Abstract
Background
Payers and regulatory bodies are increasingly placing emphasis on cost containment, quality/outcome measurement and transparent reporting. Significant cost variation occurs in many operative procedures without a clear relationship with outcomes. Clear cost-benefit associations will be necessary to justify expenditures in the era of bundled payment structures.
Methods
All laparoscopic cholecystectomies (LCCKs) performed within a single health system over a 1-year period were analysed for operating room (OR) supply cost. The cost was correlated with American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) outcomes.
Results
From July 2013 to June 2014, 2178 LCCKs were performed by 55 surgeons at seven hospitals. The median case OR supply cost was $513 ± 156. There was variation in cost between individual surgeons and within an individual surgeon's practice. There was no correlation between cost and ACS NSQIP outcomes. The majority of cost variation was explained by selection of trocar and clip applier constructs.
Conclusions
Significant case OR cost variation is present in LCCK across a single health system, and there is no clear association between increased cost and NSQIP outcomes. Placed within the larger context of overall cost, the opportunity exists for improved resource utilization with no obvious risk for a reduction in the quality of care.
Introduction
A major shift in the reimbursement mechanisms for healthcare expenditures in the last decade has been driven by a combination of legislation, media, and efforts of insurance companies and healthcare providers. The Medicare Prescription Drug, Improvement, and Modernization Act in 2003 introduced the Acute Care Episode Demonstration, a programme designed to challenge current fee-based payment systems that reward ‘quantity of services provided, rather than quality of care’.1 Five hospitals or healthcare systems participated and received global payments for all inpatient components of cardiothoracic or orthopaedic procedures over a 3-year period. With a goal of improving ’both the efficiency and quality of care provided to Medicare beneficiaries’, millions of dollars were saved without negatively impacting outcomes.2,3 The success of this programme inspired further extensions of bundled payments, which achieved slowed growth of health care spending while improving outcomes.4,5 The Center for Medicare & Medicaid Innovation's Bundled Payments for Care Improvement Initiative is the most recent, current and nation-wide project.6
The success of these programmes hinges on monitoring cost while maintaining high-quality care and preventing adverse outcomes. The interplay between cost and outcomes has been united by a popular term in this field: value. The value in healthcare has been defined as healthcare outcome per dollar spent,7 with an emphasis on beneficial outcomes. The study of outcomes has grown with large well-established reporting datasets, including the National Surgical Quality Improvement Program sponsored by the American College of Surgeons (ACS NSQIP). This dataset has been used as one component in studies examining complications in relation to the overall cost of procedures and post-operative management of patients, and has been used to study the concept of value among hospitals delivering cost-effective care while maintaining positive outcomes.8
The delivery of cost-effective care demands aggressive cost containment. Variability in the delivery of care has been shown to be the top source of excessive spending.9 Within the field of surgery, operating room and supply costs have been shown to comprise large portions of a procedural hospitalization charge.10 Common procedures with standardized operative approaches present a relatively controlled environment for the study of variability at a specific level. A cholecystectomy is one of the most common and familiar procedures for the general surgeon or hepatobiliary specialist, with over 700 000 procedures performed annually.11 Operating room (OR) and supply cost for inpatient cholecystectomy in Medicare patients represent the second- and third-largest component of total hospital charges at more than $3000 and $2000, respectively.10 Our group hypothesized that, using a robust internal data collection system for a common standardized procedure and comparing cost to outcomes utilizing ACS NSQIP data, we could identify intra-operative and surgeon-specific sources of variation in laparoscopic cholecystectomy and thus could develop strategies of cost-containment while maintaining quality.
