Abstract
Placentas associated with preeclampsia are characterized by extensive apoptosis in trophoblast lineages. Syncytin-1 (HERVWE1) mediates the fusion of cytotrophoblasts to form syncytiotrophoblasts, which assume the placental barrier, fetal–maternal exchange and endocrine functions. While decreased syncytin-1 expression has been observed in preeclamptic placentas, it is not clear if this alteration is involved in trophoblast apoptosis. In the current study, we found that siRNA-mediated knockdown of syncytin-1 led to apoptosis in choriocarcinoma BeWo, a cell line of trophoblastic origin. Characterization of the apoptotic pathways indicated that this effect does not rely on the activation of caspases. Rather, decreased syncytin-1 levels activated the apoptosis inducing factor (AIF) apoptotic pathway by inducing the expression, cleavage, and nuclear translocation of AIF. Moreover, calpain1, the cysteine protease capable of cleaving AIF, was upregulated by syncytin-1 knockdown. Furthermore, treatment with calpain1 inhibitor MDL28170 effectively reversed AIF cleavage, AIF nuclear translocation, and cell apoptosis triggered by syncytin-1 downregulation, verifying the specific action of calpain1–AIF pathway in trophoblast apoptosis. We confirmed that preeclamptic placentas express lower levels of syncytin-1 than normal placentas, and observed an inverse correlation between syncytin-1 and AIF/calpain1 mRNA levels, a result consistent with the in vitro findings. Immunohistochemistry analyses indicated decreased syncytin-1 and increased AIF and calpain1 protein levels in apoptotic cells of preeclamptic placentas. These findings have for the first time revealed that decreased levels of syncytin-1 can trigger the AIF-mediated apoptosis pathway in BeWo cells. This novel mechanism may contribute to the structural and functional deficiencies of syncytium frequently observed in preeclamptic placentas.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1533-8) contains supplementary material, which is available to authorized users.
Keywords: Syncytin-1, Trophoblast, Apoptosis, Apoptosis inducing factor, Preeclampsia
Introduction
Preeclampsia is the significant cause of pregnancy-related mortality and morbidity, occurring in 3–5 % of pregnancies [1]. The diagnostic criteria of preeclampsia include hypertension and proteinuria after the 20th week of gestation [2]. Currently, treatment of preeclampsia is limited to symptom control and/or early termination of pregnancy in cases of severe manifestations. Intrauterine growth retardation (IUGR) is another major disorder which is associated with 5–10 % of pregnancies. Preeclampsia and IUGR overlap in some patients (28.6 % of preeclamptic patients have IUGR, and 5.6 % of patients with IUGR have preeclampsia), and appear to share some common etiological backgrounds, such as maternal age, familial aggregations, and immature vasculature of the spiral arteries [3]. The precise pathological mechanism(s) of preeclampsia and IUGR is not yet fully understood. The insufficient knowledge is partially blamed for the slow progress in the development of effective prevention and treatment modalities for preeclampsia and IUGR.
While it is debatable whether preeclampsia could be defined as a “placental disease”, the intimate involvement of structural and functional alterations of placenta in the pathogenesis of preeclampsia has been well recognized [4]. Clinical investigation has shown that preterm placentas from patients with preeclampsia and IUGR are significantly smaller than those from normal pregnancies [5]. Inflammatory responses as evidenced by the infiltration of macrophages and natural killer cells are observed in preeclamptic placentas [6]. These cells release cytokines, macrophage colony stimulating factor, and tumor necrosis factor-α [7], which may further augment the inflammatory reactions. Significant alterations in placental endocrine activity were also implicated in the development of preeclampsia [8].
Notably, one of the significant observations frequently seen in preeclamptic placenta is the increased apoptosis in trophoblast lineages. These changes, as well as the ensuing secondary reactions, are considered key cellular events for the development of preeclampsia [9–11]. Syncytium, formed by a continuous layer of syncytiotrophoblasts, constitutes the placental barrier [12]. Syncytiotrophoblasts also carry out the fetal–maternal exchange and endocrine functions that are critical for the maintenance of normal pregnancy [13]. Syncytial debris is normally detected in the maternal circulation, indicating that continual shedding and replacement of syncytium is a part of normal placental physiology [14]. Preeclamptic placentas are associated with increased trophoblast deportation as well as higher levels of placental nucleic acids (DNA, RNA) in maternal serum, which reflect the increased trophoblast apoptosis as directly detected by TUNEL staining of preeclamptic placentas [15, 16]. It is thought that the increased deportation of syncytiotrophoblast in preeclampsia may lead to an increased release of human chorionic gonadotropin (hCG) and the rise of hCG levels in maternal circulation [17, 18]. Microscopic observation found that the syncytium of preeclamptic placentas is thin, vacuolated, and discontinued [19]. Moreover, Langbein et al. [20] have shown that trophoblasts isolated from preeclamptic placentas underwent active apoptosis and displayed defects in cell fusion function. Breaching of the placental barrier caused by excessive apoptosis may lead to leakage of fetal antigens into the maternal circulation, thereby activating the immune and inflammatory responses.
The fusogenic function of syncytin-1 in trophoblast differentiation has been well characterized. By binding to its membrane receptor ASCT2, syncytin-1 meditates the fusion of cytotrophoblasts to form syncytiotrophoblasts [21]. Reduced syncytin-1 expression is often found in preeclamptic placentas [22]. Moreover, in vitro studies have shown that hypoxic conditions correlate with downregulation of syncytin-1 expression in placental trophoblasts [23]. Based on these observations, the decreased levels of syncytin-1 and consequent cell fusion defects are thought to be responsible for syncytium deficiency [20]. However, recent studies suggest that syncytin-1 may also carry out nonfusogenic functions, including those involving anti-apoptotic mechanisms [24–26]. For example, Knerr et al. [24, 25] reported that ectopic overexpression of syncytin-1 in the Chinese hamster ovarian cancer cell line, CHO, resulted in increased resistance to cell death triggered by apoptosis-inducing reagents. It remains unclear if the downregulation of endogenous syncytin-1 could contribute to syncytium deficiency through a nonfusogenic pathway in placental villous trophoblasts, in which syncytin-1 is specifically expressed.
Programmed cell death can be mediated by either caspase-dependent or caspase-independent apoptosis inducing factor (AIF)-mediated pathways. First identified in hepatocytes, AIF is a dual function flavoprotein with both NADH/NADPH oxidase and apoptotic activities [27]. Upon cleavage by the cysteine protease, calpain1, AIF is released from the mitochondrial inner membranes and translocated into nuclei. Within the nucleus, AIF binds to genomic DNA, leading to DNA degradation and cell apoptosis by an unidentified mechanism [28]. Increased AIF expression and the consequent augmentation of apoptotic activities have been observed in neurologic recessive diseases. AIF-mediated apoptosis is also thought to play a critical role in autophagy during neurologic maturation and remodeling [29, 30]. Interestingly, a recent report seemed to suggest a potential involvement of AIF in trophoblast function and placental development. Riddell et al. [31] observed that AIF is expressed in trophoblast lineages. cAMP was able to induce a low level of AIF nuclear translocation, but the process did not affect trophoblast differentiation. The potential role of AIF for trophoblast cell death, however, has not been investigated. In the current study, we apply in vitro cell culture and patient placental specimens to determine whether the syncytin-1 downregulation, which has been known to be associated with preeclampsia and hypoxic conditions, could induce BeWo cell apoptosis, and how the caspase and/or calpain1–AIF pathways may be involved in this important cellular process.
Materials and methods
Collection of placental tissues
Placental tissues were collected from patients with severely preeclampsia (n = 8) and normal pregnancies (n = 8), respectively, at the Department of Obstetrics and Gynecology, Mayo Clinic, Rochester, Minnesota, USA. The term placentas were used as study subjects with the patients’ consent as well as approval by the Institutional Review Board (IRB). Preeclampsia was diagnosed following the guidelines recommended by the American College of Obstetricians and Gynecologist (http://the-medical-dictionary.com/eclampsia_article_5.htm). Normal placentas were obtained from pregnancies without maternal complications or fetal abnormalities, and 2 cm3 of placental specimens were dissected from the central part of the maternal side of the placentas. The placental tissues were washed with cold PBS, and cut into two parts. One-half of the tissue was snap frozen in liquid nitrogen and stored at −70 °C for RNA isolation. The remaining half was fixed with 4 % paraformaldehyde and paraffin-embedded. Serial sections of 4 μm thickness were prepared for immunohistochemistry analysis.
