Abstract
Background
Chemotherapy regimens for intrahepatic cholangiocarcinoma (ICC) and gallbladder adenocarcinoma (GC) remain interchangeable; however, response rates are frequently suboptimal. Biomarkers from ICC and GC patients were interrogated to identify actionable differences with potential therapeutic implications.
Methods
From 2009 to 2012, pathological specimens from 217 ICC and 28 GC patients referred to Caris Life Sciences were evaluated. Specific testing by immunohistochemical analysis for 17 different biomarkers was performed.
Results
In the collective cohort (n = 245), actionable targets included: 95% low thymidylate synthase (TS), 82% low ribonucleotide reductase subunit M (RMM) 1 and 74% low excision repair cross complementation group (ERCC) 1, indicating potential susceptibility to fluoropyrimidines/capecitabine, gemcitabine and platinum agents, respectively. Additional targets included TOPO1 (53.3% high, Irinotecan), MGMT (50.3% low, temozolomide), TOP2A (33% high, anthracyclines) and PGP (30.1% low, taxanes). Subgroup analysis by tumour origin demonstrated a differential biomarker expression pattern with a higher frequency of ICC tumours showing low levels of TS (99% versus 72%, P < 0.01), and RRM1 (85% versus 64%, P = 0.02) when compared with GC. Conversely a greater frequency of GC demonstrated high levels of TOPO1 (76% versus 50%, P = 0.02) versus ICC, indicating a potential increased benefit from irinotecan.
Discussion
Differences in the molecular profiles between ICC and GC provide evidence that the two are distinct diseases, requiring different treatment strategies to optimize a response.
Introduction
Intrahepatic cholangiocarcinoma (ICC) and gallbladder adenocarcinoma (GC) are frequently considered a similar disease in treatment planning. ICC is the second most common primary malignant liver tumour and incidence rates have been increasing in the United States and worldwide.1,2 Gallbladder adenocarcinoma, while rare among western countries, is the most common malignancy of the biliary tract and shows a geographical variance, occurring more frequently in northern India, Japan and Chile.3,4 For both ICC and GC, an R0 surgical resection is the only potentially curative treatment; however, both diseases tend to be asymptomatic in the early stages and few patients present early enough to be considered surgical candidates.5,6
For many patients diagnosed with ICC and GC, chemotherapy is the only treatment option. According to the National Comprehensive Cancer Network (NCCN), first-line regimens for both ICC and GC are interchangeable, despite the two being recognized as separate diseases. Accepted regimens include fluoropyrimidine-based, gemcitabine-based, or gemcitabine/cisplatin combination therapy for advanced or unresectable disease.7 However, suboptimal response rates as evidenced by a median survival of less than a year, underscore the need for more effective treatment regimens.8,9
Research into the molecular pathogenesis of both ICC and GC has revealed potential mechanisms contributing to tumourigenesis. Epidermal growth factor receptor activation in the setting of chronic inflammation, KRAS and IDH1 mutations, as well as epigenetic and chromosomal abnormalities have all been implicated in the development of ICC.10 While GC has not been as thoroughly studied, mutations in KRAS, p53, increased COX2, microsatellite instability and decreased adhesion molecules have all been proposed to contribute towards tumourigenesis.11 Recently, whole exome sequencing of GC showed mutations in the ErbB pathway in 36% of tumours analysed, and found the mutations correlated with a poor prognosis.12 Despite these advances, much is still unknown about the molecular profiles.
Many chemotherapeutic agents, however, have been extensively studied across multiple tumour types, yielding insight into their mechanisms of action, as well as the mechanisms of susceptibility and resistance. Clinical susceptibility to fluoropyrimidines is associated with a low expression of thymidylate synthase (TS),13 susceptibility to gemcitabine is associated with low expression levels of ribonucleotide reductase subunit M1 (RRM1) 14 and susceptibility to platinum agents, such as cisplatin, are associated with low expression of excision repair cross complementation group 1 (ERCC1).15 The use of all three of these drugs is recommended in advanced ICC and GC. Thus, information about the expression of TS, RRM1 and ERCC1 has a potential theranostic value.
