to major liver resection for colorectal liver metastases
| Characteristic | |
|---|---|
| Age, years, mean ± SD (range) | 59.7 ± 11.0 (30–80) |
| Gender, male, n (%) | 76 (65.5%) |
| Height, cm, median (range) | 170 (147–192) |
| Weight, kg, median (range) | 76.0 (47–160) |
| BMI, kg/m2, median (range) | 25.8 (15.2–52.8) |
| Neoadjuvant chemotherapy, yes, n (%) | 46 (48.9%) (n = 94) |
| Neoadjuvant cycles | – |
| Peritumoral parenchyma, n (%) | Abnormal: 37/109 (33.9%) |
| Cholestasis, 4 | |
| SOS, 10 | |
| Steatosis, 23 | |
| Fibrosis, 3 | |
| Steatohepatitis, 1 | |
| Number of liver lesions, median (range) | 2.0 (1–15) |
| Liver resection type, n (%) | |
| Three or four segments | 72 (62.6%) (n = 115) |
| Five or six segments | 43 (37.4%) (n = 115) |
| PVE, yes, n (%) | 59 (50.9%) |
BMI, body mass index; PVE, portal vein embolization; SD, standard deviation; SOS, sinusoidal occlusive syndrome.
Total liver volumes
Data for TLV in the whole cohort are shown in Table2. There was no significant difference in TLV data between the two study centres, whether measured or estimated (data not shown). A mean difference of 157 ml (P < 0.0001) was identified in a comparison of mTLV and eTLV across the whole cohort. The total range of paired differences was wide and included both positive and negative differences (−485 ml to 1693 ml), indicating that eTLV may be either greater or smaller than mTLV (Fig. 1). The mean ± SD differences for the two study centres did not differ significantly (112 ± 297 ml versus 235 ± 484 ml; P = 0.14). Spearman's correlation between mTLV and eTLV was 0.652 (P < 0.0001). Simple linear regression gave an R2 value of 0.350.
Table 2.
Total liver volume (TLV) and future liver remnant (FLR) ratio measurements and comparisons across the entire cohort (n = 116) by technique
| Item | Mean ± SD (range) | Mean difference | P-value |
|---|---|---|---|
| Measured volumetry (mTLV), ml | 1787 ± 471 (range: 1029–3409) | 157.0 (95% CI 87.4–227.0) (range: −485 to 1693) | <0.0001 |
| Estimated volumetry (eTLV), ml | 1630 ± 283 (range: 1022–2730) | ||
| Measured volumetry (mRFLR) | 36.7 ± 13.2% (range: 4.2–85.5%) | −1.7% (95% CI −3.1% to −0.4%) (range: −39.8% to 14.2%) | 0.013 |
| Estimated volumetry (eRFLR) | 38.4 ± 16.0% (range: 2.8–84.3%) |
95% CI, 95% confidence interval; eRFLR, estimated future liver remnant ratio; mRFLR, measured future liver remnant ratio.
Figure 1.
