Abstract
Aims
Loss of cardiac muscle by programmed cell death contributes to the progression of ischemic heart disease. Hypoxia, metabolite waste buildup and energy depletion are components of ischemia which may initiate caspase dependent and independent cell death pathways. Previous work from our laboratory has shown that combined hypoxia with acidosis, a hallmark of ischemia promotes cardiac myocyte injury with increasing severity as the pH declines. Hypoxia-acidosis was demonstrated to activate the pro-apoptotic Bcl-2 protein BNIP3 which initiated opening of the mitochondrial permeability transition pore and cell death in the absence of caspase activation. Because calpains are known to contribute to ischemic myocardial damage in some models, we hypothesized that they are intermediates in the BNIP3-mediated death caused by hypoxia-acidosis.
Main methods
Neonatal rat cardiac myocytes were subjected to hypoxia with and without acidosis and the contribution of calpains to hypoxia-acidosis cell death determined.
Key findings
Here we report that the death pathway activated by hypoxia-acidosis is driven by a combination of calcium-activated calpains and pro-death factors (DNases) secreted by the mitochondria. Cytochrome c accumulated in the cytoplasm during hypoxia-acidosis but caspase activity was repressed through a calpain-dependent process that prevents the cleavage of procaspase 3. Calpain inhibitors provide vigorous protection against hypoxia-acidosis-induced programmed death. Knockdown of BNIP3 with siRNA prevented calpain activation confirming a central role of BNIP3 in this pathway.
Significance
The results implicate BNIP3 and calpain as dependent components of cardiac myocyte death caused by hypoxia-acidosis.
Keywords: BNIP3, calpain, apoptosis, cardiac myocytes, caspases, heart, mitochondrial permeability transition pore, calcium, hypoxia, acidosis, ischemia
INTRODUCTION
Patient studies as well as results from animal models have confirmed that rates of apoptosis and necrosis are increased in the failing myocardium (1-4). Death of cardiac myocytes through both programmed and non-programmed pathways is a central feature of ischemic heart disease (reviewed in (5, 6)). The damage to the myocardium that ensues after ischemia-reperfusion is closely related to the duration and severity of the ischemic period, and infarction may continue to develop for days or weeks after ischemia. The relative contribution of necrosis and apoptosis to cell death during infarction is unclear (6, 7).
Hypoxia and acidosis are obligatory components of ischemia and the combination may provide a critical death signal (8, 9). Ischemic cardiac myocytes generate excess H+ through increased anaerobic metabolism, net hydrolysis of ATP, and CO2 retention (10). The excess protons are extruded from the myoplasm to the interstitial space by the combined action of three major ion-specific membrane transporters, including the Na+-H+ exchanger, the Na+-HCO3- cotransporter, and the vacuolar proton ATPase (11). Increased activity of the Na+-H+ exchanger can cause Ca2+ overload because the elevated intracellular Na+ is subsequently exchanged for Ca2+ via reversal of the Na+-Ca2+ exchanger (12). Cytoplasmic Ca2+ overload is buffered by sequestration into both the mitochondria and sarcoplasmic reticulum.
Calpains comprise a family of Ca2+-dependent cysteine proteases that have been implicated in a variety of diseases including Alzheimer’s disease, diabetes mellitus, cancer, and ischemia (13-15). Although 15 calpain gene products have been reported only µ-calpain and m-calpain are ubiquitously expressed (reviewed in (16)). Inactive calpains exists as heterodimers composed of a large catalytic subunit and a common small regulatory subunit. Each calpain differs in its Ca2+ sensitivity, with µ-calpain and m-calpain being activated by micro- and millimolar concentrations of Ca2+ respectively. Phosphorylation or intracellular localization can lower the Ca2+ concentration required for activation in vivo. Calpains are normally cytoplasmic and present in inactive forms through binding to calpastatin, an endogenous inhibitor. Following activation by Ca2+ calpains selectively degrade intracellular proteins. In the heart calpain activation accompanies pressure-overload heart failure, myocardial ischemia, and may contribute to myocardial remodeling following ischemia/reperfusion injury (17-19).
We have previously reported that hypoxia induced the expression of the pro-apoptotic, Bcl-2 family protein BNIP3, and acidosis promoted BNIP3 membrane translocation and activation of the death pathway (8). Antisense knock-down of BNIP3 or inhibitors of the mitochondrial permeability transition pore (MPTP) blocked the death pathway but caspase inhibitors did not (9). Therefore we hypothesized that caspases are not activated by hypoxia-acidosis but instead the death pathway is mediated by calpain activation. Here we confirm that caspases are not activated by hypoxia-acidosis, rather the death pathway is dependent on the activity of calpains. Calpain activity increased in parallel with increasing acidosis, and calpain inhibitors or calcium channel blockers inhibited the death pathway. Caspase activity was actively suppressed by calpains during hypoxia-acidosis, and there was evidence of calpain-mediated cleavage of procaspase 3. BNIP3 knock-down with siRNA reduced calpain activation suggesting a role for BNIP3 in this process.
METHODS
Reagents
Anti-caspase 3 antibody was from Cell Signaling. Antibodies for α-fodrin and actin were obtained from Chemicon International. Antibody specific for calpastatin was from Santa Cruz Biotechnology, Inc. Anti-COXIV antibody was from Molecular Probes. Anti-BNIP3 was from Abcam. Anti-α-tubulin antibody, ALLN, PD150606, PD151746, BocD, Hoechst 33258, and RU360 were from Calbiochem. BAPTA-AM, 5-(N-Ethyl-N-isopropyl) amiloride (EIPA), and Nifedipine were from Sigma. BHQ and KB-R7943 mesylate were from Tocris Cookson Inc. All inhibitors were dissolved in DMSO except for RU360 and Bcl-XL BH4 domain peptide (TAT-BH4) which were dissolved in water. The inhibitors were diluted in media at a concentration of 1:1000 or greater depending upon inhibitor concentration used. Vehicle treatment groups were exposed to the highest concentration of DMSO used in inhibitor treated samples.