Cell culture and siRNA transfection
BeWo cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained at 37 °C and 5 % CO2 in the RPMI 1640 medium (Thermal Scientific, Logan, UT, USA) supplemented with fetal bovine serum (10 %), streptomycin (100 μg/ml), and penicillin (100 μg/ml).
Four syncytin-1-specific siRNAs and one control siRNA were designed and synthesized by Qiagen (Valencia, CA, USA). The sequences of siRNAs are shown in Table S1. Cells were seeded at low density and transfected at 40–50 % confluence with the DharmaFECT 1 transfection reagents (Thermal Scientific, Lafayette, CO, USA) in serum-free RPMI 1640 medium. Following exposure to transfection reagents for 6 h, the serum-free medium was replaced by regular medium. mRNA,and protein levels of target genes were determined and compared at different post-transfection time points.
RNA isolation and real-time PCR
Total RNA was isolated from BeWo cells and placental tissues using the RNeasy Plus Mini Kit and RNeasy Mini Kit (Qiagen, Valencia, CA, USA), respectively. Reverse transcription was performed with High Capacity RNA-to-cDNA Kit (ABI, Foster City, CA, USA) using 1 μg RNA in 20 μl of volume. The cDNA was diluted to 100 μl for real-time PCR. Primer sequences and the sizes of amplicons for syncytin-1, AIF, calpain1, and glyceraldehyde phosphate dehydrogenase (GAPDH) are summarized in Table S2. Real-time PCR was carried out in 12 μl reactions, each containing 6 μl of 2× SYBR Green PCR Master Mix (ABI), 1 μl of forward primer, 1 μl of backward primer, 3 μl of DEPC H2O, and 1 μl of diluted cDNA templates. Following the initial denaturation at 95 °C for 10 min, 40 cycles of amplification were performed using the following conditions: denaturation at 95 °C for 30 s, annealing and extension at 60 °C for 1 min. The threshold cycles were determined in triplicate with the use of ABI 7900 Real Time PCR System. To ensure the accuracy of real-time results, the final products from real-time PCR was examined in agarose gel electrophoresis. The single DNA band at expected size indicated the achievement of high specificity and, therefore, an acceptable data reliability (Fig. S1).
Western blot analysis
Proteins were isolated from BeWo cells using RIPA buffer (Boston BioProducts, Boston, MA, USA) which was supplemented with Halt™ Protease Inhibitor Cocktail (Thermal Scientific, Rockford, IL, USA) and 1 % PMSF. Protein concentrations were measured with the use of Bradford Assay. Amounts of 20 μg of cell lysates were resolved in 8 % (for AIF, syncytin-1, calpain1) or 12 % (caspase 3, 8, 9, and β-actin) polyacrylamide SDS gels and transferred to polyvinylidene fluoride membranes using the transfer buffer (Boston BioProducts). Membranes were probed with the primary antibodies against human syncytin-1 (rabbit 1:800) (Genetex, Irvine, CA, USA), AIF (rabbit 1:800) (Epitomics, Burlingame, CA, USA), calpain1 (rabbit 1:500) (Epitomics), caspase 3 (rabbit 1:1,000) (Cell Signaling, Boston, MA, USA), caspase 8 (mouse 1:1,000) (Cell Signaling), caspase 9 (rabbit 1:1,000) (Cell Signaling), β-actin (mouse 1:3,000) (Sigma-Aldrich, St. Louis, MO, USA), and the matching secondary antibody (peroxidase-labeled anti-mouse IgG or anti-rabbit IgG; 1:4,000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Super Signal Western Femto Maximum Sensitivity Substrate (Thermal Scientific, Rockford, IL, USA) was used to enhance the detection sensitivity and densitometry analyses were performed following previously described procedures [32].
Immunohistochemistry
Serial sections of 4 μm thickness were prepared from fixed and paraffin-embedded placental tissue blocks. These sections were used in immunohistochemistry and TUNEL assays. For de-paraffinization and rehydration, the tissue sections were sequentially treated with xylene (5 min × 2), 100 % ethanol (3 min × 2), 95 % ethanol (3 min × 2), 80 % ethanol (3 min × 1), 70 % ethanol (3 min × 1), and distilled water (3 min × 2). For antigen retrieval, the slides were immersed in 10 mM citrate buffer (pH 6.0) and heated to 95 °C for 15 min in a water bath. The slides were cooled to room temperature for 30 min. Endogenous peroxidase activity was quenched with 3 % hydrogen peroxide. After incubation in blocking solution (1 % gelatin) at room temperature for 1 h, the sections were incubated overnight with rabbit antibodies against syncytin-1 (1:100), AIF (1:150), or calpain1 (1:300), respectively. Biotinylated sheep IgG raised against rabbit IgG (1:400) (Vector Laboratories, Burlingame, CA, USA) was applied as secondary antibody. Following each incubation with primary or secondary antibodies, the slides were washed with PBS containing 0.1 % Tween-20. Color development was carried out with the use of VECTASTAIN Elite ABC Kits (Vector Laboratories) and diaminobenzidine tetrahydrochloride (DAB) (Sigma-Aldrich) following the manufacturer’s instructions.
BeWo cells were seeded on the Lab-Tek II Chamber Slide (Thermal Scientific, Rochester, NY, USA). At 72 h post-transfection with syncytin-1 siRNA, cells were fixed with 4 % paraformaldehyde (Boston BioProducts). AIF and calpain1 immunostaining was performed using the same procedures as for tissue sections.
TUNEL assay
TUNEL Apoptosis Detection Kit (Millipore, Billerica, MA, USA) was used to detect apoptotic cells in placental tissue sections. Following de-paraffinization and rehydration, the slides were examined using the protocols recommended by the manufacturer. For apoptosis analysis in BeWo cell cultures, following transfection with the syncytin-1 siRNA, the cells were fixed and examined using the same procedures as those applied for placental tissue sections. Apoptotic cells and total cells were counted in ten randomly chosen microscopic fields, and the percentage of apoptotic cells was calculated and compared between the experimental and control groups.
Statistical methods and data presentation
Statistical analysis was performed with the use of SPSS 13.0 statistical package (SPSS, Chicago, IL, USA). The mRNA levels of syncytin-1, calpain1 and AIF were standardized by those of the correspondent GAPDH internal reference before calculation of their changes in the experimental groups over the control groups. Student’s t test was applied to compare between syncytin-1 siRNA knockdown and control groups. Student’s t test was also used to compare the mRNA levels between the preeclamptic and normal placentas. The relationship between the mRNA levels of AIF/calpain1 and syncytin-1 was determined by Spearman rank correlation analysis with reports on the correlation coefficient and P values. Statistical significance was set at the level of P ≤ 0.05. Quantitative data are presented in the histogram figures as the average folds relative to their correspondent controls. Standard error bars and statistical significance are presented in the figures.
