Abstract
Background
Freezing of gait is a disabling symptom of Parkinson’s disease (PD) that involves failure to initiate and continue motor activity appropriately. PD disrupts fronto-basal ganglia circuitries that also implement the inhibition of responses, leading to the hypothesis that freezing of gait may involve fundamental changes in both initiation and inhibition of motor actions.
Objective
We asked whether PD patients who show freezing of gait show selective deficits in their ability to inhibit upper and lower extremity reactions.
Method
We compared older healthy controls, older PD controls without freezing of gait, and older PD participants with freezing of gait, in stop-signal tasks that measured the initiation (go trials) and inhibition (stop trials) of both hand and foot responses.
Results
When only go trials were presented, all three groups showed similar initiation speeds across lower and upper extremity responses. When stop signal trials were introduced, both PD groups slowed their reactions nearly twice as much as healthy controls. While this adjustment helped PD controls stop their actions as quickly as healthy controls, PD patients with freezing showed significantly delayed inhibitory control of both upper and lower extremities.
Conclusions
When anticipating the need to stop their actions urgently, PD patients show greater adjustments (i.e., slowing) to reaction speed than healthy controls. Despite these proactive adjustments, PD patients who freeze show marked impairments in inhibiting both upper and lower extremity responses, suggesting that freezing may involve a fundamental disruption to the brain’s inhibitory control system.
Keywords: Cognitive Control, Freezing of Gait, Parkinson’s disease, Response Inhibition, Stop-Signal Paradigm
Introduction
Parkinson’s disease (PD) is a neurodegenerative condition disrupting multiple neurotransmitters systems and producing atrophic changes in subcortical and cortical structures (Braak and Braak 2000). The substantial loss of dopamine-producing neurons in the substantia nigra compacta of the basal ganglia is regarded as a key mechanism underlying a range of characteristic PD symptoms, including bradykinesia, tremor, postural instability, and rigidity (Davie 2008; Lang and Lozano 1998). An expanding body of literature indicates that PD, and particularly dopamine loss, also disrupts fronto-basal ganglia circuitries involved in the cognitive control of action, such as processes that implement the voluntary selection, coordination, and inhibition of actions (Cools 2006; Cooper et al. 1991; Lees and Smith 1983; Owen et al. 1993). These cognitive control circuitries are also modifiable by pharmacological or surgical therapies for PD that target modulatory neurotransmitters (e.g., dopamine) or specific nodes (e.g., subthalamic nucleus) within these circuits (Hikosaka 1998; Mink 1996).
Among the most perplexing and catastrophic failures in action control expressed in PD is the freezing of gait (FOG) phenomenon. FOG generally involves the sudden inability to walk despite the intention to move, as if the entire motor system is temporarily locked (Giladi et al. 1997; Nutt et al. 2011). FOG prevalence ranges from 6% of patients in the early stages of PD to 60–70% in the later stages and is associated with longer disease duration, longer levodopa therapy use, severity of disease, presence of midline signs, and greater overall cognitive decline (Bartels et al. 2003; Macht et al. 2007; Nutt et al. 2011). FOG responds variably to levodopa therapy and is often resistant to pharmacotherapy. While typically a lower extremity phenomenon, freezing of upper extremities (for example during fine motor tasks) and speech reveal a more complicated phenomenon that points to a more central mechanism (Achiron et al. 1993; Ackerman et al. 1993; Atchison et al. 1993; Fahn 1995; Imai et al. 1993; Lewis and Shine 2014; Moreau et al. 2007; Niewboer et al. 2009; Quinn et al. 1989).
Several putative mechanisms underlying FOG have been proposed (Nutt et al. 2011). FOG is commonly triggered in particular stimulus contexts, such as navigating narrow passageways, around obstacles, or through crowds. This has prompted theories emphasizing the role of early visual perceptual and attentional processes and the postural adjustments they trigger as contributing to FOG (Almeida and Lebold 2010). Theories emphasizing late motor processes have also been proposed, pointing to aberrant postural control dynamics, including alterations in stride variability and anticipatory postural adjustments, as key components of FOG (Hausdorff et al. 2003). More recent theories have suggested that cognitive control mechanisms between early visual processing and late motor adjustments are fundamental to understanding FOG (Giladi and Hausdorff 2006; Lewis and Barker 2009; Matar et al. 2013; Nutt et al. 2011; Vandenbossche et al. 2012; Walton et al. 2015). For example, patients with FOG show selective slowing of reaction time in times of motor system conflict (i.e., when a situation activates multiple response options or alternatives to the current motor program). This fits well with the idea that FOG represents an exacerbated disruption of basal ganglia circuitries that are implicated in coordinating action selection and inhibition. In fact, a recent formulation of this theoretical framework asserted that a variety of stimulus, emotional, and cognitive events can trigger conflict between action choices, but the final common pathway for FOG expression was a deficit in resolving the resulting overload of motor system conflict (Lewis and Barker, 2009; Lewis and Shine 2014). This overload of motor system conflict may result from dysfunctional hyperdirect fronto-STN circuitries (Lewis and Shine, 2014) that are implicated in inhibiting or delaying the selection of motor programs (Aron and Poldrack 2006; Frank 2006; Mink 1996).
