Abstract
Objectives
Fetal alcohol spectrum disorders are more common in disadvantaged populations. Environmental factors, like suboptimal nutrition, may potentiate the developmental effects of prenatal alcohol exposure. To evaluate the impact of micronutrients, including choline, on reduction of effects of exposure, we examined timing and dose of alcohol and effects of nutritional supplementation at two OMNI-Net sites in Western Ukraine that included high and low risk individuals.
Methods
Alcohol-using and nondrinking women were randomized to one of three multivitamin/mineral supplement groups: none, multivitamins/minerals (MVM), and multivitamin/minerals plus choline. Children (N = 367) were tested at 6 months with the Bayley Scales of Infant Development (2nd ED) yielding standard scores for Mental Development Index (MDI), Psychomotor Development Index (PDI) and Behavior.
Results
Generalized linear modeling was used: (1) for factorial analysis of effects of alcohol group, multivitamin/minerals, and choline supplementation; and (2) to examine the relationship between amount and timing of alcohol (ounces of absolute alcohol/day [ozAA/day] peri-conception and on average in the second trimester) and MVM supplementation on developmental outcomes while controlling sex, social class, and smoking. MDI was significantly impacted by peri-conceptual alcohol dose ( , p <.001) with more alcohol associated with lower scores and males more negatively affected than females ( , p < .002). Micronutrient supplementation had a protective effect; those receiving supplements performed better ( , p <.005). The PDI motor scores did not differ by group but were affected by peri-conceptual alcohol dose ( , p <.04).
Conclusions for Practice
Multivitamin/mineral supplementation can reduce the negative impact of alcohol use during pregnancy on specific developmental outcomes.
Keywords: Prenatal alcohol exposure, Fetal alcohol spectrum disorders, Multivitamin supplement, Choline, Infant development
Introduction
Fetal alcohol spectrum disorders (FASD) are a leading cause of developmental disabilities. Affected children have cognitive, developmental and behavior problems in addition to physical effects [1, 2]. The Centers for Disease Control and Prevention reported that 1 % of children in the USA [3] were affected by prenatal alcohol exposure (PAE) although a recent study suggested a higher rate [4]. Avoiding alcohol use during pregnancy or when planning to become pregnant is the optimum prevention strategy but alcohol-exposed pregnancies will continue as long as half of pregnancies remain unplanned. Alcohol use in the pre-conceptual and prerecognition period is most usually acknowledged and associated with negative outcomes [5]. In addition, some women are unable or unwilling to discontinue drinking either because they are problem drinkers or because they are not convinced that abstinence is necessary. Therefore, despite decades of recommendations to abstain from drinking, preventing the effects of PAE remains a public health concern.
FASD is reported more frequently among “at risk” populations and it has been difficult to rule out effects of environmental factors associated with alcohol use as alternative explanations for effects on development [6, 7]. The impact of PAE may be amplified by co-occurring risk factors including poor nutrition, tobacco use, access to resources, and other exposures. Surprisingly, there is little direct research on environmental factors associated with PAE that may contribute to negative developmental outcomes.
Thus, understanding of prenatal and postnatal factors that can modify risk in FASD is a critical need. Such knowledge could drive new prevention and intervention strategies throughout the world. For instance, the association with disadvantaged populations suggests that poor nutrition or lack of some specific nutritional elements may exacerbate the impact of alcohol. Nutritional deficits are probably more common in alcohol-abusing women and even in the United States, alcohol using women are less likely to take prescribed multivitamin supplements [8]. It is likely that this problem is more acute in more disadvantaged countries. Supplementation during pregnancy may mitigate some of the negative effects of PAE [9] prior to pregnancy recognition.
In addition to concern about failure to meet general nutritional standards, there is interest in the effects of specific nutrients. For instance, choline has been suggested as a treatment for the effects of PAE on learning [10] based on positive outcomes from animal studies [11–13]. To date, there are no human studies on choline supplementation that include PAE [14–16].
To determine whether commonly-used nutritional supplements might prevent or mitigate effects of PAE, we carried out a cohort study in Ukraine, which has a high rate of fetal alcohol syndrome [17]. Women in Ukraine drink alcohol regularly and tobacco use is common [18]. In addition, although use of prenatal vitamin and mineral supplementation is recommended, it is not universal [9]. These circumstances allowed us to examine whether the impact of moderate and higher levels of alcohol exposure reported peri-conceptually or before beginning prenatal care are related to relative deficits in infant development and to evaluate the impact of multivitamin/mineral supplementation (MVM) during gestation on exposed infants during the first year. Finally, we examined the effects of the addition of choline to the MVM regime (MVM + C) to determine whether it provided additional benefit and independently affected infant outcomes.