Cardiac Myocyte Culture
All animal protocols were approved by the Animal Care and Use Committee of the University of Miami. Primary cultures of neonatal rat cardiac myocytes were prepared from Sprague-Dawley rats as previously described (8, 20). Using this protocol approximately ten 60 mm cultures dishes or equivalent were prepared per litter. In total approximately 75 litters were used. Rat pups were euthanized by decapitation. Briefly, enriched cultures of cardiac myocytes were obtained from 1-2 day old neonatal rats by stepwise trypsin dissociation, and plated at a density of 4 × 106 cells/60 mm dish, or on 2-well glass slides from Nalgene Nunc Int. at a density of 8 × 105 cells/cm2, in minimal essential medium (MEM) supplemented with 5% fetal calf serum, penicillin, streptomycin, and BrdU. After 3-5 days, cells were rinsed three times in MEM and transferred to serum-free MEM supplemented with holo-transferrin, vitamin B12, and insulin. The final cultures contained >97% cardiac myocytes contracting at >200 beats per minute. Prior to hypoxia exposure, cells were placed in MEM with supplements and 2 g/L of glucose (8, 20).
Hypoxia
Details of our methods for exposing cells to hypoxia have been described previously (20). Acidosis was allowed to develop in the cultures through buildup of metabolic waste or in some cases by adjusting the pH with lactic and phosphoric acid (8). Oxygen was continuously monitored with an oxygen electrode inside the chamber and maintained at 0.1%. Cultures were lysed under hypoxia using ice-cold deoxygenated buffers for analyses.
Quantitative Analysis of Apoptotic Nuclei
Cells were examined for morphologic evidence of apoptosis or necrosis after staining with the fluorescent DNA-binding dyes Hoechst 33258 exactly as previously described (8, 21) . Cells were incubated with 2 μg/ml Hoechst 33258 for 15 min and visualized using a Zeiss Axiovert 200 inverted phase fluorescence microscope.
Analysis of DNA Fragmentation
Genomic DNA fragmentation (DNA ladders) was analyzed exactly as described previously (8, 21). Samples (8 µg) were subjected to electrophoresis in 2% agarose gels and imaged by ethidium bromide staining and digital photography.
Analysis of Caspase Activity
The enzymatic activity of caspases 3 and 9 were analyzed using caspase fluorometric assay from R&D Systems. Following incubation of cell lysates with the fluorometric substrate, levels of fluorescence were determined using a Victor 1420 Multi-label Counter.
Western Blot Analysis
Our procedures for Western blots have been described in detail elsewhere (8, 21). Equal amounts of protein were fractionated on 6-12% SDS-polyacrylamide gels and electroblotted to nitrocellulose (BioRad). Primary antibodies were incubated overnight at 4°C. Proteins were visualized by enhanced chemiluminescence (ECL), following incubation with horseradish peroxidase-conjugated secondary antibodies from Pierce Thermo Scientific.
Viability Assay
The end stage of the death pathway was analyzed using the LIVE/DEAD viability/Cytotoxicity kit from Molecular Probes. Briefly, cardiac myocytes were incubated aerobically or under hypoxia with 2 μM Calcein-AM or with 4 μM ethidium homodimer (EthD-1) for 20 min and visualized by fluorescence microscopy. The polyanionic dye Calcein-AM is retained in living cells and produces an intense green fluorescence while EthD-1 only enters permeabilized membranes.
Subcellular Fractionation
Cell fractionation was performed as described previously (20). After mechanical disruption, cells and nuclei were separated by centrifugation at 120 × g for 10 min; supernatants were centrifuged at 10,000 × g for 30 min to remove the heavy (mitochondrial) membrane fraction with the supernatant representing the cytoplasmic fraction.
Mitochondrial Assays
Mitochondrial membrane potential and pore transition were determined using a JC-1 Kit from Cell Technology. Briefly, cardiac myocytes plated on Nunc glass slides were incubated with JC-1 under aerobic or hypoxic environments for 15 min. Cells were immediately visualized and photographed with a dual-bandpass filter that simultaneously detects fluorescein and rhodamine. JC-1 accumulates only in the mitochondria with intact membrane potential and emits red fluorescence. In cells with collapsed mitochondrial membrane potential, JC-1 remains cytoplasmic and emits green fluorescence.
siRNA knockdown of BNIP3
BNIP3 protein expression was inhibited using rat specific BNIP3 siRNA from Dharmacon as previously described (22, 23). Cells were transfected with 10 nM BNIP3 or random sequence siRNA overnight using DharmaFECT transfection reagent. Using this protocol BNIP3 protein knock-down was greater the 95%.
Statistical analysis
All data are expressed as mean ± S.E.M. Statistical analysis was performed using Social Sciences Statistical Package (SPSS, Inc.). Statistical analysis between two groups was performed using the unpaired Student’s t-test. Statistical analysis between more than two groups was performed using a one-way ANOVA with Dunnett’s multiple comparison post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Calpain but not caspase inhibitors block death by hypoxia-acidosis
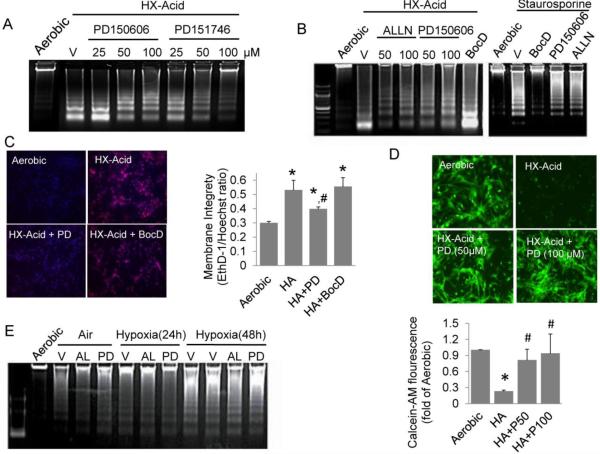
We previously reported that broad-range caspase inhibitors ZVAD and BocD did not prevent cardiac myocyte death during exposure to hypoxia-acidosis (9). As a first step to determine whether calpains were involved, cardiac myocytes were treated under hypoxia with three calpain-selective inhibitors and programmed death was monitored by analysis of DNA fragmentation. As shown in Figure 1A the calpain 1 and 2 inhibitor, PD150606, and the calpain 1 inhibitor, PD151746, blocked DNA fragmentation in a dose-dependent manner. In Figure 1B we compared the effects of PD15606 and ALLN, a cysteinyl protease inhibitor and a competitive inhibitor of both calpains, with the broad range caspase inhibitor BocD. Both calpain inhibitors significantly blocked DNA fragmentation whereas the caspase inhibitor did not. Conversely caspase, but not calpain inhibitors blocked caspase-dependent DNA fragmentation in aerobic cells driven by staurosporine (Figure 1B right panel).