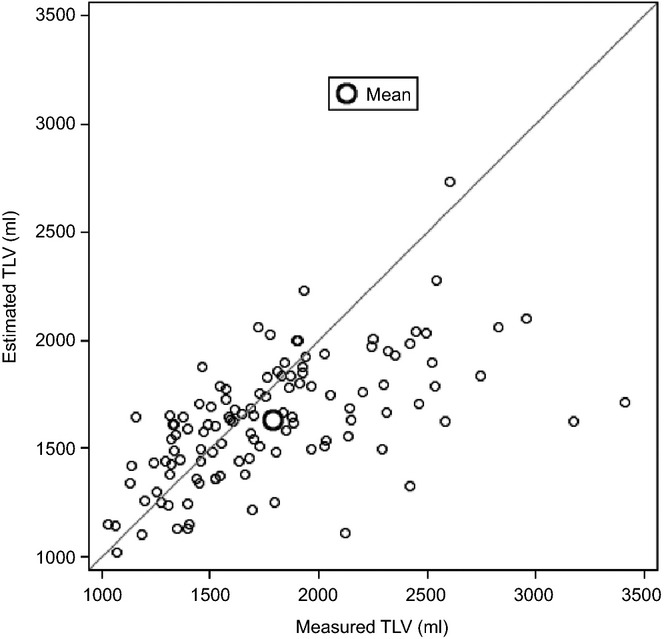
Agreement between measured and estimated total liver volume (TLV) in 116 patients submitted to major liver resection for colorectal liver metastases
In 29 patients (25.0%), the differences between mTLV and eTLV were >1 SD from the mean (≥380 ml); 82.8% of these patients were male. Measured livers in 27 (93.1%) of these 29 patients were larger than would have been predicted based on BSA, and 24 of the 27 patients demonstrated livers of >2000 ml in volume. A comparison of patients with and without a difference of >1 SD between mTLV and eTLV showed no significant difference in BMI (≥30 kg/m2: 17.2% versus 16.1%; P = 0.88), BSA (1.96 m2 versus 1.90 m2; P = 0.21) or weight (80 kg versus 77 kg; P = 0.47). However, there was a significant difference in mean height (1.75 m versus 1.70 m; P = 0.015).
Future liver remnant ratios
Future liver remnant data for the whole cohort are shown in Table2. There was no significant difference in FLR measurements between the two study centres, whether measured or estimated (data not shown). A mean difference of −1.7% (P = 0.013) was identified when comparing mRFLR and eRFLR. The total range of paired differences was wide and ranged from negative to positive values (−39.8% to 14.2%), indicating that eRFLR could be either greater or smaller than mRFLR (Fig. 2). The mean difference did not differ significantly between the two study centres (−1.2 ± 5.7% versus −2.5 ± 9.4%; P = 0.37). Spearman's correlation between the two techniques for FLR was 0.909 (P < 0.0001). Univariate linear regression gave an R2 value of 0.797.
Figure 2.
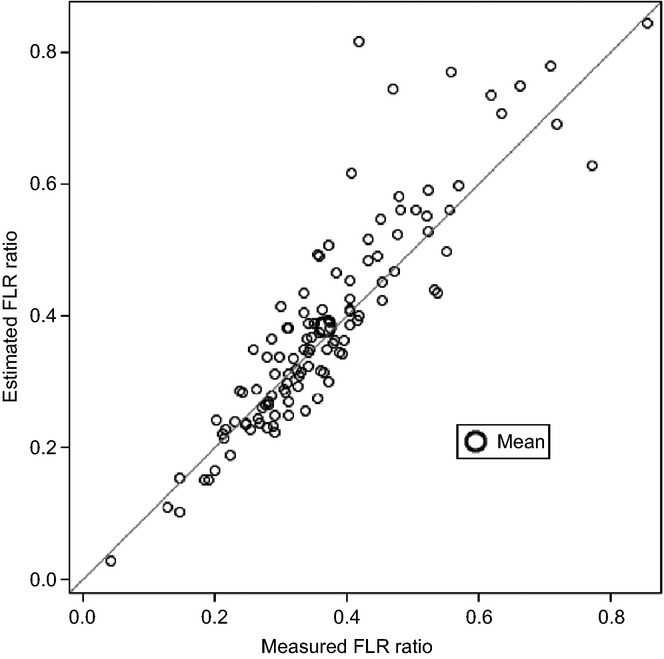
Agreement between the measured and estimated future liver remnant (FLR) ratio in 116 patients submitted to major liver resection for colorectal liver metastases
In total, 31.9% (95% CI 23.4–40.4%) of patients (n = 37) were found to have a clinically significant paired difference between mRFLR and eRFLR of ≥5% (Fig. 3). Among those, data for 51.4% (n = 19) and 24.3% (n = 9) were found to show differences that would be expected to alter preoperative decision making, assuming mRFLR decision thresholds of 40% and 30%, respectively. Among patients with differences of ≥5%, 70.3% (n = 26) had negative differences such that eRFLR overestimated mRFLR by ≥5%. In all cases of overestimation, the predicted eTLV using the formula was smaller than that measured on imaging. In the remainder (29.7%, n = 11), eRFLR underestimated mRFLR.