Biomarker analysis of actionable targets known to convey susceptibility to specific drugs has been purported to be an effective method of tailoring existing chemotherapeutic agents to exploit the specific weaknesses in individual tumours.16,17 Studies have demonstrated that molecular profile-guided therapies can provide improved response rates across multiple tumour types.18 This study sought to differentiate the molecular profiles of ICC and GC by a panel of biomarkers to evaluate the potential efficacy of current chemotherapy regimens and potentially refine current treatment strategies.
Patients and methods
From 2009 to 2012, pathological specimens from 217 ICC and 28 GC patients were referred to Caris Life Sciences, a commercial referral diagnostic laboratory, for molecular profiling aimed at providing theranostic information. The diagnoses and tissue samples were collected from referring physicians according to pathology and clinical history. This de-identified data were obtained directly from Caris Life Sciences. As the data was de-identified, patient consent was not required.
Immunohistochemistry
Specific testing by Immunohistochemistry (IHC) was performed for 17 different biomarkers using the following antibodies: AR (AR441/AR318), BCRP (6D171), cKIT (polyclonal), ERCC1 (8F1), ER (SP1), Her2 (4B5), MGMT (MT23.3), MRP1 (33A6), PGP (C494), PR (1E2/100), PTEN (6H2.1), RRM1 (polyclonal), SPARC monoclonal (122511), SPARC (polyclonal), TOPO1 (1D6), TOPO2A (3F6) and TS (TS106/4H4B1). IHC analysis was performed on formalin-fixed paraffin-embedded tumour samples using commercially available detection kits, automated staining techniques (Benchmark XT; Ventana, Tucson, AZ, USA; and AutostainerLink 48; Dako, Carpinteria, CA, USA) in a CLIA/CAP certified, ISO validated lab (Caris Life Sciences, Phoenix, AZ, USA). Staining intensity was scored 0, 1+, 2+ or 3+, and the percentage of stained cells (0–100%) was assessed by board-certified pathologists. Results were then categorized into positive or negative by defined thresholds specific to each marker based on published evidence (Supporting information).
Institutional Review Board
We obtained Institutional Review Board approval to retrospectively review and analyse the data collected from the pathological specimens described above.
Statistical analysis
Categorical variables were described as totals and frequencies. Comparison between subgroups was analysed using a two-sided, Fisher's exact test for categorical data and a two-sided Mann–Whitney U-test for continuous variables. Alpha was set at 0.05.
Results
In total, 245 tissue samples were analysed; 217 IHC and 28 GC. The median age of the total cohort was 58 years, with a slight female preponderance (n = 133, 54%). By subgroup, the median age for ICC patients was 58 years, and 59 years for GC patients (P = 0.373). Both subgroups showed a female preponderance, however, it was much more pronounced in the GC subgroup (n = 20, 71%) as compared with the ICC subgroup (n = 113, 52%; P = 0.069).
Biomarker analysis of actionable targets
IHC analysis of biomarkers associated with first-line chemotherapy agents among the total cohort found TS expression to be low in 96% (fluoropyrimidines), low RRM1 expression in 82% (gemcitabine) and low ERCC1 expression in 74% (Cisplatin, Table1). Additional non-NCCN compendium agents and their associated biomarkers were also analysed. Among the total cohort, potential susceptibility to irinotecan, temozolomide, nab-paclitaxel and epirubicin occurred at lower frequencies (Table1). Biomarkers associated with susceptibility to these drugs demonstrated an elevation in TOPO1 (irinotecan) expression in 53%, decreased expression of MGMT (temozolomide) in 50%, increased SPARC (nab-paclitaxel) in 34% and increased expression of TOP2A (epirubicin) in 30% (Fig.1).
Table 1.
