Abstract
Introduction
Midlife vascular disease risk is associated with higher incidence of cognitive impairment in late life. Regular aerobic exercise improves vascular function, which in turn may translate into better cognitive function. The purpose of this study was to determine the associations among cardiorespiratory fitness, cerebral and peripheral vascular reactivity, and cognitive function in the sedentary and endurance-trained middle-aged adults.
Methods
Thirty-two endurance-trained and 27 healthy sedentary participants aged 43–65 years underwent measurements of maximal oxygen uptake (VO2max), neurocognitive assessment, cerebrovascular reactivity to CO2 (CVR), and brachial artery flow-mediated dilation (FMD).
Results
There were no group differences in age, sex, education level, fasting blood glucose, and blood pressure. Compared with sedentary subjects, endurance-trained athletes demonstrated better cognitive performance on memory (z-score: −0.36±1.11 vs. 0.30±0.76, P<0.01), attention-executive function (z-score: −0.21±0.53 vs. 0.18±0.72, P=0.02), and total cognitive composite scores (z-score: −0.27±0.63 vs. 0.23±0.57, P<0.01). Furthermore, brachial FMD (4.70±2.50 % vs. 7.13±3.09 %, P<0.01) and CVR (4.19±0.71 %/mmHg vs. 4.69±1.06 %/mmHg, P=0.052) were greater in endurance-trained individuals than in the sedentary subjects. Total cognitive composite scores showed a significant positive association with brachial FMD (r = 0.36, P < 0.01) and CVR (r = 0.30, P = 0.03). Finally, when brachial FMD and CVR were entered as covariates, fitness-related group differences in total cognitive composite score were significantly attenuated (all P>0.05).
Conclusion
Endurance-trained middle-aged adults demonstrated better cognitive performance which may, at least in part, be mediated by their enhanced vascular function, including cerebral and endothelial-dependent vascular reactivity.
Keywords: cognitive function, cardiorespiratory fitness, cerebrovascular reactivity, endothelial function, middle age
Introduction
The prevalence of dementia is exponentially increasing due to the rapidly aging population and the lack of established treatments (23). Mounting evidence has reported that vascular disease and dysfunction elevate the risk of cognitive impairment, which indicates that vascular control of cerebral blood flow may be critical for maintaining cognitive function across the lifespan (16, 17). In this regard, vascular endothelium appears to play a key role as it governs the vasodilatory response elicited by local neuronal activations (21, 28). In comparison to healthy controls, patients with Alzheimer’s disease revealed impaired endothelium-dependent vasodilation, suggesting the potential contribution of endothelial dysfunction to cognitive deterioration (18).
Cerebral perfusion is tightly coupled with arterial level of carbon dioxide (PaCO2) such that hypercapnia increases and hypocapnia decreases cerebral blood flow (4). In clinical research settings, cerebrovascular reactivity (CVR) to changes in PaCO2 has increasingly been used as an index of cerebrovascular health and is impaired in individuals with stroke and dementia (43). In patients with Alzheimer’s disease, CVR as measured by functional MRI was attenuated in the forebrain regions compared with age-matched healthy subjects (49). Anatomically, CVR and functional hyperemia occur in the arterioles of similar size (29), suggesting that cerebrovascular health, as assessed by the reactivity to changes in PaCO2, is capable of identifying individuals at elevated risk for cognitive impairment.
Midlife vascular disease and risk factors are associated with accelerated brain aging and higher incidence of dementia in late life (17). Conversely, habitual aerobic exercise ameliorates vascular function, and its benefits for cognitive function have increasingly been recognized (32, 42). Our recent study demonstrated that exercise-related improvements in cognitive performance are, at least in part, associated with elastic property of cardiothoracic arteries (45). However, evidence is lacking as to whether vasomotor control of blood flow, as determined by CVR or endothelium-dependent vasodilation, also contributes to better cognitive performance in exercise-trained adults. Accordingly, the primary purpose of the present study was to determine the associations among cardiorespiratory fitness, cerebral and peripheral vascular reactivity, and cognitive function in middle-aged adults. Specifically, we hypothesized that CVR and endothelium-dependent vasodilation would be greater in endurance-trained adults than in the sedentary controls, and further associated with better cognitive performance.
