Abstract
Pluripotent stem cells (PSCs) such as embryonic stem cells or induced pluripotent stem cells represent a promising cell type to gain novel insights into human biology. Understanding the differentiation process of PSCs in vitro may allow for the identification of cell extrinsic/intrinsic factors, driving the specification process toward all cell types of the three germ layers, which may be similar to the human in vivo scenario. This would not only lay the ground for an improved understanding of human embryonic development but would also contribute toward the generation of novel cell types used in cell replacement therapies. In this line, especially the developmental process of mesodermal cells toward the hematopoietic lineage is of great interest. Therefore, this review highlights recent progress in the field of hematopoietic specification of pluripotent stem cell sources. In addition, we would like to shed light on emerging factors controlling primitive and definitive hematopoietic development and to highlight recent approaches to improve the differentiation potential of PSC sources toward hematopoietic stem/progenitor cells. While the generation of fully defined hematopoietic stem cells from PSCs remains challenging in vitro, we here underline the instructive role of cell extrinsic factors such as cytokines for the generation of PSC-derived mature hematopoietic cells. Thus, we have comprehensively examined the role of cytokines for the derivation of mature hematopoietic cell types such as macrophages, granulocytes, megakaryocytes, erythrocytes, dendritic cells, and cells of the B- and T-cell lineage.
Keywords: granulocytes, hematopoiesis, hematopoietic stem cells, iPSC, macrophages
Introduction
In 1961, James Till and Ernest McCulloch demonstrated the formation of hematopoietic colonies—comprising hematopoietic cells of multiple lineages—in the spleen of lethally irradiated mice that were transplanted with murine bone marrow (BM) cells. Based on these results, they postulated the existence of a clonogenic progenitor cell with multilineage developmental potential [colony-forming units, spleen (CFU-S)], later referred to as hematopoietic stem cell (HSC) (Till, 1961; Till & McCulloch, 1961). In subsequent work, they could demonstrate that these progenitor cells could (i) self-renew and (ii) reconstitute the entire hematopoietic system of a recipient (Becker et al, 1963; Siminovitch et al, 1963). Nowadays, hematopoiesis is understood as a hierarchal process, in which all the different specialized hematopoietic cell types are generated from a small number of definitive multipotent HSCs (see Fig1). Having these unique properties, HSC transplantation (HSCT) is applied in the clinics for the treatment of malignant and non-malignant hematopoietic disorders (Thomas et al, 1959; Gatti et al, 1968; Copelan, 2006). However, quantity and even quality of HSCs are currently the limiting factors for this therapeutic option. This is, on the one hand, due to insufficient sources for HSC isolation and inadequate storage of isolated cells. Furthermore, also immunological incompatibilities due to the multifaceted human leukocyte antigen system (known as HLA system) remain a major hurdle for HSCT (Copelan, 2006). In this regard, a new cell source, able to differentiate into all cell types of the endo-, ecto-, or mesodermal lineages, has been introduced by Shinya Yamanaka in 2006 (Takahashi & Yamanaka, 2006). These induced pluripotent stem cells (iPSCs) can be generated from different somatic cell sources by overexpression of specific transcription factors (TF) (Takahashi & Yamanaka, 2006; Yu et al, 2007) and may open a new chapter for the field of regenerative medicine. Although generation of iPSCs was proven for a variety of different mature cells (Takahashi et al, 2007; Aasen et al, 2008; Hanna et al, 2008; Kim et al, 2008; Haase et al, 2009), their proper differentiation toward transplantable therapeutic target cells of the hematopoietic lineage remains challenging. Therefore, this review aimed to highlight the recent progress within the field of hematopoietic differentiation of pluripotent stem cell sources and to address existing hurdles associated with the generation of HSCs capable for long-term reconstitution. By shedding light on the emerging factors that regulate both primitive and definitive hematopoietic development, we further provide insights into the differentiation potential of PSC sources toward hematopoietic stem/progenitor cells and mature hematopoietic cells, which may pave the way for innovative cell replacement therapies.
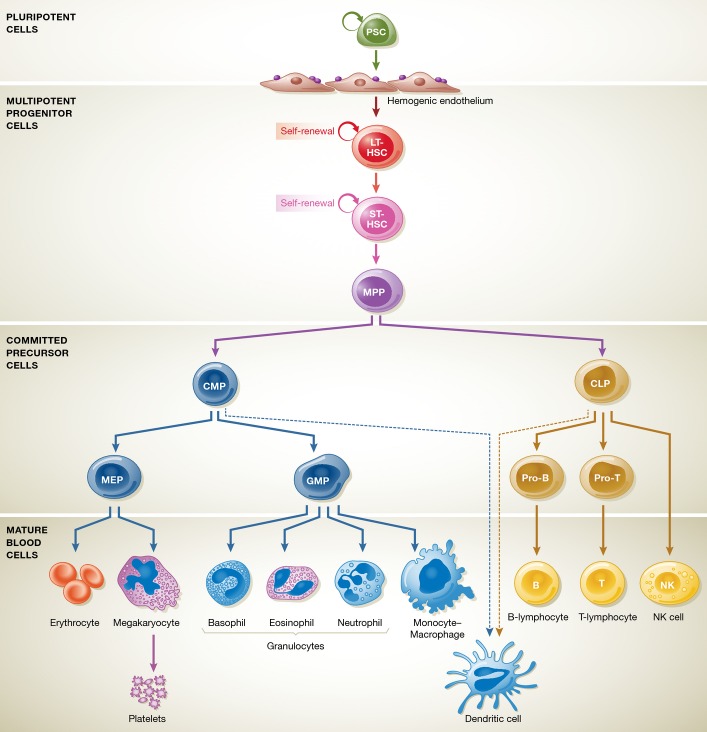
Figure 1. Classical scheme of murine adult hematopoietic development.
Multipotent LT-HSCs, with their ability for long-term reconstitution potential, can further differentiate toward ST-HSCs and also MPPs in the bone marrow. Upon subsequent differentiation, MPPs give rise to either CMPs, which have the ability to differentiate into the myeloid lineage, or CLPs, able to generate the lymphoid lineage. Following these committed progenitors, both MEPs and GMPs are able to form all differentiated cells of the myeloid lineage in the bone marrow, whereas CLPs further differentiate into pro-T cells and T cells by positive–negative selection in the thymus. Generation of B cells is ensured also by CLPs in the bone marrow following B-cell transition. Abbreviations: LT, long term; ST, short term; MPP, multipotent progenitor; HSC, hematopoietic stem cell; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; MEP, megakaryocyte erythroid progenitor; GMP, granulocyte macrophage progenitor.
Early hematopoietic development
The process of embryonic hematopoietic development is tightly regulated by the activation or repression of distinct signaling pathways. After gastrulation, cells of the epiblast ingress the primitive streak and start to differentiate toward the cells of the mesodermal lineage (Kinder et al, 1999). This initial formation of mesodermal cells resembles the first critical wave of hematopoietic development and is primarily regulated by the bone morphogenic protein 4 (BMP4), the fibroblast growth factor 2 (FGF2, also known as bFGF), as well as Wnt and Nodal signaling (Conlon et al, 1994; Flamme et al, 1995; Winnier et al, 1995; Liu et al, 1999). Further hematopoietic development is dependent on dorsal–ventral and anterior–posterior patterning as well as lateralization of the early mesoderm. In particular, the dorsal aorta, and hence also the emerging HSCs, develops from the ventro-posterior lateral plate (splanchnic) mesoderm, which is generated by the synergistic effects of Wnt ligands and BMPs (Beddington & Robertson, 1999; Langdon & Mullins, 2011). Also, FGF2 represents a key player during this early specification process, since it induces the up-regulation of the kinase inert domain-containing receptor (KDR, also known as vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2); fetal liver kinase 1 (flk1)) on meso-dermal precursor cells. Subsequently, KDR+ cells can respond to VEGF, which is produced by FOXA2- and SOX7-expressing visceral endoderm (Monaghan et al, 1993; Takash et al, 2001; Kennedy et al, 2007; Kimura-Yoshida et al, 2007). This signaling interaction between cells of the mesoderm and the visceral endoderm is critical for the development of endothelial and hematopoietic cells, which is further highlighted by the lethality of a KDR knockout caused by defects in blood vessel formation (Carmeliet et al, 1996). In line with these observations, it has been shown that exogenous substitution of VEGF, FGF2, and transforming growth factor (TGF)-β1 (Pardanaud & Dieterlen-Lievre, 1999) could mimic the endoderm interaction that induces the hemangiopoietic potential in the associated mesoderm of chick embryos. Moreover, also signaling from the hedgehog (HH) pathway is able to substitute primitive endoderm interaction and to activate murine hematopoiesis (Dyer et al, 2001) by induction of a signaling cascade that includes the downstream effectors Vegf, Notch, and Runx1 (Gering & Patient, 2005).
