Abstract
Background
Mortality after major hepatectomy remains high and is frequently related to post-hepatectomy liver failure (PHLF). Other than pre-existing liver disease and a small future liver remnant, few patient factors or early postoperative indicators identify patients at elevated risk for PHLF and mortality.
Methods
Data on demographics, comorbidities, operative procedures and postoperative laboratory trends were reviewed for patients submitted to major hepatectomy (at least three Couinaud segments) for malignancy during 1998–2013. These factors were compared among patients who died within 90 days, survivors who met the 50–50 criteria and all remaining survivors.
Results
A total of 1528 patients underwent major hepatectomy during the study period. Of these, 947 had metastatic colorectal cancer and underwent resection of a median of four segments. Overall, 49 patients (3.2%) died within 90 days of surgery and 48 patients (3.1%) met the 50–50 criteria for PHLF; 30 of these patients survived 90 days. Operative blood loss was higher in patients who died within 90 days compared with survivors (1.0 l versus 0.5 l; P < 0.001). Despite equivalent perioperative resuscitation and urine output, non-survivors had higher creatinine and phosphate levels than survivors on postoperative day (PoD) 1 (1.1 mg/dl versus 0.9 mg/dl and 4.6 mg/dl versus 3.7 mg/dl, respectively; P < 0.001).
Conclusions
Early trends in creatinine and phosphate (between the day of surgery and PoD 1) identify patients at risk for PHLF and mortality.
Introduction
Incidences of mortality following hepatectomy have fallen significantly over the last two decades. A recent study cited major morbidity and mortality rates following elective hepatectomy of as low as 20.0% and 1.6%, respectively.1 Major hepatectomy, involving the removal of three or more liver segments, carries a higher risk for post-hepatectomy liver failure (PHLF) than lesser resections. Despite careful patient selection and preoperative preparation, PHLF remains a common contributor to significant morbidity and mortality following hepatic resection.
There have been numerous attempts to identify patients at high risk for the development of PHLF and subsequent mortality in details that extend beyond recognition of the fact that more extensive resections in diseased livers carry greater risk. One series evaluating preoperative factors identified the aspartate aminotransferase to platelet count ratio as valuable in identifying patients at high risk for PHLF following surgery for hepatocellular carcinoma.2 Other investigators noted that a dampened rise in serum C-reactive protein (CRP) levels on postoperative day (PoD) 1 were predictive of PHLF.3 More recently, the lack of an early drop in serum phosphate levels was found to correlate with PHLF, morbidity and mortality.4
Efforts to identify factors that predict PHLF have been hampered by the lack of a standardized definition of PHLF. One of the more widely applied definitions of PHLF refers to the ‘50–50 criteria’, which reflect impairment in the liver's synthetic and excretory functions [as reflected by elevations in the international normalized ratio (INR) and serum bilirubin, respectively]. The original description documented an association between PHLF and a combination of a prothrombin time of <50% of normal (INR > 1.7) and a total serum bilirubin of >50 mmol/l (2.9 mg/dl) on PoD 5.5 The authors found that mortality rates after hepatectomy in patients who met both criteria exceeded 50% and subsequently confirmed these findings in a prospective study.6 When applied on PoD 5, this algorithm may identify patients at elevated risk beyond the optimal time to intervene. The early identification of patients at high risk for PHLF might trigger a careful assessment of volume status, or an aggressive search for vascular compromise or infectious complications, with the goal of preventing the worsening of hepatic insufficiency caused by secondary complications.
In 2011, taking into account the existing literature, the International Study Group of Liver Surgery (ISGLS) defined PHLF as indicated by an increased INR in the setting of hyperbilirubinaemia on or after PoD 5.7 Although this more encompassing definition identifies patients with lesser degrees of hepatic dysfunction who may be at risk for the development of more significant liver failure, the identification of these individuals still occurs late in the postoperative course (day 5). In the current study, postoperative trends in laboratory values were characterized in a large population of patients undergoing major hepatectomy. Routinely available perioperative factors were analysed in order to identify those that might facilitate the earlier identification of patients at risk for PHLF and mortality.
