Abstract
Purpose
Obese individuals vary in their experience of food cravings and tendency to engage in reward-driven eating, both of which can be modulated by the neural reward system rather than physiological hunger. We examined two predictions in a sample of obese women: (1) whether opioidergic blockade reduced food-craving intensity, and (2) whether opioidergic blockade reduced an association between food-craving intensity and reward-driven eating, which is a trait-like index of three factors (lack of control over eating, lack of satiation, preoccupation with food).
Methods
Forty-four obese, pre-menopausal women completed the Reward-based Eating Drive (RED) scale at study start and daily food-craving intensity on 5 days on which they ingested either a pill-placebo (2 days), a 25mg naltrexone dose (1 day), or a standard 50mg naltrexone dose (2 days).
Results
Craving intensity was similar under naltrexone and placebo doses. The association between food-craving intensity and reward-driven eating significantly differed between placebo and 50mg naltrexone doses. Reward-driven eating and craving intensity were significantly positively associated under both placebo doses. As predicted, opioidergic blockade (for both doses 25mg and 50mg naltrexone) reduced this positive association between reward-driven eating and craving intensity to non-significance.
Conclusions
Opioidergic blockade did not reduce craving intensity; however, blockade reduced an association between trait-like reward-driven eating and daily food-craving intensity, and may help identify an important endophenotype within obesity.
Keywords: Obesity, craving intensity, reward based eating drive, naltrexone, opioidergic blockade
The modern food environment is replete with cues to eat highly palatable foods for the rewarding properties of eating, yet individuals vary in susceptibility to hedonic eating. Susceptible individuals have poor inhibitory control and heightened cravings, which can lead to compulsive overeating and weight gain (1,2). We recently validated the Reward-Based Eating Drive (RED) Scale, which assesses a lack of control over eating, lack of satiation, and preoccupation with food. The RED scale predicts weight gain and correlates with, but is distinct from, other non-pathological eating behavior scales [e.g., Power of Food Scale (3)].
Reward-based eating is less likely to result from activation of neural circuitry of the hypothalamus (signaling hunger) than from the nucleus accumbens (signaling food reward) (1,2,4,5). Chronic consumption of highly processed, palatable, and arguably addictive foods may alter the endogenous opioid system (2,6). In turn, these alterations may increase susceptibility to, and bias experiences of, opioid-mediated food cravings, which are often such foods (7,8). Indeed, a fast-growing literature, mainly rooted in animal studies, highlights associations among food craving, reward sensitivity, and opioid-mediated pathways in the neural experience of reward (9–12).
Acute consumption of highly palatable food stimulates release of endogenous opioids (13,14). Conversely, opioid antagonists (e.g., naloxone) can suppress this action, as evidenced by reductions in rodents’ consumption of palatable food (15,16). Similarly, acute administration of opioid antagonists to people with prior opioid addiction histories (17) and obese men (17,18) can result in reduced short-term cravings for, hedonic responses to, and consumption of, highly palatable food.
Chronic over-consumption of palatable food can dampen endogenous opioid action. Rodents chronically consuming a highly palatable diet that are either removed from the diet or administered an opioid antagonist demonstrate opioid-withdrawal behavior (13). Similarly, women reporting more emotional or binge eating also report symptomology consistent with opioid withdrawal when under opioidergic blockade (19).
Probing associations between reward-driven eating and food cravings by antagonizing the endogenous opioid pathway may reveal the extent to which food cravings are opioid-mediated. This could suggest targets for pharmacological or behavioral interventions for individuals who binge eat (20,21), who are obese, or who are at risk for weight gain. Indeed, several trials indicate that although naltrexone is not an efficacious monotherapy for obesity or binge eating (22,23), the combination of naltrexone and bupropion has resulted in clinically meaningful weight loss in some patients (24,25).
In a sample of obese women, we conducted two sets of secondary analyses. First, we predicted that opioidergic blockade by naltrexone would reduce food-craving intensity relative to placebo. Second, we predicted that opioidergic blockade would reduce the association between trait-like reward-driven eating and daily food-craving intensity, relative to placebo. We also explored whether this association would differ between standard (50mg) and smaller (25mg) naltrexone doses.