Mesodermal cells that co-express KDR as well as Brachyury and are able to give rise to both the blood and the endothelial lineage are defined as the hemangioblast (Murray, 1932). The existence of this direct common precursor for endothelial and hematopoietic cells was already postulated for the first time in 1917 (Sabin, 2002). However, evidence of its existence was only given by an in vitro ESC differentiation model (Kennedy et al, 1997; Choi et al, 1998), while in vivo studies in mouse and zebrafish failed to conclusively confirm these findings (Myers & Krieg, 2013). Therefore, the hemangioblast rather represents a state of competence than a bona fide bipotential precursor cell (Amaya, 2013). During further differentiation, cells of the presumptive hemangioblast migrate to the yolk sac and contribute to the first “wave” of hematopoiesis (Ferkowicz & Yoder, 2005). This initial hematopoietic program mainly generates primitive erythroid progenitors expressing fetal hemoglobin, embryonic macrophages, and megakaryocytes. Since this phase is not able to give rise to T-lymphoid cells or even transplantable HSCs, it is defined as primitive hematopoiesis. Following this initial hemato poietic program, erythroid–myeloid progenitors (EMPs) are generated in the blood island capillaries of the yolk sac by a specialized population of endothelial cells, known as the hemogenic endothelium (HE) (Dzierzak & Speck, 2008; Lux et al, 2008; Yoder, 2014). Moreover, HE in the yolk sac as well as later in the para-aortic splanchnopleura can also develop into T-lymphoid progenitor cells (Yoshimoto et al, 2012; Boiers et al, 2013; Yoder, 2014). Since this phase is capable to generate “adult-like” blood cells from EMPs and lymphoid progenitors, it is defined as definitive hematopoiesis (Boiers et al, 2013; Yoder, 2014). However, this intermediate program does not yet generate definitive HSCs with repopulating potential.
The final step in definitive hematopoietic development is characterized by the specialization of definitive HSCs from the hemogenic endothelium that are present within the aorta-gonad-mesonephros region (AGM). Here, the proper transition from endothelial to hematopoietic cells (known as endothelial-to-hematopoietic transition (EHT)) is regulated by several specific signaling events and culminates in the formation of intra-aortic hematopoietic clusters (IAHCs) (Dzierzak & Speck, 2008). Hematopoietic transition of the HE is regulated by Notch and adenosine signaling (Gori et al, 2015; Jang et al, 2015; Jing et al, 2015; Lin et al, 2015). Although mutations in the Notch pathway lead to normal primitive hematopoiesis in the yolk sac, runx1 expression and therefore the formation of IAHC are abolished (Burns et al, 2005). In this line, Runx1 represents a crucial TF in the regulation of EHT and is highly expressed in the aortic hemogenic endothelium and IAHC (North et al, 2002). Once specified from the HE, HSCs leave the dorsal aorta and move toward the placenta and fetal liver for transient proliferation, after which they are finally able to colonize the bone marrow as the most important adult hematopoietic organ.
Hematopoietic specification of pluripotent stem cells—a mirror of development
Given the importance of intrinsic and extrinsic signals for hematopoietic development in vivo, most in vitro hematopoietic differentiation protocols for PSCs try to mimic the distinct signaling cascades active during embryonic development. Similar to the importance of BMP4, Wnt, FGF2, and VEGF signaling during early embryonic hemato-poietic development, the activation of these signaling pathways has been shown to improve hematopoietic specification also upon in vitro differentiation of hPSCs (Winnier et al, 1995; Chadwick et al, 2003; Kennedy et al, 2007; Wang & Nakayama, 2009). In this respect, Kennedy et al (2007) demonstrated that the addition of BMP4 is essential for hemangioblast development from human PSCs. Moreover, also the cooperative effect of Wnt and BMP signaling during early hematopoietic development could be recapitulated upon in vitro differentiation (Wang & Nakayama, 2009).
During early stages of hematopoietic differentiation in vitro, PSCs give rise to cell types that express typical primitive posterior mesodermal markers, such as the apelin receptor (APLNR), platelet-derived growth factor receptor (PDGFR)α/CD140a, and KDR (Shalaby et al, 1997; Slukvin, 2013b; Uenishi et al, 2014). Moreover, differentiated cells also show the up-regulation of typical meso/endodermal TFs such as Brachyury (T), MIXL1, FOXF1, and GATA2 (Slukvin, 2013a). Upon further differentiation, these cells acquire blast colony-forming cell (BL-CFC) potential in the presence of FGF2, similar to their in vivo counterparts found in the posterior region of the primitive streak, expressing KDR and T (Huber et al, 2004). Interestingly, overexpression of Gata2, Lmo2, Mycn, Pitx2, Sox17, and Tal1 in mPSCs established and subsequently maintained a proliferative state with hemangioblast potential (Vereide et al, 2014). Following in vitro differentiation, emergence of so-called hematovascular mesodermal progenitors (HVMP) that are KDRbright, APLNR+, and PDGFRαlow/− has been observed from hPSCs. Moreover, HVMPs display the down-regulation of primitive streak genes and up-regulation of genes associated with angiohematopoietic development, such as TAL1, HHEX, LMO2, GATA2, and ETV2. At this stage, the first endothelial cells characterized by the expression of CD144 (also known as VE-cadherin) and CD31 emerge (Choi et al, 2012). In addition, Choi et al (2012) were able to identify a surface marker expression profile of CD73, CD43, and CD235a that can be used to discriminate hemogenic from non-hemogenic endothelium. In their experimental setting, only CD144+/CD73−/CD235a−/CD43− cells were able to generate endothelial and definitive hematopoietic progenitors upon co-cultivation with OP9 stromal cells. Of note, Hirai et al (2003) demonstrated that the expression level of Runx1 critically defines subpopulations within the CD144+ population. This finding is in line with the observation that Runx1 is critical for the EHT during embryonic development (Chen et al, 2009). Following differentiation, Sox17 regulates hemogenic endothelium (Clarke et al, 2013; Nakajima-Takagi et al, 2013) and its hematopoietic progenitors that can be further discriminated by the expression of CD43 or the pan-hematopoietic marker CD45 with a surface marker phenotype of lin−/CD34+/CD45+/CD38− (Rafii et al, 2013).
Primitive versus definitive hematopoietic development
During mammalian embryonic development, the emergence of definitive hematopoietic “stem” cells characterized by their potential to generate B- and T-lymphoid cells as well as to repopulate an irradiated host is preceded by a wave of primitive hematopoiesis (Yoder, 2014). In this line, inducing a definitive hematopoietic program during the in vitro differentiation process of PSCs may resemble the prerequisite to generate HSCs with long-term engraftment potential. Probably, this switch from the primitive to definitive hematopoiesis represents the bottleneck that is hindering the efficient long-term engraftment potential of PSC-derived hematopoietic stem/progenitor cells (HSPCs) so far (Szabo et al, 2010; Ran et al, 2013) (see also Fig2).
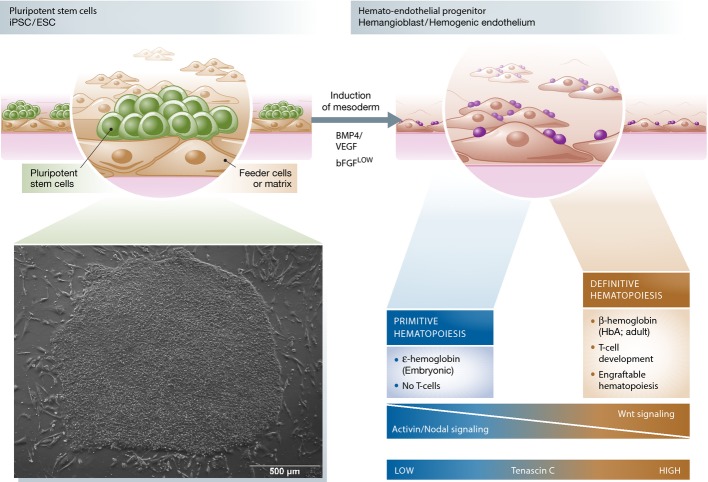
Figure 2. Primitive and definitive hematopoietic development in vitro.
Hematopoietic specification from pluripotent stem cells in vitro is primarily driven by the formation of mesodermal cells, which later gives rise to different hematopoietic cells by a hemato-endothelial progenitor. At this stage, hematopoietic differentiation in vitro can in principle generate cells of primitive or definitive hematopoiesis, which can be differentiated using specific experimental setups. Hematopoietic progenitor cells, which emerge during the differentiation process and are able to (i) give rise to erythroid cells that express adult hemoglobin (HbA or β-hemoglobin), (ii) give rise to T-lymphoid cells when cultured on NOTCH-delta ligand 1/4 (DL1 or DL4)-expressing OP9 cells, or (iii) multilineage reconstitute immunocompromised mice, are defined as cells derived from a definitive hematopoietic program. In contrast, hematopoietic progenitor cells that are not capable of fulfilling these criteria are defined as cells derived from primitive hematopoiesis. Although both programs can occur in vitro, defined signaling pathways such as Wnt, Activin/Nodal, or extracellular stimuli such as tenascin C have been proven to direct the hematopoietic program toward definitive or primitive hematopoiesis.