Direct behavioral evidence that individuals with FOG have greater deficits in inhibitory motor control (which rely on intact hyperdirect fronto-STN circuitry) than PD patients without FOG or healthy controls is extremely limited. Exacerbated conflict effects are suggestive of a possible deficit in inhibiting conflicting motor actions. However, mean conflict interference effects used in typical conflict tasks can be driven by stronger activation of conflicting responses or poorer inhibition of these activations (Ridderinkhof 2002; van den Wildenberg et al. 2010). In healthy adults, there is growing evidence that the hyperdirect fronto-STN circuitry is used for fast inhibitory control (Aron et al. 2007, 2014). Therefore, evidence of inhibitory deficits in individuals with FOG would be consistent with dysfunctional hyperdirect fronto-STN circuitry.
A powerful and theoretically rich framework for studying response initiation and inhibitory motor control is the stop-signal paradigm (Logan and Cowan 1984). Subjects perform a reaction time task (the “go” task) on every trial. On a subset of trials, a stop signal occurs and the subject must try to inhibit their go response. The Independent Race Model (Logan and Cowan 1984; Logan et al. 2014) allows computation of the latency of inhibition, the stop-signal reaction time (SSRT). Faster SSRT is evidence of better inhibitory control, because actions can be countermanded more quickly as goals change.
The stop-signal paradigm provides measures of the speed of initiating a response (go RT) and the speed of inhibiting a response (SSRT). Studies of stop-signal task performance in PD have demonstrated selective deficits in response inhibition, but less so response initiation, in PD compared to healthy controls (Gauggel et al. 2004; Obeso et al. 2011). An open question is whether stop-signal task performance differs based on the presence or absence of freezing behavior.
The first aim of the study was to determine whether PD patients with FOG showed greater deficits with initiation and inhibition of responses. We compared three groups of subjects in a stop-signal paradigm (Logan and Cowan, 1984): older healthy controls (HC), PD controls without freezing of gait (FOG−), and PD patients who have freezing of gait (FoG+). The extant cognitive control theories for freezing (Heremans et al. 2013) can be simplified to suggest that freezers may experience a failure of the initiation system, the inhibition system, or both. Freezers may experience initiation problems, as a movement is intended but initiated slowly. Support for this hypothesis would be revealed in a selective deficit in speed of initiating responses among patients with FOG compared to patients without FOG. Alternatively, freezers may experience changes in their inhibition system. Freezing often happens when hesitation is required, as when a new stimulus enters the environment or the patient is walking through a doorway. Therefore, freezers may develop deficits in control over the response inhibition system, manifesting as deficits deploying inhibition when needed, modulating movement by hesitation, or issuing complete inhibition that interferes with any and all movement initiation. In the current investigation, support for the hypothesis that freezers experience a pronounced and selective deficit in the response inhibition system would be revealed by slower inhibition (i.e., slower SSRT). This would also be consistent with the hypothesis that freezing is linked to dysfunctional frontal-STN circuitry (Lewis and Shine 2014), the putative pathway involved in response inhibition in the stop-signal paradigm (Aron et al. 2007, 2014).
Freezing is typically a gait and lower extremity problem, so we asked participants to complete two versions of the stop-signal paradigm: one requiring manual (hand) responses (as is convention in human stop-signal research) and one requiring responses with their feet. Thus, the second aim was a novel comparison of response initiation and inhibition across upper and lower extremities (effectors) in HC and in PD patients with and without FOG. Whereas response inhibition deficits in PD have been observed in manual responses, there is no evidence of generality across the skeletomotor system. Thus, we compare SSRT when making responses with hands and feet, testing the generality of SSRT deficits in PD across effectors. FOG is defined by arresting of lower extremity movements, so particularly large control deficits might be expected when engaging or stopping foot responses. Alternatively, if freezers experience a central control deficit (Lewis and Shine 2014) then SSRT deficits may be apparent when stopping either manual or foot responses.
Method
Subjects
There were three groups of subjects: FOG+, FOG−, and HC (see Table 1 for demographic). All PD patients were recruited from the Vanderbilt Parkinson’s Disease Clinic. PD (using UK brain bank criteria, Hughes et al. 1992) and FOG were diagnosed clinically by a review of clinical medical records and verification by a movement disorder specialist (CT, DC, or FP) who performed a clinical evaluation and Unified Parkinson’s Disease Rating Scale (UPDRS, Fahn et al. 1987; Goetz et al., 2007). We further included the FOG-Q to obtain a rating or freezing, festination, and gait symptom severity (Giladi et al. 2000). FOG-Q was used to further confirm that the FOG group was experiencing symptoms compatible with FOG. The PD subjects performed while on their usual medications and within 30–45 minutes of their most recent dosing. The HC group was recruited from local community advertisement and screened for medical, neurological, and psychiatric health problems.
Table 1.