Figure 1.
Calpain inhibition reduces programed cell death in cardiac myocytes exposed to hypoxia-acidosis. (A) Cardiac myocytes were exposed to hypoxia-acidosis (HX-Acid) in the presence of increasing concentrations of the calpain I and II inhibitor, PD150606, or the calpain I inhibitor, PD151746. Cells were harvested at 48 h (final pH 5.5-6.0) and DNA fragmentation determined (n = 3). In (B), myocytes were exposed to hypoxia as in (A) and treated with the calpain inhibitors ALLN, PD150606, or with the caspase inhibitor BocD (100 μM). In parallel, aerobic myocytes were treated with 100 μM ALLN, PD150606 or BocD and exposed to staurosporine (1 µM) to induce caspase dependent cell death (n = 3). In (C), EthD-1 staining was determined in cardiac myocytes treated with 100 μM PD150606, or BocD and exposed to hypoxia-acidosis for 48 h (pH < 6.0). Nuclei were visualized by Hoechst 33342 staining. EthD-1 fluorescents quantitation is shown to the right (n = 4). The effects of calpain inhibition on the viability dye, Calcein-AM, staining is shown in (D) with Calcein-AM quantitation shown below (n = 4). Both concentrations of PD150606 were protective; similar results were observed using PD151746 (not shown). To determine if calpains are activated during energy depletion, cardiac myocytes were treated with 100 μM ALLN (AL) or PD150606 (PD) under hypoxia in media containing 0.25 g/L glucose and DNA fragmentation determined (E) (n = 3 ). Results are mean ± S.E.M., (* p < 0.05 compared to aerobic controls, # p < 0.05 compared to HA), Vehicle (V)
The protection afforded by calpain inhibitors was confirmed using cell permeable and impermeable viability dyes, Calcein-AM and ethidium bromide (EthD-1), respectively. As shown in Figure 1A-C, long-term exposure to hypoxia-acidosis results in cleavage of genomic DNA predominantly into two fragments of ~ 250 and 500 bp. This coincides with a parallel enhancement of nuclear EthD-1 staining consistent with loss of membrane integrity and cell death (Figure 1C). These results are consistent with a previous report from this laboratory (9). Loss of membrane integrity indicated by intense EthD-1 staining was prevented by calpain, but not by caspase inhibition (Figure 1C). Similarly, hypoxia-acidotic cultures did not support Calcein-AM fluorescence in the absence of calpain inhibition (Figure 1D). The effects of calpain inhibitors were selective for the death pathway caused by hypoxia-acidosis because neither PD150606 nor ALLN blocked DNA fragmentation caused by hypoxia with concurrent energy depletion (Figure 1E). Under these conditions, glucose was depleted from the cultures within 12 h and cellular ATP decreased to < 20% within 24 h (data not shown; see (8, 24)). In independent experiments, these results were supported by Hoechst stain quantification of condensed nuclei (data not shown). Therefore calpain inhibitors preserved the structural and genomic integrity of cardiac myocytes even under extreme hypoxia-acidosis. In confirmation of this we found that reoxygenation supported recovery of contractility after severe hypoxia-acidosis only when calpain inhibitors were present (data not shown).
Activation of Calpains by Hypoxia-Acidosis
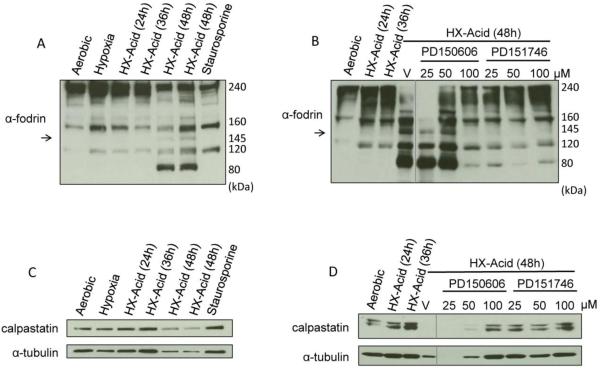
To confirm a role for calpains in hypoxia-acidosis-induced cell death we examined the cleavage products of α-fodrin in cardiac myocytes after exposure to hypoxia with progressive acidosis (Figure 2A). All samples contained a prominent ~240 kDa band that represents native α-fodrin with minor bands at 160 and 120 kDa. Caspases cleave α-fodrin into 160 and 120 kDa products whereas calpains cleave α-fodrin initially into a 160 and 145 kDa products and subsequently to 80-70 kDa. During exposure of cardiac myocytes to hypoxia-acidosis α-fodrin was cleaved into 160, 145, and 80 kDa products whereas staurosporine treatment generated only 160 and 120 kDa products (Figure 2A). α-fodrin cleavage during hypoxia-acidosis was prevented by calpain inhibitors PD150606 and PD151746 (Figure 2B). These results are consistent with activation of calpains but not caspases by hypoxia-acidosis. Calpains are normally sequestered as inactive complexes with calpastatin. During activation, calpains dissociate from calpastatin and calpastatin is degraded; therefore calpastatin levels are diagnostic of calpain activity. To confirm activation of calpains by hypoxia-acidosis, we analyzed the levels of calpastatin by western blot. As shown in Figure 2C and D, calpastatin levels and α-tubulin, a target of calpains, decreased during hypoxia-acidosis in parallel with α-fodrin cleavage which could be blocked by calpain inhibition. These results confirm that calpains are activated by hypoxia-acidosis and contribute to caspase-independent death of cardiac myocytes.
Figure 2.