Materials and methods
Participants
Fifty-nine community-dwelling adults, aged 43–65 years, were recruited through flyers and newspaper advertisements posted in Austin, Texas. These participants were recruited as a part of a larger project which investigated the relation between brain and vascular health in the sedentary and endurance-trained middle-aged adults. The similar group of subjects has been reported in our previous publication, which focused on a different aspect of vascular property (45). All subjects were nonsmoking, normotensive (<140/90 mm Hg), non-diabetic (fasting blood glucose <126 mg/dL), and free of overt cardiovascular, cerebrovascular, or neurological disease as assessed by a medical history questionnaire, blood chemistry, and hematological evaluations. None of the subjects were taking cardiovascular-acting medications, including hormone replacement therapy. Subjects were classified as endurance-trained athletes if they reported engaging in moderate or strenuous aerobic exercise at least 4 days per week on the International Physical Activity Questionnaire Short Form (15). Subjects were classified as sedentary if they reported no regular physical exercise in the past year. Physical activity status was further verified by the Godin’s physical activity questionnaire (25) and maximal oxygen uptake (VO2max) test. Endurance-trained subjects reported running, cycling, and/or swimming at moderate to vigorous exercise intensity for 7.6±0.6 hours/week. Sedentary subjects reported engaging in exercise less than once a week for the past year. Neurocognitive and vascular function assessments were conducted on separate days within a one-month period. During the period of data collection, participants reported no major changes in their physical activity or dietary habits. Vascular function measurements were conducted during the early follicular phase of the menstrual cycle in premenopausal women. Subjects fasted for >4 hours and abstained from alcohol, caffeine, and exercise for >24 hours prior to data collections. The Human Research Committee reviewed and approved all procedures, and written informed consent was obtained from all subjects.
Measurements
Body Composition and Metabolic Risk Factors
Body mass index (BMI) was calculated by dividing body mass in kilograms by height in meters squared. Body composition was measured by dual-energy X-ray absorptiometry (Lunar DPX, General Electric Medical Systems, Fairfield, CT). A blood draw was conducted after >12 hours of fasting and abstinence from alcohol, caffeine, and exercise for >24 hours. Whole blood samples were drawn from the antecubital vein. Glucose and total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterols were measured from 35 uL of whole blood using standard enzymatic technique (Cholestech LDX system, Cholestech Corporation, Hayward, CA).
Cardiorespiratory Fitness
Maximal oxygen uptake (VO2max) was determined by a continuous incremental treadmill protocol as previously described (39). After a 5-minute warm-up period, each subject ran or walked at a comfortable speed that corresponded to 70–80% of age-predicted maximal heart rate. Treadmill speed remained constant throughout the test, while the grade was increased by 2% every 2 minutes until volitional exhaustion. Each treadmill test lasted between 8 and 12 minutes. Heart rate was continuously recorded throughout the test using a chest strap monitor (Polar Electro Inc, Lake Success, NY). The rated perceived exertion (RPE) scale was measured at the end of every stage. Maximal heart rate was defined as the highest value recorded during the test. A Physio-Dyne Max-1 metabolic testing system (PHYSIO-DYNE, Quogue, NY) was used to measure flow and gas composition from expired air collected by a mouthpiece (Hans Rudolph Inc, Kansas City, MO). Before each trial, the analyzer was calibrated using a Hans Rudolph 7-liter syringe (Hans Rudolph Inc, Kansas City, MO) and against standard gases of known concentrations.