Materials and methods
A prospectively maintained database was established in 1992 for all patients submitted to hepatic resection at the Memorial Sloan–Kettering Cancer Center. Since 1998, this database has included perioperative laboratory values imported from the electronic medical record for all patients. When approval for the study had been obtained from the institutional review board, the database was queried for all patients submitted to major hepatectomy for malignancy since 1998. Major hepatectomy referred to the resection of a minimum of three segments, which is consistent with the criteria used in multiple previous studies.4,8,9
Preoperative evaluation includes standard laboratory tests, such as liver function tests, coagulation parameters, a complete blood count and serum chemistry. Preoperative volumetric analysis to calculate an estimated residual liver volume (RLV) is performed selectively, most commonly in preparation for extended hepatectomy. Hepatic resection is performed with minimal fluid resuscitation initially to allow for a low central venous pressure (CVP) during resection. At the surgeon's discretion, intermittent hepatic inflow occlusion (the Pringle manoeuvre) is used.
Postoperatively, all patients undergoing major hepatectomy are observed overnight in the post-anaesthesia care unit (PACU), and laboratory values are serially checked following surgery. Resuscitation fluids used include normal saline, lactated Ringer's solution and Normosol® (Hospira, Inc., Lake Forest, IL, USA), none of which contain phosphate. Typically, patients are transferred to the surgical ward on PoD 1. Data on complications and mortality are entered into the database by surgical housestaff and are reviewed for accuracy at twice-weekly morbidity and mortality conferences. Patient demographics, comorbidities, perioperative data, postoperative complications, patient deaths and pathology results are entered prospectively into the database by trained personnel. Patients are recorded as having cardiac disease if they carry a diagnosis of coronary artery disease, valvular disease or arrhythmia. Diagnoses of hepatitis and hepatic steatosis are based on pathological assessments. Operative details reflect the primary procedure performed, but additional procedures include wedge resections, ablations (microwave and radiofrequency), colonic resections, and the placement of hepatic artery infusion pumps for intrahepatic treatment of metastatic colorectal cancer. Renal failure is defined as indicated by either a need for renal replacement therapy or a three-fold increase in creatinine from baseline.
The patient population was broken into three groups to permit the identification of factors associated with significant PHLF and mortality. The first group of patients included those who died within 90 days of surgery and inevitably included some patients with significant liver failure who met the 50–50 criteria. The second group included patients with evidence of significant liver failure (meeting the 50–50 criteria for PHLF), but who survived. The third group of patients survived without evidence of significant PHLF (they did not meet the 50–50 criteria). Demographic information, comorbidities, operative details and outcomes were compared in univariate analyses using appropriate statistical tests. Trends in perioperative laboratory values were observed for serum albumin, creatinine, phosphate, alkaline phosphate, INR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, haemoglobin, white blood cell count, and platelet count. Univariate analyses comparing laboratory values among the different groups (both pre- and postoperatively) were performed. Multivariate analysis was performed using factors that achieved a P-value of <0.10 on univariate analysis and which were clinically relevant (gender was not deemed useful to include in the multivariate analysis). After creatinine and phosphate levels were found to be significantly different, changes in these laboratory parameters were dichotomized to create clinically relevant criteria and included in the multivariate analysis.
In order to place changes in laboratory parameters in context, a chart review was conducted to compare perioperative fluid balances between patients who died within 90 days and those with PHLF who survived. Data on crystalloid, colloid, blood product and urine output for the 24 h following the start of surgery were collected and compared among groups.
In patients for whom the requisite imaging data were available, Scout Liver (Pathfinder Therapeutics, Inc., Nashville, TN, USA) was employed to determine the predicted RLV. Comparisons were made between patients who died within 90 days of surgery and patients who met the 50–50 criteria (demonstrating significant PHLF) yet survived.
Results
A total of 1528 patients who underwent major hepatectomy for malignancy during the study period, and for whom laboratory values were available, were identified. Demographic data broken down by outcome are shown in Table1.
Table 1.