Methods
Study Design
This prospective cohort study recruited moderate to heavy drinking (n = 301), and low/unexposed comparison women (N = 313) at two sites in Western Ukraine, the Rivne Regional Medical Diagnostic Center and the Khmelnytsky Perinatal Center [19], affiliated with OMNI-Net, a network of educational and research sites focused on the prevention of birth defects. Women agreeing to participate read and signed the informed consent document, approved through the institutional review boards at the University of California San Diego and Lviv Medical University in Ukraine. Half of each of these alcohol-use groups was randomly assigned to receive a daily multivitamin and mineral (MVM) supplement (Theravit®) and half to “standard of care” in which prenatal vitamins were recommended but not provided. Further, half of the MVM-supplemented group also were provided a daily dose of 750 mg of supplemental choline. This dose was chosen as the human equivalent to that used effectively with animals [12, 13]. Women received supplements free of charge at prenatal visits and compliance with use was assessed. Of 136 women reporting, 87 % of those in the MVM and 93 % of those in the choline group took MVM supplements daily; in the MVM + C group, 80 % reported taking choline supplements daily.
When infants were born, information was collected from medical records with direct examination of growth and physical features. In the second half of the first year, children and families were invited to return to the study site for developmental assessment.
Participants’ Alcohol Use
At the first prenatal appointment (average 19 weeks gestation), nurses screened women for alcohol use [19] and provided information on risks of alcohol consumption during pregnancy. From 2007 to 2012, more than 13,000 pregnant women were screened; 94 % reported ever drinking, 45 % reported drinking when they became pregnant, 33 % continued to drink in pregnancy, and 9 % drank in a binge pattern (at least 4–5 drinks per occasion) [19]. “Heavy” drinkers—reporting at least weekly binge-drinking episodes (5+ drinks), at least five episodes of 3–4 standard drinks, or at least ten episodes of 1–2 standard drinks either in the month around conception or the most recent month of pregnancy—were recruited. The next nondrinking woman meeting screening criteria (i.e., no binge episodes, minimal or no alcohol in the month around conception, and no drinking in the most recent month of pregnancy) was recruited as a control. After enrollment, women reporting any lifetime drinking were asked about number, volume, and type of alcoholic drinks consumed on a day-by-day basis in (1) a typical week around conception and (2) the most recent 2 weeks using a time-line follow-back method [20, 21]. Quantity/frequency of alcohol consumption using a questionnaire previously validated by Barr and Streissguth [21] was summarized as: (1) average number of standard drinks per day over the reported period, reflecting overall quantity consumed (drinks/day), and (2) average number of standard drinks per day on only the days in which any alcohol was consumed (drinks/drinking day), reflecting heavier episodic or binge drinking. Results were converted to absolute ounces of alcohol (oz/AA) per time period with two standard drinks equivalent to one ounce of absolute alcohol.
Other Maternal Measures
Demographic information was obtained by maternal interview, including family (e.g., maternal age) and pregnancy (e.g., parity) characteristics. Hollingshead [22] (SES) ratings were calculated from education and occupational information. In addition to health-related activities like tobacco use and use of vitamin and mineral supplements and folic acid, maternal choline blood level were measured at recruitment and in the third trimester. Blood samples were shipped to the University of California-Davis for nutritional analysis using a UPLC-Micro triplequad MS/MS (Waters, Micromass) based on modification of the methods of Holm et al. [23] and Innis and Hasman [24]. A standardized difference score controlling for weeks of pregnancy measured changes in choline levels.
Infant Outcomes
Information about infant growth and birth outcomes was collected from medical records. The Bayley Scales of Infant Development, 2nd Ed. (BSID-II) [25] was selected for assessment of development. This widely used and well-standardized measure is used worldwide in evaluating infants and the BSID-II has better reliability and validity than the third edition [26]. In addition, a Russian translation was available that was more accessible than the English version to Ukrainian users (who are bilingual in Russian). The BSID-II measures current mental development (problem solving/prelinguistic development) and psychomotor development (fine/gross motor skills) yielding standardized scores (Mental Development Index: MDI; Psychomotor Development Index: PDI). In addition, the infant’s orientation/engagement, emotional regulation, motor quality, and total behavior quality are rated as percentiles falling into: NonOptimal (≤10th), Questionable (11th–25th) or within normal limits (≥26th) categories. The BSID-II was administered by Ukrainian child psychologists (trained and supervised by the first author) who were blind to the mother’s group status. Children were seen and tested individually in private offices while seated in their caregiver’s laps. Testing required 30–45 min.
Data Analysis
Data collected in Ukraine were entered into databases by local study staff and transmitted electronically to authors in the United States. Although 405 infants were tested, only 367 are included in this analysis. From six multiple births, one child was randomly selected from each set. Child’s age at testing was corrected for the 37 infants (9.3 %) born at <37 weeks gestational age. Only children tested at a corrected age between 20 and 44 weeks postpartum were included (n = 398). Removing infants whose mother’s report of prenatal exposure was not credible due to inconsistency yielded the final sample of 367. Generalized Linear Modeling (GLM) was used to examine first, the potential benefits of nutritional supplements including MVM and MVM + C, and secondly, the impact of alcohol dose and binge drinking at two time points (peri-conception and in mid-trimester) on infant outcomes in those who did and did not receive supplements.