To ensure that each subject attained a valid VO2max, at least two of the following three criteria were met by each subject: 1) a plateau in oxygen uptake with increasing exercise intensity, 2) a respiratory exchange ratio ≥1.1, and 3) achievement of age-predicted maximal heart rate. During the test, verbal encouragement was given if subject was not achieving the criteria of valid VO2max assessment. VO2max was determined by averaging the two consecutive highest values of oxygen uptake that were sampled every 30 seconds. Because VO2max is influenced by age and sex and potentially complicates the association with other pertinent variables, aerobic fitness percentile was calculated based on age- and sex-adjusted regression established by the American College of Sports Medicine (5).
Brachial Artery Flow-Mediated Dilation (FMD)
Endothelium-dependent vasodilation was assessed by FMD using a noninvasive, standardized procedure (14). Left (non-dominant) arm was extended and placed in a customized arm support system to prevent movement of the arm and to stabilize the position of an ultrasound transducer. Brachial artery diameter and blood flow velocity were measured 5–10 cm proximal to the antecubital fossa using a Doppler ultrasound machine equipped with a high-resolution linear array transducer (Philips iE33 Ultrasound System, Bothel, WA). A pneumatic arm cuff was placed on the forearm 3–5 cm distal to the antecubital fossa and connected to a rapid cuff inflator (E20 Rapid Cuff Inflator, D.E. Hokanson; Bellevue, WA Hokanson).
After ≥15 minutes of rest in the supine position, baseline brachial artery diameters and blood flow velocities were recorded from a longitudinal image of the brachial artery for 3 minutes. The arm cuff was then inflated to 100 mmHg above resting systolic blood pressure (measured prior to baseline image capture) for 5 minutes. Blood flow velocity was recorded for 10 seconds prior to cuff deflation and continued until 20 seconds after cuff deflation. The ultrasound was switched to 2D mode to optimize the image for brachial arterial wall measurements for the next 160 seconds. The image files were transferred to an offline computer and stored for later data analysis. Brachial arterial diameter during end-diastole, as determined by ECG, was measured by a single investigator using automated image analysis software (Brachial Analyzer, Medical Imaging Applications; Coralville, IA). FMD was calculated using the following equation: . The baseline end-diastolic diameter was calculated by taking the average of ≥20 cardiac cycles before cuff inflation. Peak end-diastolic diameter was taken from the average of 3 consecutive cardiac cycles demonstrating the largest brachial artery dilation after cuff deflation.
Cerebrovascular Reactivity to CO2 (CVR)
Blood flow velocity (BFV) of the middle cerebral artery (MCA) was measured by transcranial color-coded duplex ultrasonography (iE 33 Ultrasound System, Philips, Bothell, WA) during normocapnic, hypocapnic, and hypercapnic steady-state conditions. The MCA was insonated from the left temporal window using a 1.6 MHz transcranial Doppler probe which was mounted on a custom-made probe fixation device attached to commercially available headgear (Dia Mon, DWL Compumedics, Charlotte, NC) (1). A color-coded Doppler signal guided the investigators to find the MCA and collect the data at the constant depth, angle, and location. Subjects wore a nose clip and breathed only through a mouthpiece with a Y-way valve (Hans-Rudolph, Shawnee, KS): one end connected to a 5-liter air reservoir containing a mixture of 5 % CO2 and 21 % O2 balanced with nitrogen and another end open to room air. Breath-by-breath end-tidal CO2, an estimate of PaCO2, was measured from expired air and analyzed by a capnograph (Capnocheck Plus, Smiths Medical, Waukesha, WI).
Three minutes of baseline recording were measured during spontaneous breathing of room air after ≥15 minutes of rest in the supine position. Next, subjects underwent 1 minute of maximal voluntary hyperventilation with a cycle of 1 second using a metronome. This short period of hyperventilation was intended to reduce end-tidal CO2 without causing respiratory muscle fatigue or central hypoxia possibly associated with a prolonged hyperventilation. After reaching the steady-state, MCA-BFV was recorded during the last 20 seconds of hyperventilation. After end-tidal CO2 and MCA-BFV returned to the baseline following hyperventilation, a respiratory valve was switched to an air reservoir containing 5% CO2 and 21% O2, and the subjects were asked to breathe spontaneously for 3 minutes. The air reservoir was continuously filled from a cylinder whose air pressure was manually adjusted to subject’s respiratory volume. The MCA-BFV was recorded at steady state during the last minute of hypercapnia.