Demographic and clinical data in 1528 patients undergoing major hepatectomy
| All patients | 90-day mortality group | Survivors with PHLF | Survivors without PHLF | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors vs. 90-day mortality group | Survivors vs. survivors with PHLF | |||||||||
| Patients, n (%) | 1528 | (100%) | 49 | (3.2%) | 30 | (2.0%) | 1449 | (94.8%) | N/A | N/A |
| Age, years, median (IQR) | 60.4 | (51.0–70.2) | 70.2 | (56.5–76.2) | 68.3 | (59.9–74.1) | 60.0 | (50.9–69.6) | <0.001 | |
| Male gender, n (%) | 818 | (55.9%) | 32 | (65.3%) | 20 | (66.7%) | 766 | (52.9%) | 0.087 | 0.135 |
| Diabetes mellitus, n (%) | 70 | (4.6%) | 4 | (8.2%) | 1 | (3.3%) | 65 | (4.5%) | 0.223 | 0.767 |
| BMI, kg/m2, median (IQR) | 26.0 | (23.0–30.0) | 26.0 | (23.0–29.8) | 26 | (24.3–28.0) | 27.0 | (23.0–30.0) | 0.759 | |
| Steatosis, n (%) | 428 | (28.0%) | 11 | (22.4%) | 5 | (16.7%) | 412 | (28.4%) | 0.372 | 0.161 |
| Cardiac disease, n (%) | 94 | (6.2%) | 2 | (4.1%) | 3 | (10.0%) | 89 | (6.1%) | 0.558 | 0.381 |
| Hypertension, n (%) | 178 | (11.6%) | 5 | (10.2%) | 1 | (3.3%) | 172 | (11.9%) | 0.732 | 0.152 |
| Active smoker, n (%) | 529 | (37.9%) | 21 | (42.9%) | 11 | (36.7%) | 497 | (34.3%) | 0.205 | 0.770 |
| COPD, n (%) | 53 | (3.5%) | 1 | (2.0%) | 0 | 52 | (3.6%) | 0.568 | 0.292 | |
| Hepatitis, n (%) | 61 | (3.9%) | 7 | (14.3%) | 0 | 54 | (3.7%) | <0.001 | 0.283 | |
| Neoadjuvant chemotherapy, n (%) | 550 | (36.0%) | 12 | (24.5%) | 7 | (23.3%) | 531 | (36.6%) | 0.087 | 0.139 |
| Cholestasis (preop bilirubin >1.5), n (%) | 86 | (5.6%) | 8 | (16.3%) | 9 | (30.0%) | 69 | (4.8%) | <0.001 | <0.001 |
P-values are based on pairwise chi-squared analyses for categorical variables and Kruskal–Wallis tests for continuous variables.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; N/A, not applicable; PHLF, post-hepatectomy liver failure.
Operative indications and outcomes for surgery are listed in Table2. Patients submitted to surgery for primary malignancies of the liver were over-represented in the 90-day mortality group in comparison with the overall population (55.1% versus 29.1%). Specifically, mortality was nearly three-fold higher among patients submitted to surgery for primary hepatic malignancies compared with patients submitted to surgery for metastasis (6.0% versus 2.1%; P < 0.001).
Table 2.
Indications for surgery in 1528 patients undergoing major hepatectomy
| All patients (n = 1528) | 90-day mortality group (n = 49) | Survivors with PHLF (n = 30) | Survivors without PHLF (n = 1449) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors vs. 90-day mortality group | Survivors vs. survivors with PHLF | |||||||||
| Malignancy, n (%) | 1528 | (100%) | 49 | 30 | 1449 | |||||
| Primary, n (%) | 442 | (28.9%) | 27 | (55.1%) | 13 | (43.3%) | 402 | (27.7%) | <0.001 | 0.0637 |
| HCC, n (%) | 154 | (10.1%) | 13 | (26.5%) | 4 | (13.3%) | 137 | (9.5%) | <0.001 | 0.495 |
| Cholangio, n (%) | 206 | (13.5%) | 12 | (24.5%) | 9 | (30.0%) | 185 | (12.8%) | 0.016 | 0.005 |
| Hilar, n (%) | 104 | (6.8%) | 9 | (18.4%) | 6 | (20.0%) | 89 | (6.1%) | <0.001 | 0.001 |
| Intrahepatic, n (%) | 102 | (6.7%) | 3 | (6.1%) | 3 | (10.0%) | 96 | (6.6%) | 0.897 | 0.458 |
| Other, n (%) | 82 | (5.4%) | 2 | (4.1%) | 0 | 80 | (5.5%) | 0.663 | 0.186 | |
| Metastatic, n (%) | 1070 | (70.0%) | 22 | (44.9%) | 17 | (56.7%) | 1031 | (71.2%) | <0.001 | 0.085 |
| CRC, n (%) | 947 | (62.0%) | 18 | (36.7%) | 15 | (50.0%) | 914 | (63.1%) | <0.001 | 0.154 |
| Other, n (%) | 123 | (8.0%) | 4 | (8.2%) | 2 | (6.7%) | 117 | (8.1%) | 0.982 | 0.683 |
| Extrahepatic tumours, n (%) | 16 | (1.0%) | 0 | 0 | 16 | (1.1%) | 0.461 | 0.564 | ||
Pairwise comparisons between survivors and both the 90-day mortality group and patients who survived with PHLF were performed using chi-squared analysis.