Demographic Data for the FOG+, FOG−, and HC Groups
| Measure | FOG+ | FOG− | HC | p-value |
|---|---|---|---|---|
| N | 20 | 22 | 21 | |
| Age (years) | 63.6 (10.3) | 63.2 (6.0) | 65 (8.3) | .76 |
| Education (years) | 15.1 (2.6) | 15.6 (3.0) | 16.7 (2.5) | .17 |
| Gender (M:F) | 13:7 | 11:11 | 15:7 | |
| Depression Rating (CES-D) | 17.8 (7.0) | 11.2 (9.1) | 6.9 (6.2) | <.001a |
| Cognitive Impairment (MOCA) | 24.4 (4.1) | 25.5 (3.0) | 27.1 (2.1) | .03b |
| Years Since Diagnosis | 8.6 (5.3) | 5.5 (3.2) | .03 | |
| UPDRS III | 25.4 (10.8) | 22 (12.7) | .36 | |
| FOG-Q | 14.2 (2.9) | 1.7 (2.0) | <.001 | |
| Levodopa Dose (mg) | 1263 (578) | 742 (361) | .001 |
Note. Standard deviation presented in parentheses. P-value is output of one-way ANOVA when comparing 3-groups and two sample t-test when 2 groups.
Post-hoc Tukey’s HSD showed FOG+ < FOG− (p=.02) and FOG+ < HC (p<.001) but FOG− and HC did not differ (p=.16).
Post-hoc Tukey’s HSD showed FOG+ did not differ from FOG− (p=.53), FOG+ < HC (p=.02), and FOG-did not differ from HC (p=.22).
All participants had normal or corrected-to-normal vision. Participants were screened to ensure that they did not have a history of any neurological, mood, affective, or cognitive condition other than PD. All participants provided written informed consent, which was compliant with standards of ethical conduct in human investigation as regulated by the Vanderbilt institutional review board. Table 1 describes demographic data for the three groups. The FOG− group differed from the FOG+ group in CES-D, Years Since Diagnosis, FOG-Q, and Levodopa Dose, but did not significantly differ in Age, Education, MOCA, and UPDRS III. The FOG− group did not differ form the HC group in any measure (Age, Education, CES-D, or MOCA). The CES-D rating cutoff for clinical depression has been argued to be between >=20 and >=25 (Haringsma, Engels, Beekman, and Spinhoven, 2004; Himmelfark and Murrell, 1983). Both HC (M = 6.9) and FOG− (M = 11.2) groups showed mean depression ratings that were well below the cutoffs indicated clinical depression, but the FOG+ group (M = 17.8) was approaching the cutoff for clinical depression, suggesting the likely presence of mild depression in the FOG+ group. At the end of the Results section we will argue that our results cannot be explained by any group differences in demographic data.
Apparatus and Stimuli
The experiment was run on a laptop PC running E-Prime 2 (pstnet.com). The go stimulus on each trial was a single gray arrow on a black background presented in the center of the screen. On some trials, the gray shape changed to red, which was the stop signal. When responding with their hands, subjects responded with two custom-made response devices that were held within the palm of each hand. Subjects responded by pressing a key with their right thumb to a right arrow and a key with their left thumb to a left arrow. When responding with their feet, subjects responded with two foot pedals, one under the heel of each foot. They depressed both foot pedals in anticipation of each trial, and then lifted either their right or left heel to signal a right or left response, respectively.
Procedure
Each trial began with a 500ms fixation cross, followed immediately by the presentation of the go stimulus for 1300ms, and then followed immediately by a 500ms blank-screen inter-trial interval. The go task was to respond quickly and accurately based upon the direction of the centrally presented arrow.
Before subjects were introduced to the stop signal, they completed approximately 40 trials responding with hands to go stimuli and 40 trials responding with feet to go stimuli (PreStop Block). A total of nine subjects completed a different number of these PreStop trials (across the entire group of 63 subjects the mean number of hands trials = 39.4, mean number of feet trials = 37.8).
On 40% of trials, the gray shape changed to red after the stop-signal delay (SSD). SSD was initially set at 250ms and was adjusted based upon a staircase tracking algorithm designed to yield a .5 probability of responding given a stop signal (Levitt 1971). When subjects succeeded at stopping to a stop signal, SSD increased by 50ms, handicapping the race in favor of the go process. When subjects failed at stopping, SSD decreased by 50ms, handicapping the race in favor of the stop process. This staircase tracking algorithm yields accurate SSRT estimates (Band et al. 2003), particularly when used with the integration method for SSRT computation (Verbruggen et al. 2013), as we used here.
At the beginning of the simple stop condition, subjects completed 40 trials of practice either responding with hands or feet with 40% stop signals. They next completed the main task 250 trials using whichever effector they practiced. Then they had 20 trials of practice using whichever effector they did not practice at the beginning of the simple stop condition. Then they completed the main task of 250 trials with the effector that they practiced second. Each 250 trial section was separated into five, 50 trial blocks. At the end of each block, subjects were given a rest period and received feedback on their go RT and accuracy from the preceding block.
Dependent Measures and Analyses
To assess the ability to initiate a response without the requirement to balance the demands of inhibiting a response, we looked at reaction time and accuracy in the initial block before stop signals were introduced. To assess the ability to initiate a response with the requirement to balance the demands of inhibiting response, we looked at reaction time and accuracy of the trials in the stop-signal task in which a stop signal did not occur (go trials). Subjects tend to proactively slow RT when stop signals are introduced (Chikazoe et al. 2009; Verbruggen and Logan 2009). This has been discussed as a shift in strategy from initiation toward inhibition as subjects expect stop signals (Bissett and Logan 2011). We assessed proactive slowing by comparing go RT before stop signals were introduced (PreStop Block) to go RT during the stop task. Longer RT during the stop task than the PreStop block indicates proactive slowing, and therefore greater shifts in priority from initiation towards inhibition in preparation to stop more effectively.