Methods
Participants
We recruited 44 obese community dwelling, English speaking, healthy, pre-menopausal women using flyers displaying a study website and phone number (M age=32.7 y, SD=7.6 y; M BMI=34.5; SD=3.3). Participants were 34.1% White, 31.8% Black, 15.9% Asian, 11.4% Mixed Race/Other, and 6.8% Hispanic. At a university medical center, potential participants completed laboratory blood and urine screens for pregnancy, diabetes, anemia, and liver function, and a psychological screen for mental disorders. Inclusion criteria included female sex, overweight status (30≤BMI≤40), and age of 20–45 years. Exclusion criteria included diabetes, current pregnancy or breastfeeding, smoking, medication, historical or current mental disorder or mental health treatment1, kidney or liver disease, illegal drug use or substance misuse, or contraindications to naltrexone (26). Participants were financially compensated for participating. This trial is registered at clinicaltrials.gov (NCT01175512).
Procedure
The UCSF Institutional Review Board approved all study procedures. All participants provided written informed consent. Participants gave consent and completed questionnaires assessing demographics and eating behavior on day 0. We gave participants five identical-appearing pills labeled A thru E: two each of placebo and 50mg naltrexone, and one of 25mg naltrexone. Study staff and participants were told that pill ordering was randomized and were masked to pill ordering. Participants were instructed which pill to take as close as possible to 1:00 PM (after lunch) on days 1 (placebo), 4 (25mg naltrexone), 7 (placebo), 10 (50mg naltrexone), and 4 weeks after day 10 (50mg naltrexone). Participants recorded their exact time of ingestion and completed self-report items in paper logbooks, which they returned by mail.
Materials and measures
Study drug
Participants ingested either 25mg or 50mg of naltrexone hydrochloride, (ReVia; Teva, North Wales, PA), or a placebo pill. The FDA-approved dose for treatment of alcohol and opioid dependence is 50mg, which has been recently used to investigate eating behavior (26). Naltrexone has a mean eliminiation half life (T−1/2) of approximately 4 hours. Participants recorded ingestion time on each study day in their logbook.
Reward-Based Eating Drive (RED) Scale
Participants completed the 9-item RED scale, which assesses a loss of control over eating, a lack of satiety, and preoccupation with food. Sample items include: When I start eating, I just can’t seem to stop (lack of control); and I don't get full easily (lack of satiety). Participants rated items on a scale from 1 (strongly disagree) to 5 (strongly agree). Items were summed, with higher scores reflecting higher reward-based eating drive (M RED=8.21, SD=5.03, Cronbach α=.90).
Food-craving intensity
Just before bedtime each study day, participants recorded the time and retrospectively reported in their logbook their food-craving intensity that day. Participants first completed a dichotomous screening item, “Did you experience a craving for a certain food today?” If the participant endorsed “yes,” she then answered the craving intensity item, “how strong was your craving?” on a Likert scale from 1 (very weak) to 5 (very strong).
Nausea
Nausea symptoms can follow naltrexone ingestion (27) with peak concentrations 2–3 hours after administration. We therefore included nausea as a covariate. Participants self-reported nausea on a scale from 0 (none) to 3 (severe) hourly from 1:00–5:00 PM and then at bedtime each day in their logbooks. We coded women as experiencing nausea if they endorsed nausea at any timepoint (e.g., 26).
Statistical analyses
We used repeated-measures ANOVA to test for differences in craving intensity between all three study doses (average across the two placebo days vs. 25mg naltrexone day vs. average across the two 50mg naltrexone days). We used a generalized estimating equations (GEE) model to test whether dose (placebo vs. 25mg naltrexone vs. 50mg naltrexone) moderated an association between RED and craving intensity. GEE analysis can tolerate missing data and allows for maximum data retention. Our GEE analysis utilized an exchangeable correlation structure, which takes into account within-person dependence (29). We used a series of dummy codes for dose (0,1). We used multiple linear regression to conduct follow-up tests of associations between RED and craving intensity on each study day. All analyses accounted for age, BMI, and nausea2. We conducted all analyses in SPSS Statistics Version 22.
Results
Descriptive statistics appear in Table 1. Of 44 women who enrolled in the study, 5 provided no pill ingestion times or logbook responses, leaving an analytic sample of 39. On average, participants reported ingesting study pills at 1:07 PM (SD=13 min). As directed, participants recorded craving intensity on each study evening (on average, between 10:00–10:15 PM). Approximately one-third or fewer participants endorsed any nausea on any study day (Table 1), which is similar to previous reports of naltrexone nausea responses (27,30,31).
Table 1.