CRC, C-reactive protein; HCC, hepatocellular carcinoma; PHLF, post-hepatectomy liver failure.
Outcomes by operative variables are listed in Table3. The mortality rate in patients undergoing resection of fewer than four segments was 1.4%, whereas that in patients undergoing resection of at least four segments was 3.7% (P = 0.034). A total of 49 patients (3.2%) died within 90 days after surgery. Of these, two patients who died on the day of surgery (DoS) and two who died shortly afterwards (<PoD 5) were diagnosed with PHLF according to the death summary. In an additional two patients who died without overt evidence of liver failure, laboratory values beyond day 4 to determine whether they met criteria for PHLF were not available. Of the remaining 43 patients, 39 (90.7%) met the ISGLS criteria for PHLF. Of the entire cohort, 581 (38.0%) had a postoperative complication. The most common complications were infection-related (Table S1, online). Of the 49 patients who died within 90 days of surgery, 12 (24.5%) developed an intra-abdominal infection, as did 76 of the 1479 (5.1%) patients who survived (P < 0.001). Patients in the 90-day mortality group were also much more likely to develop renal failure [six of 49 (12.2%) patients versus nine of 1457 (0.6%) patients; P < 0.001] than those in the group that survived.
Table 3.
Operative data in 1528 patients undergoing major hepatectomy
| All patients (n = 1528) | 90-day mortality group (n = 49) | Survivors with PHLF (n = 30) | Survivors without PHLF (n = 1449) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors vs. 90-day mortality group | Survivors vs. PHLF survivors | |||||||||
| Patients, n | 1528 | 49 | 30 | 1449 | ||||||
| Preop PVE, n (%) | 188 | (12.3%) | 6 | (12.2%) | 4 | (13.3%) | 178 | (12.3%) | 0.995 | 0.853 |
| Estimated RLV, median (IQR) | N/A | 32.0% | (22.8–41.5) | 31.1% | (23.8–33.6) | N/A | 0.761a | |||
| Repeat resection, n (%) | 119 | (7.7%) | 2 | (4.1%) | 3 | (10.0%) | 114 | (7.9%) | 0.334 | 0.661 |
| Segments resected, median (IQR) | 4.0 | (4.0–5.0) | 4.3 | (4.0–5.0) | 4.1 | (4.0–5.0) | 4.0 | (4.0–5.0) | 0.043 | |
| Type of surgery, n (%) | ||||||||||
| Right lobectomy | 576 | (35.8%) | 18 | (36.7%) | 16 | (53.3%) | 542 | (37.4%) | 0.901 | 0.078 |
| Right trisectionectomy | 441 | (28.7%) | 19 | (38.8%) | 10 | (33.3%) | 412 | (28.4%) | 0.110 | 0.543 |
| Left lobectomy | 273 | (17.9%) | 7 | (14.3%) | 2 | (6.7%) | 264 | (18.2%) | 0.485 | 0.104 |
| Left trisectionectomy | 99 | (6.5%) | 2 | (4.1%) | 2 | (6.7%) | 95 | (6.6%) | 0.474 | 0.998 |
| Non-standard resection | 73 | (4.8%) | 1 | (2.0%) | 0 | 72 | (5.0%) | |||
| Biliary reconstruction, n (%) | 78 | (5.1%) | 8 | (16.3%) | 4 | (13.3%) | 66 | (4.6%) | <0.001 | 0.024 |
| Ablation, n (%) | 84 | (5.5%) | 5 | (10.2%) | 4 | (13.3%) | 75 | (5.2%) | 0.121 | 0.048 |
| HAI pump, n (%) | 197 | (12.9%) | 2 | (4.1%) | 3 | (10.0%) | 192 | (13.3%) | 0.062 | 0.610 |
| Other intra-abdominal procedure, n (%) | 390 | (25.5%) | 13 | (26.5%) | 6 | (20.0%) | 371 | (25.6%) | 0.866 | 0.496 |
| Inflow occlusion, n (%) | 1361 | (89.1%) | 47 | (95.9%) | 25 | (83.3%) | 1289 | (89.0%) | 0.105 | 0.385 |
| Duration, min, median (IQR) | 34 | (20–49) | 35 | (21–45) | 35 | (20–60) | 33 | (20–48) | 0.603 | |
| EBL, l, median (IQR) | 0.5 | (0.3–0.9) | 1.0 | (0.6–1.4) | 0.6 | (0.4–1.1) | 0.5 | (0.3–0.9) | <0.001 | |
| OR time, min, median (IQR) | 257 | (202–325) | 274 | (204–384) | 291 | (200–372) | 256 | (202–323) | 0.088 | |
Comparison between 90-day mortality group and survivors with PHLF.