In order to assess inhibitory control, we computed SSRT. SSRT is a measure of the latency of the inhibitory process, with shorter SSRT indicated faster, more effective inhibition. SSRT was computed with the integration method (Logan and Cowan 1984). The integration method involves rank ordering all N go RTs from fastest to slowest, then finding the Mth go RT, where M = N × the probability of responding given a stop signal, and then subtracting mean SSD from the Mth go RT to get SSRT. We ran two planned comparisons on SSRT: FOG− versus HC and FOG− versus FOG+. The former comparison is motivated by the literature that has established an inhibition deficit in PD (Gauggel et al. 2004; Obeso et al. 2011). The latter comparison is motivated by the hypothesis that freezers may experience dysfunction in inhibitory control circuitry (Lewis and Shine 2014).
Results
Go Responses and Proactive Slowing
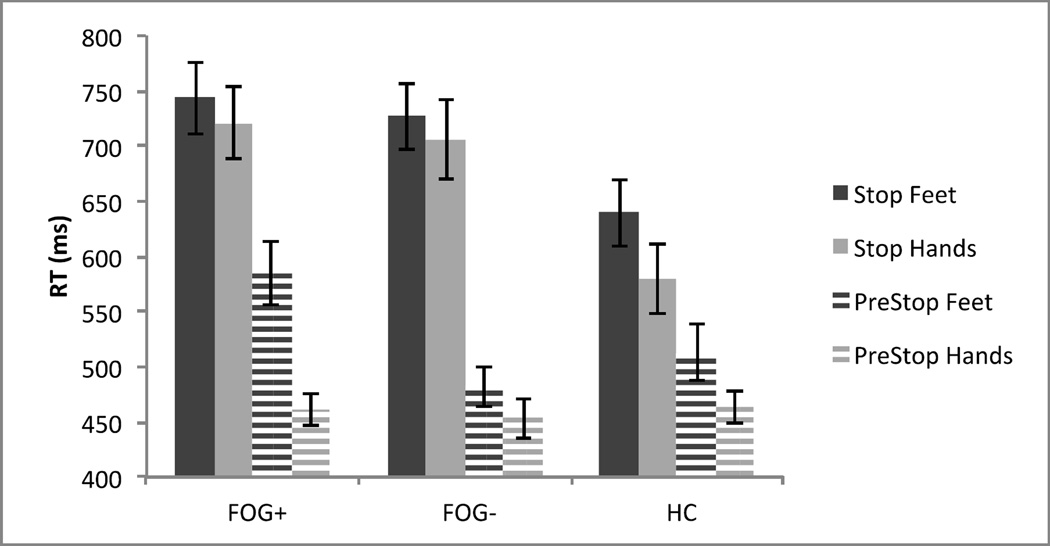
The RT for the three groups were compared with a 2 (Effector: Hands Vs. Feet) × 2 (Block: PreStop Vs. Stop) × 3 (Group: FOG+, FOG−, and HC) mixed ANOVA, with the first two factors within subjects and the last factor between subjects (see Figure 1). This revealed a main effect of Effector, F(1,60)=32.89, MSE=5009, p<.001, with feet RT (M=615ms) slower than hands RT (M=564ms). There was also a main effect of Block, F(1,60)=191.38, MSE=12217, p<.001, with Stop Block RT slower (M = 686 ms) than PreStop Block RT (M=493ms). This is evidence of large proactive slowing. However, there was no main effect of Group, F(2,60)=3.14, MSE=39930, p>.05. Therefore, there was not evidence of slower response initiation in FOG− or FOG+ than in HC. There was a significant interaction of Block X Group, F(2,60)=7.18, MSE=12217, p=.002. There were no other interactions (all p’s >.05).
Figure 1.
Mean reaction times across Group (FOG+, FOG−, and HC), Effector (Hands Vs. Feet), and Block (PreStop Vs. Stop) on correct go trials.
Note. Error bars are one standard error of the mean.
The significant interaction of Block X Group suggests that the introduction of stop signals influenced the groups differently. Simple effects analyses showed that the groups did not differ in PreStop Block RT, F(2,60)=1.97, MSE=6795, p=.15, but they did differ in Stop Block RT, F(2,60)=4.83, MSE=19279, p=.01. In the stop block, FOG+ RT (M=732ms) and FOG− RT (M=716ms) were considerably longer than HC RT (M=609ms). Taken together, the RT results show that the process of initiating speeded responses is not impaired in FOG+ or FOG− (as evidenced by similar PreStop Block RT), but FOG+ and FOG− show larger proactive slowing than HC (as evidenced by the different Stop Block RT) when stop signals can occur unpredictably. Therefore, FOG+ and FOG− more drastically engage proactive slowing, shifting priority towards inhibition when stop signals are introduced.