Potentially confounding factors were identified by correlating the following measures with both independent and dependent variables: SES, parental ages, cigarette use, parity, maternal pre-enrollment vitamin and folic acid use, choline levels, child sex, birth weight, child’s age at testing and test site. Factors correlating with significance at the 0.10 level with either independent variables or developmental outcomes were retained.
Results
Sample Characteristics
Attrition. We compared those included in this analysis with those who were not either because they did not return for follow up (n = 240) or because they were excluded as noted above, using the following variables: test site, alcohol group, MVM group, parental ages, marital status, SES, parity, alcohol amount peri-conceptually and during mid-trimester, cigarette use, child sex, gestational age at recruitment and birth, growth (birth weight, length, head circumference, percentiles). Analysis, using Chi Square or One-way Analysis of Variance, found no group differences on: test site, MVM group, parents’ ages, marital status, parity, amount of alcohol use at any time point, and cigarette use. However, those in the alcohol (45.2 %) were more likely than those in the contrast group (34.8 %), , p < .009, to be lost to follow-up, and those included in the study had higher SES, M = 37.68 (11.77) versus M = 33.34 (12.95), F(1,595) = 18.02, p < .001. There were more preterm births in those not included, , p < .03; thus, birth growth variables were also significantly lower. Difference in child sex approached significance, (More females (62.8 %) than males (58.7 %) tested, , p = .059). Therefore, those tested were less at risk due to social factors, preterm delivery and alcohol-exposure, factors that could limit power to find significant group differences.
Participant Characteristics
Tables 1 and 2 show maternal and infant characteristics of study participants. Note that because the MVM + choline group did not differ from the MVM group in any analyses, Tables are shown collapsed across choline with the MVM and MVM + C group means shown in the italicized values. Except for alcohol use, SES and paternal age, there were no differences on demographic characteristics. Alcohol group was associated with infant growth and developmental status as anticipated. The range of alcohol use reported peri-conceptually by drinking women in this study was from 0.87 to 5.15 oz/AA per day (1.5–10 drinks/day), with the majority of women reporting drinking daily at low to “moderate” levels.
Table 1.
Characteristics of mothers by alcohol use (EtOH) and supplement group (MVM)a (N = 367): MVM and MVM + choline (italicized values)
| Alcohol use (n = 163)
|
Contrast (n = 204)
|
p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No supplement (n = 78) | Supplement (n = 85) | MVM (n = 38) | MVM + choline (n = 47) | No supplement (n = 98) | Supplement (n = 106) | MVM (n = 58) | MVM + choline (N = 48) | Statisticc | |||
| Maternal age (years) | 25.47 (5.67) | 26.12 (6.05) | 26.03 (5.91) | 26.19 (6.23) | 26.63 (4.75) | 26.32 (4.14) | 26.12 (3.95) | 26.56 (4.39) | EtOH: F(1,359) < 1 MVM: F(1,359) < 1 |
NS NS |
|
| Paternal age (years) | 29.97 (6.87) | 30.34 (6.72) | 29.47 (6.12) | 30.85 (7.17) | 28.64 (5.45) | 28.36 (4.73) | 28.07 (4.52) | 28.71 (4.99) | EtOH: F(1,359) = 6.25 MVM: F(1,306) < 1 |
.01 NS |
|
| Marital status (% with partner) | 88.42 % | 85.90 % | 84.2 % | 87.2 % | 96.9 % | 99.1 % | 98.3 % | 100 % |
|
NS | |
| Parity-number of children | 0.51 (0.73) | 0.74 (1.1) | 0.66 (0.99) | 0.81 (1.19) | 0.77 (1.21) | 0.64 (0.83) | 0.72 (0.93) | 0.54 (0.68) | EtOH: F(1,359) < 1 MVM: F(1,359) < 1 |