Time course of calpain activation during hypoxia-acidosis. Cardiac myocytes were exposed to hypoxia-acidosis for the times indicated or to staurosporine (1 μM) for 8 h. Western blots were probed for the calpain substrates α-fodrin (A) (n = 5) and calpastatin (C) (n = 4). Arrow indicates calpain specific α-fodrin cleavage. The effect of calpain inhibition on calpain activation was determined in cardiac myocytes exposed to hypoxia-acidosis. Calpain activity was determined by α-fodrin, and calpastatin cleavage (B and D) (n = 4). Vehicle (V).
Cytochrome c is released but caspases are not activated during hypoxia-acidosis
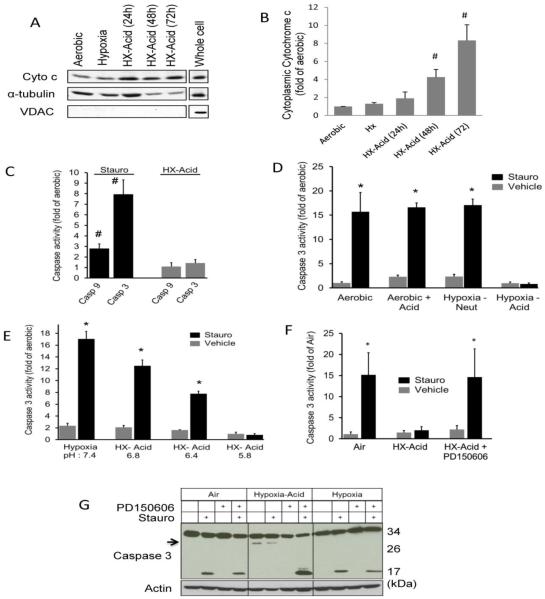
Hypoxia-acidosis promotes opening of the MPTP and the release of pro-apoptotic factors from the mitochondria that are blocked by MPTP inhibitors thereby conferring protection (9, 20). This is predicted to support activation of caspase-dependent apoptosis. Cytochrome c release is required for caspase activation; therefore we measured cytoplasmic cytochrome c levels. As illustrated in Figure 3A and B, there is a significant accumulation of cytoplasmic cytochrome c which accompanies hypoxia-acidosis exposure. To determine whether the intrinsic pathway of apoptosis is activated as predicted, we measured the activities of caspases 3 and 9. As shown in Figure 3C, both caspases were activated in aerobic cells by staurosporine treatment, but not in cells exposed to hypoxia-acidosis. Therefore we addressed this apparent anomaly by determining whether hypoxia-acidosis precluded caspase activation. To determine this, caspase 3 activity was quantified during staurosporine treatment with simultaneous exposure to hypoxia and/or acidosis. As shown in Figures 3D and E, staurosporine-mediated caspase 3 activity was not influenced by either acidosis or hypoxia alone but it was abrogated by combined hypoxia-acidosis.
Figure 3.
Caspase activation is inhibited by calpains during hypoxia-acidosis. (A) Cardiac myocytes were subjected to hypoxia or hypoxia with progressive acidosis and the cytoplasmic cytochrome c protein levels determined. VDAC was used to determine the purity of the cytoplasmic fraction. Quantitation of cytoplasmic cytochrome c is shown in (B) (n = 5). Caspase 9 and 3 activities in cardiac myocytes exposed to hypoxia-acidosis or 1 μM staurosporine is shown in (C) (n = 4). In (D), the effect of hypoxia, acidosis, and hypoxia-acidosis on caspase 3 activity was determine in cardiac myocytes after 8 h exposure to 1 μM staurosporine in neutral, acidic (pH 6.5), or hypoxia-acidic cultures as indicated (n = 4). In (E), cardiac myocytes were exposed to hypoxic-acidosis with and without 1 μM staurosporine for 8 h at progressively lower pH and caspase 3 activity determined (n = 4). In (F), staurosporine (1 μM) induced caspase 3 activity was determined in cardiac myocytes subjected to aerobic incubation, hypoxia-acidosis, or hypoxia-acidosis with 100 μM PD150606 (n = 4). Western blot analysis of caspase 3 protein activation is shown in (G). Inactivated procaspase 3 migrates at 35 kDa, activated caspase 3 migrates at 17 kDa, and a 30 kDa product (arrow) was observed only under hypoxia-acidosis. The 30 kDa product disappeared with PD150606 (100 μM) treatment (n = 3). Results are mean ± S.E.M., ( # p < 0.05 compared to aerobic controls; * p<0.05 compared to vehicle controls).
Calpains block caspases during hypoxia-acidosis
Previous studies have demonstrated cross-talk between caspases and calpains (25). To determine whether calpains regulate caspases activity during hypoxia-acidosis, cardiac myocytes were pretreated with calpain inhibitors PD150606 and exposed to staurosporine and hypoxia-acidosis. As shown in Figure 3F, caspase 3 was activated by staurosporine only when calpains were inhibited with PD150606. To investigate the mechanism by which calpains suppress caspase 3 activation we analyzed the cleavage products of procaspase 3 in the presence and absence of staurosporine, PD150606, and hypoxia-acidosis (Figure 3G). Staurosporine treatment of aerobic or hypoxic cultures generated the predicted 17 kDa activated caspase 3 cleavage product that was unaffected by PD150606 treatment. In contrast staurosporine treatment did not generate a 17 kDa-cleavage product under conditions of hypoxia-acidosis, but rather a larger fragment of about 30 kDa was produced. When myocytes were pretreated with PD150606 before exposure to staurosporine and hypoxia-acidosis, the 30 kDa product was eliminated and the 17 kDa product reappeared confirming that hypoxia-acidosis inhibits processing of procaspase 3 in a calpain-dependent manner.