Time-averaged peak velocity (i.e., area under curve of BFV waveform) recorded from >10 cardiac cycles in normocapnic, hypocapnic, and hypercapnic steady-states were averaged and reported with the corresponding averages of end-tidal CO2. Because transcranial Doppler does not measure blood flow per se, CVR was calculated as a percent change in MCA-BFV over an absolute change in end-tidal CO2. It has been reported that the percent changes in MCA-BFV are strongly correlated with absolute changes in cerebral blood flow measured by intravenous Xenon dilution technique (9). The CVR was calculated from the three different ranges of end-tidal CO2 levels: normocapnia to hypocapnia (NORM-HYPO), normocapnia to hypercapnia (NORM-HYPER), and hypocapnia to hypercapnia (HYPO-HYPER). The CVR (HYPO-HYPER) represents cerebrovascular responsiveness to a wider change in end-tidal CO2 and to minimize the potential effects of baseline cerebral blood flow velocity.
Neurocognitive Assessment
Participants completed a comprehensive battery of neurocognitive assessments, including standard clinical neurocognitive instruments with established reliability and validity. In order to reduce the number of multiple comparisons, neurocognitive measures were grouped into one of the two domains: memory or attention-executive function. These cognitive domains were selected a priori as they have been reported to benefit from aerobic exercise training (13). For the domain scores, raw test scores were converted to z-scores based on the study sample’s mean and standard deviation. Timed-test scores (i.e., Trail making test) were multiplied by −1 so that higher scores indicate better performance. As previously described (45), domain scores were calculated using z-scores of the following tests: 1) memory: California Verbal Learning Test (CVLT)-II immediate recall and delayed recall (19) and 2) attention-executive function: Trail making B time to completion (40), Controlled Oral Word Association Test (COWAT) (41), and Wechsler Adult Intelligence Scale (WAIS)-III Digit Span Subtest (47). A total cognitive composite score was also calculated by averaging the z-scores of all the individual tests. All tests were administered and scored by a trained research assistant using standard administration and scoring criteria.
Statistical Analyses
All continuous variables were examined for normality by the Shapiro-Wilk test and the visual inspections of histogram and Q-Q plot. Group differences in demographic and physiological variables were assessed by the Mann-Whitney U test or independent t-test according to the variable distributions. Chi-square test was used to compare group differences in categorical variables. Hierarchical multiple linear regression analysis was used to determine the impact of vascular reactivity (i.e., CVR and FMD) on group-level differences in cognitive function. In this analysis, vascular reactivity measurements were entered in the first model and then cognitive domain scores in the second model. Pearson’s product moment and partial correlation analyses examined the associations among cardiorespiratory fitness, cognitive function, and vascular reactivity measurements, after the variables with skewed distributions had been transformed to achieve normal distribution. In partial correlation analyses, age, sex, and education were entered as covariates. All statistical analyses were performed using SPSS 19 (SPSS inc., Chicago, IL). An α-level of 0.05 was set as the criterion for statistical significance. All data are expressed as means ± standard deviation.
Results
Sedentary versus Endurance-Trained Participants
Subject characteristics are presented in Table 1. There were no group differences in age, sex, ethnicity, and educational level. As expected, endurance-trained subjects demonstrated lower BMI and body fat percentage and greater cardiorespiratory fitness, as assessed by both subjective (i.e., Godin physical activity score) and objective (i.e., VO2max and aerobic fitness percentile) measures. Endurance-trained athletes also showed higher levels of HDL-cholesterol than sedentary subjects. As presented in Table 2, cognitive performance on memory, attention-executive function, and total composite scores were higher in endurance-trained athletes than in the sedentary subjects.