Multiple regression analyses predicting food-craving intensity from reward-driven eating.
| Day | β | b (95% CI) | SE(b) | p |
n (%) endorsing craving |
n (%) endorsing nausea (of those endorsing craving) |
M (SD) craving intensity° |
|---|---|---|---|---|---|---|---|
| Placebo (1) | 0.57 | 0.03 (0.020, 0.178) | 0.03 | .017 | 21 (53.8%) | 5 (12.8%) | 3.24 (1.00) |
| Placebo (2) | 0.56 | 0.04 (0.008, 0.185) | 0.04 | .034 | 21 (53.8%) | 2 (5.1%) | 3.22 (1.06) |
| Naltrexone 25mg | 0.20 | 0.04 (−0.066, 0.143) | 0.04 | .443 | 18 (46.2%) | 7 (17.9%) | 3.14 (0.85) |
| Naltrexone 50mg (1) | 0.33 | 0.03 (−0.028, 0.108) | 0.03 | .230 | 18 (46.2%) | 14 (35.9) | 3.33 (0.69) |
| Naltrexone 50mg (2) | 0.33 | 0.03 (−0.034, 0.125) | 0.03 | .215 | 19 (48.7%) | 12 (30.8%) | 3.05 (0.71) |
Note. All models account for nausea (present vs. absent on that day), body mass index (BMI), and age. Significance and pattern of results do not change when we omit covariates.
Craving intensity did not significantly differ between days, per repeated-measures ANCOVA.
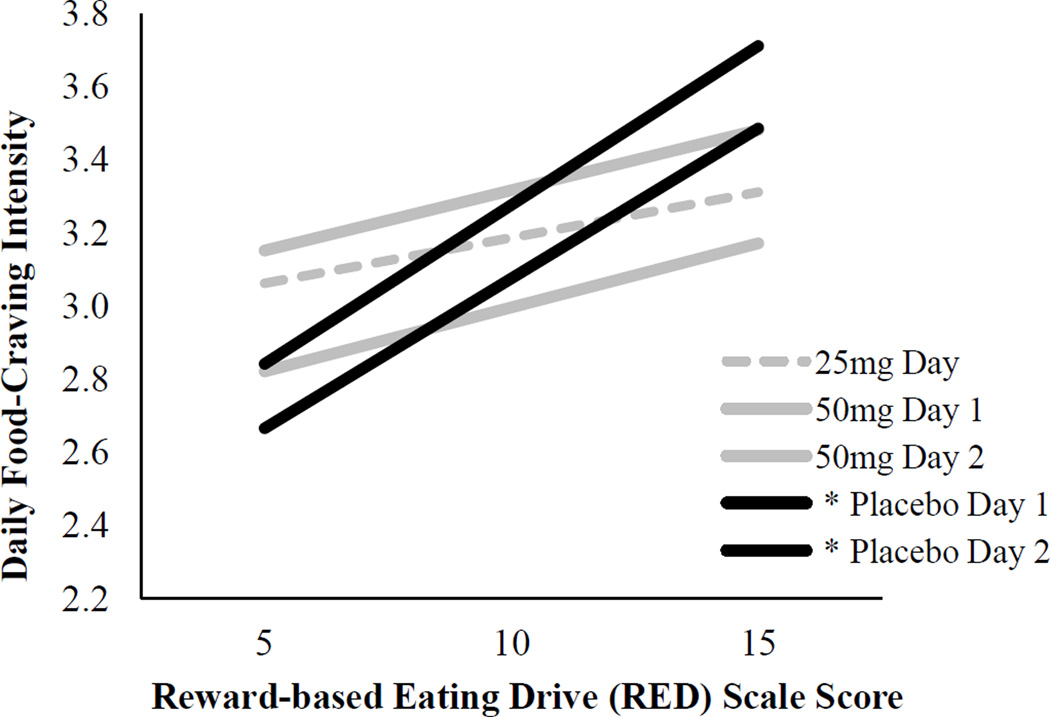
Repeated measures analysis showed that placebo, 25mg, and 50mg doses did not differentially impact craving intensity, F(2, 5.82)=1.19, p=.441. GEE analysis; however, revealed a dose × RED interaction such that the association between RED and craving intensity differed between the placebo and 50mg doses [b=−0.06, SE(b)=0.02, 95% CI (−0.099, −0.012), p=.012]. The association between RED and craving intensity did not significantly differ between the placebo and 25mg doses [b=0.05, SE(b)=0.05, 95% CI (−0.033, 0.142), p=.224] or the 25mg and 50mg doses [b=−0.01, SE(b)=0.04, 95% CI (−0.084, 0.082), p=.977]. Multiple linear regression analyses examining each study day (Table 1; Figure 1) revealed significant positive associations between RED and craving intensity on each placebo day (p=.017; p=.034) and non-significant associations on naltrexone days (p=.433; p=.230; p=.215).