Whereas the distinct waves of hematopoiesis are temporally and spatially separated during embryonic development in vivo, culture systems do not allow this clear separation, so that both developmental processes simultaneously coexist in vitro. It has been shown that both hematopoietic programs involve the formation of hemogenic endothelium and give rise to CD34+/CD45+ hematopoietic precursor cells. Once differentiated, primitive and definitive hematopoietic progenitor cells cannot be distinguished by differential surface marker expression but only by functional criteria, such as the generation of T lymphocytes. While in principle both programs can occur in vitro, the Activin/Nodal pathway triggers the development of primitive but not definitive hematopoiesis (Kennedy et al, 2012), leading to the hypothesis that appropriate markers can discriminate progenitors of both hematopoietic programs at very early stages of mesoderm development (see also Fig2). Indeed, a recent antibody screen from Sturgeon et al (2014) identified glycophorin A (CD235a) as such a marker. While KDR+/CD235a+ mesodermal cells give rise to primitive hematopoiesis, KDR+/CD235a− cells represent precursors of a definitive hematopoietic program that are able to generate T cells upon OP9 co-cultivation. Importantly, the authors also demonstrate that the induction of a definitive hematopoietic program was driven by the Wnt–catenin signaling pathway, whereas primitive hematopoiesis was dependent on Activin/Nodal signaling (see also Fig2). Modulation of these pathways and specifically the use of Wnt agonists (CHIR99021) and Activin/Nodal antagonists led to the generation of a selected population of definitive hematopoietic progenitors (Sturgeon et al, 2014). Given the instructive role of Wnt signaling during early mesodermal patterning in embryonic development, the importance during in vitro differentiation of PSCs is not unexpected. However, it remains elusive whether its mode of action is also in vitro mediated by the activation of caudal-type homeobox (Cdx) genes, which regulate homeobox (Hox) expression during in vivo development (Ikeya & Takada, 2001; Shimizu et al, 2005; Pilon et al, 2006; Lengerke et al, 2008). Overall, this example clearly demonstrates the importance of understanding the physiological hematopoietic development in order to improve the in vitro differentiation of PSCs.
In addition to the activation of signaling cascades, TFs, or other signaling molecules, also components of the extracellular matrix (ECM) contribute to the stem cell niche and modulate in vivo HSC fate and development. In this respect, tenascin C (TenC) has been demonstrated to be one important component of the ECM. TenC is a highly conserved glycoprotein, mainly expressed during embryonic development and it possesses a multiplicity of binding sites for integrin cell surface receptors, proteoglycans, as well as cell adhesion molecules. In addition, it also interacts with other ECM components such as heparin, fibronectin, and collagen, suggesting complex functionality (Hsia & Schwarzbauer, 2005). In adult bone marrow, it is expressed in the endosteal regions and presumably plays a role in stress conditions (Ohta et al, 1998). Furthermore, TenC has been shown to improve in vitro maintenance of HSPCs (Zuckerman & Wicha, 1983). Although its role during embryonic development is unclear, it has recently been reported that TenC promotes hemato-endothelial development of hPSCs upon in vitro differentiation and—even more importantly—uniquely supports T-lymphoid commitment (Chen et al, 2011). The effect of extracellular factors, such as components of the ECM or blood flow, is further supported by the generation of engraftable HSCs from hiPSCs by teratoma formation (Amabile et al, 2013; Suzuki et al, 2013). Based on the necessity to provide a suitable microenvironment, developing HSCs from the teratoma were able to migrate toward the bone marrow with engraftment potential.
Improving the generation of long-term engrafting HSPCs from hPSCs
In order to improve the poor engraftment ability of in vitro iPSC- or ESC-derived hematopoietic stem or progenitor cells, researchers have made big attempts to find key factors involved in HSC specification from hematopoietic mesoderm. It has been suggested that members of the HOX gene cluster exert an important molecular switch mediated via downstream factors such as BMP4, Activin A, or VEGF. HOX proteins are a group of highly conserved TFs, which are characterized by a DNA-binding motif termed “homeobox.” The HOX genes are organized into four major clusters and were shown to play important roles in embryonic organogenesis. Clusters A–C have been implicated in HSC self-renewal and regulation as well as being dysregulated in the context of several leukemia subtypes (Antonchuk et al, 2002; Peters et al, 2010). Members of the CAUDAL family of genes (CDX1, CDX2, and CDX4) tightly regulate HOX gene expression during early anterior–posterior embryonic patterning. In the zebrafish, cdx4 is expressed in the posterior mesoderm priming hematopoietic commitment by up-regulating target HOX genes. Further, cdx4 initiates the generation of runx1a+ definitive HSCs derived from the AGM (Davidson & Zon, 2006). This implicates a strong instructive role of CDX4 during HSC specification from the hemangioblast. Indeed, the ectopic overexpression of HoxB4 in murine HSCs leads to enhanced ex vivo and in vivo expansion, while maintaining their normal differentiation and long-term repopulation potential (Antonchuk et al, 2001, 2002; Kyba et al, 2002; Krosl et al, 2003; Tashiro et al, 2011). In normal adult hematopoiesis, HoxB4 enhances the proliferation of early cells, including HSCs, and is expressed through early stages of erythroid and granulocytic differentiation (Giampaolo et al, 1995; Sauvageau et al, 1995; Pineault et al, 2002). Furthermore, the overexpression of HoxB4 and Cdx4 in murine ESC-derived hematopoietic progenitors promotes hematopoietic mesoderm specification, increases hematopoietic progenitor formation, and enhances multilineage hematopoietic engraftment of cells in lethally irradiated adult mice (Wang et al, 2005b). Moreover, when iPSC-derived CD41+, c-Kit+ cells were transduced with adenoviral vectors containing HoxB4, the number of hematopoietic progenitor cells with colony-forming potential was significantly increased (Tashiro et al, 2011). In addition, early embryonic development and patterning of murine CCE-ESCs shows the activation of genes targeted by HoxB4 such as Dll1, Gli2, Nodal, Sox2, Sim2, Smad2, or Tbx3, whereas at later time points, HoxB4 targets genes important for specific functions, such as myeloid and lymphocyte proliferation, differentiation, and activation (Fan et al, 2012). Therefore, HoxB4 favors the engraftment of CCE-derived hematopoietic stem and progenitor cell in immunocompetent mice (Lesinski et al, 2012).
Considering the instructive role of HoxB4 in the murine system, transient overexpression of HOXB4 does not improve the features of human ESC- or iPSC-derived hematopoietic progenitors toward a transplantable cell population (Wang et al, 2005a). Unlike HOXB4, overexpression of the RUNX1a isoform improves the in vitro generation of hematopoietic progenitors from human ESCs and iPSCs by regulating Brachyury, KDR, SCL, GATA2, and PU.1 (Ran et al, 2013). Runx1 is expressed in the HE and important for endothelial-to-hematopoietic transition (EHT) (Chanda et al, 2013). In this line, ectopic expression of RUNX1a in human PSCs leads to hPSC-derived hematopoietic progenitors that are able for multilineage reconstitution of irradiated NOD-scid IL2rγnull (NSG) mice for more than 9 weeks (Ran et al, 2013). Moreover, the combined over-expression of GATA2/ETV2, GATA2/TAL1, or ER71/GATA2/SCL can lead to the formation of endothelial cells with hemogenic potential from PSC sources (Liu et al, 2013; Elcheva et al, 2014; Shi et al, 2014). Alternatively, the addition of HOXA9, ERG, RORA, SOX4, and MYB in human PSCs favors the direct differentiation into CD34+/CD45+ progenitors with multilineage potential (Doulatov et al, 2013).
While defined factors such as HOXB4, CDX4, SCL/TAL1, or RUNX1a have been proven to support the hematopoietic program in murine or human PSCs, further factors have been identified to instruct terminally differentiated cells toward an immature hematopoietic phenotype. In this line, hematopoietic progenitor cells have been generated from blood or endothelial cells by the ectopic overexpression of key TFs.
While the first attempt used one TF to reprogram fibroblast to immature hematopoietic precursor cells (Szabo et al, 2010), the combined overexpression of the transcription factors Gata2, Gfi1b, cFos, and Etv6 in mouse fibroblasts efficiently induced an endothelial-like cell population (Pereira et al, 2013) (see Fig 3). The transient overexpression of Run1t1, Hlf, Lmo2, Pbx1, Prdm5, and Zfp37 in committed myeloid and lymphoid progenitors triggered the formation of so-called induced HSCs (iHSC), which possess multilineage reconstitution potential, while being serially transplantable and showing a gene expression profile similar to in vivo HSCs (Riddell et al, 2014). Given a specific vascular niche, human endothelial cells overexpressing the TFs FOSB, GFI1, RUNX1, and SPI1 are able to generate hematopoietic colonies resembling multipotent progenitor cells (MPPs), when cultured on E4EC vascular niche cells. These so-called rEC-hMPPs show colony cell-forming potential in vitro and faithfully engraft and reconstitute primary and secondary immune-deficient recipients (Sandler et al, 2014). More recently, also the ectopic expression of the TFs Erg, Gata2, Lmo2, Runx1, and Scl was proven to efficiently reprogram murine fibroblasts into blood cells through a hemogenic stage even without the need for a co-culture system (Batta et al, 2014) (see Fig3). Another interesting approach was introduced by Pulecio et al, who utilized miR-125b, a non-coding RNA, in conjunction with the TF SOX2 to convert human fibroblasts into engraftable hematopoietic progenitor cells with mainly monocytic potential (Pulecio et al, 2014).
Figure 3. Early hematopoietic development and transprogramming strategies.