P-values are based on pairwise chi-squared analysis for categorical variables and Kruskal–Wallis tests for continuous variables.
EBL, estimated blood loss; HAI, hepatic artery infusion; IQR, interquartile range; N/A, not available; OR, operating room; PHLF, post-hepatectomy liver failure; PVE, portal vein embolization; RLV, remnant liver volume.
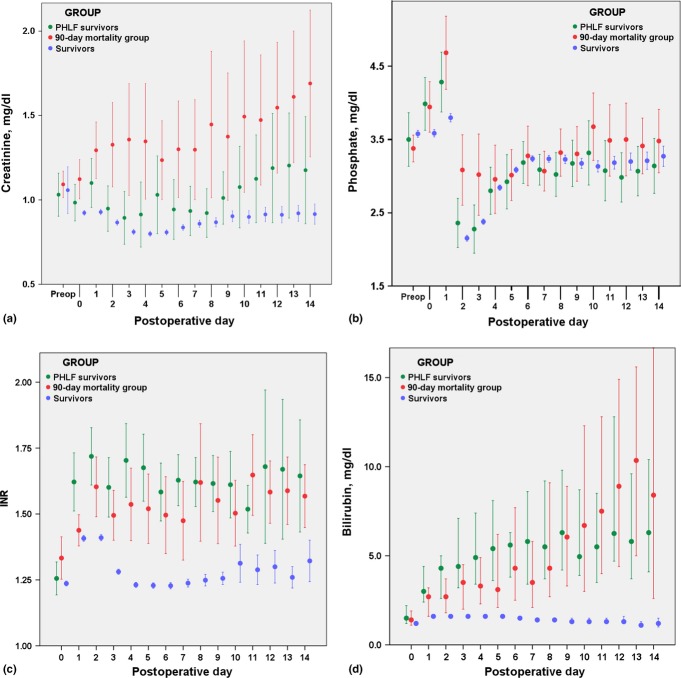
Of the 12 laboratory parameters measured perioperatively, 10 were statistically equivalent among the groups preoperatively. Preoperative serum total bilirubin levels were significantly higher among patients who died within the first 90 days than in survivors [0.7 mg/dl, interquartile range (IQR) 0.5–1 mg/dl versus 0.6 mg/dl, IQR 0.4–0.8 mg/dl; P = 0.018]. Preoperative albumin levels were significantly lower among patients who died within 90 days in comparison with survivors [3.9 mg/dl (IQR: 3.7–4.2 mg/dl) versus 4.2 mg/dl (IQR: 3.9–4.4 mg/dl); P < 0.001]. The ISGLS definition of postoperative hepatic insufficiency, specifically an abnormal bilirubin and INR on or after PoD 5, applied to 460 of the 1528 patients (30.1%). Of these patients with abnormal synthetic and excretory function, 39 of 460 (8.5%) died within the first 90 days postoperatively. Calculation of the ISGLS grade of PHLF was not possible with the database fields available. An examination of postoperative laboratory trends in all patients showed postoperative INR and serum bilirubin values to be significantly higher in the group who died than in patients who survived (Fig. S1 online), coinciding with a higher incidence of PHLF in this population. The changes in both serum phosphate and creatinine levels over time by outcome group are shown in Fig.1. Whereas estimated blood loss was higher among patients who died, as described above, fluid resuscitation [median 7.7 l (IQR: 6.1–8.4 l) versus 6.5 l (IQR: 5.6–8.3 l); P = 0.160] and blood product use (eight of 47 patients versus nine of 30 patients; P = 0.457) were similar. However, patients who died had urine output equivalent to that of patients in the group that survived [median 1.5 l (IQR: 1.1–1.9 l) versus 1.5 l (IQR: 1.1–2.0 l); P = 0.886] during the 24 h from the time of surgical incision, which suggests that resuscitation was appropriate.