Figure 3.
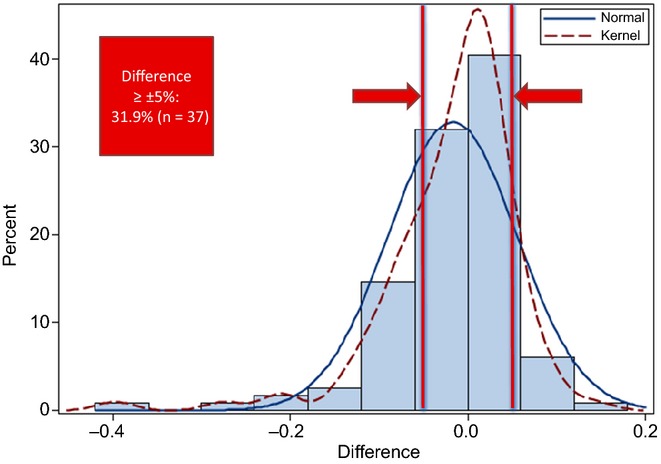
Distribution of paired differences in the future liver remnant (FLR) between measurement techniques
The effect of TV on these findings was evaluated by repeating the calculation, but with TV included within TLV (i.e. mRFLR = FLR volume/mTLV × 100). The mean difference between mRFLR and eRFLR was −3.5% (P < 0.0001). In this scenario, 39.7% of patients (n = 46) had a paired difference between mRFLR and eRFLR of ≥5%.
These calculations were repeated using five other published formulae to estimate TLV.10,13–16 The findings were consistent across formulae (data not shown), with all formulae yielding a difference between mRFLR and eRFLR of ≥5% in 32–60% of patients (Table3).
Table 3.
Absolute differences of ≥5% in paired comparisons of future liver remnant ratio based on different techniques (whole cohort, n = 116)
| Comparison | Absolute difference ≥5% a | ||
|---|---|---|---|
| n | % | 95% CI | |
| Measured/BSA Vauthey et al. (2002)10 | 37 | 31.9% | 23.4–40.4 |
| Measured/weight Vauthey et al. (2002)10 | 45 | 38.8% | 29.9–47.7 |
| Measured/BSA Heinemann et al. (1999)15 | 41 | 35.3% | 26.7–44.0 |
| Measured/BSA Deland et al. (1968)13 | 41 | 35.3% | 26.7–44.0 |
| Measured/BSA Urata et al. (1995)14 | 70 | 60.3% | 51.4–69.3 |
| Measured/age and weight Sinai et al. (2001)16 | 55 | 47.4% | 38.3–56.5 |
Binomial proportions for one-way tables, asymptotic (Ward) confidence limits.
95% CI, 95% confidence interval; BSA, body surface area.
Discussion
This work has examined the two techniques most commonly used to determine the FLR ratio. Using measured volumetry as the comparative standard, estimated volumetry has been shown to lead to a clinically significant over- or underestimation of the FLR ratio by ≥5% in 31.9% of patients undergoing major liver resection for colorectal liver metastases.
The current findings are significant because differences in preoperative estimations of the FLR ratio may lead to changes in patient management. In the event of overestimation of the FLR ratio, the surgeon may be misled into thinking that a given patient has sufficient FLR to allow for major liver resection, which may potentially put the patient at risk for liver insufficiency. By contrast, with underestimation, the surgeon may prefer to induce further hypertrophy with potentially unnecessary preoperative PVE, thus placing the patient at additional risk for the occurrence of complications and delaying surgery. Both over- and underestimations of the FLR ratio may lead to a modification of the surgical plan, including the utilization of techniques to induce contralateral liver hypertrophy such as PVE or ligation, the modification of the surgical resection plan, the utilization of parenchyma-sparing ablative techniques, as well as the cancellation of surgery in the case of an insufficient FLR ratio. In either direction, inaccurate estimation of the FLR ratio can have significant implications for patient care.