Materials and methods
A retrospective review of laparoscopic cholecystectomy case costs from OR records within a multi-hospital healthcare system was performed for cases in the 12-month period July 2013 to June 2014. Included procedures were limited to laparoscopic cholecystectomy and laparoscopic cholecystectomy with an intra-operative cholangiogram. Excluded procedures were any laparoscopic cholecystectomy converted to a laparotomy or during which any additional procedure was billed. Administrative data from seven hospitals, all serving populations >50 000, were used to determine intra-operative supply cost, defined as the estimated acquisition cost for instruments on the surgical field as recorded in the intra-operative electronic case log by an OR nurse. This cost is exclusive of charges for anaesthesia supplies, radiology support for cholangiograms and physician or facility fees. The estimated cost was used instead of the exact cost to account for inter-facility differences and the impact of purchasing agreements including tiered pricing, incentives and rebates. Comparative outcomes data for surgeons and institutions were obtained using cases that met the above inclusion criteria and were included within the ACS NSQIP during 2012 and 2013. Five of the seven examined hospitals participated in ACS NSQIP during this interval. References to the complication rate pertain to any one or more standard ACS NSQIP-specified postoperative complications occurring in the 30-day post-procedure period for a patient, as per standard ACS NSQIP program protocol. Note that cost assessments and quality assessments were not matched on a case-by-case basis but were instead derived from separate but overlapping 12-month periods with robust data for either cost or complications.
Results
A total of 2178 laparoscopic cholecystectomies were performed by 55 surgeons at seven hospitals during the 12-month review period July 2013 to June 2014. The total intra-operative supply cost for the seven hospitals was $1 169 000 (mean $537/case). The grand median case supply cost was $513, with some variability in median per hospital cost as well as full case distribution per hospital (Fig.1). Surgeon-specific expenditure demonstrated variability both across and within a surgeon's practice, without any correlation to case volume (Fig.2).
Figure 1.
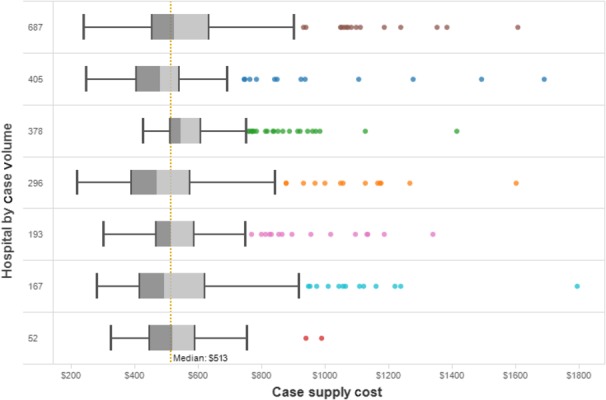
Intra-operative supply cost per case. The distribution of case costs for each of seven hospitals is outlined by row. Box plots depict 25th percentile, median and 75th percentile. Whiskers represent 1.5× interquartile (IQR), and dots represent high outliers. The system median cost of $513 is depicted as a dotted vertical line. Hospitals are sorted by case volume in the period as per the numbers in the first column. Investigation of high outlier cases identified the use of energy devices as a common cause of high case cost
Figure 2.
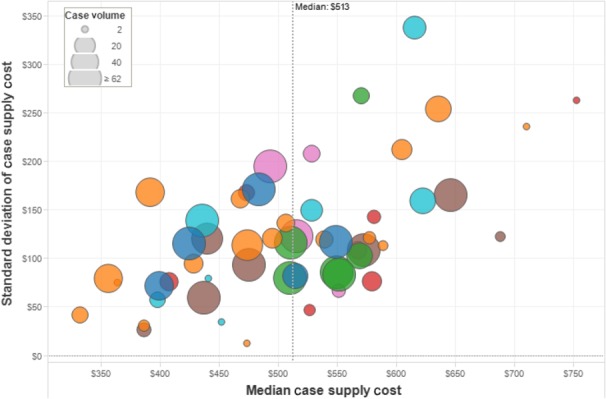
Intra-operative supply cost variability among surgeons. Each dot represents one surgeon. Dot colour represents one specific hospital. Dot size corresponds to case volume per surgeon. Intra-operative supply cost varies across surgeons and within a single surgeon's practice (y-axis ‘standard deviation’) for both high- and low-volume surgeons
The overall ACS NSQIP ’any ’ complication rate for laparoscopic cholecystectomies at our hospitals was 3%; the average nationwide ACS NSQIP complication rate for the same period and procedure types was 3.5%. Surgeon expenditure relative to outcomes failed to demonstrate any association (Fig.3). Surgeons who have median costs lower than the healthcare system median of $513 with operative outcomes lower than the healthcare system average of 3% would be considered ’high-value’ surgeons, whereas those with cost and outcomes above these benchmarks would be considered ‘low-value’ surgeons.