Figure 1.
Perioperative laboratory trends, encompassing preoperative (within 2 weeks prior to surgery) data, day of surgery [postoperative day (PoD) 0] data, and data to 2 weeks following surgery, for (a) serum creatinine, (b) serum phosphate, (c) international normalized ratio (INR) and (d) total bilirubin. Values for each day were compared between the groups using the Mann–Whitney U-test. *P ≤ 0.002 for each day the significance star refers to Figure 1(b) phosphate pod 1, 2, and 3. (b) *P < 0.001 for PoD 1 and PoD 2, P = 0.007 for the day of surgery, P = 0.034 for PoD 3, and non-significant for all other days
In efforts to create a clinically useful cut-off point to stratify patients at higher risk for PHLF and mortality, an increase in creatinine between the DoS and PoD 1 in combination with a failure of the serum phosphate level to drop by 20% from the DoS to PoD 1 were found to optimize sensitivity and specificity. These criteria selected 24 of the 46 (52.2%) patients who died within 90 days of surgery, 17 of the 30 (56.7%) patients who met the 50–50 criteria and survived, and 342 of the 1397 (24.5%) patients who survived without significant evidence of PHLF. (In the present study, data for all four laboratory values with which to evaluate the criteria were available for 90.0% of patients.) A comparison of patients who died within 90 days and survivors without PHLF indicated that clinically relevant factors to be included in a multivariate analysis were patient age, diagnosis (primary versus metastatic malignancy), estimated blood loss, preoperative albumin levels, and the combination of changes in creatinine and phosphate previously noted. As only 30 patients met the 50–50 criteria and survived, multivariate analyses comparing these patients with survivors without PHLF were limited to the following variables: diagnosis (primary versus metastatic); preoperative cholestasis (total bilirubin >1.5 mg/dl), and creatinine and phosphate changes. The results of the multivariate analysis are shown in Table4.
Table 4.
Multivariate analysis of risk factors associated with mortality and post-hepatectomy liver failure (survivors without liver failure represent the reference group)
| Factor | Odds ratio (95% CI) | P-value |
|---|---|---|
| Risk factors associated with mortality | ||
| EBL (OR associated with each 100-ml increase) | 1.08 (1.04–1.11) | <0.001 |
| Primary versus metastatic disease | 2.66 (1.43–5.00) | 0.002 |
| Age (OR associated with 1-year increase) | 1.04 (1.01–1.07) | 0.005 |
| Preop albumin (OR associated with 0.1-mg/dl decrease) | 1.11 (1.05–1.18) | 0.001 |
| Creatinine increase (PoD 1 > DoS and phosphate fails to decrease by 20% from DoS to PoD 1 | 2.53 (1.36–4.71) | 0.003 |
| Risk factors associated with PHLF | ||
| Primary versus metastatic disease | 1.19 (0.49–2.72) | 0.69 |
| Preoperative cholestasis (total bilirubin >1.5) | 7.08 (2.68–17.8) | <0.001 |
| Creatinine increase (PoD 1 > DoS and phosphate fails to decrease by 20% from DoS to PoD 1 | 3.89 (1.85–8.37) | <0.001 |
95% CI, 95% confidence interval; DoS, day of surgery; EBL, estimated blood loss; OR, odds ratio; PHLF, post-hepatectomy liver failure; PoD, postoperative day.
Discussion
In this series of 1528 major hepatectomies, outcomes were comparable with those in other modern series, with a 90-day mortality rate of 3.2% and a morbidity rate of 37.8%. Although no novel preoperative factors associated with PHLF or mortality following major hepatectomy were identified, the data confirmed older age, preoperative hyperbilirubinaemia (total bilirubin >1.5 mg/dl), lower preoperative albumin levels, and primary liver malignancies to be associated with higher incidences of mortality and PHLF (significant for only the first three risk factors).