Role of the mitochondria
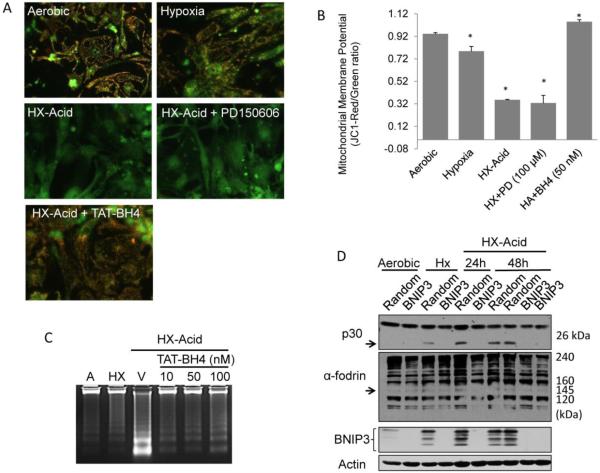
The dominant role of calpains over caspases in hypoxia-acidosis mediated cell death raises the question of the relevance of the MPTP and the mitochondrial membrane potential to this atypical apoptotic death pathway. We previously demonstrated that this pathway was blocked by antisense BNIP3 and MPTP inhibitors (9). Therefore we asked whether calpains provide signals for mitochondrial dysfunction. To test this we used JC-1 to monitor mitochondrial membrane potential during hypoxia-acidosis (Figure 4A and B). In aerobic myocytes JC-1 is accumulated in the mitochondria producing a punctate red fluorescence. This pattern was maintained in hypoxic-neutral cultures, but was lost in favor of a more punctate green fluorescence during hypoxia-acidosis indicating loss of membrane potential. The membrane potential was not preserved by calpain inhibition with PD150606, suggesting that calpain activation is downstream of mitochondrial dysfunction. Pre-treatment with TAT-BH4, a selective MPTP inhibitor peptide (26-28) prior to hypoxia-acidosis preserved the membrane potential and prevented DNA fragmentation (Figure 4C). These results indicate that MPTP opening is independent of calpain activation but is a required step in the hypoxia-acidosis cell death pathway.
Figure 4.
Calpain activation is dependent upon BNIP3 following mitochondrial dysfunction. (A) Mitochondrial membrane potential was determined by JC-1 staining in cardiac myocytes treated with 100 µM PD150606 or 50 nM TAT-BH4 peptide following 24 h hypoxia or hypoxia-acidosis (pH 6.0) exposure. The red punctate staining indicates intact mitochondrial membrane potential were as predominantly green staining indicates collapsed mitochondrial membrane potential. JC1 quantitation is shown in (B) (n = 8). The effects of TAT-BH4-peptide on DNA fragmentation is shown in (C) (n = 3). In (D), BNIP3 protein was knocked-down using BNIP3 specific siRNA and the autolytic degradation product of the small calpain subunit p30 (arrow) and the 145 kDa α-fodrin specific calpain cleavage product (arrow) determined. Parallel plates were treated with random sequence siRNA controls which did not affect BNIP3 expression or calpain activation (n = 3). Results are mean ± S.E.M., (* p<0.05 compared to aerobic controls).
Role of BNIP3 in calpain activation
We previously demonstrated that BNIP3 over-expression induces the opening of the MPTP under conditions of acidosis and that acidosis increases BNIP3 binding to membranes (9). Therefore we asked whether calpain activation during hypoxia-acidosis requires BNIP3. To test this we used a BNIP3-specific siRNA to knock-down BNIP3 expression, and examined the cleavage products of the calpain small subunit p30, and α-fodrin. As shown in Figure 4D, both the calpain small subunit and α-fodrin cleavage products (145 kDa), both indicator of calpain activation, were blocked by BNIP3-specific siRNA but not by random sequence siRNA consistent with a role for BNIP3 in the mitochondria-dependent activation of calpain during exposure of cardiac myocytes to hypoxia-acidosis.
Calcium is required for hypoxia-acidosis cell death
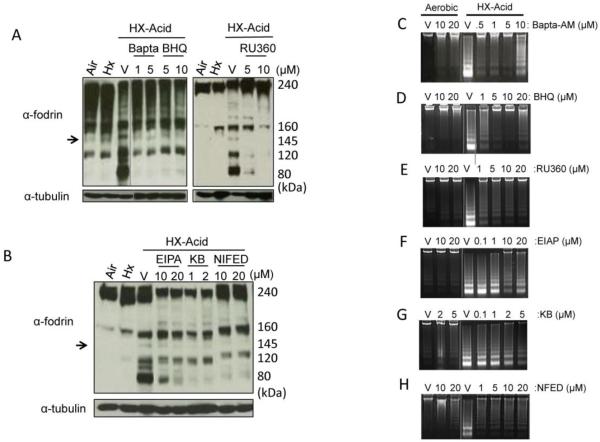
Because calpains require calcium for activity and because it has been demonstrated that BNIP3 alters endoplasmic reticulum and mitochondrial calcium levels (29), we investigated the possible intracellular source(s) of calcium using chelators and channel-selective inhibitors. Treatment of cells with BAPTA-AM (intracellular calcium chelator), BHQ (SR-calcium channel blocker), or RU360 (mitochondrial calcium uptake inhibitor) prevented the calpain-mediated cleavage of α-fodrin and effectively blocked DNA fragmentation during hypoxia-acidosis (Figure 5 A, C, D, E). The increase levels of DNA fragmentation observed at the higher concentrations of BAPTA-AM and BHQ have been previously reported (30, 31) and may be the result of ER calcium depletion leading to the activation of ER stress pathways. EPIA (Na+/H+ channel blocker), KB-R7943 (reverse mode Na+/Ca2+ blocker) and nifedipine (voltage-dependent Ca2+ channel blocker) also blocked α-fodrin cleavage and reduced DNA fragmentation although not as effectively as the former reagents (Figure 5B, F, G, H). These results indicate that calcium is integral to this pathway and chelation of intracellular calcium or inhibition of calcium transport from the mitochondria or sarcoplasmic reticulum (SR) block calpain activation and DNA fragmentation. The results are also consistent with the known cross-talk between the mitochondria and SR (32) and the localization of BNIP3 to non-mitochondrial as well as mitochondrial sites (29). These results also indicate that Ca2+ release from the ER/SR may be more important in the activation of calpains then Ca2+ influx by plasma membrane mechanisms. Pre-treatment of myocytes with RU360 did not affect apoptosis caused by staurosporine (data not shown).
Figure 5.
Effect of calcium modulators on calpain activation. Hypoxic cardiac myocytes were pretreated with 1 or 5 μM of BAPTA-AM, or BHQ or with 5 or 10 μM RU360 in (A), 10 and 20 μM of EIPA, 1 and 2 μM KB-R743 (KB), or 10 and 20 μM of nifedipine (NIFED) in (B) for 1.5 h then subjected to hypoxia-acidosis (pH <6.0) for 5.5 h. Western blots were probed for α-fodrin and α-tubulin as indicators of calpain activity. Arrow represents the 145 kDa calpain specific cleavage product. The effect of calcium modulators on DNA fragmentation is shown in C-H. All results are representative of three separate experiments.