This work has considered measured volumetry to represent the reference standard with which to compare estimated volumetry because not only is it the most widely used method, but Vauthey et al.'s original description utilized measured volumetric data from four centres to generate a linear regression equation, from which TLV can be estimated based on BSA.10 In this context, the present results are not surprising as the original linear regression equation yielded an R2 value of 0.46, indicating that Vauthey et al.'s regression model10 can explain only 46% of the variability in mTLV. Thus, there remains much variability in predicting a given patient's TLV, which cannot simply be estimated based on BSA. Similar results were obtained in the current study, in which the R2 for TLV was only 0.35, such that the eTLV explains only 35% of the variability in mTLV. Moreover, it is important to note that the current work excluded any patients with chronic liver disease or any patient who may have had prior liver surgery. In those patients, pre-existing parenchymal remodelling is expected and it is likely that estimations of TLV based on BSA would be much less accurate. Indeed, a patient's eTLV based on BSA is expected to remain constant over time (except with significant weight variations), whereas it is fair to say that TLV is a dynamic value that may change based on the health of the liver and any prior interventions. Thus, a measurement of the TLV that reflects its current state rather than a theoretical constant value is likely to be superior.
Numerous factors can influence the accuracy of preoperative liver volumetry that relies upon measured tracings. These include the phase of contrast administration, cross-section slice thickness, the use of CT versus MRI, varying hardware computer platforms, varying image processing or radiological software, and inter-user variability, as well as the degree to which non-parenchymal structures (e.g. intrahepatic bile ducts or tumours) are erroneously included within the functional liver volume.17 In the present work, significant efforts were made to standardize the measurement techniques across the two study sites. The study group chose to remain pragmatic with respect to imaging phase selection, slice thickness and MRI use. The present authors argue that observed differences in measured and estimated volumetry are unlikely to relate to differences in tracing methodology because the general technique used was the same as that employed in the original derivation study.10 Tumour volume was excluded from measurements, which, in fact, appears to render the present estimate more conservative.
Ribero et al.18 also examined this question. They reviewed 242 patients without cirrhosis who underwent major hepatectomy for metastatic and primary liver tumours. They found a small difference between measured and estimated TLV of 92 ml (P < 0.001), and noted that this difference became more pronounced in patients with a BMI of >30 kg/m2.18 This group also reported that the proportions of patients in whom FLR was found to be inadequate on measured and estimated volumetry, respectively, differed significantly (19.3% versus 30.0%; P = 0.006).18 In the current paper, estimated volumetry was more frequently associated with an underestimation of the eTLV and a resulting overestimation of the eRFLR. By contrast, Ribero et al.18 reported the opposite pattern, whereby estimated volumetry was more frequently associated with overestimation of the eTLV and underestimation of the eRFLR. Although this difference would appear surprising, the present authors argue that it simply reflects inherent differences in the distribution of patients in each study. As Fig. 3 shows, paired differences between mTLV and eTLV and between mRFLR and eRFLR are normally distributed, which allows for both the over- and underestimation of the TLV and FLR ratio in any given individual patient.
Examination of the patterns of difference between mTLV and eTLV suggests that there exists a subgroup of patients in whom estimated volumetry performs poorly. This was particularly evident in a subgroup of patients with livers that were significantly larger than might have been expected based on their body habitus. Although these patients were generally taller than the remainder of the cohort, this discrepancy remains incompletely explained at present and will require further investigation.