Results
Establishment of syncytin-1 knockdown assay
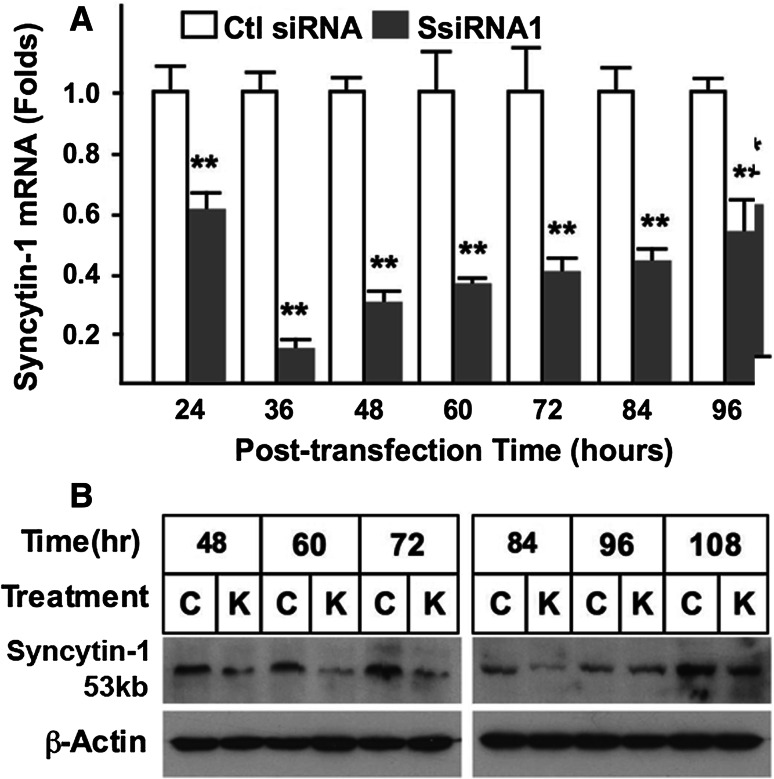
BeWo is a choriocarcinoma cell line that maintains several morphological and functional features characteristic of placental trophoblasts. Expressing human chorionic gonadotropin (hCG) and human chorionic somatomammotropin (hCS), as well as receptors for estrogen [33] and progesterone [26], these cells have been frequently used as an in vitro model for studying placental gene regulation and trophoblast cell differentiation. Syncytin-1 expression in this cell line has been confirmed, and a small percentage of cells (less than 5 %) constantly undergo cell fusion [34]. In this study, we used BeWo cells as a study model for initial characterization of syncytin-1 function. Four candidate syncytin-1 siRNAs were synthesized and tested to identify the most effective siRNA species for syncytin-1 knockdown. Following cell transfection, syncytin-1 mRNA levels were examined by real-time PCR at 48 h post-transfection. When a final concentration of 100 nM was applied, only syncytin-1 siRNA1 was able to significantly reduce the syncytin-1 mRNA level (Fig. S2). Using this siRNA, we subsequently performed a time course study and observed syncytin-1 mRNA levels to be decreased starting at 24 h post-transfection, and reaching the lowest levels (approximately 10 % of the controls) at 36 h post-transfection (Fig. 1a). Following the 36 h time-point, syncytin-1 mRNA levels gradually increased, but remained significantly lower than controls even at 96 h post-transfection. The syncytin-1 knockdown of protein levels was determined by Western blot analysis (Fig. 1b), in which the unglycosylated 53-kDa syncytin-1 was detected. Decreased syncytin-1 protein expression was readily detected at 48 h post-transfection, and lower levels were observed at 60, 72, and 84 h. These results established the efficacy of siRNA-mediated syncytin-1 knockdown in BeWo cells.
Fig. 1.
Confirmation of syncytin-1 knockdown by siRNA in BeWo cells. a BeWo cells were transfected with 100 nM (final concentration) of syncytin-1 specific siRNA (SsiRNA1) or control siRNA (Ctl siRNA). At various post-transfection time points, syncytin-1 mRNA levels were measured with real-time PCR. A significant decrease of syncytin-1 mRNA levels was observed following transfection with SsiRNA1 (**P < 0.01). b Western blot analysis. Syncytin-1 protein (57 kDa) was detected with syncytin-1-specific antibody at different post-transfection time points. β-actin was detected in the same blots for protein loading controls. Compared to the cells transfected with control siRNA (C), cells transfected with SsiRNA1 (K) contain decreased levels of syncytin-1
Decreased levels of syncytin-1 were accompanied by cell apoptosis
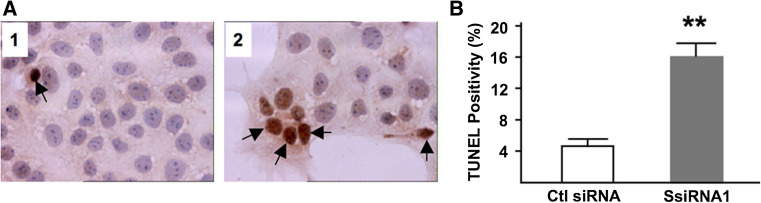
During syncytin-1 knockdown, BeWo cell culture was closely monitored under phase contrast microscope. While no significant morphological alteration was observed following the knockdown of syncytin-1, siRNA1-transfected cultures appeared to contain more floating cells and cell debris than did the culture transfected with control siRNA, suggesting the presence of cell apoptosis. Consequently, TUNEL staining was performed and the number of apoptotic cells were counted and compared. Fourfold more TUNEL-positive cells were found in the syncytin-1 knockdown culture than the control group (Fig. 2; 15.9 ± 1.4 vs. 4.3 ± 0.5 %, P < 0.01). Experiments with varied syncytin-1 siRNA1 concentrations and post-transfection time indicated cohesive time- and dose-dependent responses by BeWo cells to decreased endogenous syncytin-1 levels (supplemental data, Figs. S3, S4). In addition to the brown-colored staining, many TUNEL-positive cells exhibited other features characteristic of apoptosis, including condensed and occasionally disintegrated nuclei (Fig. 2), as well as large vacuoles in the cytoplasm under phase-contrast microscope (data not shown).
Fig. 2.
Syncytin-1 knockdown-induced BeWo cell apoptosis. a Representative results of TUNEL assay (×40). BeWo cells transfected with control (panel 1) or syncytin-1 specific siRNA (panel 2) were examined with TUNEL for apoptosis at 48 h post-transfection. Nuclei were counter stained with hematoxylin. Brown staining in the nuclei indicated cell apoptosis (arrows). b Apoptotic and total cells were counted in ten randomly selected microscopic areas and the percentage of apoptotic cells was calculated for the control (Ctl siRNA) and syncytin-1 knockdown groups (SsiRNA1). A significant increase in apoptotic cells was observed in the syncytin-1 knockdown group compared to the control group (**P < 0.01). Note the condensed and disintegrated nuclei in some cells following syncytin-1 knockdown
Apoptosis caused by decreased levels of syncytin-1 is independent of the caspase pathway
Caspase activation has been well recognized for its critical role in the cell death process. Previous immunohistochemistry analysis of preeclamptic placentas showed an increase of TUNEL-positivity in the trophoblastic lineage. This alteration coincided with an elevated caspase 3 expression, pointing to the involvement of caspase-mediated cell death in preeclamptic placentas [35–38]. Another study demonstrated that, when treated with nimbolide, BeWo cells underwent apoptosis through initiation of the caspase pathway [39]. Consequently, we performed Western blotting on caspases 8, 9, and 3 to assess the involvement of both intrinsic and extrinsic apoptotic pathways. To our surprise, following syncytin-1 knockdown, no change in either the cleavage or the expression of caspases was found at any time point examined (Fig. 3). These results suggest that the syncytin-1 knockdown-triggered apoptosis is independent of the classic caspase pathway.
Fig. 3.