DISCUSSION
We describe a novel pathway of caspase-independent cardiac myocyte death that is caused by a combination of hypoxia and acidosis and driven by activated calpains. The pathway may be relevant to myocardial infarction caused by prolonged ischemia where hypoxia and acidosis are obligatory. These results support the pathway illustrated in Figure 6. We found that combined hypoxia and acidosis promoted a progressive pH-dependent fragmentation of genomic DNA and loss of sarcoplasmic membrane integrity that was prevented by calpain inhibitors, calcium blockers, MPTP inhibition, and knock down of BNIP3 by siRNA. The results are consistent with our previous report that hypoxia-acidosis promotes opening of the MPTP and BNIP3-dependent but caspase-independent death of cardiac myocytes. Our results further show that caspase 3 activation was prevented in a calpain-dependent manner during exposure to hypoxia-acidosis despite MPTP opening and release of pro-apoptotic mediators. The mechanism by which caspases are inhibited during hypoxia-acidosis involves calpain-dependent cleavage of procaspase 3 that prevents its activation. Procaspase 3 cleavage was prevented and caspase 3 activated when cardiac myocytes were exposed to hypoxia-acidosis in the presence of calpain inhibitor. These results also support our previous report that cardiac myocyte loss caused by hypoxia-acidosis is caspase-independent but BNIP3, and MPTP-dependent (9). It seems probable that the decline in intracellular pH causes the reversal of plasma membrane Na+-Ca2+ exchangers resulting in increased cytoplasmic Ca2+ which is rapidly buffered by the mitochondria and SR. Concurrently, low pH stabilizes BNIP3 and in parallel may promote integration into the mitochondrial membranes where it causes opening of the MPTP and subsequent release of cytochrome c and excessive Ca2+, an initiating step in both calpain and caspase activation (9, 22, 33, 34). Our results are consistent with a role for the endoplasmic reticulum which may release additional excessive calcium in a BNIP3-dependent manner. The results are also consistent with our previous reports that this death pathway involves the nuclear accumulation of multiple caspase-independent DNases (20). The death pathway is BNIP3-dependent and insensitive to pan-caspase inhibitors. We found no evidence for changes in abundance or intracellular localization of Bax, BAD or Bid (data not shown).
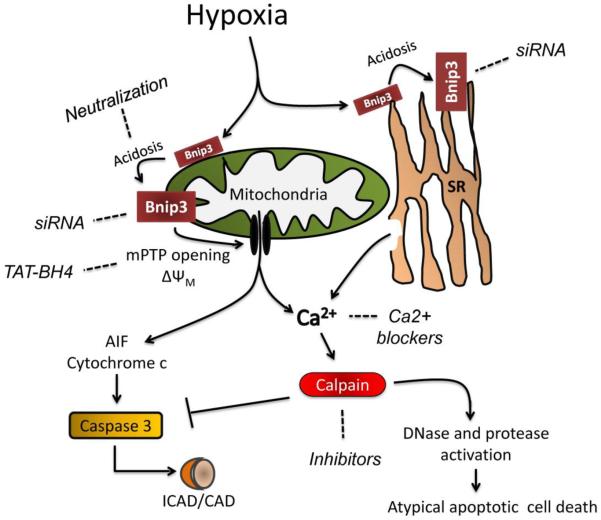
Figure 6.
Pathway of hypoxia-acidosis mediated cell death in cardiac myocytes. BNIP3 protein is expressed by hypoxia but accumulates and induces mitochondrial dysfunction as the pH declines. Calcium release from both the mitochondria and sarcoplasmic reticulum activates calpains which leads to caspase inactivation and protease and DNase activation and cell death. Broken lines indicate where the death pathway can be inhibited. SR is sarcoplasmic reticulum.
Calpains are a family of calcium-dependent serine proteases ubiquitously present, usually in an inactive form in the cell cytoplasm. Apoptotic stimuli that increase cytosolic Ca2+ are expected to simultaneously increase the activity of calpains. Sarcoplasmic Ca2+ levels increase during both the ischemic and reperfusion phases of myocardial ischemia, and are predicted to activate calpains (32, 35). Mitochondria and the SR are major intracellular storage sites for Ca2+. We found that calpain inhibitors blocked cell death but did not block MPTP opening; suggesting that MPTP opening precedes calpain activation. The death pathway was also blocked by inhibiting the SERCA2 pump with BHQ or the plasma membrane L-type Ca2+ channel with nifedipine. These results implicate multiple intracellular sources of calcium as well as accumulation from the extracellular compartment. Calcium can accumulate during ischemia by the combination of Na+/H+ and Na+/Ca2+ exchangers (36). Inhibition of the Na+/H+ exchanger with EPIA or the reverse mode of the Na+/Ca2+ channel by KB was protective but less so than the other channel inhibitors.
Inhibition of the MPTP with the TAT-BH4 peptide was highly protective suggesting a critical role of the mitochondrial pore in activating calpain and promoting the death pathway. Previous work from this and other groups indicate that BNIP3 targets mitochondrial and non-mitochondrial intracellular sites (9, 29, 37). Recently it was demonstrated that localization of BNIP3 to the endoplasmic reticulum (ER) initiates a Ca2+-dependent, caspase-independent death pathway which could be blocked by inhibiting mitochondrial uptake of Ca2+ with RU360 (29). It was demonstrated that ER localized BNIP3 facilitates the release of Ca2+ from the ER which is subsequently taken up by the mitochondria thereby inducing mitochondrial dysfunction and caspase-independent cell death. As discussed above, an increase in Ca2+ influx during hypoxia-acidosis overloads the ER and the mitochondria thereby exacerbating possible ER to mitochondrial Ca2+ transport. BNIP3 appears to mediate Ca2+ release from the ER and mitochondria during hypoxia-acidosis because knock-down of BNIP3 expression with siRNA also blocked calpain activation. The reversal of plasma membrane Ca2+ transporters during hypoxia-acidosis would result in enhanced mitochondrial and SR Ca2+ levels which may enhance calpain activation upon release by BNIP3. In a pressure overload model of heart failure, BNIP3 was demonstrated to induce ER to mitochondrial Ca2+ shuttling via VDAC oligomerization (38) and to increase the expression of the ER stress (p-eIF2a) and apoptotic (CHOP) markers. Therefore the protective effect of TAT-BH4, which blocks VDAC channels (26), in this study may also exert its protective effect through the inhibition of ER-mitochondrial Ca2+ shuttling in conjunction with regulating MPTP opening.