Trends in biochemical tests assessing liver function following major hepatectomy have not been well described. One of the larger reviews included 835 patients, of whom only 384 (46.0%) underwent major hepatectomy.10 Although INR values did return to normal in most of the present patients (1068/1528, 69.9%) by PoD 5, total serum bilirubin was above the upper limit of normal in 1204 of 1528 patients (78.8%), which is similar to trends documented by Roberts et al.11 Consequently, 460 of 1528 patients (30.1%) met the ISGLS definition for PHLF. However, only 8.3% of these patients died within 90 days of surgery, which suggests that the liver dysfunction was predominantly mild and reversible. By contrast, 3.1% of patients in the study, of whom 37.5% died, met the 50–50 criteria. Thus, both the ISGLS and 50–50 criteria identify patients with PHLF on PoD 5, which may make effective intervention challenging.
This analysis demonstrated an early increase in creatinine and a delay in the typical drop in phosphate early in the postoperative period that were more pronounced in patients who died within 90 days of surgery than in those who survived. Although the association between an increase in creatinine and postoperative mortality is novel, the adverse prognostic significance of a delayed nadir of phosphate levels has been recently described.4
Hypophosphataemia following hepatic resection is a common phenomenon12–14 and was originally thought to be solely a consequence of consumption during liver hypertrophy. Although the active incorporation of phosphate into the liver has been documented to peak in the first 72 h following hepatectomy,15 this does not appear to explain hypophosphataemia. A study by Salem and Tray demonstrated a significant increase in the urinary excretion of phosphate following hepatectomy, leading its authors to postulate that an unidentified phosphaturic protein leads to renal wasting of phosphate postoperatively.16 This observation has been validated, with increases in urinary phosphate occurring in the first few hours following surgery.17 Recently, a phosphaturic protein responsible for urinary wasting of phosphate following hepatectomy was identified in an animal model.18 Regardless of the cause of the hypophosphataemia, the data demonstrate that the lack of a drop in serum phosphate levels in the early postoperative period is associated with a higher risk for mortality following hepatectomy, as well as non-fatal postoperative hepatic dysfunction (as evidenced by the failure of serum phosphate levels to drop soon after surgery among patients who met the 50–50 criteria but survived).
The increase in creatinine levels among patients who developed PHLF and who died within 90 days after surgery (many of whom developed multi-system organ failure) stood out as another prominent laboratory trend and has not been previously described. Within the limits of a retrospective review, this did not appear to be a consequence of inadequate resuscitation. Whether the rise in creatinine represents a similar but less severe reflection of the complex interplay between hepatic and renal function seen in hepatorenal syndrome is unknown.
A simultaneous increase in creatinine and the failure of phosphate to drop 20% between the DoS and PoD 1 identified more than 50% of patients who went on to develop significant PHLF or die within 90 days, and was found in fewer than 25% of patients who survived. These criteria are highly associated with both PHLF and mortality on multivariate analysis. As a very early indicator of patients at risk for PHLF and death following hepatectomy, this may permit the development of interventions to avoid further physiologic insults that might worsen hepatic insufficiency. The determination of exactly which interventions might avoid progression to PHLF is unclear; however, the associations between renal function, infection and PHLF suggest some basic interventions. For example, close attention to volume status might correct mild renal insufficiency that develops perioperatively. As an arterial line is placed for surgery in most patients undergoing major hepatectomy, those patients meeting these criteria might be better served in an environment in which continued goal-directed resuscitation based on arterial line parameters can take place. Furthermore, although a 0.1-mg/dl increase in creatinine can easily be dismissed as insignificant, this trend may be more significant in these patients, warranting strict avoidance of any nephrotoxic medications. As infectious complications (particularly intra-abdominal abscesses) were far more common among patients who died within 90 days, an aggressive search for and treatment of intra-abdominal fluid collections might avoid the development of more significant infectious sequelae. Finally, these patients may be referred for early postoperative ultrasonography to rule out any abnormalities in hepatic arterial and venous flow or biliary obstruction that might contribute to low-grade hepatic dysfunction. Prospective studies will be necessary to determine if such targeted interventions will successfully reduce the incidence of PHLF in patients identified as being at higher risk.