NS NS |
|
| Social class (SES)b | 33. 91 (10.59) | 31.96 (11.51) | 32.89 (12.02) | 31.21 (11.16) | 41.94 (10.38) | 41.17 (11.32) | 40.19 (12.05) | 42.33 (10.39) | EtOH: F(1,357) = 52.88 MVM: F(1,357) < 1 |
.001 NS |
|
| Alcohol use: peri-conception | |||||||||||
| OzAA/day: M (SD) | 0.64 (0.66) | 0.77 (0.84) | 0.67 (0.69) | 0.85 (0.95) | 0.00 (0.016) | 0.00 (0.006) | 0.00 (0.01) | 0.00 (0.00) | EtOH: F(1,357) = 166.63 MVM: F(1,357) = 1.34 |
.001 NS |
|
| OzAA/per dking day: M (SD) | 1.74 (1.48) | 1.96 (2.65) | 1.70 (1.32) | 2.18 (3.37) | 0.020 (0.11) | 0.001 (0.04) | 0.01 (0.06) | 0.00 (0.00) | EtOH: F(1,357) = 135.67 MVM: F(1,357) < 1 |
.001 NS |
|
| Alcohol use: mid-trimester | |||||||||||
| OzAA/day: M (SD) | 0.19 (0.48) | 0.16 (0.37) | 0.10 (0.25) | 0.20 (0.45) | 0 | 0 | 0 | 0 | EtOH: F(1,357) = 26.74 MVM: F(1,357) < 1 |
.001 NS |
|
| OzAA/per dking day: M (SD) | 0.73 (1.15) | 0.61 (0.98) | 0.45 (0.69) | 0.74 (1.56) | 0 | 0 | 0 | 0 | EtOH: F(1,357) = 67.77 MVM: F(1,357) = 1.33 |
.001 NS |
|
| Cigarettes/week/pregnancy | 11.94 (15.96) | 12.15 (16.42) | 13.78 (16.82) | 10.83 (16.14) | 1.36 (6.85) | 0.04 (0.42) | 0.00 (0.00) | 0.09 (0.62) | EtOH: F(1,359) = 87.90 MVM: F(1,359) < 1 |
.001 NS |
|
| Choline blood level at recruitment (μmol/L)d (N = 271) | 14.39 (2.71) | 15.15 (3.24) | 15.40 (3.72) | 14.90 (2.74) | 15.35 (3.74) | 14.79 (2.89) | 14.86 (2.99) | 14.69 (2.78) | EtOH: F(1,266) < 1 MVM: F(2,266) < 1 |
NS NS |
|
| Choline blood level in 3rd trimester (μmol/L)d (N = 201) | 16.35 (3.95) | 15.79 (4.19) | 16.06 (4.38) | 15.57 (4.11) | 15.58 (3.35) | 15.50 (3.50) | 15.21 (3.24) | 16.12 (4.02) | EtOH: F(1,195) < 1 MVM: F(2,195) < 1 |
NS NS |
|
| Choline standardized change score (N = 201) | 1.46 (3.93) | 0.59 (4.21) | 0.59 (5.39) | 0.58 (3.00) | −0.12 (4.08) | 0.63 (3.83) | 0.09 (3.97) | 1.77 (3.31) | EtOH: F(1,195) < 1 MVM: F(2,195) < 1 |
NS NS |
|
| % Folate supplement | 31.6 % | 32.9 % | 31.6 % | 34.0 % | 42.9 % | 44.3 % | 45.8 % | 43.6 % |
|
NS | |
Collapsing across choline; italicized values without collapsing across choline
Hollingshead Scale [22]—includes measures of educational attainment and occupation
Statistic reported on uncollapsed supplement variable (i.e., none, MVM, MVM + choline) as most conservative. Collapsing across choline does not change the pattern of results or the statistical significance
Choline blood levels are standardized to control for differences in gestational age at recruitment. No group differences were found for unstandardized values
Table 2.
Group means for infant developmental outcomes by alcohol use (EtOH) and supplement status (MVM)a (N = 367): MVM and MVM + C (italicized values)
| Alcohol exposed (n = 163)
|
Unexposed (n =204)
|
Statistic | p values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No supplement (n = 78) |
Supplement (n = 85) |
MVM (N = 37) |
MVM + C (n = 47) |
No supplement (n = 98) |
Supplement (n = 106) |
MVM (n = 58) |
MVM + C (n = 48) |
|||
| Child sex (% male) | 42.3 % | 48.24 % | 50 % | 46.8 % | 52.04 % | 58.49 % | 58.6 % | 58.3 % | EtOH:
MVM: |
NS NS |
| Birth weight (gms)b | 3093.27 (547.74) | 3180.05 (616.1) | 3218.38 (603.64) | 3150.43 (630.59) | 3389.94 (441.84) | 3403.87 (444.72) | 3470.52 (507.07) | 3323.33 (343.37) | EtOH: F(1,361) = 21.47 MVM: F(1,361) < 1 |
.001 NS |
| Birth length (cm)b | 50.62 (3.28) | 51.05 (3.66) | 51.08 (4.02) | 51.02 (3.40) | 51.97 (2.27) | 51.88 (2.25) | 52.03 (2.57) | 51.69 (1.81) | EtOH: F(1,361) = 11.65 MVM: F(1,361) < 1 |
.001 NS |
| Birth head circumference (cm)b | 33.81 (1.95) | 33.98 (1.94) | 34.19 (1.45) | 33.81 (2.23) | 34.51 (1.59) | 34.66 (1.46) | 34.81 (1.59) | 34.48 (1.27) | EtOH: F(1,361) = 13.07 MVM: F(1,361) < 1 |