A role for intracellular acidosis in promoting cellular damage during myocardial ischemia has been recognized for some time (39, 40). Acidosis is an obligatory component of ischemia and can promote cell death by activating acid-regulated DNases, promoting Ca2+ overload, and stimulating multiple protease and kinase pathways (11, 35). During experimental myocardial ischemia in rodents, the left ventricular pH drops to approximately 6.5 within 10 min of ischemia and falls below 6.0 after 20-30 min (41, 42). Interestingly only the longer ischemic period that involves extreme low pH promotes infarction during reperfusion. Cardiac biopsies taken from pigs and humans after cross clamping undergo a pH-dependent programmed death that is most severe at pH below 6.0 (43). The low pH values observed in ischemic tissues described in these and other studies are well within the range for BNIP3 activation and initiation of this death pathway.
Death of cardiac myocytes contributes significantly to the progression of ischemic heart disease but the extent of death that is attributed to programmed pathways and the relative contributions of apoptosis, necrosis, and autophagic pathways are not clear. Olivetti et al (1) first described a dramatic increase (232-fold) of apoptosis in the hearts of patients with heart failure. They used multiple parameters including DNA strand breaks, DNA fragmentation and nuclear morphology. These results were generally supported by Narula et al. (44) and Seleste et al. (45) . Kajstura et al. demonstrated that apoptosis was the major form of cardiac myocyte loss during ischemia/reperfusion in rats as assessed by terminal deoxynucleotidyl transferase analysis and DNA laddering (7), but necrosis vastly exceeded apoptosis in hearts subjected to coronary constriction (2) . There has been ongoing debate over whether histological or fragmentation measurements including TUNEL and DNA ladders accurately reflect programmed death. The concept of necrosis as an unregulated chaotic event has also changed, and pathways expressing multiple markers of apoptosis, necrosis, and autophagic death have been described (46). The pathway activated by BNIP3 may best be described as an intrinsic pathway of programmed necrosis. Because it is caspase negative and only weakly TUNEL positive (20), this form of cell loss may have been overlooked in some previous analyses of ischemic tissues that leant heavily on such parameters. Our previous findings in human tissues, as well as the results from ischemic rat hearts suggest that BNIP3 may contribute significantly to the loss of myocardium in patients with ischemic heart disease (22).
ACKNOWLEDGEMENTS
This work was supported by grants (HL44578 and HL072924 to KAW) from the National Institutes of Health by an American Heart Association (Florida Affiliate) postdoctoral fellowship (RMG) by an American Heart Association (Florida Affiliate) predoctoral fellowship (JWT) and by a Walter G. Ross endowed chair in vascular biology (KAW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
REFERENCES
- 1.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. The New England journal of medicine. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Liu Y, Baldini A, Li B, Olivetti G, Leri A, Anversa P. Coronary artery constriction in rats: necrotic and apoptotic myocyte death. The American journal of cardiology. 1998;82:30K–41K. doi: 10.1016/s0002-9149(98)00535-9. [DOI] [PubMed] [Google Scholar]
- 3.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. Journal of molecular and cellular cardiology. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 4.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. The New England journal of medicine. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 5.McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochemical and biophysical research communications. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- 6.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease--a novel therapeutic target? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- 7.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Laboratory investigation; a journal of technical methods and pathology. 1996;74:86–107. [PubMed] [Google Scholar]
- 8.Webster KA, Discher DJ, Kaiser S, Hernandez O, Sato B, Bishopric NH. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. The Journal of clinical investigation. 1999;104:239–252. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis SC, Gevers W, Opie LH. Protons in ischemia: where do they come from; where do they go to? Journal of molecular and cellular cardiology. 1991;23:1077–1086. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- 11.Karwatowska-Prokopczuk E, Nordberg JA, Li HL, Engler RL, Gottlieb RA. Effect of vacuolar proton ATPase on pHi, Ca2+, and apoptosis in neonatal cardiomyocytes during metabolic inhibition/recovery. Circulation research. 1998;82:1139–1144. doi: 10.1161/01.res.82.11.1139. [DOI] [PubMed] [Google Scholar]
- 12.Pierce GN, Czubryt MP. The contribution of ionic imbalance to ischemia/reperfusion-induced injury. Journal of molecular and cellular cardiology. 1995;27:53–63. doi: 10.1016/s0022-2828(08)80007-7. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira A. Calpain dysregulation in Alzheimer's disease. ISRN biochemistry. 2012;2012:728571. doi: 10.5402/2012/728571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World journal of cardiology. 2014;6:638–652. doi: 10.4330/wjc.v6.i7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan TT, Li XF, Sun YM, Li YB, Su Y. Role of the calpain on the development of diabetes mellitus and its chronic complications. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2015;74:187–190. doi: 10.1016/j.biopha.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Lopatniuk P, Witkowski JM. Conventional calpains and programmed cell death. Acta biochimica Polonica. 2011;58:287–296. [PubMed] [Google Scholar]
- 17.Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, Fritz H, Siebeck M. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. European journal of pharmacology. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovascular research. 2012;96:38–45. doi: 10.1093/cvr/cvs099. [DOI] [PubMed] [Google Scholar]