Caspase expression and cleavage are not affected by syncytin-1 knockdown. At different time points, BeWo cells transfected with control siRNA (C) or syncytin-1-siRNA (K) were examined for caspase 3, 8, and 9 cleavage in Western blot analysis. β-Actin was detected in the same blot for protein loading controls. Although there appeared to be some variation in the expression and cleavage across different time points, comparison between the two groups at the same time point did not find significant change in the expression or cleavage of caspases
Syncytin-1 knockdown activates AIF through increased AIF cleavage and upregulation of AIF expression
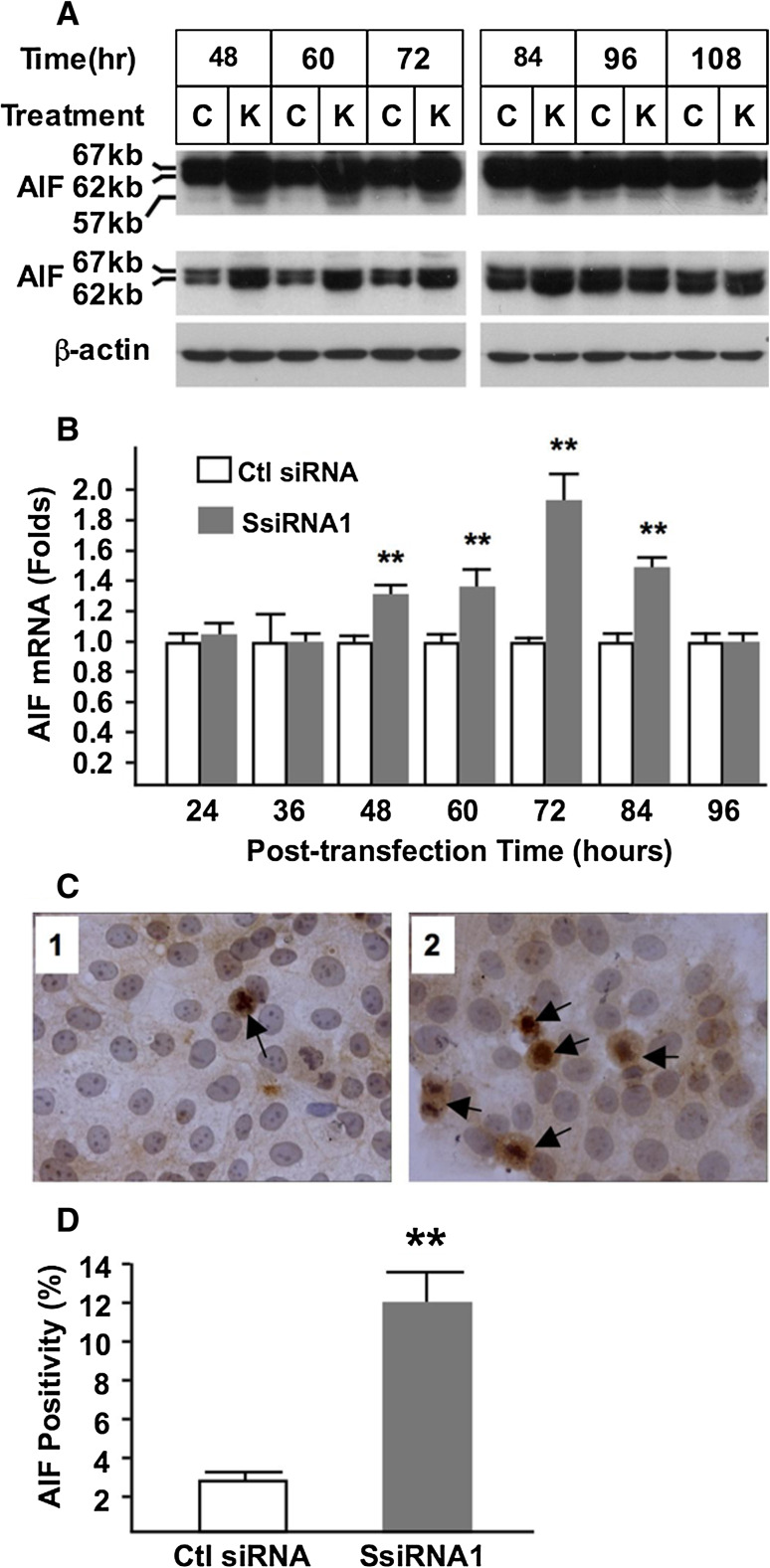
Since syncytin-1 knockdown-triggered BeWo cell apoptosis could not be explained by caspase activation, we searched for other apoptotic pathways that could potentially be involved. AIF is a dual function flavoprotein involved in mitochondrial respiratory function [40]. Its cleavage by calpain1, releasing from the inner membrane of mitochondria to the cytoplasm and subsequent translocation into nuclei, was reported to lead to chromatin condensation and DNA degradation in non-trophoblast cells [28]. Therefore, we examined AIF cleavage and its involvement in trophoblast apoptosis triggered by syncytin-1 knockdown. Results from Western blot analysis confirmed the expression of AIF in BeWo cells (Fig. 4a, top panel). Importantly, the same experiments also indicated that upon syncytin-1 knockdown, AIF cleavage is significantly increased at 48–84 h post-transfection time points compared to the control groups (Fig. 4a, mid-panel). Real-time PCR results showed that AIF mRNA levels were elevated in the same time window (Fig. 4b). Moreover, immunostaining using AIF antibody demonstrated AIF nuclear translocation in many cells at 72 h post-transfection following syncytin-1 knockdown (Fig. 4c, d). Counting of AIF-positive cells with visible AIF nuclear translocation showed an increased AIF positivity in syncytin-1 knockdown cells. A close inspection of the AIF-positive cells also led to the realization that AIF staining often concentrates in the nucleus, an observation consistent with the reported AIF-binding to genomic DNA as a key step for DNA degradation and cell death [28]. These findings, together with the increased cell apoptosis observed at the same time points, strongly suggested the involvement of AIF cleavage and nuclear translocation in the apoptosis triggered by syncytin-1 knockdown.
Fig. 4.
Activation of AIF by syncytin-1 knockdown. a BeWo cells were examined for AIF cleavage by Western blot analysis at various post-transfection time points. Positions and sizes of uncleaved and cleaved AIF were indicated. Top panel long exposure, middle panel short exposure, bottom panel β-actin. At 48, 60, 72, and 84 h post-transfection, increased total AIF protein as well as increased AIF cleavage were detected in the cells transfected with SsiRNA1 (K) compared to cells transfected with control siRNA (C). b AIF mRNA levels were determined by real-time PCR. At 48, 60, 72, and 84 h post-transfection, significantly increased AIF mRNA levels were found in the syncytin-1 knockdown (SsiRNA1) groups compared to control groups (Ctl siRNA) (**P < 0.01). c BeWo cells transfected with control siRNA (panel 1) or syncytin-1-siRNA (panel 2) were examined by immunocytochemistry using the AIF-specific antibody. Nuclei were counterstained with hematoxylin. Increased AIF translocation was observed in a number of cells, which are defined as AIF-positive (arrows). d AIF-positive and total cells were counted and the AIF-positivities were compared. A significant increase in the percentage of AIF-positive cells was observed in the syncytin-1 knockdown group (SsiRNA1) compared to the control group (Ctl siRNA) (**P < 0.01). Note the concentrated staining of nucleolus in some AIF-positive cells
One intriguing observation is the increase in both uncleaved and cleaved AIF from 48–84 h post-transfection. Thus, syncytin-1 knockdown may upregulate AIF through promotion of AIF gene transcription and/or the enhancement of AIF mRNA stability. The exact mechanism responsible for AIF upregulation is unclear at this time. Nevertheless, our results indicated that AIF activation by syncytin-1 knockdown is accomplished by AIF upregulation, increased cleavage, and increased nuclear translocation.
AIF cleavage is accompanied by calpain1 activation
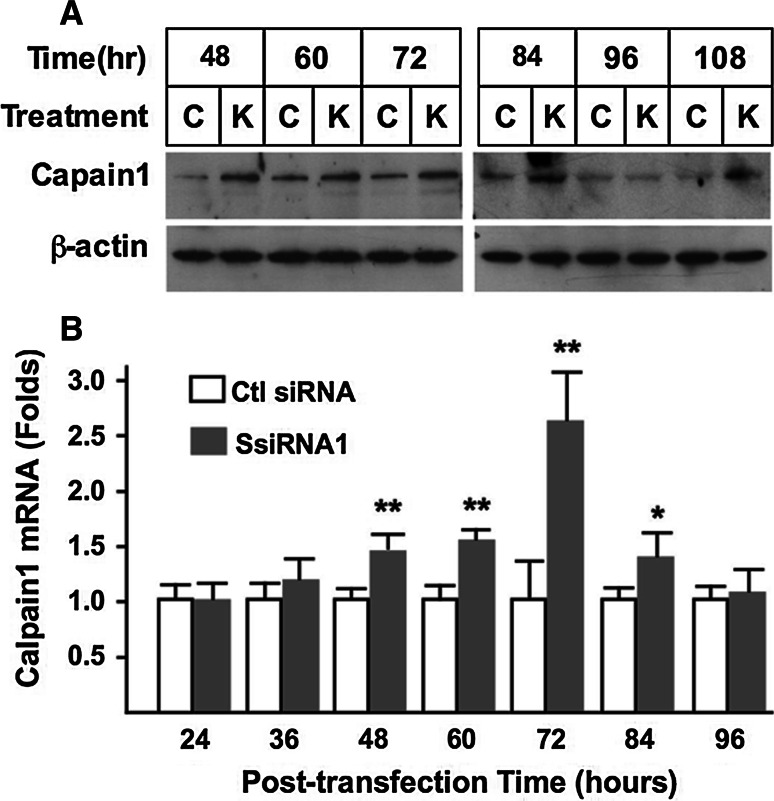
To investigate the mechanism involved in the increased AIF cleavage following syncytin-1 knockdown, we assessed changes in calpain1, the protease implicated in AIF cleavage in previous studies [41]. As shown in Fig. 5a, syncytin-1 knockdown caused an increase of calpain1 protein levels from 48 to 84 h post-transfection, the same time window for increased AIF cleavage (Fig. 4a). We subsequently measured calpain1 mRNA levels, which were also elevated at 48–84 h post-transfection. Thus, as demonstrated in Fig. 5b, syncytin-1 knockdown, through upregulation of calpain1 steady-state mRNA levels, was able to induce calpain1 protein expression, leading to increased AIF cleavage and nuclear translocation (Fig. 9, for pathway illustration).