Figure 3.
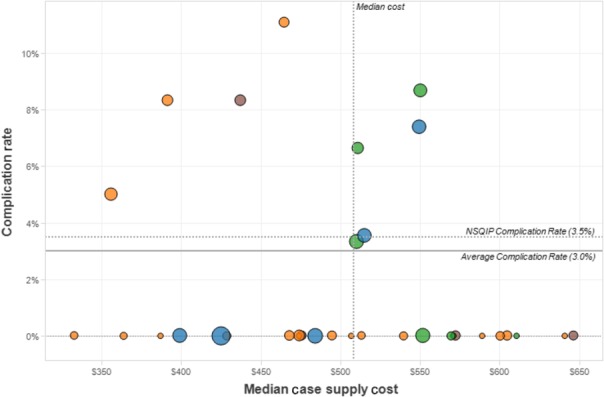
Surgeon-specific median expenditure versus any National Surgical Quality Improvement Program (NSQIP) adverse events. Surgeon expenditure is compared with the health system complication rate (‘average rate’ = 3%) and the national American College of Surgeons (ACS) NSQIP complication rate (‘NSQIP rate’ = 3.5%) during the study period. The area of circles represents surgeon case volume and colour represents hospital affiliation. There is no obvious negative or positive correlation between cost and outcomes
Of the total operative supply costs, the largest charges came from three categories of disposable items: laparoscopic trocar bundles (33.4%), packs and drapes (26.8%), and clip appliers (16%). Packs and drapes are largely standardized across this health system's ORs, but the stock and utilization of laparoscopic trocar bundles and clip appliers exhibited great variation. Laparoscopic trocar bundles consisting of one 10- to 12-mm umbilical port and three 5-mm ports are offered from three different manufacturers with costs differing as much as $138. Disposable clip appliers are available from two manufacturers, with variation in the price of a few dollars for 10-mm clip appliers and $60 for 5-mm clip appliers. The most expensive 5-mm clip applier was used 781 times in the review period, and the less expensive 5-mm clip applier was used 500 times in the same period. The most expensive 10-mm clip was used 670 times and the less expensive 10-mm clip was used 231 times.
Finally, using a line-item analysis comparing low-cost to high-cost laparoscopic cholecystectomies, it was determined that inclusion of an intra-operative cholangiogram was not a significant contributor to excess cost but is a significant contributor to cost variation, with cholangiogram-specific supply cost ranging from $18 to over $150.