The frequency of actionable targets along with associated therapies in the total cohort (n = 245)
| Target biomarker | % | N | Associated agent |
|---|---|---|---|
| TS (−) | 96 | 199 | Fluoropyrimidine, capecitabine |
| RRM1 (−) | 82 | 197 | Gemcitabine |
| ERCC1 (−) | 74 | 197 | Cisplatin |
| TOPO1 (+) | 53 | 199 | Irinotecan |
| MGMT (−) | 50 | 203 | Temozolomide |
| SPARC Monoclonal (+) | 34 | 213 | Nab-paclitaxel |
| TOP2A (+) | 33 | 191 | Epirubicin |
| PGP (−) | 30 | 176 | Taxane |
| MRP1 (−) | 30 | 177 | Etoposide |
| SPARC Polyclonal (+) | 19 | 213 | Nab-paclitaxel |
| PTEN (+) | 19 | 209 | Erlotnib, Cetuximab |
| PR (+) | 6 | 201 | Hormonal therapy |
| BCRP (−) | 5 | 58 | Doxorubicin |
| C-KIT (+) | 4 | 175 | Sorafenib, sunitinib, imatinib |
| Her2/Neu (+) | 2 | 207 | Trastuzumab |
| AR (+) | 1 | 197 | Hormonal therapy |
| ER (+) | 1 | 203 | Hormonal therapy |
(−) = decreased expression, (+) = increased expression.
Figure 1.
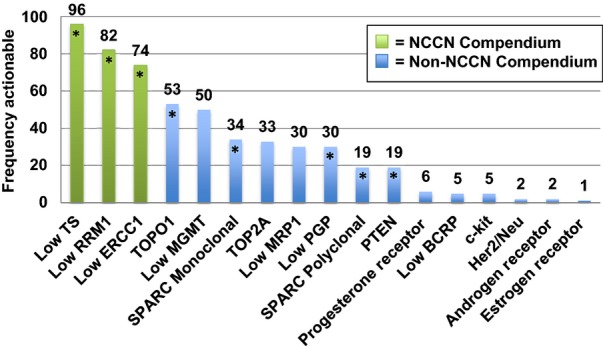
The overall frequency of actionable targets with potential susceptibility to established chemotherapeutic agents among all tumours tested by immunohistochemistry (n = 245)
Subgroup analysis by tumour origin
When comparing the molecular profiles based on site of origin, six of the 17 biomarkers demonstrated a differential expression pattern (Table2). In particular, TS (99% versus 72%; P < 0.01), RRM1 (85% versus 64%; P = 0.021) and MRP1 (33% versus 9%; P = 0.024) were all shown to be actionable more frequently in ICC, which indicates a greater potential benefit from fluoropyrimidines, gemcitabine and etoposide, respectively. Conversely, TOPO1 (76% versus 50%; P = 0.018) and PTEN (36% versus 16%; P = 0.027) were shown to be actionable more frequently in GC indicating a greater potential benefit from irinotecan and erlotnib, respectively.
Table 2.
Biomarker comparison of intrahepatic cholangiocarcinoma and gallbladder cancer by immunohistochemistry
| Target Biomarker | Associated agent | ICC (%) n = 217 | GC (%) n = 28 | P-value | Increased benefit for |
|---|---|---|---|---|---|
| TS | Fluoropyrimidines, capecitabine | 99 | 72 | <0.01 | ICC |
| RRM1 | Gemcitabine | 85 | 64 | 0.021 | ICC |
| MRP1 | Etoposide | 33 | 9 | 0.024 | ICC |
| TOPO1 | Irinotecan | 50 | 76 | 0.018 | GC |
| PTEN | Erlotnib, Cetuximab | 16 | 36 | 0.027 | GC |
| HER2/Neu | Trastuzumab | 1 | 12.5 | <0.01 | GC |
ICC, intrahepatic cholangiocarcinoma; GC, gallbladder adenocarcinoma.
Discussion
The NCCN currently recommends fluoropyrimidine-based, gemcitabine-based or gemcitabine/cisplatin combination therapy for the treatment of advanced ICC and GC. However, the use of gemcitabine/cisplatin therapy is the only treatment option backed by category 1 evidence. This was based primarily on the findings of a phase III clinical trial, which demonstrated that gemcitabine/cisplatin increased median progression-free survival from 5 to 8 months for advanced biliary tract cancers, compared with gemcitabine alone.19 Among patients included in the study were those with ICC or GC, which resulted in the dual agent regimen being applied to either tumour type.