To assess go accuracy, we ran a mixed ANOVA with the same structure as the ANOVA on RT. There was a main effect of Effector, F(1,60)=87.89, MSE=.002, p<.001, with feet responses less accurate (M=93%) than hand responses (M=98%). There was also a main effect of Block, F(1,60)=13.09, MSE=.003, p=.001. Accuracy before stop signals were introduced (M=94%) was lower than after stop signals were introduced (M=96%). There was also a main effect of Group, F(2,60)=3.91, MSE=.004, p=.03. Post-hoc Tukey’s HSD tests revealed lower accuracy in the FOG+ (94%) than the HC Group (96%), p=.03, but no other pairwise differences (all other p’s >.05). FOG− accuracy was 96%. Finally, there was a significant interaction of Effector X Block, F(1,60)=4.04, MSE=.002, p=.05. The hands – feet accuracy difference was larger in the PreStop Block (M=3%) than the Stop Block (M=1%).
SSRT
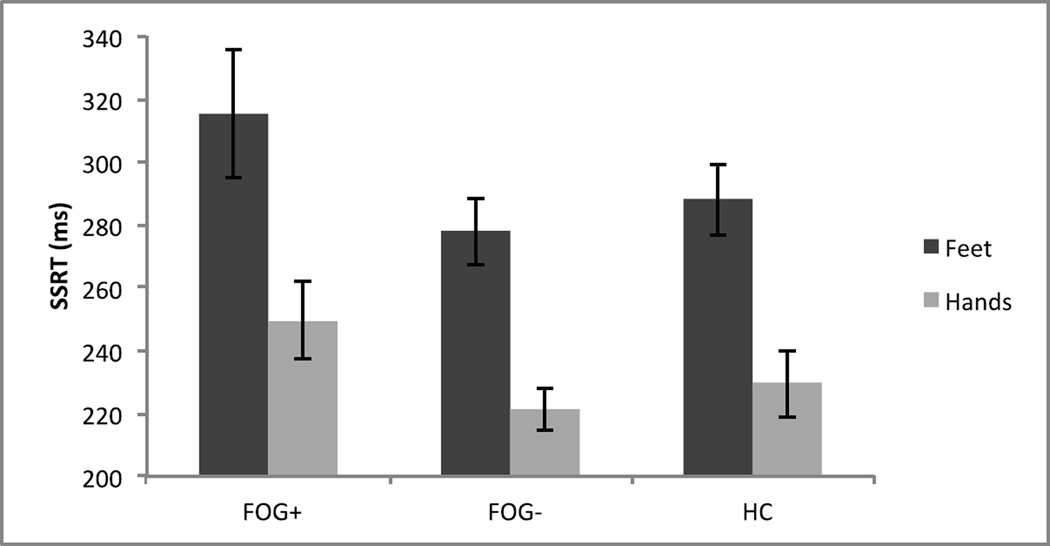
We then compared the three groups in SSRT, the measure of the speed of inhibition (see Figure 2). We ran a 2 (Effector: Hands Vs. Feet) X 3 (Group: FOG+, FOG−, and HC) mixed ANOVA on SSRT. This revealed a main effect of Effector, F(1,60)=74.58, MSE=1525, p<.001. Feet SSRT (M=293ms) was longer than hands SSRT (M=233ms). This is in line with previous work showing longer SSRT in feet than hands (Tabu et al. 2012). The main effect of Group did not reach significance (p=.11), nor did the interaction (p=.86). We ran two planned comparisons on SSRT, the first between FOG+ and FOG−, and the second between FOG− and HC. FOG+ SSRT was longer than FOG− SSRT, t=2.11, p=.04. FOG− SSRT did not differ from HC SSRT, t<1. These SSRT results suggest that there is no inhibitory control deficit for PD patients without freezing. The inhibitory deficit was specific to PD subjects with freezing.
Figure 2.
Stop-Signal reaction times (SSRT) across Group (FOG+, FOG−, and HC) and Effector (Hands Vs. Feet).
Note. Error bars are one standard error of the mean.
Relationship Between Demographics and Dependent Variables
In order to assess the relationship between our demographic variables (see Table 1) and our dependent variables (PreStop Block RT, PreStop Block accuracy, Stop Block RT, Stop Block accuracy, SSRT) we ran a series of correlations across groups between each demographic variable and each dependent measure (8 demographics X 5 dependent variables = 40 correlations). No correlation reached significance after Bonferroni correction for multiple comparisons, suggesting that the results presented above cannot be explained by demographic differences across groups.1
Discussion
We compared performance in a stop-signal paradigm between three groups: FOG+, FOG−, and HC. Before stop signals were introduced, RT did not differ between groups. This suggests that FOG+ and FOG− do not have a deficit when initiating speeded responses. When we introduced stop signals, we found that the FOG+ and FOG− groups had longer go RT in the stop-signal task than the HC group. This suggests that when stop signals are introduced the FOG+ and FOG− groups engage more proactive slowing than HC to prepare for the stop signals.
Comparing SSRT across groups showed that FOG+ had longer SSRT than FOG−, but FOG-did not differ from HC. This is evidence that a deficit in response inhibition in PD is clearly expressed in patients with PD who also exhibit freezing. Additionally, though foot responses were slower to engage and slower to inhibit, this effect did not interact with group. Taken together, these results suggest that freezers have an inhibition deficit that results in poor stopping control of both hand and foot movements.