.001 NS |
| Corrected age at test (weeks)c | 27.99 (4.19) | 28.44 (4.34) | 28.48 (5.14) | 28.17 (4.52) | 28.09 (4.35) | 28.55 (3.97) | 29.43 (4.49) | 27.48 (2.97) | EtOH: F(1,363) < 1 MVM: F(1,363) < 1 |
NS NS |
| Bayley Scales-IId | ||||||||||
| MDI | 88.21 (9.84) | 89.21 (10.88) | 91.37 (10.16) | 87.47 (11.23) | 90.65 (8.39) | 91.48 (6.37) | 91.05 (7.08) | 92.00 (5.46) | EtOH: F(1,362) = 6.82 MVM: F(1,362) < 1 |
.01 NS |
| PDI | 88.60 (13.42) | 88.00 (13.96) | 90.26 (13.81) | 86.17 (13.96) | 90.99 (10.26) | 89.77 (10.52) | 90.71 (10.93) | 88.65 (10.02 | EtOH: F(1,362) = 2.72 MVM: F(1,362) < 1 |
.10 NS |
| Orientation/engagement (n = 349) | 20.93 (14.25) | 24.54 (18.43) | 24.68 (15.51) | 24.43 (20.49) | 26.48 (17.24) | 25.79 (17.22) | 24.69 (17.12) | 27.15 (17.44) | EtOH: F(1,347) = 3.47 MVM: F(1,347) = 1.09 |
.06 NS |
| Emotional reactivity (n = 349) | 43.65 (26.39) | 46.20 (25.68) | 45.97 (25.11) | 46.37 (26.37) | 48.13 (25.49) | 45.42 (24.82) | 47.12 (25.81) | 43.32 (23.65) | EtOH: F(1,347) < 1 MVM: F(1,347) < 1 |
NS NS |
| Motor quality | 16.36 (13.54) | 17.53 (15.76) | 18.94 (16.82) | 15.20 (14.62) | 20.18 (15.49) | 20.91 (16.61) | 20.24 (15.82) | 21.96 (17.83) | EtOH: F(1,363) = 4.99 MVM: F(1,363) < 1 |
.03 NS |
| Total behavior quality | 20.58 (16.42) | 24.34 (19.32) | 24.74 (18.17) | 22.67 (19.84) | 25.16 (17.18) | 24.68 (18.16) | 24.43 (18.26) | 25.06 (18.3) | EtOH: F(1,363) = 1.75 MVM: F(1,363) < 1 |
NS NS |
Collapsing across choline; italicized values: without collapsing across choline
Multivariate analysis controlling for child sex [birthweight: F(1,361) = 4.55, p < .03; birth length: F(1,361) = 4.41, p < .04; birth head circumference: F(1,361) = 4.44, p < .05]
Corrected for gestational age at birth
Multivariate analysis of variance
Choline Status
A separate multivariate analysis using alcohol group and Supplement status (None, MVM and MVM + choline) found no significant differences among maternal choline blood levels pre and post supplementation or on the standardized difference score. Correlations of choline variables with newborn growth measures, with cigarette smoking as a covariate, were all nonsignificant.
Impact of Supplement Use
Generalized Linear Regression (GLR) was used to evaluate the impact of three group factors, (1) alcohol use, (2) MVM supplementation, and (3) choline administration, on BSID-II outcomes. The factorial design allowed examination of interaction of these factors as well as their direct effects. Note that, because choline was given only in the group receiving MVM, this was an incomplete factorial design. Of covariates tested for inclusion in the model only site, SES, paternal age, child sex, cigarette use and folic acid use contributed significant variance and were retained. Bayley MDI, PDI and behavioral ratings were considered separately. For PDI, the final model was significant ( , p <.001) with testing site and SES contributed significant variance. None of the factors, alcohol group, MVM or choline supplementation, contributed significantly to differences in the PDI. Choline approached significance with those taking choline having lower scores (no choline M = 89.98 (0.71); choline M = 87.92 (1.11); , p = .10).
For the MDI, the final model was significant ( , p < .001) with testing site, child sex, paternal age and SES all significant contributors to the outcome. For the MDI, there was a significant effect for both alcohol group (alcohol exposed had lower scores, , p < .05) and MVM group ( , p <.03) with the supplement group having higher outcomes. There was no effect of choline or interaction of choline with alcohol group.