Discussion
A seemingly endless number of sources of variation exist within a single operative and post-operative hospitalization, but one that is most intimately and actively controlled by surgeons is the intra-operative supply cost. We have demonstrated that intra-operative cost, particularly through the expense of disposable items such as trocar sets and clip appliers, varies greatly among surgeons and have presented a data set that failed to identify a relationship between cost, case volume and observed ACS NSQIP outcomes. The purpose of this study was to identify, within our single healthcare system, sources of cost variability to develop affordable, streamlined systems for intra-operative cost containment while maintaining quality. The data herein have been presented to working groups including the individuals surgeons included in the study, who now know where their cost and performance assessment, and thus are given some sense of their ‘value’, relative to others (as shown in Fig.3). The initial reception by our surgeons to this data was favourable and, in early follow-up, some resource-favourable practices have been observed. The metric of value is expected to be a key component of referral and reimbursement methods from insurers, with legislation requiring a value-based payment modifier for Medicare reimbursement set to go into effect in 2015, with extension to all physicians by 2017.12
The first anticipated outcome of this study was to stimulate discussion regarding the cost of and options associated with intra-operative supplies. Disposables were the leading source of cost variability and have previously been the target of cost-saving strategies in a laparoscopic appendectomy.13 With this approach, we can release to each surgeon not only their total case volume and associated costs, but also the exact number of times they used a disposable item and how much that item costs. Intra-operative bar codes displaying cost are fixed to each disposable item, with the intent to educate surgeons and OR staff of these costs at the time of opening the item. Interventions to educate surgeons on intra-operative supply cost and cost-effective alternatives in a laparoscopic cholecystectomy have reduced cost by 10% in a recent study.14
Our second goal was to optimize intra-operative supply utilization by monitoring intra-operative utilization variability. Demonstrating that spending has no evident association with outcomes should allow us contemplation of these issues without raising concern for risking adverse outcomes. For example, these data demonstrate that our surgeons are frequently selecting the most expensive model of both 5- and 10-mm clip appliers. If surgeons instead selected the less expensive manufacturer in each size, this would have saved 4% of the entire laparoscopic cholecystectomy budget, over $52 000, in 12 months. Resource utilization interventions planned include standardization of instrument sets, which have been shown to result in a cost reduction as high as 20% in a laparoscopic appendectomy.15
Finally, we are using this same analytic approach to study cost reduction strategies for other types of surgical cases, including laparoscopic colectomies and the associated variability in the utilization of available stapling devices.
In maximizing value, the hospital and the surgeon would be expected to benefit in a bundled payment system, ideally without compromising the care of the patient. Unfortunately, this drive towards resource preservation has several associated considerations that could threaten its success. The first is that surgeons might be convinced that using familiar instruments – or not being restricted to the use of alternative lower-cost instruments – is safer and results in quicker surgeries, and there is every reason to believe that this should be true. Our current study excluded the many costs associated with operating room time and thus is unable to address this idea. As we study the impact of cost-conscious practices and interventions, we will carefully consider and explore the limitations of surgeon preference and stances towards resource stewardship. A second concern is that the current reimbursement model does not adequately support a system in which cost-conscious surgeons are rewarded. Savings are primarily realized by hospitals or payers; cost savings do not necessarily return to the surgeon who created them. The motivation for using a lower-cost clip applier, at least at this stage, must instead be primarily based on an obligation to be responsible resource stewards for a society without compromising patient care. Alternative payment mechanisms that can directly reward surgeons for resource stewardship are growing but not yet widespread. Finally, we must be careful when promoting increasing value through controlling resource utilization; in doing so, we must always guard against unintended effects or unsafe practices. Surgeons may become more risk averse, refusing to care for patients whose pathophysiology or comorbidities are such that even adjusted reimbursement are not likely to cover the anticipated cost of an expectedly complicated surgery 16 or, alternatively, surgeons may remain motivated by preference or patient satisfaction to offer surgeries with advanced yet more expensive equipment (i.e. smaller instruments, robotic procedures, etc.).
Limitations of this study should be mentioned. The first is that the cost and quality data were not matched case by case. Both internal cost and outcome ACS NSQIP data were collected from separate but overlapping 12-month periods as these time periods were the most recent available data for each data source and thus were used to present the most recent insights. Despite this, this study is the largest for laparoscopic cholecystectomy intraoperative cost and the only one utilizing ACS NSQIP data. An additional limitation is that we did not establish a system to adjust for a surgeon's ‘value’ based on the complexity of his or her patients. We believe that patient-specific complexity is likely to have more of an impact on OR time and post-operative care, which are excluded from the present study. This is an area of ongoing investigation beyond the scope of this current work. Exclusions of complex case types from this current work also help to standardize against extremely complex cases.