The Influence of Stop Context
The stop-signal paradigm involves competing goals of initiation and inhibition. As subjects expect stop signals to occur, they shift priority from initiating reactions quickly (i.e., go priority) to preparing to inhibit (i.e., stop priority), which slows go RT (Bissett and Logan 2011, 2012a) but speeds SSRT (Bissett and Logan 2012b). In this study, 40% of all trials were stop trials, which is higher than the usual 25% or 33%. When stop signals occur at higher probabilities, subjects tend to shift their task priority from going to stopping more drastically (Bissett and Logan 2011; Logan 1981; Logan and Burkell 1986). Compared to the HC group, both the FOG+ and FOG− slowed go RT more when stop signals were introduced, as evidenced by significantly larger proactive slowing. For FOG−, this drastic shift in priority from going to stopping control was accompanied by normal SSRT latencies, suggesting that they may have effectively eliminated the response inhibition deficit that is usually observed in PD patients. This suggests that an effective strategy for mitigating inhibitory control deficits in PD may be to enter a slower, more deliberate response control mode.
This is consistent with recent work in PD investigating strategic adjustments in control during response conflict resolution (van Wouwe et al. 2014; Wylie et al. 2009). When PD subjects prioritized speed over accuracy, they had greater difficulty suppressing incorrect response impulses compared to HC subjects. However, when PD subjects prioritized accuracy over speed, they showed a marked reduction in the suppression deficit. Together with the current results, these patterns suggest that cognitive control deficits in PD can be modulated by strategies that de-emphasize speed of reacting and shift priority toward greater inhibition control. Encouraging more cautious strategies may be an effective way to ameliorate action control deficits in PD.
Future work will hopefully distinguish the hypotheses that a stop-focused context can compensate for the inhibitory deficit in FOG− from the hypothesis that PD in the absence of freezing is not accompanied by an inhibitory deficit.
Response Inhibition Deficit in FOG
One challenge to addressing FOG, which is a costly and debilitating symptom of PD, is understanding the basic mechanisms of freezing. Some progress has been made addressing mechanisms (Lewis and Barker, 2009; Lewis and Shine 2014; Shine et al. 2013), but the diversity of conditions that provoke and relieve freezing make it difficult to address the underlying mechanisms. In this report, we ask whether FOG+ patients have a deficit in initiating or inhibiting a response. Freezers may experience alterations in their ability to initiate a response when desired, or an inability to engage and then disengage inhibition when necessary.
Our results show that freezers are able to engage speeded responses about as quickly as FOG− and HC groups before stop signals were introduced. Once stop signals were introduced, much like the FOG− group, the FOG+ group engaged drastic proactive slowing, entering into a stop focused context that traded priority in the go task for priority in the stop task. Despite this drastic adjustment, SSRT was still slowed in FOG+ compared to FOG− and HC. This suggests that individuals with freezing of gait experience a deficit in inhibitory control that cannot be overcome by strategically prioritizing inhibition. This is consistent with a recent theory proposing that FOG involves dysfunction in the hyperdirect fronto-STN pathway (Lewis and Shine 2014) that is putatively engaged in response inhibition (Aron et al. 2007, 2014).
SSRT is a measure of the latency of the inhibitory process, so prolonged SSRT in FOG+ suggests that inhibitory control is less efficient and effective. Current theories suggest that the hyperdirect fronto-STN pathway (Aron et al., 2007; Aron et al., 2014) underlies the inhibitory process measured by SSRT. Therefore, longer SSRT in FOG+ is consistent with the theory that FOG is linked to dysfunctional hyperdirect fronto-STN circuitry.
If the hyperdirect pathway engages inhibition, dysfunctional hyperdirect circuitry may be expected to produce more movement, not the apparent arresting of movement despite the intention to move in FOG. However, there is often continued moving or festination when stopping or freezing (Naismith & Lewis, 2010), suggesting that freezing may involve incomplete or ineffective inhibitory control over some movement options. Also, if inhibition is less effectively engaged in FOG it may also be less effectively disengaged, resulting in freezing episodes.
Inhibitory Deficit in PD-FOG is not Specific to Lower Extremities
The SSRT patterns across groups did not differ when subjects were stopping hands or feet. Irrespective of effector, SSRT in FOG+ was longer than FOG−, which did not differ from HC. This is evidence that the inhibitory control problems in PD subjects who exhibit freezing are not exclusive to the lower extremities. Lower extremity freezing may be most commonly observed clinically because they are particularly debilitating, as they can result in dangerous falls. However, the SSRT results suggest that freezers have similar difficulty inhibiting hand and foot responses. This shows that the control deficit in FOG+ is a central inhibitory control deficit, not a peripheral deficit that manifests specifically in the lower extremities.
Conclusion
We compared the speed to initiate and inhibit responses in the stop-signal paradigm in three groups: FOG+, FOG−, and HC. Previous work showed impaired response inhibition in PD, a neurodegenerative disorder that results in basal ganglia dysfunction. Our results revealed that, compared to HC, PD patients with and without FOG responded similarly quickly until stop signals were introduced, and then became considerably slower, revealing greater proactive slowing in anticipation of the need to stop. This adjustment facilitated normal stopping speeds among FOG−, but FOG+ showed inhibition deficits despite shifting priority toward stopping control. The inhibition impairment in PD-FOG was general to hands and feet, revealing a central inhibitory control deficit in PD-FOG. These results are consistent with a recent theory of FOG that postulates dysfunction in the hyperdirect fronto-STN circuitry that is engaged during response inhibition (Lewis and Shine 2014).