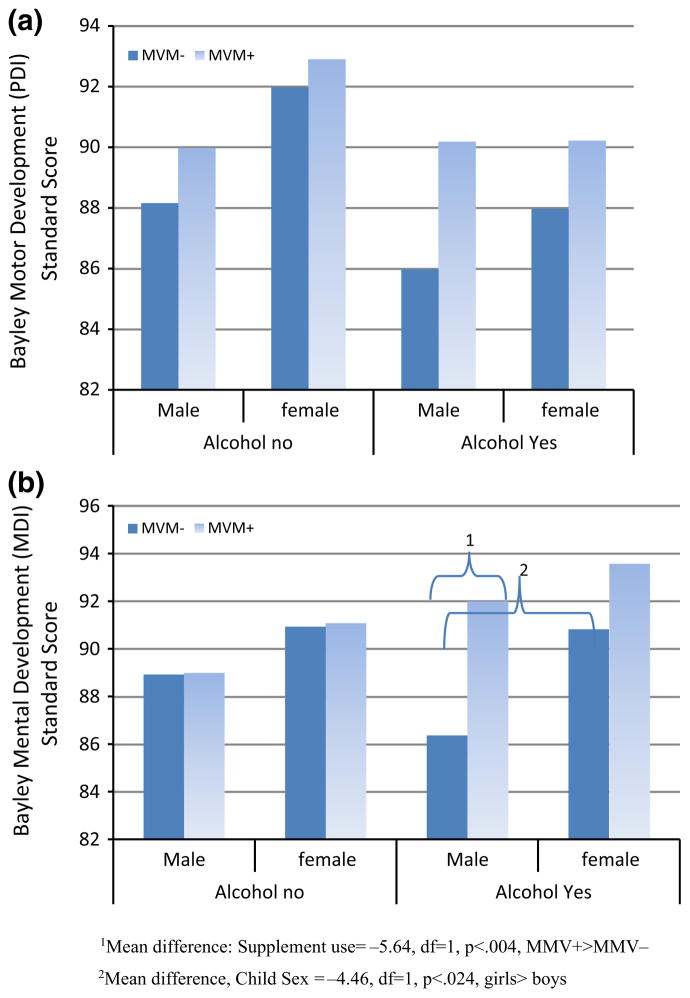
To examine effect of higher doses of alcohol and binge drinking at different points during gestation on development, a second set of models used alcohol dose rather than alcohol group status. We included the same potential covariates in this analysis to determine whether supplements were more effective with higher doses of alcohol or during a particular time period. Alcohol variables used included oz/AA/day and oz/AA/per drinking day (binge variables) peri-conceptually and during the two weeks prior to enrollment. In all analyses, binge variables were not as strongly related to outcomes as oz/AA/day and were not significant with both measures included. For the PDI, the model was significant ( , p < .001) with testing site and SES related to results. Higher AA/day in the peri-conception period was related to poorer outcomes ( , p = .04) but MVM and choline use were not significant. A three-way interaction of child sex, MVM use and oz/AA/day was found ( , p <.02), but pairwise comparisons of group means were not significant (Fig. 1a).
Fig. 1.
Bayley infant development, 2nd edition psychomotor and mental development scores at a mean of 6 months of age as a function of child sex, alcohol exposure in gestation and multivitamin/mineral supplementation. a Psychomotor Development Index (PDI) (M = 100, SD = 15). No groups means significantly different. b Mental Development Index (MDI) (M = 100, SD = 15)
For the MDI, the model (Table 3) was significant ( , p < .001) with site, SES, paternal age, and child sex related to outcome. Alcohol use peri-conceptually was related to lower ( , p < .001) and supplement (MVM) use, to higher ( , p <.005) MDI, with no effect of choline. When peri-conceptual alcohol use was included, mid-trimester use did not add significant variance. A significant three-way interaction was found among alcohol dose, supplement group and child sex ( , p < .002) (Fig. 1b). Post hoc examination (Least Significant Difference), indicated that alcohol-exposed males without MVM supplementation had the poorest performance (In alcohol exposed males: supplement use = −5.64, DF = 1, p <.004,) (Fig. 1b).
Table 3.
Generalized linear regression model for Bayley Mental Development Index (MDI) ( , p < .001)
| Variables | β | χ2 | p value |
|---|---|---|---|
| Testing sitea | −6.68 | 62.35 | .001 |
| Child sexb | −3.52 | 14.86 | .001 |
| MVM groupc | −2.97 | 8.03 | .005 |
| Choline supplement | 0.345 | <1 | NS |
| SESd | 0.145 | 15.14 | .001 |
| Cigarettes per daye | 0.183 | 3.40 | .065 |
| Father’s agef | −0.155 | 4.64 | .03 |
| Folate use before recruitmentg | −1.54 | 3.21 | .07 |
| Alcohol use around conceptionh | −5.28 | 8.54 | .001 |
| Alcohol use in mid-trimester | −2.25 | 1.71 | .19 |
| Interaction: alcohol × MVM × child sexi | 11.04 | 14.75 | .002 |
Rivne >Khmelnytsky
Females > males
MVM+ > MVM−
Higher SES associated with higher MDI scores
More cigarettes associated with lower MDI scores
Greater age associated with lower MDI scores
Folate use associated with higher scores
More alcohol reported associated with lower MDI scores
Alcohol-exposed, MVM− males more affected
Behavioral ratings on the BSID-II included orientation/engagement, emotional reactivity, motor quality and total behavior with GLR used to evaluate the percentile score for each outcome. There were no effects of the interventions and no interactions. For orientation/engagement, alcohol exposure peri-conceptually or mid-trimester was significant, with other factors not contributing to outcomes. For emotional reactivity, only alcohol peri-conceptually was significant ( , p < .03), although cigarette use approached significance ( , p = .07). Motor quality was affected by SES ( , p < .006) and by peri-conceptual alcohol exposure ( , p < .03). Total behavior quality was significantly impacted by alcohol peri-conceptually ( , p <.012).