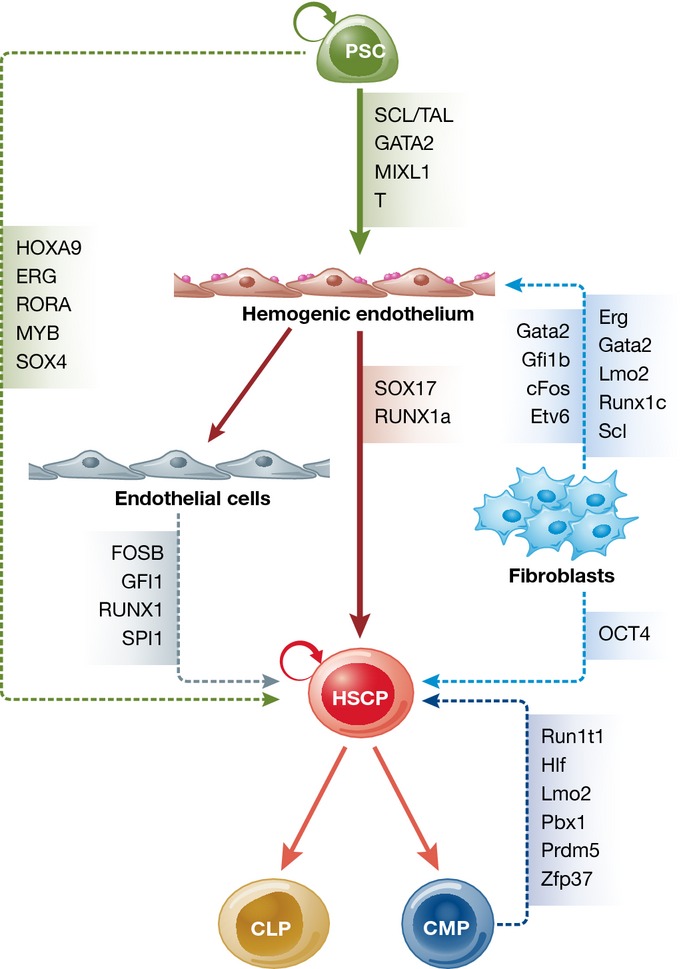
During hematopoietic differentiation in vitro, PSCs differentiate through mesodermal cells into cells of hemogenic endothelium capable to form either HSPCs or endothelial cells. This process is regulated by defined transcription factors, such as SCL/TAL, GATA2, MIXL1, T, SOX17, and RUNX1a. Further differentiation of HSPCs gives rise to CLPs or CMPs, respectively. Overexpression of Erg, Gata2, Lmo2, Runx1c, and Scl or Gata2, Gfi1b, cFos, or Etv in murine fibroblasts allows for the direct reprogramming of fibroblasts into HSPCs by a hemogenic endothelium intermediate. Alternatively, murine CMPs can be directly reprogrammed toward HSPCs by overexpression of Run1t1, Hlf, Lmo2, Pbx1, Prdm5, or Zfp37. Moreover, also endothelial cells can be directed toward HSPCs by the expression of FOSB, GFI1, RUNX1, or SPI1, whereas direct induction of HSPCs from PSCs was shown by overexpression of HOXA9, ERG, RORA, MYB, and SOX4. Abbreviations: PSC, pluripotent stem cell; HSPC, hematopoietic stem/progenitor cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor.
PSC-derived hematopoiesis and the instructive role of cytokines
Pluripotent stem cells have not only been used in differentiation protocols toward hematopoietic stem and progenitor cell, but also directed toward mature blood cells. Whereas the generation of terminally differentiated hematopoietic cells has been proven for many different lineages (macrophages, monocytes, megakaryocytes, platelets, dendritic cells, T and natural killer cells as discussed below), bona fide HSCs—which engraft and show long-term multilineage reconstitution—have not been obtained using in vitro protocols so far.
The induction of specific signaling pathways by the addition of key cytokines and/or co-cultivation with instructive feeder cells is substantial for the generation of hematopoietic progenitors and mature blood cells from human PSC sources, which are usually cultured under serum-free conditions. In normal adult hematopoiesis in vivo, the specification of mature cell types from multipotent HSCs is positively regulated by instructive cytokine signals, that is by the activation of crucial TFs (Zhu & Emerson, 2002). It is still under debate, whether cytokines have a rather selective than a lineage-instructive function during the commitment of multipotent hematopoietic progenitors (reviewed in Endele et al, 2014). However, TPO and erythropoietin (EPO) were shown to support erythro-megakaryocytic lineage commitment of a common TER119+/4A5+ precursor (Vannucchi et al, 2000), while macrophage colony-stimulating factor (M-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) signals drive the differentiation toward monocytes/granulocytes from multipotent progenitors (Kondo et al, 2000; Pawlak et al, 2000; Niwa et al, 2011). It seems reasonable that any blood cell derived from PSC has to pass through a transient progenitor cell state. Protocols for the expansion of HSPCs have therefore been adapted for the derivation of in vitro HSPCs from PSCs. Cytokines, such as SCF, TPO, FLT3-L, IL-6, and aryl hydrocarbon receptor signaling antagonizers (i.e., the small molecule StemRegenin), drive the expansion of cord blood CD34+ HSPCs while maintaining their CFU potential over a period of 10 days in culture (Haemmerle et al, 2014). The addition of IL-3, however, favors the expansion and differentiation of CD34+ HSPCs on the expense of engraftment and reconstitution in vivo (Du et al, 2014). Similarly, these cytokines have been utilized to support the generation of HSPCs from PSCs in vitro (Uenishi et al, 2014).
For early differentiation of hiPSCs and hESCs, most protocols rely on the addition of SCF, BMP4, or VEGF for the induction of mesodermal differentiation or the spontaneous differentiation within embryoid bodies (EBs) (Olivier et al, 2006; Pick et al, 2007; Yokoyama et al, 2009; Niwa et al, 2011; Ferrell et al, 2015; Toscano et al, 2015). Generated EBs can be subsequently either plated in co-culture systems with, for example, OP9, AFT024, or C3H10T1/2, or further differentiated with the help of special media, such as APEL, BPEL, or StemSpan (Chang et al, 2006; Olivier et al, 2006; Pick et al, 2013; Ferrell et al, 2015; Vanhee et al, 2015) (summarized in Table1).
Table 1.
Generation of mature hematopoietic cells from pluripotent stem cell sources.
| Cell type | PSC source | Embryoid bodies | FCS | Feeder cells | Cytokines | Reference | |
|---|---|---|---|---|---|---|---|
| Myeloid cells | Erythrocytes | hESC (H1) | Yes | Yes | No | bFGF, VEGF, EPO, SCF, nFlt3-L, IL-3, IL-6, G-CSF, TPO | Chang et al (2006) |
| hESC (H1) | No | Yes | S17 and FH-B-hTERT (mFL stroma cells) | Clonogenic assay: SCF, GM-CSF, IL-3, EPO | Qiu et al (2005) | ||
| hESC | No | Yes | mFL stroma cells | Clonogenic assay: SCF, IL-3, IL-6, TPO, G-CSF, EPO | Ma et al (2008) | ||
| hESC (H1), hiPSC | No | Yes | OP9, MS5 | TPO, IL-3, IL-6, Flt3-L, SCF, EPO | Dias et al (2011) | ||
| hESC (H1) | No | Yes | FH-B-hTERT, MS5 | IL-3, BMP4, Flt3-L, SCF, EPO, IGF-1 | Olivier et al (2006) | ||
| Megakaryocytes/platelets | hESC (HES3, Envy, MEL1) | Yes | No | No | BMP4, VEGF, bFGF, SCF, TPO, IL-3 | Pick et al (2013) | |
| hESC (WA01) | Yes | Yes | OP9 | BMP4, VEGF, IL-3, Flt3-L, TPO, SCF, EPO | Vanhee et al (2015) | ||
| hESC (MA09, NED07), hiPSC | No | No | No | BMP4, VEGF, bFGF, TPO, SCF, Flt3-L, IL-3, IL-6, IL-9 | Feng et al (2014) | ||
| hESC (HuES3, MA01, MA09) | No | No | OP9, C3H | BMP4, VEGF, IL-6, IL-9, IL-11, bFGF, TPO, SCF | Lu et al (2011) | ||
| Granulocytes | hESC (KhES-3) | Yes | Yes | No | IGF-II, VEGF, SCF, Flt3-L, TPO, G-CSF | Saeki et al (2009) | |
| hESC (KhES-3) | Yes | Yes | OP9 | BMP4, SCF, Flt3-L, IL-6, TPO, G-CSF | Yokoyama et al (2009) | ||
| hESC (KhES-1, 3), hiPSC | Yes | No | No | BMP4, VEGF, SCF, TPO, Flt3-L, IL-3, G-CSF | Niwa et al (2011) | ||
| hiPSC | Yes | No | No | IL-3, G-CSF or GM-CSF | Lachmann et al (2015) | ||
| hESC (H1, H9), hiPSC | No | Yes | OP9 | GM-CSF, G-CSF, IL-3, IL-5 | Choi et al (2009) | ||
| MΦ | hESC (HUES-2, KCL001, 002) | Yes | No | No | IL-3, M-CSF | Karlsson et al (2008), van Wilgenburg et al (2013) | |
| hESC (H9), hiPSC | Yes | No | No | IL-3, M-CSF, or GM-CSF | Lachmann et al (2015) | ||
| hESC (H1, H9), hiPSC | No | Yes | OP9 | GM-CSF, M-CSF, IL-1b | Choi et al (2009) | ||
| DCs | hESC (H9) | Yes | hAS | No | SCF, Flt3-L, GM-CSF, IL-3, TPO, IL-4, TNF-α | Su et al (2008) | |
| hIPSC | No | Yes | OP9 | GM-CSF, M-SCF, IL-4, TNF-α | Senju et al (2011) | ||
| hESC (H1, H9), hiPSC | No | Yes | OP9 | GM-CSF, IL-4, TNF-α | Choi et al (2009) | ||
| Lymphoid cells | NK cells | hESSC (H1, HES-2), hiPSC | Yes | Yes | OP9-DL4 | BMP4, bFGF, Activin A, VEGF, IGF-1, IL-6, IL-11, SCF, IL-3, EPO, TPO, IL-13, Flt3-L, IL-15 | Sturgeon et al (2014) |
| hESC (H9), hiPSC | Yes | hAS | OP9-DL1 | BMP-4, VEGF, SCF, IL-3, Il-6, TPO, EPO, IL-7, Flt3-L, IL-15 | Ferrell et al (2015) | ||
| hESC (H9) | No | Yes | S17, AFT024 (mFL cells) | IL-15, IL-3, IL-7, SCF, Flt3-L | Woll et al (2005) | ||
| T cells | hESC (H1) | Yes | Yes | OP9-DL4 | BMP-4, bFGF, Activin A, VEGF, IL-6, IGF-1, IL-11, SCF, EPO, TPO, Flt3-L, IL-7, IL-15 | Kennedy et al (2012) | |
| hESSC (H1, HES-2), hiPSC | Yes | Yes | OP9-DL4 | BMP4, bFGF, Activin A, VEGF, IGF-1, IL-6, IL-11, SCF, IL-3, EPO, TPO, IL-3, Flt3-L, IL-7 | Sturgeon et al (2014) | ||
| hESC (H1, H9), hiPSC | No | Yes | OP9-DL1, OP9-DL4 | BMP-4, bFGF, VEGF, TPO, SCF, IL-6, IL-3, IL-7, Flt3-L | Uenishi et al (2014) | ||