Figure 1.
Associations between reward-based eating drive (RED) and food-craving intensity by study day.
Note. * p <.05. See Table 1 for multiple regression model results.
Discussion
We used self-reported reward-driven eating (1) and a biological probe (19) as a novel assessment method to identify obese women with increased opioid-mediated food cravings. We first found that naltrexone did not alter craving intensity in comparison to placebo: That is, craving intensity did not significantly differ between placebo, 25mg naltrexone, and 50mg naltrexone doses. Second, we found that opioidergic blockade reduced the association between reward-driven eating and craving intensity. Specifically, reward-driven eating and craving intensity were positively associated under placebo, and not significantly associated under 25mg or 50mg naltrexone. This suggests that reward-based eating may index an endophenotype reflecting greater opioid-mediated reward circuitry. We found some evidence of a dose-response effect of naltrexone such that the association between reward-driven eating and craving intensity did not significantly differ between (1) 25mg and 50mg or (2) placebo and 25mg conditions; however, this association differed between (3) placebo and 50mg conditions. Given these findings, we suggest that the standard 50mg dose of naltrexone (versus placebo) may more clearly identify individuals with opioid-mediated food cravings.
That craving intensity did not significantly differ between placebo, 25mg naltrexone, and 50mg naltrexone doses suggests that craving intensity may not be predominantly opioid-mediated across all obese women. Rather, only obese women who report higher levels of reward-driven eating may have opioidergic alterations that underlie food cravings. These findings highlight the utility of characterizing individuals based on reward-driven eating. We note, however, that these results do not necessarily reflect changes in actual eating behavior in response to cravings. Assessing actual food intake will be key to determining clinical importance of these results.
Food cravings may be similar in intensity and neurobiological pathways to cravings observed in the context of drug addiction (6,32). Individuals who tend toward reward-based eating may experience opioid-mediated cravings due to adaptations in reward-related brain circuitry (33–35) and fare worse in the modern obesogenic food environment (36,37), which continuously provides cues to overeat calorically dense, hyper-palatable foods. These results have implications for treatment matching: behavioral interventions targeting craving-related eating might be more effective among obese women who endorse more reward-driven eating.
This study has several limitations. Although the sample size is small, repeated measures analysis substantially increases statistical power to identify significant effects. We used an all-female sample, and although we did not control for menstrual phase or oral contraceptive status, recent data report that food cravings are similar across menstrual phases (38). Future research could consider targeting women in the luteal phase (39,40) to increase consistency and likelihood of observing craving experiences. Of the 39 participants who provided craving intensity data, three used oral contraceptives on three to five of five study days; however, data suggest that oral contraceptives do not significantly impact food cravings (41,42). Additionally, we did not evaluate participants for the DSM-5 diagnosis of Binge Eating Disorder (BED); future studies should do so.
The out-of-laboratory study design assessed women among the food cues that they most encounter in their typical environments; future research could incorporate both in-laboratory and out-of-laboratory components. We did not randomize study medication days so as to limit attrition due to nausea or other side effects, and nausea experiences may have allowed participants to suspect they were receiving an active dose. Future studies should incorporate randomization while also considering potential attrition effects. As in daily diary methodologies (43), we used a single, face-valid item amenable to repeated assessments that minimized participant burden, as we are not aware of a validated single-item measure of food-craving intensity.
Individuals with greater tendencies to eat for hedonic reward may experience cravings that are more difficult to resist. This is among the first studies to pair self-reported reward-driven eating with a biological index of hedonic eating drive (19). Opioidergic blockade reduced a positive association between reward-driven eating and daily craving intensity, suggesting that food craving may be partially opioid-mediated. This unique methodology may help identify women who are particularly vulnerable to intense food cravings and highlight a potential neurobehavioral pathway contributing to obesity.
Highlights.