Fig. 5.
Induction of calpain1 expression by syncytin-1 knockdown. a Western blot analysis. At various post-transfection time points BeWo cells were examined for calpain1 expression levels. At 48, 60, 72, and 84 h post-transfection, increased calpain1 protein levels were detected in the cells transfected with syncytin-1-siRNA (SsiRNA1, marked as K) compared to cells transfected with control siRNA (marked as C). β-Actin was detected in the same blot as protein loading controls. b Calpain1 mRNA levels were determined by real-time PCR. At 48, 60, 72, and 84 h post-transfection, significantly increased calpain1 mRNA levels were found in the syncytin-1 knockdown (SsiRNA1) groups compared to the correspondent control (Ctl siRNA) groups (**P < 0.01, *P < 0.05)
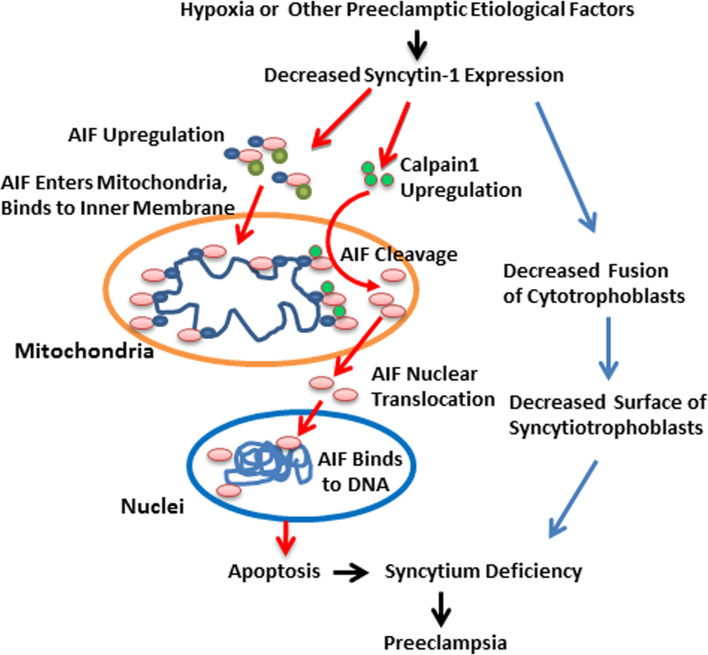
Fig. 9.
Schematic illustration of how AIF-mediated trophoblast apoptosis induced by decreased levels of syncytin-1 may be involved in the pathogenesis of preeclampsia. The left side of the chart (connected with red arrows) illustrates the nonfusogenic effect of syncytin-1, which is the focus of current study. Decreased syncytin-1 levels induced by hypoxia or other preeclamptic etiological factors can upregulate the expression of calpain1 and AIF. Calpain1 cleaves AIF located on the inner membrane of mitochondria, promoting its release from mitochondria and translocation to nuclei. Within nuclei AIF binds to genomic DNA, which leads to chromatin condensation and genomic DNA fragmentation by some unknown mechanisms, resulting in cell apoptosis. Ultimately the excessive apoptosis may cause syncytium deficiency through the depletion of cytotrophoblasts. The right side of the chart (connected with blue arrows) illustrates the fusogenic activity of syncytin-1. By this classic pathway, decreased levels of syncytin-1 would affect the formation of syncytiotrophoblasts, which may also lead to the syncytium deficiency. Syncytium deficiency, as manifested by the disruption of placental barrier, endocrine, and fetal–maternal exchange, functions, may contribute to the development of preeclampsia
Calpain1 inhibitor, but not caspase inhibitor, specifically reversed the syncytin-1 knockdown-induced AIF cleavage, nuclear translocation, and cell apoptosis
To verify the role(s) of calpain1 and caspases in the cell apoptosis caused by syncytin-1 knockdown, we took advantage of the calpain1-specific inhibitor, MDL28170, and a pan caspase inhibitor, Z-VAD-fmk. BeWo cells were transfected with syncytin-1 siRNA1, and at 24 h post-transfection were treated with MDL28170 or Z-VAD-fmk for 48 h. Cell apoptosis was assessed with the use of TUNEL assay. Consistent with earlier results showing the absence of changes in caspases 3, 8, and 9 protein levels (Fig. 3), treatment with caspase inhibitor did not affect the cell apoptosis induced by syncytin-1 knockdown. In contrast, treatment with calpain1 inhibitor effectively reversed cell apoptosis to the baseline level of around 2.5 % (Fig. 6a, b). AIF immunostaining of BeWo cells showed that AIF nuclear translocation was also reversed by calpain1 inhibitor, but not by capase inhibitor (Fig. 6c, d). These results confirmed the insignificance of the caspase pathway, and underscore the role of calpain1 in AIF-mediated cell apoptosis triggered by syncytin-1 knockdown.
Fig. 6.
Syncytin-1 knockdown-induced AIF activation and cell apoptosis were reversed by calpain1 inhibitor, but not caspase inhibitor. BeWo cells transfected with syncytin-1 siRNA were treated at 24 h post-transfection with the pan caspase inhibitor Z-VAD-fmk (100 μM, SsiRNA1 + Z-VAD), calpain1 inhibitor MDL28170 (20 μM, SsiRNA1 + MDL) or DMSO solvent as control (SsiRNA1). a Representative results of TUNNEL assay showing transfected cells treated with DMSO solvent (panel 1), caspase inhibitor (panel 2), or calpain1 inhibitor (panel 3). Apoptotic cells (arrows) were stained dark brown. b Compared to the solvent control, caspase inhibitor did not affect the TUNEL positivity. Calpain1 inhibitor significantly reversed the TUNEL positivity (**P < 0.01). c AIF immunostaining of BeWo cells at 72 h post-transfection time treated with DMSO solvent (panel 1), caspase inhibitor (panel 2), or calpain1 inhibitor (panel 3). Increased AIF protein and AIF nuclear translocation (dark brown) were detected and defined as AIF-positive (arrows). d Compared to the solvent control, caspase inhibitor (Z-VAD-fmk) did not affect the AIF positivity. Calpain1 inhibitor (MDL28170) significantly reversed the AIF positivity (**P < 0.01). e Western blot analysis. Left panel post-transfection treatment with caspase inhibitor or calpain1 inhibitor did not affect the caspase 3 cleavage pattern, middle panel treatment with calpain1 inhibitor, but not caspase inhibitor, significantly decreased the calpain1 protein level, right panel treatment with caspase inhibitor did not affect the expression level or cleavage of AIF. Treatment with calpain1 inhibitor did not change the AIF protein level, but inhibited the AIF cleavage. f, g Results of real-time PCR showed that treatment with caspase or calpain1 inhibitor did not change calpain1 and AIF mRNA levels
To further delineate the mechanism underlying the distinct effects of caspase and calpain1 inhibitors, we examined the changes at the mRNA and protein levels of both calpain1 and AIF following syncytin-1 knockdown and drug treatment at 72 h post-transfection. Treatment with caspase inhibitor did not significantly alter calpain1 or AIF expression on either mRNA or protein levels, again indicating the insignificance of the caspase pathway in syncytin-1 knockdown-induced apoptosis. On the other hand, treatment with the calpain1 inhibitor MDL28170 led to a series of molecular alterations associated with the syncytin-1 knockdown-triggered apoptotic events. First, cell treatment with calpain1 inhibitor resulted in a significant decrease in calpain1 protein levels (Fig. 6e) but did not affect calpain1 mRNA levels (Fig. 6f). Indeed, it was reported that MDL28170 binds to calpain1 and destabilizes the protein [42]. Second, treatment with the calpain1 inhibitor resulted in a reduction of AIF cleavage (Fig. 6e). This result provided a mechanistic explanation for its reversal of the syncytin-1 knockdown-induced apoptosis (Fig. 6a, b). Third, results of real-time PCR showed that the calpain1 inhibitor did not affect AIF mRNA levels (Fig. 6g). Therefore, the increased AIF mRNA levels following syncytin-1 knockdown was unlikely related to calpain1, but possibly due to an alternative, unidentified regulatory mechanism. In spite of this uncertainty, these experiments clearly demonstrate the specific role of calpain1 in AIF cleavage triggered by syncytin-1 knockdown. Thus, as illustrated in Fig. 9, syncytin-1 knockdown appears to elicit cell apoptosis through two actions: one involving upregulation of calpain1 expression, which may lead to increased AIF cleavage; the other involving upregulation of AIF expression levels, as evidenced by increased AIF mRNA levels as well as total AIF protein expression.