| hESC (H1) | No | Yes | OP9, OP9-DL1 | Flt3-L, IL-7, SCF | Timmermans et al (2009) | ||
| B cells | hESC (H1, H9, ES03), | Yes | Yes | OP9 | BMP4, VEGF, FGF1, bFGF, SCF, Flt3-L, TPO, GM-CSF, IL-2, IL-4, IL-15, G-CSF, IL-3, IL-6, IL-7 | Zambidis et al (2008) | |
| hIPSC | No | Yes | OP9, MS5 | IL-7, IL-3, SCF, Flt3-L | French et al (2015) |
PSC, pluripotent stem cells; hESC, human embryonic stem cells; hiPS, human-induced pluripotent stem cells; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; EPO, erythropoeitin; SCF, stem cell factor; Flt3-L, FMS-like tyrosine kinase 3 ligand; IL-3, interleukin-3; IL-6, interleukin-6; G-CSF, granulocyte colony-stimulating factor; TPO, thrombopoietin; FL, fetal liver; GM-CSF, granulocyte–macrophage colony-stimulating factor; BMP4, bone morphogenic protein 4; IGF-1, insulin-like growth factor 1; IL-9, interleukin-9; IL-11, interleukin-11; IGF-II, insulin like-growth factor II; M-CSF, macrophage colony-stimulating factor; IL-1b, interleukin-1b; IL-4, interleukin-4; TNF-α, tumor necrosis factor-alpha; IL-7, interleukin-7; FGF1, fibroblast growth factor 1; IL-15, interleukin-15; hAS, human antibody serum; MΦ, macrophages; DCs, dendritic cells; NK, natural killer cells.
Different studies have shown the production of erythrocytes from hiPSCs or hESCs using VEGF, SCF, BMP4, Flt3-L, IL-3, IL-6, and EPO, while some protocols additionally used TPO, hydrocortisone, or insulin-like growth factor 1 (IGF-1) (Qiu et al, 2005; Chang et al, 2006; Olivier et al, 2006; Ma et al, 2008; Dias et al, 2011). Although the generation of such cells has been proven, successful transfusion of mature red blood cells (RBC) is still hampered primarily due to the low efficiency of the differentiation process. For clinical application, one unit should at least contain 1010 in vitro derived cells, whereas 1012 RBCs are desirable, a cell number that is not reached by far with the current protocols from PSCs (Dorn et al, 2015). Likewise, the resulting erythrocytes were mostly nucleated and contained only embryonic or fetal, but not adult hemoglobin, favoring the concept of primitive hematopoiesis. Alternatives, such as immortalized iPSC-derived erythroblast (imERYPCs), are highly promising and maybe used as a safe and constant supply for RBC transfusion (Hirose et al, 2013).
Similarly, TPO, SCF, or IL-3 also seem to be essential for the development of megakaryocytes and platelets from peripheral blood-derived CD34+ cells as well as hESCs/hiPSCs (Pick et al, 2013; Nakamura et al, 2014; Vanhee et al, 2015). Moreover, also interleukin-9 (IL-9) and interleukin-11 (IL-11) were reported to improve the quality of the produced platelets (Lu et al, 2011). Most recently, a feeder- and serum-free protocol with collagen IV was established, which yields highly pure CD41a+/CD42b+ double-positive mature megakaryocyte and platelet populations from hiPSCs and hESCs (Feng et al, 2014). Human iPSC-derived platelets can be generated as HLA-ABC negative, can be frozen and share functional features with peripheral blood-derived platelets both in vitro and in vivo (Feng et al, 2014; Nakamura et al, 2014). As the efficient dose for a transfusion would be approximately 300–600 × 109 platelets, yields of 6 platelets per megakaryocyte progenitor from iPSC cultures are clearly too low for a clinical translation (mature MKs produce 2,000–10,000 platelets) (Feng et al, 2014). However, it might be possible to further improve the current protocols by altering sheer stress and matrix interactions in combination with GMP-compliant media or expandable iPSC-derived megakaryocytic cell lines (Moreau et al, 2013; Nakamura et al, 2014).
Peripheral blood-derived CD34+ cells can be efficiently differentiated into neutrophil or eosinophil granulocytes by the use of granulocyte colony-stimulating factor (G-CSF) and IL-6 or the combination of IL-3 and IL-5 on OP9 feeder cells, respectively (Choi et al, 2009). A similar protocol was used to generate neutrophils and eosinophils from human ESCs and iPSCs via a GM-CSF-expanded intermediate CD235a−/CD41a−/CD34+/CD45+ cell type. The resulting populations were essentially pure and similar to peripheral blood-derived granulocytes regarding marker expression, function, and morphology. Another approach using an EB-based, feeder-free protocol with the addition of insulin-like growth factor II (IGF-II), VEGF, SCF, Flt3-L, TPO, and G-CSF could only generate neutrophils from hESCs with lower efficiencies (Saeki et al, 2009). Recently, the combination of SCF, TPO, IL-3, or G-CSF was shown by several groups to faithfully induce mature neutrophils from human pluripotent cell sources (Yokoyama et al, 2009; Niwa et al, 2011; Lachmann et al, 2015). Limitations to most systems comprise the low yields, especially in feeder-free conditions, the dependence on expensive cytokine cocktails, or the selection of a suitable ESC/iPSC line that efficiently differentiates into myeloid cells. Although PSC-derived granulocytes show similarities to their in vivo counterparts, discrepancies in the formation of neutrophil extracellular traps (NETs) or migration toward hIL-8 were reported (Lachmann et al, 2015), arguing for cells in different maturation stages within granulocytic differentiation. IPSC-derived granulocytes hold great potential for the treatment of infections and septicemia in neutropenic patients. However, it is still unclear whether the in vitro derived granulocytes are fully functional after transfusion. In addition, suitable cell numbers for individual granulocyte transfusions and storage conditions remain critical obstacles that have not been addressed to date. In particular, the latter is of interest, as granulocytes are known to have a short life span after leukapheresis. Since granulocytes are generated at 37°C using cell culture media, it remains elusive whether PSC-derived granulocytes can be stored in aliquots ready for on-demand therapeutical use.
Since macrophages and granulocytes descend from a common progenitor (i.e., GMP), their early differentiation steps from HSCs or PSCs toward the myeloid lineage are principally guided by the same molecular cues. In order to obtain monocytes and macrophages in vitro, most protocols use a combination of SCF, IL-3, M-CSF, or GM-CSF (Karlsson et al, 2008; Choi et al, 2009; van Wilgenburg et al, 2013; Lachmann et al, 2015) (see also Table1). In vitro derived macrophages show high functional and morphological similarities to PB-derived counterparts, but again their in vivo function upon transfusion has to be evaluated using suitable animal models. Here, even smaller numbers of monocytes or macrophages might be sufficient for a clinical benefit as a bridging therapy for infectious diseases or as tissue resident macrophages in organotropic transplantation scenarios (Happle et al, 2014; Lachmann et al, 2014; Suzuki et al, 2014a,b).
Dendritic cells (DCs) are antigen-presenting cells, which can be derived from both myeloid and lymphoid developmental pathways. DCs have been differentiated from bone marrow or peripheral blood CD34+ cells using GM-CSF, Flt3-L, SCF, IL-4, and tumor necrosis factor-α (TNF-α) (Lutz et al, 1999; Choi et al, 2009). Similarly, hESC/hiPCS-derived DCs can be generated with the help of mainly GM-CSF, IL-4, or TNF-α through a transient myeloid intermediate (Su et al, 2008; Choi et al, 2009; Senju et al, 2011) (see also Table1).