Opioidergic blockade does not uniformly reduce food-craving intensity
Reward-based eating drive (RED) correlates with daily food-craving intensity
Opioidergic blockade reduces an association between RED and food-craving intensity
Together, opioidergic blockade and RED may identify an obesity endophenotype
Acknowledgements
Ashley E. Mason was supported by the National Institutes of Health (NCCIH T32AT003997). The authors wish to thank Michael Cohn who assisted in proofreading several drafts of the manuscript.
Role of Funding Sources
This research was supported by funding from the National Institutes of Health (NHLBI 1U01HL097973-01) to Elissa S. Epel, Nancy Adler, and Barbara Laraia. NHLBI had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Though we excluded participants who met DSM-IV-TR criteria for bulimia, we did not exclude potential participants who reported binge eating.
Results are unchanged regardless of covariate inclusion.
Contributors
Authors Epel, Adler, Kiernan, Laraia, and Gearhardt designed the study and wrote the protocol. Authors Mason, Puterman, and Daubenmier conducted the statistical analysis and interpreted the data. Authors Mason, Epel, and Daubenmier conducted literature searches. Author Mason wrote the first complete manuscript draft. Authors Adler, Epel, and Laraia obtained funding for the execution of this study. Authors Lustig, Dallman, and Hecht provided critical feedback and consultation on several drafts of the manuscript. Author Lustig prescribed study medication and was available throughout the study for medication-related issues.
Conflict of Interest
Ashley E. Mason, Barbara Laraia, Jennifer Daubenmier, Frederick M. Hecht, Robert H. Lustig, Eli Puterman, Nancy Adler, Mary Dallman, Michaela Kiernan, Ashley Gearhardt, and Elissa S. Epel declare that they have no conflict of interest.
References
- 1.Epel E, Tomiyama AJ, Mason AE, Laraia BA, Hartman W, Ready K, et al. The Reward-Based Eating Drive Scale: A Self-Report Index of Reward-Based Eating. PloS One. 2014;9(6):e101350. doi: 10.1371/journal.pone.0101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes. 2009;33(8):913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- 4.Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45(2):198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang G, Fowler J, Tomasi D, Baler R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev Off J Int Assoc Study Obes. 2013 Jan;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen L. Craving for sweet carbohydrate and fat-rich foods–possible triggers and impact on nutritional intake. Nutr Bull. 2007;32(s1):43–51. [Google Scholar]
- 8.Gearhardt AN, Rizk MT, Treat TA. The association of food characteristics and individual differences with ratings of craving and liking. Appetite. 2014 Aug 1;79:166–173. doi: 10.1016/j.appet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: The molecular face of hedonism? Brain Res Rev. 2006 Jun;51(1):85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Kirkham TC. Cannabinoids and appetite: Food craving and food pleasure. Int Rev Psychiatry. 2009 Jan 1;21(2):163–171. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- 11.Schneider M, Heise V, Spanagel R. Differential involvement of the opioid receptor antagonist naloxone in motivational and hedonic aspects of reward. Behav Brain Res. 2010;208(2):466–472. doi: 10.1016/j.bbr.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Tetley AC, Brunstrom JM, Griffiths PL. The role of sensitivity to reward and impulsivity in food-cue reactivity. Eat Behav. 2010 Aug;11(3):138–143. doi: 10.1016/j.eatbeh.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10(6):478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 14.Peciña S, Smith KS. Hedonic and motivational roles of opioids in food reward: implications for overeating disorders. Pharmacol Biochem Behav. 2010;97(1):34–46. doi: 10.1016/j.pbb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119(5):1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- 16.Pijlman FT, Wolterink G, Van Ree JM. Physical and emotional stress have differential effects on preference for saccharine and open field behaviour in rats. Behav Brain Res. 2003;139(1):131–138. doi: 10.1016/s0166-4328(02)00124-9. [DOI] [PubMed] [Google Scholar]
- 17.Langleben DD, Busch EL, O’Brien CP, Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology (Berl) 2012;220(3):559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel TA, Stunkard AJ, Shrager EE, O’Brien CP, Morrison MF, Stellar E. Effect of naltrexone on food intake, hunger, and satiety in obese men. Physiol Behav. 1987;40(2):135–141. doi: 10.1016/0031-9384(87)90198-3. [DOI] [PubMed] [Google Scholar]