Acknowledgments
The authors greatly appreciate the assistance provided by Anne Eaton, MS (Dept of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY) in the statistical analyses performed for this project.
Funding sources
This study was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Postoperative complications.
Figure S1. Perioperative laboratory trends from the day of surgery (postoperative day 0), through the second week following surgery for (a) international normalized ratio (INR) and (b) total bilirubin. The differences between survivors and patients in each of the other two groups were statistically significant for each day (Mann–Whitney U-test, P ≤ 0.001), except for INR on the day of surgery (P = 0.030).
References
- Kingham TP, Correa-Gallego C, D'Angelica MI, Gonen M, DeMatteo RP, Fong Y, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg. 2015;220:471–479. doi: 10.1016/j.jamcollsurg.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Uenishi T, Takemura S, Oba K, Ogawa M, Kodai S, et al. A simple, noninvasively determined index predicting hepatic failure following liver resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;16:42–48. doi: 10.1007/s00534-008-0003-4. [DOI] [PubMed] [Google Scholar]
- Rahman SH, Evans J, Toogood GJ, Lodge PA, Prasad KR. Prognostic utility of postoperative C-reactive protein for posthepatectomy liver failure. Arch Surg. 2008;143:247–253. doi: 10.1001/archsurg.2007.75. [DOI] [PubMed] [Google Scholar]
- Squires MH, Dann GC, Lad NL, Fisher SB, Martin BM, Kooby DA, et al. Hypophosphataemia after major hepatectomy and the risk of post-operative hepatic insufficiency and mortality: an analysis of 719 patients. HPB. 2014;16:884–891. doi: 10.1111/hpb.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50–-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the ‘fifty–fifty’ criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124–128. doi: 10.1097/SLA.0b013e31819279cd. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836–844. doi: 10.1002/bjs.7459. [DOI] [PubMed] [Google Scholar]
- Roberts KJ, Bharathy KGS, Lodge JPA. Kinetics of liver function tests after a hepatectomy for colorectal liver metastases predict post-operative liver failure as defined by the International Study Group for Liver Surgery. HPB. 2013;15:345–351. doi: 10.1111/j.1477-2574.2012.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposelli JJ, Pomfret EA, Burns DL, Lally A, Sorcini A, Gordon FD, et al. Life-threatening hypophosphatemia after right hepatic lobectomy for live donor adult liver transplantation. Liver Transpl. 2001;7:637–642. doi: 10.1053/jlts.2001.26287. [DOI] [PubMed] [Google Scholar]
- Buell JF, Berger AC, Plotkin JS, Kuo PC, Johnson LB. The clinical implications of hypophosphatemia following major hepatic resection or cryosurgery. Arch Surg. 1998;133:757–761. doi: 10.1001/archsurg.133.7.757. [DOI] [PubMed] [Google Scholar]
- Wrighton LJ, O'Bosky KR, Namm JP, Senthil M. Postoperative management after hepatic resection. J Gastrointest Oncol. 2012;3:41–47. doi: 10.3978/j.issn.2078-6891.2012.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooby DA, Zakian KL, Challa SN, Matei C, Petrowsky H, Yoo HH, et al. Use of phosphorous-31 nuclear magnetic resonance spectroscopy to determine safe timing of chemotherapy after hepatic resection. Cancer Res. 2000;60:3800–3806. [PubMed] [Google Scholar]
- Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafidi O, Lepage R, Lapointe RW, D'Amour P. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2007;245:1000–1002. doi: 10.1097/SLA.0b013e31805d0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Tatsumi S, Miyagawa A, Shiozaki Y, Sasaki S, Kaneko I, et al. Hepatectomy-related hypophosphatemia: a novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephr. 2014;25:761–772. doi: 10.1681/ASN.2013060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Postoperative complications.
Figure S1. Perioperative laboratory trends from the day of surgery (postoperative day 0), through the second week following surgery for (a) international normalized ratio (INR) and (b) total bilirubin. The differences between survivors and patients in each of the other two groups were statistically significant for each day (Mann–Whitney U-test, P ≤ 0.001), except for INR on the day of surgery (P = 0.030).