The in vitro differentiation of hESCs/hiPSCs into cells of the lymphoid lineage is strictly dependent on the co-culture with stromal cells, such as OP9, AFT024, or MS-5, and much lower in efficiency compared to the in vitro generation of myeloid or erythro-megakaryocytic cells. In this line, several protocols have been established to generate mature natural killer cells (NK) from human iPSCs or ESCs, commonly using the hematopoietic cytokines SCF, Flt3-L, IL-3, IL-7, and IL-15 (Woll et al, 2005; Sturgeon et al, 2014; Ferrell et al, 2015) (see also Table1).
As Notch signaling was shown to be essential for T lymphopoiesis (Mohtashami et al, 2010), derivation of T cells from hiPSCs/hESCs was achieved with the help of OP9 stromal cells constantly overexpressing the Notch ligand Delta-like 1 or 4 (DL1 or DL4, respectively). A combination of different cytokines was shown to faithfully induce T-cell differentiation using either thymopentin, OP9 co-culture, or transplantation-based differentiation protocols starting from in vivo progenitor populations or ESCs (Timmermans et al, 2009; Kennedy et al, 2012; Sturgeon et al, 2014; Uenishi et al, 2014; Zhu et al, 2015) (see also Table1). Early protocols for the generation of in vitro B cells used OP9 stromal cells and a combination of Flt3-L and IL-7 (Cho et al, 1999; Zambidis et al, 2008). When cultured on MS-5 stroma in the presence of IL-7, SCF, Flt3-L, and IL-3, hiPSC-derived CD34+ cells differentiated into CD19+CD10+ B cells that undergo in vitro VDJ recombination and express cell surface IgM (French et al, 2015).
Taken together, current protocols for the in vitro generation of mature blood cells from human pluripotent cells demonstrate the importance of the key cytokines SCF, IL-3, and IL-6 for myeloid differentiation, whereas SCF, TPO, and EPO seem to be instructive for a rather erytho-megakaryocytic lineage decision. In contrast, the generation of lymphoid progenitors from PSCs involves signaling by SCF, Flt3-L, IL-3, IL-7, and/or IL-15 and additionally relies on co-culture systems with OP9 cells. This dependency might reflect the fact that the current protocols for the derivation of hematopoietic cells from PSCs are not yet able to mimic the complex cues needed for the induction of a definitive hematopoietic program. Further improvement of environmental and intrinsic signaling pathways could lead to an enhanced, large-scale production of fully functional PSC-derived blood cells.
While large-scale generation of suitable iPSC-derived cells under GMP-compliant conditions remains the next hurdle for the successful transfer toward the clinics, also the functionality of iPSC-derived cells in suitable in vivo mouse models remains elusive. Different mouse models favoring the engraftment of human hematopoietic cells have been developed, either expressing mutants of the Kit receptor (Cosgun et al, 2014) or different human cytokines such as CSF1 alone (Rathinam et al, 2011) or in combination with IL3, CSF2, and THPO (Rongvaux et al, 2014). While current protocols have shown the functionality of mature cells mostly in vitro, transfer of cells into the aforementioned humanized mouse models is still hampered by the rather low output of differentiated cells from PSC sources. Investigating new ways of differentiation to either increase the yield or quality of cells may pave the way for innovative cell replacement strategies using iPSC-derived mature hematopoietic cells.
Conclusion
A multitude of differentiation protocols has proven the efficient generation of mature hematopoietic cells from PSC sources. However, the generation of lymphoid cells or erythrocytes still remains challenging and highly inefficient. Moreover, the directed differentiation of PSCs into HSPC, with the ability to efficiently reconstitute xenograft models long term, is still hampered. This indicates that most protocols direct the differentiation process toward primitive hematopoietic development. Here, a better understanding of defined factors regulating both the primitive and the definitive hematopoietic development in vivo might also help to further fine-tune the in vitro differentiation process. Considering the hematopoietic differentiation from PSC as a finely orchestrated and dynamic process, the use of defined cytokines only may skew or bias the in vitro hematopoietic differentiation toward cell types not applicable for clinical use. Here, cell–cell interactions, the extracellular matrix (ECM), or cytokines from cell niches acting either in an autocrine or paracrine fashion during the in vitro differentiation process are rarely considered yet. As an example, Sturgeon et al (2014) elegantly demonstrated that modulation of the Wnt pathway during hematopoietic specification in vitro can lead to the generation of definitive hematopoiesis, highlighting the importance of an improved understanding. Further understanding of the finely tuned influence from niche and stromal cells on hematopoietic progenitors (i.e., via up-regulation of TF) will be essential for the improved, large-scale production of PSC-derived blood cells in vitro.
Pending issues.
What factors (intrinsic and extrinsic) are regulating primitive versus definitive hematopoietic development?
Will the identification of those factors lead to improved differentiation of PSCs into long-term engrafting HSPCs?
Do we bias in vitro hematopoietic differentiation, resulting in hematopoietic cells not similar to their in vivo counterparts?
How can we improve protocols for the generation of functional lymphoid cells and red blood cells from PSCs?
Do suitable xenograft models that are able to support multilineage engraftment of human iPSC-derived hematopoiesis help us to understand the human hematopoietic differentiation?
Where can we apply PSC-derived mature hematopoietic cells for innovative treatment options and how should we proceed?
What are the obstacles associated with up-scaling of GMP-compliant hematopoietic differentiation protocols for regenerative therapies?
Acknowledgments
The authors thank Prof. Thomas Moritz, Sebastian Brennig, Jessica Fritsch, and Prof. Axel Schambach (all Hannover Medical School, Hannover, Germany) for critical comments on the review. Moreover, the authors thank Cornelia Richter for helping in preparing figures. This work was supported by grants from the Else Kröner-Fresenius Foundation, the Deutsche Forschungsgemeinschaft (DFG, Cluster of Excellence REBIRTH; Exc 62/1), German José Carreras Leukemia Foundation (R 13/09), and Hannover Medical School (“Young Academy” Fellowship award).
Glossary
- Engraftment
Incorporation of donor cells, e.g. HSC, into a new host, thereby giving rise to donor-derived hematopoiesis.
- Epiblast
During early embryonic development, the inner cell mass (ICM) of the blastocyst segregates into the bilaminar disc, which comprises the epiblast and the primitive endoderm. The epiblast (primitive ectoderm) further undergoes gastrulation and is able to form the three germ layers (endoderm, ectoderm and mesoderm) and some extra-embryonic components.
- Hematopoietic stem cell (HSC)
The concept of blood formation is a hierarchical process referred to as hematopoiesis that is based on the multi-potent hematopoietic stem cell (HSC), which is able to reconstitute the entire hematopoietic system.
- Hemogenic endothelium
A specialized population of endothelial cells present in the developing embryo able to give rise to hematopoietic cells.
- Induced pluripotent stem cells (iPSC)
A pluripotent cell-type with the capacity to differentiate towards all cells of the three germ-layers (ectoderm, mesoderm, endoderm) and induced by reprogramming of a somatic cell by defined factors.
- Mesoderm
One of the three germ layers formed upon gastrulation during early embryonic development. Mesodermal progenitors give rise to e.g. cartilage, muscles, connective tissues, blood, heart and kidney.
- Transdifferentiation
Describes the direct conversion of a somatic cell-type into another somatic cell, without going through an intermediate pluripotent status. This process can occur naturally during development or be induced by overexpression of lineage-specific transcription factors
Conflict of interest
The authors declare that they have no conflict of interest.