Discussion
It is well established that PAE is associated with risks to offspring that include growth retardation, birth defects and deficits in cognitive and motor development [27]. In this study, we hypothesized an association between relative deficits on developmental outcomes and higher levels of alcohol use peri-conceptually and during mid-pregnancy. We also hypothesized that MVM and MVM + C during gestation would be associated with higher scores on infant measures and that such intervention would mitigate effects of alcohol exposure. Finding effects of nutritional supplementation raises the possibility of preventing or ameliorating the teratogenic effects of early alcohol exposure through interventions during pregnancy. Since many women become pregnant unintentionally or continue drinking during the “prerecognition” period, interventions that can be applied after pregnancy identification would be of considerable value, particularly in populations that are at risk not only due to PAE but also because of environmental or social factors.
The study found that, in addition to the expected negative effects of PAE, there are small but significant positive effects of nutritional intervention. However, the pattern of effects was not simple. MVM supplementation was associated with higher scores on cognitive but not psychomotor development. There was no effect of choline on cognitive scores while there was a trend for more negative motor outcomes. Thus, at 6 months, intervention effects appear to be specific to the improvement in problem solving and prelinguistic skills that are measured on the cognitive scale.
It is encouraging that nutritional intervention that improves outcome of alcohol-exposed children is possible and that the effects of this intervention can be detected this early in development when it is difficult to measure cognition efficiently. However, at 6 months of age, the BSID-II, and indeed all infant tests, are relatively insensitive to all but the most serious developmental deficits. This is true, in general, but is certainly the case when evaluating the impact of PAE. Many problems identified later in development cannot be measured at 6 months [2]. Thus, the current results are suggestive but not definitive in understanding either the impact of early alcohol exposure or of the potential for nutritional supplementation to ameliorate these effects.
It is also important that while these are statistically significant results, the alcohol-exposed group, as a whole, was not different from the contrast group who were born to non-drinking women. It was women reporting high dosages in the peri-conceptual period whose children had lower scores at this age. It is important to discriminate these statistical significant differences from possible clinical differences that may become more apparent with time. Early infant assessment does not discriminate children who have subtle developmental problems. To adequately characterize the impact of alcohol as well as the potential benefits of the interventions, longer-term follow-up is required.
In the current study, reported peri-conceptual drinking was found to be a better predictor of outcome than reported drinking during the 2 weeks preceding recruitment, a pattern that has been found previously [5]. In addition, average oz/AA per day was a better predictor than oz/AA per drinking day, which is assumed to reflect binge drinking. These results may be accurate but may also reflect inaccuracies in reporting alcohol use during pregnancy.
Developmental improvement associated with choline seen in animal models was not observed in this human study, the first to evaluate the effect of this nutrient in relation to PAE. This result may be related to the insensitivity of infant tests at 6 months; however, this finding is similar to several other recent publications that evaluated samples of older children without PAE. Strain et al. [14] reported no relationship between outcomes at 5 years and plasma concentrations of free choline, betaine, dimethylglycine (DMG), methionine and homocysteine. Similarly, Villamor et al. [15] found no effects of maternal supplementation with choline, betaine, and methionine on cognition at 3 years. While it is important not to over-interpret nonsignificant results, it is also a concern that the psychomotor scores were mildly depressed in the MVM + C groups. These results suggest that a more rigorous evaluation of the impact of choline supplementation is warranted, including both a focus on functions, like memory that are known to be impacted by choline, as well as any potential negative effects that may be observed. Some of these outcomes will be addressed in future research. The current sample was followed to 12 months and a preschool assessment is underway and should provide a much more nuanced understanding of effects of both MVM and MVM + C in relation to alcohol use early in pregnancy.
The results of this study should be interpreted in light of potential limitations. The Ukrainian sample is predominantly Caucasian, so it may not be possible to generalize the results to other groups. It also appears likely that those women not returning for follow-up were more high-risk drinkers with more social and emotional problems than those bringing children for testing. Thus, potential effects may have been attenuated. In addition, although we measured maternal nutritional status at recruitment and following delivery, there was no direct measure of child nutritional status during the first year. Future research should consider investigation of nutritional supplementation in other populations and within the context of child nutritional status.
Despite these limitations, this study of women and infants at risk due to alcohol use and environmental risk provides insight into the factors that determine the impact of PAE on child outcomes. Most important, is the potential benefit of appropriate nutritional prenatal care for women worldwide. Although there can be no better strategy than abstinence during pregnancy, these findings suggest that identification of women at risk due to drinking and nutritional insufficiencies when they come for prenatal care may reduce some of the burden of prenatal alcohol exposure on their offspring.