Increased trophoblast apoptosis and altered expression levels of syncytin-1, calpain1, and AIF in preeclamptic placentas
One important pathologic change in placentas associated with preeclampsia is the massive apoptosis [20, 43] and decreased syncytin-1 expression in the trophoblast lineage [22]. These previous observations, together with the current findings from in vitro experiments, raise the question of whether the AIF-mediated apoptotic pathway is activated in preeclamptic placentas. To seek an answer to this question, we examined clinical specimens for AIF mRNA/protein levels.
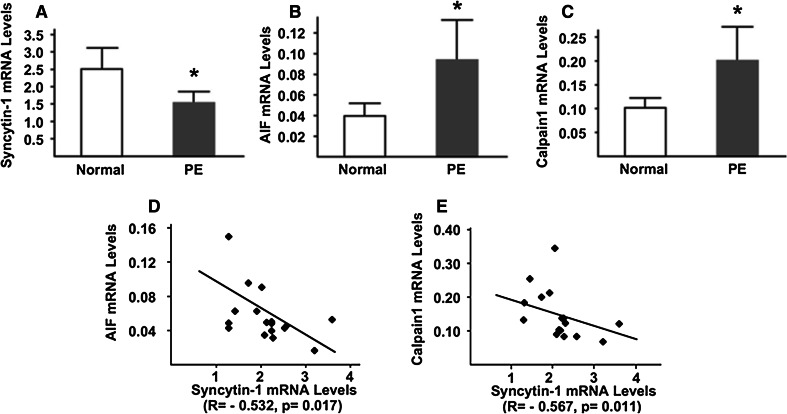
Real-time PCR results confirmed the previously reported lower levels of syncytin-1 mRNA levels in preeclamptic than in normal placentas (Fig. 7a). Importantly, we found that, despite the relatively large variations among individual samples, average mRNA levels of calpain1 and AIF are significantly higher in preeclamptic placentas compared to normal controls (Fig. 7b, c). Moreover, regression analysis among individual normal and preeclamptic placental samples indicated that decreased mRNA levels of syncytin-1 were inversely correlated with increased mRNA levels of AIF and calpain1, respectively (Fig. 7d, e). These results are in agreement with those from BeWo cells and revealed concomitant changes among syncytin-1, AIF and calpain1 mRNA levels in patients’ samples.
Fig. 7.
Quantitative analysis of syncytin-1, AIF and calpain1 mRNA levels in placental tissues. a–c Syncytin-1, AIF, and calpain1 mRNA levels were measured with real-time PCR. Compared to the levels in placentas from normal pregnancies (Normal), syncytin-1 mRNA was significantly decreased, whereas AIF and calpain1 mRNA levels were significantly increased (*P < 0.05), in the preeclamptic placentas (PE). Regression analysis of individual normal and preeclamptic placentas indicated that syncytin-1 mRNA levels were inversely correlated to those of the AIF (d) or calpain1 (e)
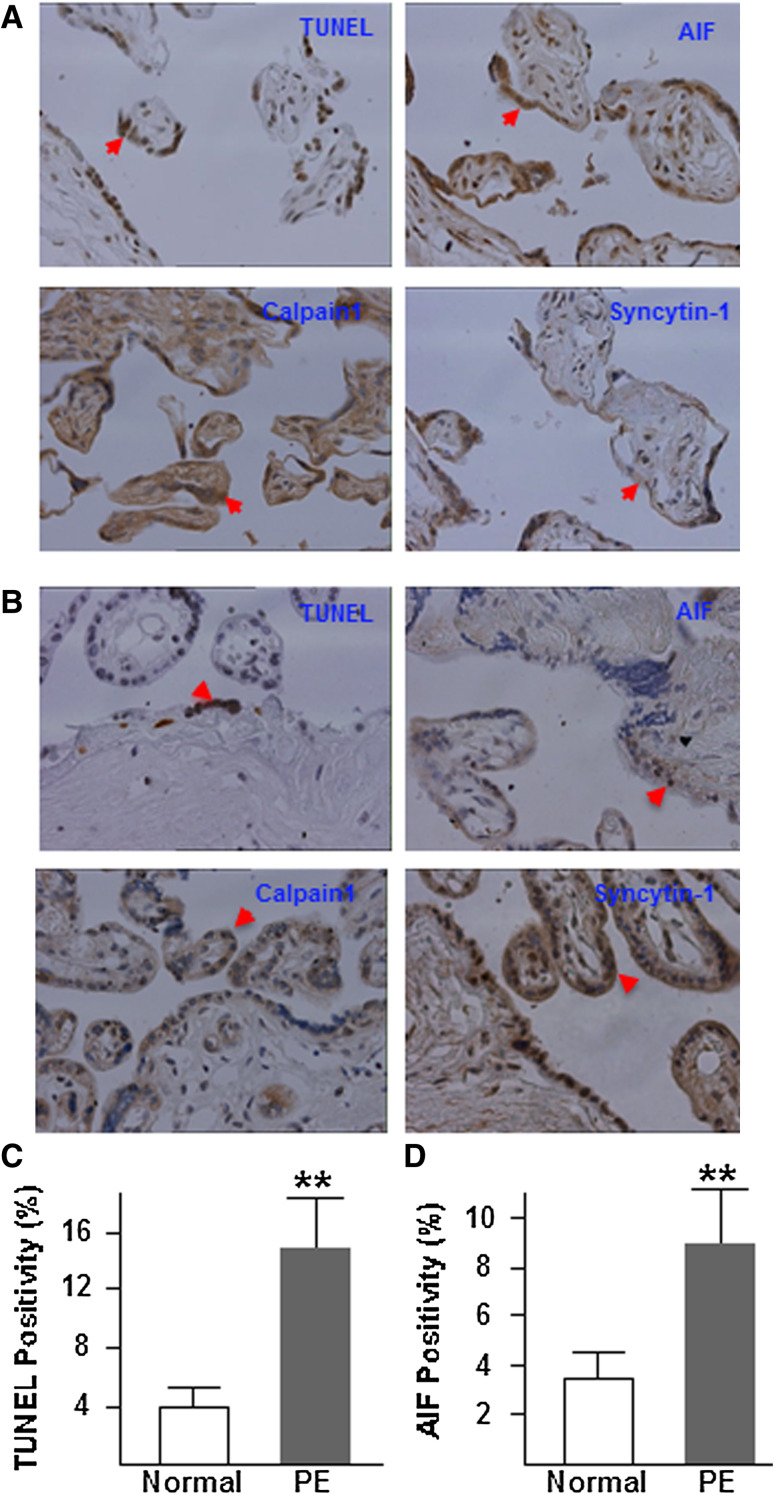
To examine the changes in cell apoptosis as well as the protein expression alterations of syncytin-1, calpain1, and AIF in vivo, TUNEL and immunohistochemistry were performed in tissue sections of preeclamptic placentas. Consistent with previous reports, decreased levels of syncytin-1 protein and increased apoptotic trophoblasts were observed in preeclamptic placentas (Fig. 8a) compared to placentas from normal pregnancies (Fig. 8b). Moreover, increased levels of AIF expression, AIF nuclear translocation, and calpain1 were found in preeclamptic placentas compared to control placentas. Counting of TUNEL-positive and AIF nuclear translocation-positive trophoblasts indicated higher levels of cell apoptosis (Fig. 8c) and AIF expression (Fig. 8d) in preeclamptic placentas than in normal controls. These data from clinical samples corroborated the in vitro findings from the syncytin-1 knockdown experiments (Figs. 4, 5), and validated a close relationship among the decreased syncytin-1 levels, upregulated calpain1 and AIF expression, and increased trophoblast apoptosis under the preeclamptic condition.
Fig. 8.