- 19.Daubenmier J, Lustig RH, Hecht FM, Kristeller JL, Woolley J, Adam T, et al. A new biomarker of hedonic eating? A preliminary investigation of cortisol and nausea responses to acute opioid blockade. Appetite. 2014;74:92–100. doi: 10.1016/j.appet.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McElroy SL, Guerdjikova AI, Mori N, O’Melia AM. Pharmacological management of binge eating disorder: current and emerging treatment options. Ther Clin Risk Manag. 2012;8:219–241. doi: 10.2147/TCRM.S25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G-J, Tomasi D, Volkow ND, Wang R, Telang F, Caparelli EC, et al. Effect of combined naltrexone and bupropion therapy on the brain’s reactivity to food cues. [cited 2014 Jan 3];Int J Obes. 2013 Sep 10; doi: 10.1038/ijo.2013.145. [Internet]. Available from: http://www.nature.com/ijo/journal/vaop/ncurrent/full/ijo2013145a.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, et al. Comparison of Combined Bupropion and Naltrexone Therapy for Obesity with Monotherapy and Placebo. J Clin Endocrinol Metab. 2009 Dec 1;94(12):4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- 23.McElroy SL, Guerdjikova AI, Winstanley EL, O’Melia AM, Mori N, McCoy J, et al. Acamprosate in the treatment of binge eating disorder: A placebo-controlled trial. Int J Eat Disord. 2011;44(1):81–90. doi: 10.1002/eat.20876. [DOI] [PubMed] [Google Scholar]
- 24.Caixàs A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Des Devel Ther. 2014 Sep 18;8:1419–1427. doi: 10.2147/DDDT.S55587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015 Mar 24;313(12):1213–1214. doi: 10.1001/jama.2015.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Center for Substance Abuse. Incorporating alcohol pharmacotherapies into medical practice. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. Oral naltrexone. [PubMed] [Google Scholar]
- 27.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26(6):713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 28.Mason AE, Daubenmier J, Lustig R, Moran P, Adler N, Brown RR, et al. Acute responses to opioidergic blockade as a biomarker of hedonic eating among obese women enrolled in a mindfulness-based weight loss intervention trial. Under Review. doi: 10.1016/j.appet.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19(8):769–776. doi: 10.1023/b:ejep.0000036572.00663.f2. [DOI] [PubMed] [Google Scholar]
- 30.Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiol Behav. 1996;60(2):439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 31.Yeomans MR, Gray RW. Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav. 1997;62(1):15–21. doi: 10.1016/s0031-9384(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 32.Gearhardt A, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68(8):808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010 Sep 2;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes. 2009 Jul 21;33(9):1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 35.Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: A model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007 Jan;48(1):12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health. 2006;126(6):262–267. doi: 10.1177/1466424006070487. [DOI] [PubMed] [Google Scholar]
- 37.Schäfer Elinder L, Jansson M. Obesogenic environments–aspects on measurement and indicators. Public Health Nutr. 2009;12(03):307–315. doi: 10.1017/S1368980008002450. [DOI] [PubMed] [Google Scholar]
- 38.McVay MA, Copeland AL, Newman HS, Geiselman PJ. Food cravings and food cue responding across the menstrual cycle in a non-eating disordered sample. Appetite. 2012 Oct;59(2):591–600. doi: 10.1016/j.appet.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. 1991;17(3):199–212. doi: 10.1016/0195-6663(91)90022-k. [DOI] [PubMed] [Google Scholar]
- 40.Tomelleri R, Grunewald KK. Menstrual cycle and food cravings in young college women. J Am Diet Assoc. 1987;87(3):311–315. [PubMed] [Google Scholar]
- 41.Michener W, Rozin P, Freeman E, Gale L. The role of low progesterone and tension as triggers of perimenstrual chocolate and sweets craving: some negative experimental evidence. Physiol Behav. 1999;67(3):417–420. doi: 10.1016/s0031-9384(99)00094-3. [DOI] [PubMed] [Google Scholar]
- 42.Tucci SA, Murphy LE, Boyland EJ, Dye L, Halford JCG. Oral contraceptive effects on food choice during the follicular and luteal phases of the menstrual cycle. A laboratory based study. Appetite. 2010 Dec;55(3):388–392. doi: 10.1016/j.appet.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Iida M, Shrout PE, Laurenceau J-P, Bolger N. Using diary methods in psychological research. In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA handbook of research methods in psychology, Vol 1: Foundations, planning, measures, and psychometrics. Washington, DC, US: American Psychological Association; 2012. pp. 277–305. [Google Scholar]