Table 1.
Subject characteristics
| Sedentary | Endurance-trained | P-values | |
|---|---|---|---|
| Men/Women (n) | 10/17 | 11/21 | 0.83 |
| Age (year) | 54±5 | 52±6 | 0.30 |
| Education (year) | 16±2 | 17±2 | 0.29 |
| Ethnicity (%) | 0.85 | ||
| Caucasian | 69 | 78 | |
| African-American | 8 | 3 | |
| Hispanic | 8 | 3 | |
| Asian | 4 | 3 | |
| Other | 11 | 13 | |
| Body Composition & Metabolic Risk Factors | |||
| Height (cm) | 170±12 | 167±8 | 0.40 |
| Body mass (kg) | 76±16 | 65±10 | 0.001 |
| Body mass index (kg/m2) | 26±5 | 23±2 | 0.001 |
| Body fat (%) | 36.7±8.2 | 23.4±8.6 | <0.001 |
| Lean tissue mass (kg) | 45.6±10.7 | 47.3±10.1 | 0.48 |
| Total cholesterol (mg/dl) | 205±37 | 191±36 | 0.16 |
| HDL-cholesterol (mg/dl) | 60±23 | 72±21 | 0.03 |
| LDL-cholesterol (mg/dl) | 123±30 | 108±24 | 0.10 |
| Glucose (mg/dl) | 91±11 | 93±9 | 0.54 |
| Cardiorespiratory Fitness Measures | |||
| Godin physical activity score (AU) | 21±21 | 64±20 | <0.001 |
| VO2max (mL/min/kg) | 26±5 | 43±9 | <0.001 |
| Aerobic fitness percentile (%ile) | 15±20 | 93±29 | <0.001 |
| Maximal heart rate (bpm) | 169±15 | 170±12 | 0.85 |
| Maximal respiratory exchange ratio (AU) | 1.10±0.06 | 1.08±0.05 | 0.19 |
Bold P-values represent <0.05. Values are mean ± standard deviation. Aerobic fitness percentile was calculated based on age- and sex-adjusted regression established by the American College of Sports Medicine. AU=arbitrary unit, HDL=high density lipoprotein, LDL=low density lipoprotein, VO2max=maximal oxygen uptake. Data from the similar group of subjects were reported in our previous publication (44), except for 1 additional participant included in the current study. Copyright © 2013 Wolters Kluwer Health and Lippincott Williams & Wilkins. Used with permission.
Table 2.
Neurocognitive assessment results
| Sedentary | Endurance-trained | P-values | |
|---|---|---|---|
| Mini-Mental State Exam | 28.7±1.3 | 29.0±1.2 | 0.35 |
| Wechsler Test for Adult Reading | 41.2±9.7 | 44.5±4.5 | 0.09 |