- 19.Trumbeckaite S, Neuhof C, Zierz S, Gellerich FN. Calpain inhibitor (BSF 409425) diminishes ischemia/reperfusion-induced damage of rabbit heart mitochondria. Biochemical pharmacology. 2003;65:911–916. doi: 10.1016/s0006-2952(02)01610-6. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JW, Graham RM, Webster KA. DNase activation by hypoxia-acidosis parallels but is independent of programmed cell death. Life sciences. 2012;91:223–229. doi: 10.1016/j.lfs.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dougherty CJ, Kubasiak LA, Prentice H, Andreka P, Bishopric NH, Webster KA. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. The Biochemical journal. 2002;362:561–571. doi: 10.1042/0264-6021:3620561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of Bnip3 death pathways by calcium, phosphorylation, and hypoxia-reoxygenation. Antioxidants & redox signaling. 2007;9:1309–1315. doi: 10.1089/ars.2007.1726. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JW, Wei J, Appau K, Wang H, Yu H, Spiga MG, Graham RM, Webster KA. Bnip3 Binds and Activates p300: Possible Role in Cardiac Transcription and Myocyte Morphology. PloS one. 2015;10:e0136847. doi: 10.1371/journal.pone.0136847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster KA, Discher DJ, Bishopric NH. Induction and nuclear accumulation of fos and jun proto-oncogenes in hypoxic cardiac myocytes. The Journal of biological chemistry. 1993;268:16852–16858. [PubMed] [Google Scholar]
- 25.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. The Journal of cell biology. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin LJ, Adams NA, Pan Y, Price A, Wong M. The mitochondrial permeability transition pore regulates nitric oxide-mediated apoptosis of neurons induced by target deprivation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:359–370. doi: 10.1523/JNEUROSCI.2225-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. The Journal of biological chemistry. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Li L, Liu H, Borowitz JL, Isom GE. BNIP3 mediates cell death by different pathways following localization to endoplasmic reticulum and mitochondrion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3405–3414. doi: 10.1096/fj.08-124354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frosali S, Leonini A, Ettorre A, Di Maio G, Nuti S, Tavarini S, Di Simplicio P, Di Stefano A. Role of intracellular calcium and S-glutathionylation in cell death induced by a mixture of isothiazolinones in HL60 cells. Biochimica et biophysica acta. 2009;1793:572–583. doi: 10.1016/j.bbamcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Zhu WH, Loh TT. Roles of calcium in the regulation of apoptosis in HL-60 promyelocytic leukemia cells. Life sciences. 1995;57:2091–2099. doi: 10.1016/0024-3205(95)02202-t. [DOI] [PubMed] [Google Scholar]
- 32.Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochemical and biophysical research communications. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 33.Frazier DP, Wilson A, Graham RM, Thompson JW, Bishopric NH, Webster KA. Acidosis regulates the stability, hydrophobicity, and activity of the BH3-only protein Bnip3. Antioxidants & redox signaling. 2006;8:1625–1634. doi: 10.1089/ars.2006.8.1625. [DOI] [PubMed] [Google Scholar]
- 34.Graham RM, Frazier DP, Thompson JW, Haliko S, Li H, Wasserlauf BJ, Spiga MG, Bishopric NH, Webster KA. A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. The Journal of experimental biology. 2004;207:3189–3200. doi: 10.1242/jeb.01109. [DOI] [PubMed] [Google Scholar]
- 35.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS letters. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 36.Salameh A, Dhein S, Beuckelmann DJ. Role of the cardiac Na(+)/H(+)exchanger in [Ca(2+)](i)and [Na(+)](i)handling during intracellular acidosis. Effect of cariporide (Hoe 642) Pharmacological research : the official journal of the Italian Pharmacological Society. 2002;45:35–41. doi: 10.1006/phrs.2001.0908. [DOI] [PubMed] [Google Scholar]
- 37.Ray R, Chen G, Vande Velde C, Cizeau J, Park JH, Reed JC, Gietz RD, Greenberg AH. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. The Journal of biological chemistry. 2000;275:1439–1448. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 38.Chaanine AH, Gordon RE, Kohlbrenner E, Benard L, Jeong D, Hajjar RJ. Potential role of BNIP3 in cardiac remodeling, myocardial stiffness, and endoplasmic reticulum: mitochondrial calcium homeostasis in diastolic and systolic heart failure. Circulation. Heart failure. 2013;6:572–583. doi: 10.1161/CIRCHEARTFAILURE.112.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottlieb RA, Gruol DL, Zhu JY, Engler RL. Preconditioning rabbit cardiomyocytes: role of pH, vacuolar proton ATPase, and apoptosis. The Journal of clinical investigation. 1996;97:2391–2398. doi: 10.1172/JCI118683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisenholder GW, Martin SJ, Green DR, Nordberg J, Babior BM, Gottlieb RA. Events in apoptosis. Acidification is downstream of protease activation and BCL-2 protection. The Journal of biological chemistry. 1996;271:16260–16262. doi: 10.1074/jbc.271.27.16260. [DOI] [PubMed] [Google Scholar]
- 41.Murphy E, Cross H, Steenbergen C. Sodium regulation during ischemia versus reperfusion and its role in injury. Circulation research. 1999;84:1469–1470. doi: 10.1161/01.res.84.12.1469. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer S, Carr LJ, Prussel E, Ramasamy R. Effects of glycogen depletion on ischemic injury in isolated rat hearts: insights into preconditioning. The American journal of physiology. 1995;268:H935–944. doi: 10.1152/ajpheart.1995.268.3.H935. [DOI] [PubMed] [Google Scholar]
- 43.Thatte HS, Rhee JH, Zagarins SE, Treanor PR, Birjiniuk V, Crittenden MD, Khuri SF. Acidosis-induced apoptosis in human and porcine heart. The Annals of thoracic surgery. 2004;77:1376–1383. doi: 10.1016/j.athoracsur.2003.07.047. [DOI] [PubMed] [Google Scholar]
- 44.Narula J, Hajjar RJ, Dec GW. Apoptosis in the failing heart. Cardiology clinics. 1998;16:691–710. doi: 10.1016/s0733-8651(05)70045-x. ix. [DOI] [PubMed] [Google Scholar]
- 45.Saraste A, Pulkki K, Kallajoki M, Heikkila P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM. Cardiomyocyte apoptosis and progression of heart failure to transplantation. European journal of clinical investigation. 1999;29:380–386. doi: 10.1046/j.1365-2362.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 46.Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM. Apoptotic versus autophagic cell death in heart failure. Cardiovascular research. 2001;51:304–312. doi: 10.1016/s0008-6363(01)00290-5. [DOI] [PubMed] [Google Scholar]