Acknowledgements
This research was supported by grant number BCS-0957074 and BCS-1257272 from the National Science Foundation and grant number R01-EY021833-01 from the National Eye Institute.
Footnotes
We considered the appropriateness of ANCOVA for our analyses, especially for our critical analyses of SSRT across groups. However, two assumptions for ANCOVA are violated: (1) given the significant relationship between multiple demographic variable (Depression Rating, Years Since Diagnosis, FOG-Q, and Levodopa Dose) and the independent variable Group, the assumption of independence between independent variables and covariates is violated (Miller and Chapman 2001), and (2) given the significant correlation between several pairs of covariates (e.g., UPDRS and Years Since Diagnosis, Levodopa Dose and Years Since Diagnosis, Levodopa Dose and FOG-Q, CES-D and FOG-Q, and a negative correlation between MOCA and Age), the assumption that covariates are not overly correlated with each other is violated. Additionally, given the lack of a significant correlation between any demographic variables and dependent measures, the assumption of a linear relationship between covariate and dependent variable may be violated. In spite of these violations that we believe undermine any conclusions that can be drawn from ANCOVA, we ran a 2 (Effector: Hands Vs. Feet) X 2 (Group: PD-FoG Vs. PD-C) mixed ANCOVA on SSRT with all 8 demographic variables from Table 1 as covariates. The group effect on SSRT remained significant (p < .05).
No Financial Disclosure/Conflict of Interest
Financial Disclosures of All Authors (for the preceding 12 months)
P.G.B.:
None
G.D.L.:
Grants: NSF, NEI
N.C.V.W.:
None
C.M.T.:
None
F.T.P.:
Consultancies: Medtronic, Boston Scientific
Grant: Medtronic
D.O.C.:
Consultancies: Lundbeck
Advisory Boards: Teva Neuroscience, Lundbeck
Honoraria: Lundbeck, Teva Neuroscience
Grants: K23 NS080988, Michael J Fox Foundation, Auspex, Teva Neuroscience, Lundbeck
S.A.W:
Grant: K23 K23AG028750
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Achiron A, Ziv I, Goren M, Goldberg H, Zoldan Y, Sroka H, Melamed E. Primary progressive freezing gait. Mov Disord. 1993;8:293–297. doi: 10.1002/mds.870080307. [DOI] [PubMed] [Google Scholar]
- Ackerman H, Grone BF, Hoch G, Schonle PW. Speech freezing in Parkinson’s disease: A kinematric analysis of orofacial movements by means of electromagnetic articulography. Folia Phoniatr. 1993;45:84–89. doi: 10.1159/000266222. [DOI] [PubMed] [Google Scholar]
- Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2010;81:513–518. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Frank MJ, Smith S, Poldrack RA. Triangulating a cognitive control network with diffusion weighted MRI and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamatic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Atchison PR, Thompson PD, Frackowiak RSJ, Marsden CD. The syndrome of gait ignition failure: a report of six cases. Mov Disord. 1993;8:285–292. doi: 10.1002/mds.870080306. [DOI] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD. Horse-race model simulations studies of the stop signal procedure. Acta Psychol. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Balash Y, Gurevich T, Schaafsma JD, Hausdorff JM, Giladi N. Relationship between freezing of gait (FOG) and other features of Parkinson’s: FOG is not correlated with bradykinesia. J Clin Neurosci. 2003;10:584–588. doi: 10.1016/s0967-5868(03)00192-9. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Balancing cognitive demands: Control adjustments in the Stop-signal paradigm. J Exp Psychol Learn Mem Cogn. 2011;37:392–404. doi: 10.1037/a0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Post-stop-signal slowing: Strategies dominate reflexes and implicit learning. J Exp Psychol Hum Percept Perform. 2012a;38:746–757. doi: 10.1037/a0025429. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Post-stop-signal adjustments: Inhibition improves subsequent inhibition. J Exp Psychol Learn Mem Cogn. 2012b;38:955–966. doi: 10.1037/a0026778. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247 doi: 10.1007/PL00007758. II/3-II/10. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K-I, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- Fahn S. The freezing phenomenon in parkinsonism. Adv Neurol. 1995;65:53–63. [PubMed] [Google Scholar]
- Fahn S, Elton RL . Members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. 3–163. Vol. 2. 1987. pp. 293–304. [Google Scholar]
- Frank MJ. Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoof T-A. Inhibition of ongoing responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:539–544. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. J Neurol Sci. 2006;248:173–176. doi: 10.1016/j.jns.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Giladi NN, Kao RR, Fahn S. Freezing phenomenon in patients with parkinsonian syndromes. Mov Disord. 1997;12:302–305. doi: 10.1002/mds.870120307. [DOI] [PubMed] [Google Scholar]
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with parkinsonism. Parkinsonism Relate Disord. 2000;6:165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Goetz C, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (mds-updrs): process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman ATF, Spinhoven P. The criterion validity of the center for epidemiological studies depression scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149:187–194. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson’s disease: Where are we now? Curr Neurol Neurosci Rep. 2013;13:350. doi: 10.1007/s11910-013-0350-7. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Neural systems of control of voluntary action-A hypothesis. Adv Biophys. 1998;35:81–102. [PubMed] [Google Scholar]