Significance.
What is known about nutrition and effects of prenatal alcohol exposure?
Negative effects of prenatal alcohol exposure are reported more frequently in disadvantaged populations; however, the basis for this discrepancy has not been identified. Animal models suggest that nutritional deficiencies may contribute to negative outcomes but there have been no studies of nutritional supplementation in pregnancy or their effect on child development.
What does this study add?
Supplementation after pregnancy recognition with multivitamins/minerals resulted in improved cognitive performance in 6-month olds exposed to alcohol. This result illustrates the importance of early prenatal care and appropriate nutrition in high risk populations.
Acknowledgments
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at http://cifasd.org/. Research described in this manuscript was supported by Contract #U01AA014835 funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the NIH Office of Dietary Supplements (ODS). We wish to acknowledge the contribution of: OMNI-Net, Ukraine, Participating families and staff in Rivne and Khmelnytsky, Ukraine.
References
- 1.Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: An overview. Neuropsychology Review. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Fetal alcohol syndrome—Alaska, Arizona, Colorado, and New York, 1995–1997. Morbidity and Mortality Reports. 2002;51(20):433–435. [PubMed] [Google Scholar]
- 4.May PA, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134(5):855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson SW, et al. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 6.Niclasen J. Drinking or not drinking in pregnancy: The multiplicity of confounding influences. Alcohol and Alcoholism. 2014;49(3):349–355. doi: 10.1093/alcalc/agt141. [DOI] [PubMed] [Google Scholar]
- 7.Abel EL. Fetal alcohol abuse syndrome. New York, NY: Plenum Press; 1998. [Google Scholar]
- 8.Weiss LA, Chambers CD. Associations between multivitamin supplement use and alcohol consumption before pregnancy: Pregnancy risk assessment monitoring system, 2004 to 2008. Alcoholism, Clinical and Experimental Research. 2013;37(9):1595–1600. doi: 10.1111/acer.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keen CL, et al. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: The potential influence of zinc status as an example. BioFactors. 2010;36(2):125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, West AA, Caudill MA. Maternal choline supplementation: A nutritional approach for improving offspring health? Trends in Endocrinology and Metabolism. 2014;25(5):263–273. doi: 10.1016/j.tem.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicology and Teratology. 2009;31(5):303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas JD, et al. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(10):827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JD, et al. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology and Teratology. 2000;22(5):703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 14.Strain JJ, et al. Choline status and neurodevelopmental outcomes at 5 years of age in the Seychelles Child Development Nutrition Study. British Journal of Nutrition. 2013;110(2):330–336. doi: 10.1017/S0007114512005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villamor E, et al. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatric and Perinatal Epidemiology. 2012;26(4):328–335. doi: 10.1111/j.1365-3016.2012.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheatham CL, et al. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. American Journal of Clinical Nutrition. 2012;96(6):1465–1472. doi: 10.3945/ajcn.112.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.E.s.o.c anomalies. EUROCAT Prevalence Data Tables: A5—Fetal alcohol syndrome § (per 10, 000 births) for the following registries: All Registries, from 2008–2012 2014 [Google Scholar]
- 18.Bakhireva LN, et al. Paternal drinking, intimate relationship quality, and alcohol consumption in pregnant Ukrainian women. Journal of Studies on Alcohol and Drugs. 2011;72(4):536–544. doi: 10.15288/jsad.2011.72.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers CD, et al. Prevalence and predictors of maternal alcohol consumption in 2 regions of Ukraine. Alcoholism, Clinical and Experimental Research. 2014;38(4):1012–1019. doi: 10.1111/acer.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobell LC, Sobell MB. American Psychiatric Association, editor. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000. Alcohol timeline follow-back (TLFB) pp. 477–479. [Google Scholar]
- 21.Barr HM, Streissguth AP. Identifying maternal self-reported alcohol use associated with fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2001;25(2):283–287. [PubMed] [Google Scholar]
- 22.Hollingshead AB. Four factor index of social status. Yale Journal of Sociology. 2011;8:21–51. [Google Scholar]
- 23.Holm PI, et al. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clinical Chemistry. 2003;49(2):286–294. doi: 10.1373/49.2.286. [DOI] [PubMed] [Google Scholar]
- 24.Innis SM, Hasman D. Evidence of choline depletion and reduced betaine and dimethylglycine with increased homocysteine in plasma of children with cystic fibrosis. Journal of Nutrition. 2006;136(8):2226–2231. doi: 10.1093/jn/136.8.2226. [DOI] [PubMed] [Google Scholar]
- 25.Bayley N. Bayley scales of infant development (BSID-2) San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 26.Moore T, et al. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. Journal of Pediatrics. 2012;160(4):553–558. doi: 10.1016/j.jpeds.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Jones KL, et al. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]