Conclusion
Intra-operative supply cost for a laparoscopic cholecystectomy varies greatly among surgeons and does not correlate with ACS NSQIP outcomes. Variability from supply cost is largely observed in the use of disposable items including trocar sets and clips. Resource stewardship has become a clear and present obligation for our profession, and these results present opportunities to increase value by reducing variability and reducing cost while maintaining outcome quality for our patients.
Conflicts of interest
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
References
- Center for Medicare and Medicaid. Acute Care Episode Demonstration. Baltimore: . Available at: https://www.cms.gov/Medicare/Demonstration-Projects/DemoProjectsEvalRpts/downloads/ACEFactSheet.pdf (last accessed 10 March 2015) [Google Scholar]
- Herman B. 2 major lessons from CMS’ bundled payment ACE demonstration. Becker's Hospital Review. Available at: http://www.beckershospitalreview.com/hospital-physician-relationships/2-major-lessons-from-CMS-bundled-payment-ace-demonstration.html (last accessed 10 March 2015)
- Vesely R. An ACE in the deck? Bundled-payment demo shows returns. Mod Healthc. 2011;41:32–33. [PubMed] [Google Scholar]
- Casale AS, Paulus RA, Selna MJ, Doll MC, Bothe AE, Jr, McKinley KE, et al. “ProvenCareSM”: a provider-driven pay-for-performance program for acute episodic cardiac surgical care. Ann Surg. 2007;246:613–621. doi: 10.1097/SLA.0b013e318155a996. [DOI] [PubMed] [Google Scholar]
- Song Z, Safran DG, Landon BE, Landrum MB, He Y, Mechanic RE, et al. The ‘Alternative Quality Contract’, based on a global budget, lowered medical spending and improved quality. Health Aff. 2012;31:1885–1894. doi: 10.1377/hlthaff.2012.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Medicare and Medicaid Services. Bundled Payments for Care Initiative (BCPI) Overview: General Information. Baltimore. Available at: http://innovation.cms.gov/initiatives/bundled-payments/ (last accessed 22 April 2015)
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- Lawson EH, Zingmond DS, Stey AM, Hall BL, Ko CY. Measuring risk-adjusted value using Medicare and ACS-ACS NSQIP. Ann Surg. 2014;260:668–679. doi: 10.1097/SLA.0000000000000931. [DOI] [PubMed] [Google Scholar]
- Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307:1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- Stey AM, Brook RH, Needleman J, Hall BL, Zingmond DS, Lawson EH, et al. Hospital costs by cost center of inpatient hospitalization for Medicare patients undergoing major abdominal surgery. J Am Coll Surg. 2015;220:207–217. doi: 10.1016/j.jamcollsurg.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st Century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- Center for Medicare and Medicaid Services. Summary of 2015 Physician Value-based Payment Modifier Policies. Baltimore. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/CY2015ValueModifierPolicies.pdf (last accessed 22 April 2015)
- Chu T, Chandhoke RA, Smith PC, Schwaitzberg SD. The impact of surgeon choice on the cost of performing laparoscopic appendectomy. Surg Endosc. 2011;25:1187–1191. doi: 10.1007/s00464-010-1342-1. [DOI] [PubMed] [Google Scholar]
- Gitelis M, Vigneswaran Y, Ujiki MB, Denham W, Talamonti M, Muldoon JP, et al. Educating surgeons on intraoperative disposable supply costs during laparoscopic cholecystectomy: a regional health system's experience. Am J Surg. 2015;209:488–492. doi: 10.1016/j.amjsurg.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Avansino JR, Goldwin AB, Risley R, Waldhausen JHT, Sawin RS. Standardization of operative equipment reduces cost. J Pediatr Surg. 2013;48:1843–1849. doi: 10.1016/j.jpedsurg.2012.11.045. [DOI] [PubMed] [Google Scholar]
- Naylor MD, Kurtzman ET, Grabowski DC, Harrington C, McClellan M, Reinhard SC. Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff. 2012;31:1623–1632. doi: 10.1377/hlthaff.2012.0110. [DOI] [PubMed] [Google Scholar]