Consistent with these recommendations, we found that the three biomarkers most frequently actionable across the entire cohort were TS (96%), RRM1 (82%) and ERCC1 (74%), inferring susceptibility to fluoropyrimidines, gemcitabine and platinum agents, and supporting their use as first-line agents. Supporting evidence for the use of these agents in the treatment of ICC is even stronger, as TS, RRM1 and ERCC1 are were found to be actionable in 99%, 85% and 75% of samples. Furthermore, low ERCC1 is associated with susceptibility to platinum-based agents, and the high frequency that ERCC1 was actionable further supports the efficacy of gemcitabine/oxaliplatin, which has been demonstrated by several phase II clinical trials.20,21
However, when compared with the ICC subgroup, TS, RRM1 and ERCC1 were actionable at a lower frequency among the GC samples. TS was actionable in 72%, and both RRM1 and ERCC1 were actionable in 64% of samples each, suggesting that there is a large subgroup of patients who may not respond to the recommended first-line therapies. Furthermore, the most frequently actionable target for GC is TOPO1 (76%) associated with a susceptibility to irinotecan22 suggesting that its use in the treatment of GC could prove beneficial and should be investigated. Indeed there are currently several phase II clinical trials that are looking into its use in various combinations to treat advanced gallbladder and biliary tract cancers (SCH01, GAMBIT201201).
In the entire cohort, actionable targets associated with non-NCCN compendium drugs include MGMT (50%) and SPARC monoclonal (34%) which have been associated with susceptibility to temozolomide23 and nab-paclitaxel.24 While we did not find these biomarkers to be actionable as frequently as the first-line agents, they suggest alternative treatment options that should be further explored in a clinical setting. In fact, drugs associated with eight of the 17 biomarkers analysed are currently being investigated, in various combinations, in ongoing clinical trials.
Comparative analysis by tumour type provides evidence that ICC and GC are molecularly distinct diseases and merit different treatments. We found a differential expression pattern in the six of the 17 biomarkers analysed. TS (99% versus 72%; P < 0.01) as well as RRM1 (85% versus 64%; P = 0.021) were significantly decreased in GC as compared with the ICC. The suggested potential decrease in tumour response to fluoropyrimidines and gemcitabine, in patients with GC, is particularly noteworthy as these drugs represent two of the three first-line drugs.
The differences in the molecular profiles of ICC and GC suggest that response rates to the first-line agent can be variable and supports a more targeted therapeutic approach to these diseases. The concept of using a tumour's specific molecular characteristics is being used with increased frequency. Molecular profiling has been used to guide treatment in breast cancer25 and to identify site-specific therapy in carcinoma of an unknown primary site.26 Von Hoff et al. used molecular profiling to guide the treatment of 66 patients with refractory metastatic cancer and found that 47% of patients showed some reduction in tumour size. Furthermore, an increase in the progression-free survival (PFS) of 30% compared with the PFS of the most recent failed regimen was demonstrated in 20 patients (27% of the entire cohort) – five of whom had GI cancer, one of which was cholangiocarcinoma.18 Currently, a phase II clinical trial is investigating the use of molecular profiling to prospectively guide neoadjuvant and adjuvant therapy in patients with pancreatic cancer (NCT01726582).