For more information
For more information on pluripotent stem cells and multipotent stem cells please visit:
Stem Cell Information, National Institute of Health (NIH)
http://stemcells.nih.gov/info/basics/Pages/Default.aspx
International Society for Stem Cell Research
PSC-derived hematopoietic cells and their use for rare diseases
http://www.rebirth-hannover.de/en/research/research-groups/area-b1/unit-68.html
References
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Amabile G, Welner RS, Nombela-Arrieta C, D’Alise AM, Di Ruscio A, Ebralidze AK, Kraytsberg Y, Ye M, Kocher O, Neuberg DS, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E. The hemangioblast: a state of competence. Blood. 2013;122:3853–3854. doi: 10.1182/blood-2013-10-533075. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp Hematol. 2001;29:1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Batta K, Florkowska M, Kouskoff V, Lacaud G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Rep. 2014;9:1871–1884. doi: 10.1016/j.celrep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Boiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, Woll PS, Mead AJ, Hultquist A, Swiers G, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013;155:215–227. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- Chang K-H, Nelson AM, Cao H, Wang L, Nakamoto B, Ware CB, Papayannopoulou T. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SK, Webber TD, Carlyle JR, Nakano T, Lewis SM, Zúñiga-Pflücker JC. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:9797–9802. doi: 10.1073/pnas.96.17.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Choi K-D, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RL, Yzaguirre AD, Yashiro-Ohtani Y, Bondue A, Blanpain C, Pear WS, Speck NA, Keller G. The expression of Sox17 identifies and regulates haemogenic endothelium. Nat Cell Biol. 2013;15:502–510. doi: 10.1038/ncb2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Cosgun KN, Rahmig S, Mende N, Reinke S, Hauber I, Schafer C, Petzold A, Weisbach H, Heidkamp G, Purbojo A, et al. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell. 2014;15:227–238. doi: 10.1016/j.stem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Zon LI. The caudal-related homeobox genes cdx1a and cdx4 act redundantly to regulate hox gene expression and the formation of putative hematopoietic stem cells during zebrafish embryogenesis. Dev Biol. 2006;292:506–518. doi: 10.1016/j.ydbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dias J, Gumenyuk M, Kang H, Vodyanik M, Yu J, Thomson JA, Slukvin II. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn I, Klich K, Arauzo-Bravo MJ, Radstaak M, Santourlidis S, Ghanjati F, Radke TF, Psathaki OE, Hargus G, Kramer J, et al. Erythroid differentiation of human induced pluripotent stem cells is independent of donor cell type of origin. Haematologica. 2015;100:32–41. doi: 10.3324/haematol.2014.108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S, Vo LT, Chou SS, Kim PG, Arora N, Li H, Hadland BK, Bernstein ID, Collins JJ, Zon LI, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13:459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Jin H, Cai H, Yang S, Tan W-S. Hematopoietic repopulating ability of CD34(+) progenitor cells ex vivo expanded with different cytokine combinations. Artif Cells Nanomed Biotechnol. 2014;43:398–402. doi: 10.3109/21691401.2014.897630. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elcheva I, Brok-Volchanskaya V, Kumar A, Liu P, Lee J-H, Tong L, Vodyanik M, Swanson S, Stewart R, Kyba M, et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 2014;5:4372. doi: 10.1038/ncomms5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endele M, Etzrodt M, Schroeder T. Instruction of hematopoietic lineage choice by cytokine signaling. Exp Cell Res. 2014;329:207–213. doi: 10.1016/j.yexcr.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Fan R, Bonde S, Gao P, Sotomayor B, Chen C, Mouw T, Zavazava N, Tan K. Dynamic HoxB4-regulatory network during embryonic stem cell differentiation to hematopoietic cells. Blood. 2012;119:e139–e147. doi: 10.1182/blood-2011-12-396754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Shabrani N, Thon JN, Huo H, Thiel A, Machlus KR, Kim K, Brooks J, Li F, Luo C, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014;3:817–831. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ferrell PI, Xi J, Ma C, Adlakha M, Kaufman DS. The RUNX1 + 24 enhancer and P1 promoter identify a unique subpopulation of hematopoietic progenitor cells derived from human pluripotent stem cells. Stem Cells. 2015;33:1130–1141. doi: 10.1002/stem.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169:699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- French A, Yang C-T, Taylor S, Watt SM, Carpenter L. Human induced pluripotent stem cell-derived B lymphocytes can express sIgM and can be generated via a hemogenic endothelium intermediate. Stem Cells Dev. 2015;24:1082–1095. doi: 10.1089/scd.2014.0318. [DOI] [PubMed] [Google Scholar]
- Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Giampaolo A, Pelosi E, Valtieri M, Montesoro E, Sterpetti P, Samoggia P, Camagna A, Mastroberardino G, Gabbianelli M, Testa U. HOXB gene expression and function in differentiating purified hematopoietic progenitors. Stem Cells. 1995;13(Suppl 1):90–105. [PubMed] [Google Scholar]
- Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, Nolan DJ, Elemento O, Wood BL, Adair JE, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Investig. 2015;125:1243–1254. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Haemmerle R, Phaltane R, Rothe M, Schröder S, Schambach A, Moritz T, Modlich U. Clonal dominance with retroviral vector insertions near the ANGPT1 and ANGPT2 genes in a human Xenotransplant mouse model. Mol Ther Nucleic Acids. 2014;3:e200. doi: 10.1038/mtna.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happle C, Lachmann N, Skuljec J, Wetzke M, Ackermann M, Brennig S, Mucci A, Jirmo AC, Groos S, Mirenska A, et al. Pulmonary transplantation of macrophage progenitors as effective and long-lasting therapy for hereditary pulmonary alveolar proteinosis. Sci Transl Med. 2014;6:250ra113. doi: 10.1126/scitranslmed.3009750. [DOI] [PubMed] [Google Scholar]
- Hirai H, Ogawa M, Suzuki N, Yamamoto M, Breier G, Mazda O, Imanishi J, Nishikawa S. Hemogenic and nonhemogenic endothelium can be distinguished by the activity of fetal liver kinase (Flk)-1 promoter/enhancer during mouse embryogenesis. Blood. 2003;101:886–893. doi: 10.1182/blood-2002-02-0655. [DOI] [PubMed] [Google Scholar]
- Hirose S, Takayama N, Nakamura S, Nagasawa K, Ochi K, Hirata S, Yamazaki S, Yamaguchi T, Otsu M, Sano S, et al. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Rep. 2013;1:499–508. doi: 10.1016/j.stemcr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- Jang IH, Lu YF, Zhao L, Wenzel PL, Kume T, Datta SM, Arora N, Guiu J, Lagha M, Kim PG, et al. Notch1 acts via Foxc2 to promote definitive hematopoiesis via effects on hemogenic endothelium. Blood. 2015;125:1418–1426. doi: 10.1182/blood-2014-04-568170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Tamplin OJ, Chen MJ, Deng Q, Patterson S, Kim PG, Durand EM, McNeil A, Green JM, Matsuura S, et al. Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J Exp Med. 2015;212:649–663. doi: 10.1084/jem.20141528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KR, Cowley S, Martinez FO, Shaw M, Minger SL, James W. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol. 2008;36:1167–1175. doi: 10.1016/j.exphem.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zuniga-Pflucker JC, Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, Ruau D, Han DW, Zenke M, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Tian E, Nakano H, Amazaki S, Shimokawa K, Rossant J, Aizawa S, Matsuo I. Crucial roles of Foxa2 in mouse anterior-posterior axis polarization via regulation of anterior visceral endoderm-specific genes. Proc Natl Acad Sci USA. 2007;104:5919–5924. doi: 10.1073/pnas.0607779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RCR, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lachmann N, Happle C, Ackermann M, Luttge D, Wetzke M, Merkert S, Hetzel M, Kensah G, Jara-Avaca M, Mucci A, et al. Gene correction of human induced pluripotent stem cells repairs the cellular phenotype in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2014;189:167–182. doi: 10.1164/rccm.201306-1012OC. [DOI] [PubMed] [Google Scholar]
- Lachmann N, Ackermann M, Frenzel E, Liebhaber S, Brennig S, Happle C, Hoffmann D, Klimenkova O, Lüttge D, Buchegger T, et al. Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 2015;4:282–296. doi: 10.1016/j.stemcr.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet. 2011;45:357–377. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JB, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Lesinski DA, Heinz N, Pilat-Carotta S, Rudolph C, Jacobs R, Schlegelberger B, Klump H, Schiedlmeier B. Serum- and stromal cell-free hypoxic generation of embryonic stem cell-derived hematopoietic cells in vitro, capable of multilineage repopulation of immunocompetent mice. Stem Cells Transl Med. 2012;1:581–591. doi: 10.5966/sctm.2012-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MI, Price EN, Boatman S, Hagedorn EJ, Trompouki E, Satishchandran S, Carspecken CW, Uong A, DiBiase A, Yang S, et al. Angiopoietin-like proteins stimulate HSPC development through interaction with notch receptor signaling. Elife. 2015;4:e05544. doi: 10.7554/eLife.05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Liu F, Bhang SH, Arentson E, Sawada A, Kim CK, Kang I, Yu J, Sakurai N, Kim SH, Yoo JJW, et al. Enhanced hemangioblast generation and improved vascular repair and regeneration from embryonic stem cells by defined transcription factors. Stem Cell Rep. 2013;1:166–182. doi: 10.1016/j.stemcr.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S-J, Li F, Yin H, Feng Q, Kimbrel EA, Hahm E, Thon JN, Wang W, Italiano JE, Cho J, et al. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011;21:530–545. doi: 10.1038/cr.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rößner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, Zaike Y, Tsuchida E, Nakahata T, Nakauchi H, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zúñiga-Pflücker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010;185:867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Moreau T, Colzani M, Arumugam M, Evans A, Tijssen M, Trotter M, Ouwehand W, Pedersen R, Ghevaert C. In vitro production of megakaryocytes and platelets from human induced pluripotent cells by GMP compatible methods. Blood. 2013;122:2401. [Google Scholar]
- Murray PDF. 1932. The development in vitro of the blood of the early chick embryo.