According to a review by Johnson et al.,19 at least 12 groups have reported different formulae for estimating TLV based on various parameters such as age, gender, weight, height and BSA. All formulae were derived from different patient populations, some of which focused on specific ethnicities such as ‘Japanese’, ‘North American’ or ‘North European’. This abundance of formulae aimed at estimating the same parameter would suggest that no single formula relying on traditional morphologic values is likely to successfully account for the full range of variability observed in TLV. Lim et al.17 also argued this point, demonstrating that, in an average 60-kg adult patient, estimations of TLV derived from the various formulae available would range from 1024 ml to 1302 ml. This could result in an FLR ratio of 23–29%, assuming a measured remnant of 300 ml. These results are comparable with those of the present study. Clearly, for liver volumetry to be useful to surgeons, it must be more consistently accurate and reliable. This notion also underscores the need for quantitative liver function tests in the assessment of the FLR in patients requiring major liver resection.20 The findings of this study would suggest that TLV is best evaluated by direct radiologic measurement rather than by indirect estimation if a more accurate assessment of the FLR ratio is to be generated.
The limitations of the current study include its retrospective nature and moderate sample size. In addition, this work did not examine the functional consequences of the stated differences between the volumetry techniques in terms of postoperative outcomes. Despite the sample size, the present authors argue that the current work is valid as its patient population was homogeneous and typical of patients who undergo major hepatectomy for colorectal cancer metastases. Further, the patient population was not contaminated by other types of pathology, such as biliary tract cancers, as this might have influenced the findings and affected the volumetric analysis in the context of biliary dilation. Finally, this work was intended to report upon the accuracy of the two most commonly used methods of volumetry and specifically did not address outcomes such as hepatic insufficiency because volumetric differences were felt to be an important finding in and of themselves.
Conclusions
Measured and estimated volumetry yielded differences in the FLR ratio of ≥5% in almost one-third of patients, potentially affecting clinical decision making. Estimated volumetry should thus be used cautiously and cannot be recommended for general use.
Conflicts of interest
RL has received honoraria from Roche (Canada) and Sanofi (Canada). GM is the recipient of a clinical and research bursary from Covidien Canada and has received honoraria from Sanofi (Canada).
References
- Clavien PA, Oberkofler CE, Raptis DA, Lehman K, Rickenbacher A, El-Badry AM. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology. 2010;52:715–729. doi: 10.1002/hep.23713. [DOI] [PubMed] [Google Scholar]
- Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188–1200. doi: 10.1002/bjs.7630. [DOI] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindl MJ, Redhead DN, Fearon KCH, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definitions, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–864. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. ‘State of the art’ in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg. 2009;33:797–803. doi: 10.1007/s00268-008-9878-0. [DOI] [PubMed] [Google Scholar]
- Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–681. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- Couinaud C. Le Foie: Études Anatomiques et Chirurgicales. Paris: Masson & Cie; 1957. [Google Scholar]
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- DeLand FH, North WA. Relationship between liver size and body size. Radiology. 1968;91:1195–1198. doi: 10.1148/91.6.1195. [DOI] [PubMed] [Google Scholar]
- Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- Heinemann A, Wischhusen F, Puschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366–368. doi: 10.1002/lt.500050516. [DOI] [PubMed] [Google Scholar]
- Emre S, Soejima Y, Altaca G, Facciuto M, Fishbein TM, Sheiner PA, et al. Safety and risk of using pediatric donor livers in adult liver transplantation. Liver Transpl. 2001;7:41–47. doi: 10.1053/jlts.2001.20940. [DOI] [PubMed] [Google Scholar]
- Lim MC, Tan CH, Cai J, Zheng J, Kow AW. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 2014;69:887–895. doi: 10.1016/j.crad.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Ribero D, Amisano M, Bertuzzo F, Langella S, Tesoriere RL, Ferrero A, et al. Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg. 2013;258:801–807. doi: 10.1097/SLA.0000000000000213. [DOI] [PubMed] [Google Scholar]
- Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481–1493. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- Cieslak KP, Runge JH, Heger M, Stoker J, Bennink RJ, van Gulik TM. New perspectives in the assessment of the future liver remnant. Dig Surg. 2014;31:255–268. doi: 10.1159/000364836. [DOI] [PubMed] [Google Scholar]