- Himmelfarb S, Murrell SA. Reliability and validity of five mental health scales in older persons. J Gerontol. 1983;38:333–339. doi: 10.1093/geronj/38.3.333. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Nakamura T, Kondo T, Narabayashi H. Dopa-unresponsive pure akinesia or freezing: A condition within a wide spectrum of PSP? Adv Neurol. 1993;60:622–625. [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down method in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- Lewis SJG, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:333–338. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Shine JM. The next step: A common neural mechanism for freezing of gait. Neuroscientist. Published online before print. 2014 doi: 10.1177/1073858414559101. [DOI] [PubMed] [Google Scholar]
- Logan GD. In: Attention, automaticity, and the ability to stop a speeded choice response. Long J, Baddeley AD, editors. Attention and Performance IX; 1981. [Google Scholar]
- Logan GD, Burkell J. Dependence and independence in responding to double stimulation: A comparison of stop, change, and dual-task paradigms. J Exp Psychol Hum Percept Perform. 1986;12:549–563. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action. A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Van Zandt T, Verbruggen F, Wagenmakers E-J. On the ability to inhibit thought and action: General and special theories of an act of control. Psychol Rev. 2014;121:66–95. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Macht M, Kaussner Y, Moller JC, Stiasny-Kolster K, Eggert KM, Kruger H-P, Ellgring H. Predictors of freezing in Parkinson’s disease: A survey of 6,620 patients. Mov Disord. 2007;15:953–956. doi: 10.1002/mds.21458. [DOI] [PubMed] [Google Scholar]
- Matar E, Shine JM, Naismith S, Lewis SJG. Using virtual reality to explore the role of conflict resolution and environmental salience in freezing of gait in Parkinson’s disease. Parkinsonism Relate Disord. 2013;19:937–942. doi: 10.1016/j.parkreldis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moreau C, Ozsancak C, Blatt J-L, Derambure P, Destee A, Defebvre L. Oral festination in Parkinson’s disease: Biomechanical analysis and correlation with festination and freezing of gait. Mov Disord. 2007;22:1503–1506. doi: 10.1002/mds.21549. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Lewis SJG. A novel paradigm for modeling freezing of gait in Parkinson’s disease. J Clin Neurosci. 2010;17:984–987. doi: 10.1016/j.jocn.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci. 2009;29:1422–1430. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Casabona E, Bringas ML, Alvarez M, Alvarez L, Pavon N, Rodriguez-Oroz M-C, Macias R, Obeso JA, Jahanshahi M. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp Brain Res. 2011;212:371–384. doi: 10.1007/s00221-011-2736-6. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain. 1993;116:1159–1179. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Quinn NP, Luthert P, Marsden CD. Pure akinesia due to Lewy body Parkinson’s disease: A case with pathology. Mov Disord. 1989;4:85–89. doi: 10.1002/mds.870040112. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: Activation and suppression in conflict tasks. Psychol Res. 2002;66:312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Shine JM, Moustafa AA, Matar E, Frank MJ, Lewis SJG. The role of frontostriatial impairment in freezing of gait in Parkinson’s disease. Front Syst Neurosci. 2013;7:61. doi: 10.3389/fnsys.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabu H, Mima T, Aso T, Takahashi R, Fukuyama H. Common inhibitory prefrontal activation during inhibition of hand and foot responses. Neuroimage. 2012;59:3373–3378. doi: 10.1016/j.neuroimage.2011.10.092. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Wylie SA, Forstmann BU, Burle B, Hasbroucq T, Ridderinkhof KR. To head or to heed? Beyond the surface of selective action inhibition: A review. Front Hum Neurosci. 2010;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wouwe NC, van den Wildenberg WPM, Claassen DO, Kanoff K, Bashore TR, Wylie SA. Speed pressure in conflict situations impedes inhibitory action control in Parkinson’s disease. Biol Psychol. 2014;101:44–60. doi: 10.1016/j.biopsycho.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbossche J, Deroost N, Soetens E, Zeischka P, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E. Conflict and freezing of gait in Parkinson’s disease: Support for a response conflict deficit. Neuroscience. 2012;206:144–154. doi: 10.1016/j.neuroscience.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD. Fictitious inhibitory differences: How skewness and slowing distort the estimation of stopping latencies. Psychol Sci. 2013;24:352–362. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform. 2009;35:835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CC, Shine JM, Mowszowski L, Gilat M, Hall JM, O’Callaghan C, Naismith SL, Lewis SJG. Impaired cognitive control in Parkinson’s disease patient with freezing of gait in response to cognitive load. J Neural Transm. 2015;122:653–660. doi: 10.1007/s00702-014-1271-6. [DOI] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WPM, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF. The effect of speed-accuracy strategy on response interference control in Parkinson’s disease. Neuropsychologia. 2009;47:1844–1853. doi: 10.1016/j.neuropsychologia.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]