- Myers CT, Krieg PA. BMP-mediated specification of the erythroid lineage suppresses endothelial development in blood island precursors. Blood. 2013;122:3929–3939. doi: 10.1182/blood-2013-03-490045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Takagi Y, Osawa M, Oshima M, Takagi H, Miyagi S, Endoh M, Endo TA, Takayama N, Eto K, Toyoda T, et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121:447–458. doi: 10.1182/blood-2012-05-431403. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, Fujita K, Koike T, Harimoto K, Dohda T, et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14:535–548. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Niwa A, Heike T, Umeda K, Oshima K, Kato I, Sakai H, Suemori H, Nakahata T, Saito MK. A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS One. 2011;6:e22261. doi: 10.1371/journal.pone.0022261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- Ohta M, Sakai T, Saga Y, Aizawa S, Saito M. Suppression of hematopoietic activity in tenascin-C-deficient mice. Blood. 1998;91:4074–4083. [PubMed] [Google Scholar]
- Olivier EN, Qiu C, Velho M, Hirsch RE, Bouhassira EE. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Dieterlen-Lievre F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development. 1999;126:617–627. doi: 10.1242/dev.126.4.617. [DOI] [PubMed] [Google Scholar]
- Pawlak G, Grasset MF, Arnaud S, Blanchet JP, Mouchiroud G. Receptor for macrophage colony-stimulating factor transduces a signal decreasing erythroid potential in the multipotent hematopoietic EML cell line. Exp Hematol. 2000;28:1164–1173. doi: 10.1016/s0301-472x(00)00522-1. [DOI] [PubMed] [Google Scholar]
- Pereira C-F, Chang B, Qiu J, Niu X, Papatsenko D, Hendry CE, Clark NR, Nomura-Kitabayashi A, Kovacic JC, Ma’ayan A, et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 2013;13:205–218. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Burridge PW, Pryzhkova MV, Levine MA, Park T-S, Roxbury C, Yuan X, Péault B, Zambidis ET. Challenges and strategies for generating therapeutic patient-specific hemangioblasts and hematopoietic stem cells from human pluripotent stem cells. Int J Dev Biol. 2010;54:965–990. doi: 10.1387/ijdb.093043ap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- Pick M, Azzola L, Osborne E, Stanley EG, Elefanty AG. Generation of megakaryocytic progenitors from human embryonic stem cells in a feeder- and serum-free medium. PLoS One. 2013;8:e55530. doi: 10.1371/journal.pone.0055530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, Lohnes D. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pineault N, Helgason CD, Lawrence HJ, Humphries RK. Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Exp Hematol. 2002;30:49–57. doi: 10.1016/s0301-472x(01)00757-3. [DOI] [PubMed] [Google Scholar]
- Pulecio J, Nivet E, Sancho-Martinez I, Vitaloni M, Guenechea G, Xia Y, Kurian L, Dubova I, Bueren J, Laricchia-Robbio L, et al. Conversion of human fibroblasts into monocyte-like progenitor cells. Stem Cells. 2014;32:2923–2938. doi: 10.1002/stem.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Hanson E, Olivier E, Inada M, Kaufman DS, Gupta S, Bouhassira EE. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Rafii S, Kloss CC, Butler JM, Ginsberg M, Gars E, Lis R, Zhan Q, Josipovic P, Ding BS, Xiang J, et al. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood. 2013;121:770–780. doi: 10.1182/blood-2012-07-444208. [DOI] [PubMed] [Google Scholar]
- Ran D, Shia W-J, Lo M-C, Fan J-B, Knorr DA, Ferrell PI, Ye Z, Yan M, Cheng L, Kaufman DS, et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121:2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Poueymirou WT, Rojas J, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rongvaux A, Eynon EE, Manz MG, Flavell RA. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118:3119–3128. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- Riddell J, Gazit R, Garrison BS, Guo G, Saadatpour A, Mandal PK, Ebina W, Volchkov P, Yuan G-C, Orkin SH, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157:549–564. doi: 10.1016/j.cell.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res. 2002;11:5–7. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- Saeki K, Saeki K, Nakahara M, Matsuyama S, Nakamura N, Yogiashi Y, Yoneda A, Koyanagi M, Kondo Y, Yuo A. A feeder-free and efficient production of functional neutrophils from human embryonic stem cells. Stem Cells. 2009;27:59–67. doi: 10.1634/stemcells.2007-0980. [DOI] [PubMed] [Google Scholar]
- Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, Butler JM, Scandura JM, Rafii S. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511:312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, Humphries RK. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Senju S, Haruta M, Matsumura K, Matsunaga Y, Fukushima S, Ikeda T, Takamatsu K, Irie A, Nishimura Y. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–883. doi: 10.1038/gt.2011.22. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shi X, Richard J, Zirbes KM, Gong W, Lin G, Kyba M, Thomson JA, Koyano-Nakagawa N, Garry DJ. Cooperative interaction of Etv2 and Gata2 regulates the development of endothelial and hematopoietic lineages. Dev Biol. 2014;389:208–218. doi: 10.1016/j.ydbio.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Siminovitch L, McCulloch EA, Till JE. The Distribution of Colony-Forming Cells among Spleen Colonies. J Cell Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Slukvin II. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle. 2013a;12:720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin II. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013b;122:4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Frye C, Bae K-M, Kelley V, Vieweg J. Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin Cancer Res. 2008;14:6207–6217. doi: 10.1158/1078-0432.CCR-08-0309. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, Otsu M, Nakauchi H. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013;21:1424–1431. doi: 10.1038/mt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Arumugam P, Sakagami T, Lachmann N, Chalk C, Sallese A, Abe S, Trapnell C, Carey B, Moritz T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014a;514:450–454. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mayhew C, Sallese A, Chalk C, Carey BC, Malik P, Wood RE, Trapnell BC. Use of induced pluripotent stem cells to recapitulate pulmonary alveolar proteinosis pathogenesis. Am J Respir Crit Care Med. 2014b;189:183–193. doi: 10.1164/rccm.201306-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takash W, Canizares J, Bonneaud N, Poulat F, Mattei MG, Jay P, Berta P. SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res. 2001;29:4274–4283. doi: 10.1093/nar/29.21.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K, Kawabata K, Omori M, Yamaguchi T, Sakurai F, Katayama K, Hayakawa T, Mizuguchi H. Promotion of hematopoietic differentiation from mouse induced pluripotent stem cells by transient HoxB4 transduction. Stem Cell Res. 2011;8:300–311. doi: 10.1016/j.scr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Jr, Cannon JH, Sahler OD, Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–1716. doi: 10.1172/JCI103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE. Radiation effects on the division cycle of mammalian cells in vitro. Ann N Y Acad Sci. 1961;95:911–919. doi: 10.1111/j.1749-6632.1961.tb50086.x. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Van Coppernolle S, Taghon T, Moore HD, Leclercq G, Langerak AW, Kerre T, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182:6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- Toscano M, Navarro-Montero O, Ayllon V, Ramos-Mejia V, Guerrero-Carreno X, Bueno C, Romero T, Lamolda M, Cobo M, Martin F, et al. SCL/TAL1-mediated Transcriptional Network Enhances Megakaryocytic Specification of Human Embryonic Stem Cells. Mol Ther. 2015;23:158–170. doi: 10.1038/mt.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenishi G, Theisen D, Lee JH, Kumar A, Raymond M, Vodyanik M, Swanson S, Stewart R, Thomson J, Slukvin I. Tenascin C Promotes Hematoendothelial Development and T Lymphoid Commitment from Human Pluripotent Stem Cells in Chemically Defined Conditions. Stem Cell Rep. 2014;3:1073–1084. doi: 10.1016/j.stemcr.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhee S, De Mulder K, Van Caeneghem Y, Verstichel G, Van Roy N, Menten B, Velghe I, Philippe’ J, De Bleser D, Lambrecht BN, et al. In vitro human embryonic stem cell hematopoiesis mimics MYB-independent yolk sac hematopoiesis. Haematologica. 2015;100:157–166. doi: 10.3324/haematol.2014.112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi AM, Paoletti F, Linari S, Cellai C, Caporale R, Ferrini PR, Sanchez M, Migliaccio G, Migliaccio AR. Identification and characterization of a bipotent (erythroid and megakaryocytic) cell precursor from the spleen of phenylhydrazine-treated mice. Blood. 2000;95:2559–2568. [PubMed] [Google Scholar]
- Vereide DT, Vickerman V, Swanson SA, Chu LF, McIntosh BE, Thomson JA. An Expandable, Inducible Hemangioblast State Regulated by Fibroblast Growth Factor. Stem Cell Rep. 2014;3:1043–1057. doi: 10.1016/j.stemcr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, Cerdan C, Levac K, Bhatia M. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005a;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005b;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nakayama N. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Res. 2009;3:113–125. doi: 10.1016/j.scr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- van Wilgenburg B, Browne C, Vowles J, Cowley SA. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One. 2013;8:e71098. doi: 10.1371/journal.pone.0071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- Yoder MC. Inducing definitive hematopoiesis in a dish. Nat Biotechnol. 2014;32:539–541. doi: 10.1038/nbt.2929. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Suzuki T, Sakata-Yanagimoto M, Kumano K, Higashi K, Takato T, Kurokawa M, Ogawa S, Chiba S. Derivation of functional mature neutrophils from human embryonic stem cells. Blood. 2009;113:6584–6592. doi: 10.1182/blood-2008-06-160838. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH, Yoder MC. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, Pryzhkova M, Peault B. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 2002;21:3295–3313. doi: 10.1038/sj.onc.1205318. [DOI] [PubMed] [Google Scholar]
- Zhu M-X, Wan W-L, Li H-S, Wang J, Chen G-A, Ke X-Y. Thymopentin enhances the generation of T-cell lineage derived from human embryonic stem cells in vitro. Exp Cell Res. 2015;331:387–398. doi: 10.1016/j.yexcr.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Zuckerman KS, Wicha MS. Extracellular matrix production by the adherent cells of long-term murine bone marrow cultures. Blood. 1983;61:540–547. [PubMed] [Google Scholar]