TUNEL and immunohistochemistry analysis of cell apoptosis, and syncytin-1, AIF, and calpain1 in tissue sections from preeclamptic (a) and normal (b) placentas. Brown indicates the staining signals for target factors. The nuclei were counterstained with hematoxylin. Compared to placentas from normal pregnancies, preeclamptic placentas displayed increased cell apoptosis, decreased syncytin-1 expression, increased calpain1, and increased AIF protein expression and nuclear translocation, in syncytiotrophoblasts as well as cytotrophoblasts. Cell counting indicated a significantly higher TUNEL positivity (c) as well as AIF positivity (d) in the preeclamptic placentas (PE) than normal placentas (Normal) (**P < 0.01)
Discussion
Under normal physiological conditions, syncytin-1 expression is restricted to the placental trophoblast lineage, and its levels continue to rise along the progression of pregnancy [44]. Recent studies suggest that, in non-trophoblastic cells, syncytin-1 may have anti-apoptotic activity. Knerr et al. [24] observed that in CHO-52, a Chinese hamster ovarian cancer cell line, ectopic overexpression of syncytin-1 protected cells from apoptosis induced by staurosporine, and increased caspase 3 cleavage was observed following staurosporine induction. The same group also reported that syncytin-1 was able to inhibit the BCL-XL-mediated apoptosis induced by actinomycin A [25]. While these studies pointed to the nonfusogenic function of syncytin-1, syncytin-1 involvement in the apoptosis of placental trophoblasts, where it is normally expressed, remains unclear. Findings from the current study have provided the first evidence that a sufficient level of syncytin-1 expression is required for trophoblast survival. Further analysis indicated that this protective effect was independent of caspase activation. Rather, it was specifically mediated by the calpain1–AIF pathway. Subsequent observations in the preeclamptic placentas provided support for the clinical relevance of these in vitro findings.
AIF-mediated apoptosis has been implicated in autophagy, tissue remodeling [29, 45], hypoxia-induced cell death, and embryo development [46, 47]. It should be pointed out that our results, while underscoring the significance of AIF activation in the syncytin-1 downregulation-triggered apoptosis, do not reject a potential role of the classic caspase pathway for syncytium deficiency. Preeclampsia is a complicated syndrome related to divergent factors including genetic background, malnutrition, and aberrant maternal immune responses against the semi-allergenic fetus [48]. Indeed, immunohistochemistry analysis showed that preeclamptic placentas contain more apoptotic cells than AIF-positive cells (Fig. 7f, g; 15.2 ± 2.4 vs. 9.0 ± 1.8 %), which suggested the existence of apoptotic events unrelated to AIF activation. Thus, it is highly possible that caspase and AIF pathways could coexist in preeclamptic placentas, and when activated by distinct etiological factors, both may contribute to the trophoblast apoptosis and placental dysfunction observed in preeclampsia.
Several intriguing observations of the calpain1–AIF pathway deserve special attention. First, the calpain1 inhibitor MDL28170, but not the caspase inhibitor, was able to specifically reverse the cleavage (Fig. 6e) and nuclear translocation of AIF (Fig. 6c, d), as well as the cell death induced by syncytin-1 knockdown. This finding confirms the dominant role of this pathway for the syncytin-1 knockdown-triggered apoptosis. Second, previous studies of calpain1 activation were limited to the observation of its increased protein levels [41, 49]. In the current study, we determined both calpain1 mRNA and protein levels at various post-transfection time points, and found that calpain1 activation may be initiated on the mRNA level (Fig. 5). And third, comparison of AIF mRNA and protein levels revealed a two-level control on AIF activation. On the first level, syncytin-1 knockdown is able to induce calpain1 expression, which leads to increased AIF cleavage; on the second level, syncytin-1 knockdown resulted in elevated expression of AIF. Thus, syncytin-1 appears to regulate both calpain1 and AIF by transcriptional activation and/or enhancement of these factors’ mRNA stability. These findings have built a foundation for future investigations on the specific mechanism by which syncytin-1 regulates calpain1 and AIF expression in trophoblasts.
The syncytium, being the interface between maternal and fetal circulation, is exposed to oxidative stress [50] and attack by the maternal immune system [6]. Indeed, cell debris as the result of trophoblast deportation is found in maternal circulation, indicating constant trophoblast death and regeneration [51]. Although our in vitro studies demonstrated a protective role of syncytin-1 in cytotrophoblasts, the same function may be present in syncytiotrophoblasts. Results from this as well as previous studies indicated that the syncytium expresses the highest levels of syncytin-1 (Fig. 8b) [52]. It has been found that cell fusion induced by ectopic expression of various viral envelope proteins quickly leads to cell death [53, 54]. In fact, the cell killing effect by viral fusogenic proteins has been explored as a gene therapy modality for cancer treatment. From this point of view, syncytiotrophoblasts need a protective mechanism to counteract the adverse effects of cell fusion. Syncytin-1, through its high expression in syncytiotrophoblasts, may meet this need by serving as a guardian during and after cell fusion. Thus, syncytin-1 qualifies as a dual function protein to mediate cell fusion and protect cells from apoptosis. This hypothesis concerning the fusogenic and nonfusogenic functions of syncytin-1 is supported by recent findings in primary trophoblast cultures. Lee et al. [22] observed that trophoblasts isolated from preeclamptic placentas are deficient in cell fusion, presumably secondary to decreased expression of syncytin-1. Most interestingly, these cells also showed a higher tendency for apoptosis than those isolated from normal placentas. The nonfusogenic functions of syncytin-1 revealed in the current study have offered a plausible explanation for this phenomenon.
The syncytium of preeclamptic placentas exhibits recessive morphological changes including discontinuation, prominent vacuoles, and bulbous structures [19]. Since syncytium has endocrine functions, the lower levels of hCS observed in the maternal circulation of preeclamptic patients may be attributed to syncytium deficiency [55]. These changes are accompanied by increased apoptotic activities in syncytiotrophoblast, as well as elevated syncytium deportation from preeclamptic placentas to maternal circulation [14, 56]. While hypoxia secondary to immature vasculature of spiral arteries is considered a culprit for preeclampsia, the cellular and molecular events involved in the development of preeclampsia are not clear [48]. Term placentas associated with preeclampsia express lower levels of syncytin-1 compared to placentas from normal pregnancy [22]. Independent in vitro studies using either the BeWo cell line or trophoblast primary culture have shown that hypoxia inhibited syncytin-1 expression [23, 57]. Our data confirm the decreased syncytin-1 expression in preeclamptic placentas on both mRNA and protein levels. Comparison between placentas from normal and preeclamptic pregnancies indicated that the decreased levels of syncytin-1 is correlated with increased AIF mRNA levels as well as increased AIF-positive trophoblasts in preeclamptic placentas, either by groups or by individual patients. Furthermore, immunohistochemistry and TUNEL analysis demonstrated increased calpain1 and AIF proteins and apoptotic cells in preeclamptic placentas (Fig. 8). These data from clinical samples have provided circumstantial evidence in support of the in vivo significance of syncytin-1 in maintenance of the syncytium integrity.
The major findings of this study and their clinical implications are summarized in Fig. 9. The decreased levels of syncytin-1, potentially caused by etiological factors for preeclampsia such as hypoxia, may lead to syncytium deficiency via triggering cell apoptosis through the nonfusogenic pathway. At the same time, through the fusogenic pathway, decreased syncytin-1 will affect the formation of syncytiotrophoblasts, which may also contribute to syncytium deficiency. Taken together, our findings have implicated the syncytin-1-triggered, calpain1-AIF-mediated apoptosis in the pathogenesis of preeclampsia. Further mechanistic studies along this line can be performed for a better understanding of the development of preeclampsia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to express their gratitude for the strong support by Mercer University School of Medicine, where most of the study is performed. Shi-Wen Jiang is supported by the Distinguished Cancer Scholarship of Georgia Cancer Coalition (GCC). This work was partially funded by research grants from National Institute of Health (NIH) (R01 HD 41577, Shi-Wen Jiang), NIH/NCI MD Anderson Uterine Cancer SPORE (Jinping Li, Shi-Wen Jiang), and research supplements from the Department of Obstetrics and Gynecology, Mayo Clinic and Foundation (Shi-Wen Jiang, Brian Brost), and Seed Grants from the Mercer University School of Medicine (Jinping Li, Shi-Wen Jiang).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Q. Huang and H. Chen contributed equally to this work.
Contributor Information
Ya Gao, Email: ygao@mail.xjtu.edu.cn.
Shi-Wen Jiang, Phone: +1-912-3500411, FAX: +1-912-3501281, Email: jiang_s@mercer.edu.