This study has several limitations; first, this was a retrospective analysis of de-identified data compiled from tissue samples sent for biomarker analysis. Consequently, clinical and disease related data were obtained solely from referring physicians and was verified by board-certified pathologists at Caris, and clinical information regarding prior therapy, decisions that resulted, or final outcomes are unknown. Obtaining prospective data is currently the goal of an ongoing study by Caris Life Sciences. In addition, the number of available GC samples was limited and may have affected the precision of the analysis. While there is growing evidence to suggest that biomarker analysis can be used to identify actionable targets for treatment, to date, there are no large, prospective studies proving this for ICC or GC. Additionally, our analysis of the molecular profiles of these two diseases was based solely on immunohistochemical data and did not evaluate other methods of molecular profiling such as Sanger or NextGen sequencing. Lastly, while the present study demonstrated a high percentage of samples possessing a molecular profile consistent with susceptibility to first-line agents, the poor response rates noted in clinical trials to these drugs, underscores additional factors such as the tumour microenvironment as having a potential role in affecting drug efficacy.27
In conclusion, ICC and GC continue to carry a poor prognosis, thereby highlighting the need for more effective treatment regimens. The use of molecular profiling to guide treatment has shown promising results in other tumour types and warrants further investigation. While our analysis supports the use of fluoropyrimidines, gemcitabine and cisplatin as first-line agents to treat ICC and GC, we also identified agents, such as irinotecan, that could be beneficial in subsets of patients unresponsive to first-line drugs. The molecular characteristics described, in addition to the differential expression pattern found between ICC and GC provides compelling evidence that future investigations should approach these diseases independently.
Funding sources
None.
Conflicts of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Thresholds to categorize the immunohistochemical staining as high (positive) or low (negative).
References
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liv. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181. doi: 10.1634/theoncologist.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakić M, Patrlj L, Kopljar M, Kliček R, Kolovrat M, Loncar B, et al. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3:221–226. doi: 10.3978/j.issn.2304-3881.2014.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers. Available at: http://www.NCCN.org (last accessed 13 February 2015)
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–750. doi: 10.1016/j.jamcollsurg.2013.05.021. .e4. [DOI] [PubMed] [Google Scholar]
- Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218–229. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46:872–876. doi: 10.1038/ng.3030. [DOI] [PubMed] [Google Scholar]
- Qiu LX, Tang QY, Bai JL, Qian XP, Li RT, Liu BR, et al. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. Int J Cancer. 2008;123:2384–2389. doi: 10.1002/ijc.23822. [DOI] [PubMed] [Google Scholar]
- Gong W, Zhang X, Wu J, Chen L, Li L, Sun J, et al. RRM1 expression and clinical outcome of gemcitabine-containing chemotherapy for advanced non-small-cell lung cancer: a meta-analysis. Lung Cancer. 2012;75:374–380. doi: 10.1016/j.lungcan.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang J, Wang R, Luo X, Chen H. The platinum-based treatments for advanced non-small cell lung cancer, is low/negative ERCC1 expression better than high/positive ERCC1 expression? A meta-analysis. Lung Cancer. 2010;70:63–70. doi: 10.1016/j.lungcan.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Stenvang J, Kümler I, Nygård SB, Smith DH, Nielsen D, Brünner N, et al. Biomarker-guided repurposing of chemotherapeutic drugs for cancer therapy: a novel strategy in drug development. Front Oncol. 2013;3:313. doi: 10.3389/fonc.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi R, Rossana B, Scartozzi M, Mario S, Freddari F, Federica F, et al. Biliary tract cancers: molecular profiling as a tool for treatment decisions. A literature review. Cancer Treat Rev. 2006;32:333–347. doi: 10.1016/j.ctrv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Stephenson JJ, Rosen P, Loesch DM, Borad MJ, Anthony S, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- Marino D, Leone F, Cavalloni G, Cagnazzo C, Aglietta M. Biliary tract carcinomas: from chemotherapy to targeted therapy. Crit Rev Oncol Hematol. 2013;85:136–148. doi: 10.1016/j.critrevonc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- Gilbert DC, Chalmers AJ, El-Khamisy SF. Topoisomerase I inhibition in colorectal cancer: biomarkers and therapeutic targets. Br J Cancer. 2012;106:18–24. doi: 10.1038/bjc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- Elsadek B, Kratz F. Impact of albumin on drug delivery–new applications on the horizon. J Control Release. 2012;157:4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Rubin MS, Spigel DR, Boccia RV, Raby S, Quinn R, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013;31:217–223. doi: 10.1200/JCO.2012.43.3755. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581–4586. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Thresholds to categorize the immunohistochemical staining as high (positive) or low (negative).