| Memory (z-score) | −0.36±1.11 | 0.30±0.76 | <0.01 |
| CVLT-II immediate recall | 10.6±3.2 | 12.4±2.1 | |
| CVLT-II delayed recall | 10.8±3.2 | 12.7±2.3 | |
| Attention-executive function (z-score) | −0.21±0.53 | 0.18±0.72 | 0.02 |
| Trail Making Test B (sec) | 70.8±20.8 | 58.2±19.4 | |
| COWAT | 43.1±8.8 | 46.7±14.7 | |
| WAIS-III Digit Span Subtest | 18.0±3.6 | 18.9±3.7 | |
| Total composite score (z-score) | −0.27±0.63 | 0.23±0.57 | <0.01 |
Bold P-values represent <0.05. Values are mean ± standard deviation. COWAT=Controlled Oral Word Association Test, CVLT-II=California Verbal Learning Test-II, WAIS-III=Wechsler Adult Intelligence Scale-III
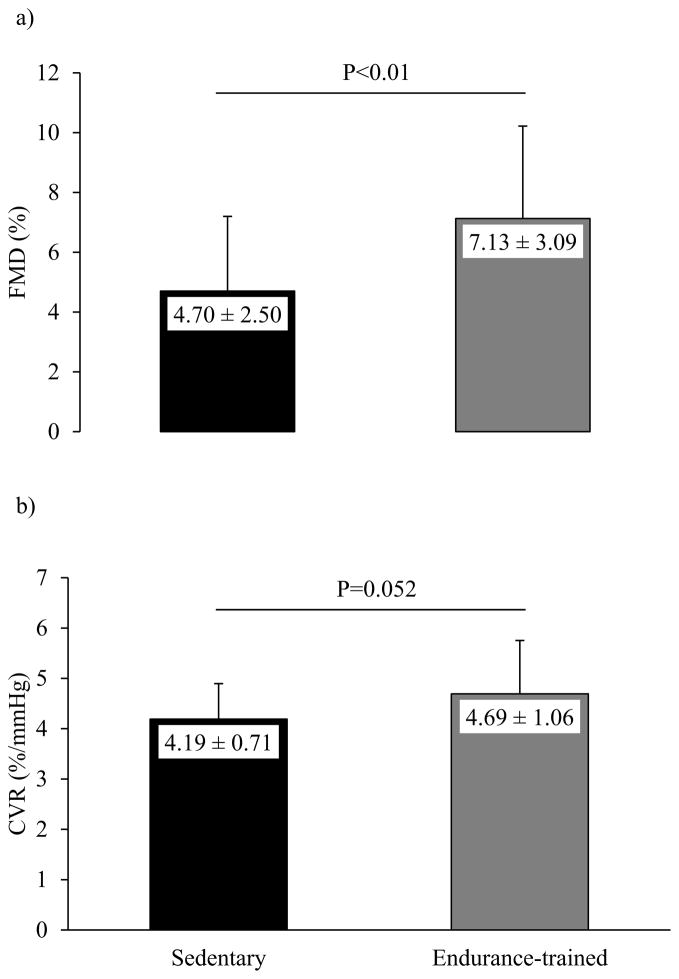
Endothelium-dependent vasodilation, as assessed by brachial artery FMD, was higher in endurance-trained athletes than in the sedentary group (Figure 1a). Table 3 shows cardiovascular and cerebrovascular parameters measured during normocapnia, hypocapnia, and hypercapnia. During all of the conditions, blood pressure was similar between the groups. Compared with the sedentary subjects, endurance-trained athletes showed lower heart rate and higher MCA-BFV and end-tidal CO2 at normocapnia. The CVR (HYPO-HYPER) showed a strong trend towards higher levels in endurance-trained athletes compared with the sedentary peers (Figure 1b), while CVR (NORM-HYPO) (2.45±0.50 vs. 2.21±0.43 %/mmHg, P=0.14) and CVR (NORM-HYPER) (3.42±1.56 vs. 3.54±1.17 %/mmHg, P=0.31) were not different between the groups. There was a positive correlation between the levels of HDL-cholesterol and CVR (HYPO-HYPER) (r=0.30, P=0.03).
Figure 1.
Vascular endothelial function assessed by brachial artery flow-mediated dilation (FMD) (Figure 1a) and cerebrovascular reactivity (CVR) to changes in end-tidal CO2 from hypo- to hypercapnia (Figure 1b) in the sedentary and endurance-trained middle-aged participants. Values in columns are mean ± standard deviation.
Table 3.