References
- 1.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MA, Lindheimer MD, de Swiet M, Assche AV, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20(1):ix–xiv. doi: 10.3109/10641950109152635. [DOI] [PubMed] [Google Scholar]
- 3.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, Campodonico L, Al-Mazrou Y, Lindheimer M, Kramer M. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194(4):921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Trudinger BJ, Duarte N, Wilcken DE, Wang XL. Elevated circulating homocyst(e)ine levels in placental vascular disease and associated pre-eclampsia. BJOG. 2000;107(7):935–938. doi: 10.1111/j.1471-0528.2000.tb11095.x. [DOI] [PubMed] [Google Scholar]
- 6.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 7.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37(3):240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Merviel P, Muller F, Guibourdenche J, Berkane N, Gaudet R, Breart G, Uzan S. Correlations between serum assays of human chorionic gonadotrophin (hCG) and human placental lactogen (hPL) and pre-eclampsia or intrauterine growth restriction (IUGR) among nulliparas younger than 38 years. Eur J Obstet Gynecol Reprod Biol. 2001;95(1):59–67. doi: 10.1016/S0301-2115(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 9.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96(2):271–276. doi: 10.1016/S0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 10.Austgulen R, Isaksen CV, Chedwick L, Romundstad P, Vatten L, Craven C. Pre-eclampsia: associated with increased syncytial apoptosis when the infant is small-for-gestational-age. J Reprod Immunol. 2004;61(1):39–50. doi: 10.1016/j.jri.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64(3):159–169. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavicchia JC. Junctional complexes in the trophoblast of the human full term placenta. J Anat. 1971;108(Pt 2):339–346. [PMC free article] [PubMed] [Google Scholar]
- 13.Guller S. Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb Res. 2009;124(4):389–392. doi: 10.1016/j.thromres.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59(2):153–160. doi: 10.1016/S0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 15.Huppertz B, Kingdom JC. Apoptosis in the trophoblast—role of apoptosis in placental morphogenesis. J Soc Gynecol Invest. 2004;11(6):353–362. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Gupta AK, Holzgreve W, Huppertz B, Malek A, Schneider H, Hahn S. Detection of fetal DNA and RNA in placenta-derived syncytiotrophoblast microparticles generated in vitro. Clin Chem. 2004;50(11):2187–2190. doi: 10.1373/clinchem.2004.040196. [DOI] [PubMed] [Google Scholar]
- 17.Kalinderis M, Papanikolaou A, Kalinderi K, Ioannidou E, Giannoulis C, Karagiannis V, Tarlatzis BC. Elevated serum levels of interleukin-6, interleukin-1 beta and human chorionic gonadotropin in pre-eclampsia. Am J Reprod Immunol. 2011;66(6):468–475. doi: 10.1111/j.1600-0897.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 18.Basirat Z, Barat S, Hajiahmadi M. Serum beta human chorionic gonadotropin levels and preeclampsia. Saudi Med J. 2006;27(7):1001–1004. [PubMed] [Google Scholar]
- 19.MacLennan AH, Sharp F, Shaw-Dunn J. The ultrastructure of human trophoblast in spontaneous and induced hypoxia using a system of organ culture. A comparison with ultrastructural changes in pre-eclampsia and placental insufficiency. J Obstet Gynaecol Br Commonw. 1972;79(2):113–121. doi: 10.1111/j.1471-0528.1972.tb15763.x. [DOI] [PubMed] [Google Scholar]
- 20.Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckmann MW, Schild RL. Impaired cytotrophoblast cell–cell fusion is associated with reduced syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75(1):175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 21.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74(7):3321–3329. doi: 10.1128/JVI.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22(10):808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 23.Kudo Y, Boyd CA, Sargent IL, Redman CW. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim Biophys Acta. 2003;1638(1):63–71. doi: 10.1016/S0925-4439(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 24.Knerr I, Schnare M, Hermann K, Kausler S, Lehner M, Vogler T, Rascher W, Meissner U. Fusiogenic endogenous-retroviral syncytin-1 exerts anti-apoptotic functions in staurosporine-challenged CHO cells. Apoptosis. 2007;12(1):37–43. doi: 10.1007/s10495-006-0329-9. [DOI] [PubMed] [Google Scholar]
- 25.Knerr I, Soder S, Licha E, Aigner T, Rascher W. Response of HEK293 and CHO cells overexpressing fusiogenic syncytin-1 to mitochondrion-mediated apoptosis induced by antimycin A. J Cell Biochem. 2008;105(3):766–775. doi: 10.1002/jcb.21874. [DOI] [PubMed] [Google Scholar]
- 26.Strick R, Ackermann S, Langbein M, Swiatek J, Schubert SW, Hashemolhosseini S, Koscheck T, Fasching PA, Schild RL, Beckmann MW, Strissel PL. Proliferation and cell–cell fusion of endometrial carcinoma are induced by the human endogenous retroviral syncytin-1 and regulated by TGF-beta. J Mol Med (Berlin) 2007;85(1):23–38. doi: 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 27.Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84(2–3):215–222. doi: 10.1016/S0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 28.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G, Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol. 2002;9(9):680–684. doi: 10.1038/nsb836. [DOI] [PubMed] [Google Scholar]
- 29.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 30.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410(6828):549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 31.Riddell MR, Winkler-Lowen B, Guilbert LJ. The contribution of apoptosis-inducing factor (AIF) to villous trophoblast differentiation. Placenta. 2012;33(2):88–93. doi: 10.1016/j.placenta.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Xiong Y, Dowdy SC, Podratz KC, Jin F, Attewell JR, Eberhardt NL, Jiang SW. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 2005;65(7):2684–2689. doi: 10.1158/0008-5472.CAN-04-2843. [DOI] [PubMed] [Google Scholar]
- 33.Jiang SW, Lloyd RV, Jin L, Eberhardt NL. Estrogen receptor expression and growth-promoting function in human choriocarcinoma cells. DNA Cell Biol. 1997;16(8):969–977. doi: 10.1089/dna.1997.16.969. [DOI] [PubMed] [Google Scholar]
- 34.Orendi K, Gauster M, Moser G, Meiri H, Huppertz B. The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction. 2010;140(5):759–766. doi: 10.1530/REP-10-0221. [DOI] [PubMed] [Google Scholar]
- 35.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155(1):293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cindrova-Davies T. Gabor Than award lecture 2008: pre-eclampsia—from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30(suppl A):S55–S65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Aban M, Cinel L, Arslan M, Dilek U, Kaplanoglu M, Arpaci R, Dilek S. Expression of nuclear factor-kappa B and placental apoptosis in pregnancies complicated with intrauterine growth restriction and preeclampsia: an immunohistochemical study. Tohoku J Exp Med. 2004;204(3):195–202. doi: 10.1620/tjem.204.195. [DOI] [PubMed] [Google Scholar]
- 38.Aschkenazi S, Straszewski S, Verwer KM, Foellmer H, Rutherford T, Mor G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod. 2002;66(6):1853–1861. doi: 10.1095/biolreprod66.6.1853. [DOI] [PubMed] [Google Scholar]
- 39.Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27(3):246–252. doi: 10.1007/s10637-008-9170-z. [DOI] [PubMed] [Google Scholar]
- 40.Hangen E, Blomgren K, Benit P, Kroemer G, Modjtahedi N. Life with or without AIF. Trends Biochem Sci. 2010;35(5):278–287. doi: 10.1016/j.tibs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol. 2007;27(13):4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem. 2003;278(16):14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]
- 43.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186(1):158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 44.Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG. 2006;113(2):152–158. doi: 10.1111/j.1471-0528.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 45.Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C. Poly(ADP-ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312(3):891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 46.Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G, Hagberg H, Blomgren K. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem. 2003;86(2):306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 47.Brown D, Yu BD, Joza N, Benit P, Meneses J, Firpo M, Rustin P, Penninger JM, Martin GR. Loss of AIF function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc Natl Acad Sci USA. 2006;103(26):9918–9923. doi: 10.1073/pnas.0603950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–1375. doi: 10.1016/S0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 49.Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280(8):6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- 50.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 51.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178(9):5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 52.Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, Jr, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403(6771):785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 53.Lifson JD, Feinberg MB, Reyes GR, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer KS, Engleman EG. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 54.Higuchi H, Bronk SF, Bateman A, Harrington K, Vile RG, Gores GJ. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res. 2000;60(22):6396–6402. [PubMed] [Google Scholar]
- 55.Huppertz B, Sammar M, Chefetz I, Neumaier-Wagner P, Bartz C, Meiri H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn Ther. 2008;24(3):230–236. doi: 10.1159/000151344. [DOI] [PubMed] [Google Scholar]
- 56.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 57.Knerr I, Schubert SW, Wich C, Amann K, Aigner T, Vogler T, Jung R, Dotsch J, Rascher W, Hashemolhosseini S. Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett. 2005;579(18):3991–3998. doi: 10.1016/j.febslet.2005.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.