Cardiovascular and cerebrovascular measures during normocapnia, hypocapnia, and hypercapnia
| Sedentary | Endurance-trained | P-values | |
|---|---|---|---|
| Cardiovascular measures during normocapnia | |||
| Heart rate (bpm) | 64±7 | 52±8 | <0.001 |
| Systolic BP (mm Hg) | 120±9 | 118±10 | 0.32 |
| Mean BP (mm Hg) | 90±8 | 88±8 | 0.28 |
| Diastolic BP (mm Hg) | 73±8 | 70±6 | 0.11 |
| Pulse pressure (mm Hg) | 48±6 | 48±6 | 0.77 |
| Brachial artery mean diameter (mm) | 3.54±0.73 | 3.61±0.70 | 0.69 |
| Cerebrovascular measures | |||
| End-tidal CO2 (mm Hg) | |||
| Normocapnia | 42±5 | 45±4 | 0.01 |
| Hypocapnia | 26±4 | 28±4 | 0.08 |
| Hypercapnia | 51±3 | 53±2 | 0.01 |
| Mean arterial pressure (mm Hg) | |||
| Normocapnia | 90±9 | 87±9 | 0.22 |
| Hypocapnia | 80±11 | 79±12 | 0.79 |
| Hypercapnia | 95±10 | 92±11 | 0.46 |
| Mean blood flow velocity (cm/sec) | |||
| Normocapnia | 59±21 | 67±13 | 0.03 |
| Hypocapnia | 38±14 | 40±9 | 0.34 |
| Hypercapnia | 77±23 | 84±15 | 0.20 |
Bold P-values represent <0.05. Values are mean ± standard deviation. BP=blood pressure, CO2=carbon dioxide
Based on hierarchical multiple linear regression analysis, FMD and CVR (HYPO-HYPER) accounted for 14% (ΔR2=0.14, P=0.03) and 12% (ΔR2=0.12, P=0.049) of variances in total cognitive composite and attention-executive function scores respectively. Furthermore, group differences in these cognitive performance scores were abolished (total composite: ΔR2=0.04, P=0.16 and attention-executive function: ΔR2=0.03, P=0.22) after controlling for the vascular reactivity measurements.
Associations among Cardiorespiratory Fitness, Cognitive Function, and Vascular Reactivity
Table 4 presents simple correlations among cardiorespiratory fitness, cognitive performance, and vascular reactivity measures in all subjects. Aerobic fitness percentile and brachial FMD were positively correlated with better performance on memory, attention-executive function, and total cognitive composite scores. The CVR (HYPO-HYPER) was also positively correlated with better performance on memory and total cognitive composite scores. After adjusting for age, sex, and education, aerobic fitness percentile and CVR (HYPO-HYPER) remained significantly correlated with memory (r=0.42, P<0.01 and r=0.29, P=0.04 respectively) and total cognitive composite (r=0.42, P<0.01 and r=0.34, P=0.02 respectively) scores.
Table 4.
Pearson’s product moment correlation coefficients and (P-values) showing the associations among cardiorespiratory fitness, cognitive function, and cerebral and peripheral vascular reactivity measures
| VO2max | Aerobic fitness percentile | Total cognitive composite | Memory | Attention-executive function | FMD | CVR | |
|---|---|---|---|---|---|---|---|
| VO2max | 0.93 | 0.24 | 0.18 | 0.21 | 0.33 | 0.20 | |
| Aerobic fitness percentile | (<0.001) | 0.40 | 0.36 | 0.29 | 0.37 | 0.27 | |
| Total cognitive composite | (0.07) | (<0.01) | 0.82 | 0.82 | 0.36 | 0.30 | |
| Memory | (0.17) | (<0.01) | (<0.001) | 0.34 | 0.27 | 0.27 | |
| Attention-executive function | (0.11) | (0.03) | (<0.001) | (<0.01) | 0.31 | 0.22 | |
| FMD | (0.01) | (<0.01) | (<0.01) | (0.04) | (0.02) | −0.02 | |
| CVR | (0.15) | (0.055) | (0.03) | (0.049) | (0.11) | (0.90) |
Bold values represent P-value<0.05. Aerobic fitness percentile was calculated based on age- and sex-adjusted regression established by the American College of Sports Medicine. CVR=cerebrovascular reactivity to changes in end-tidal CO2 from hypo- to hypercapnia, FMD=brachial artery flow-mediated dilation, VO2max=maximal oxygen uptake
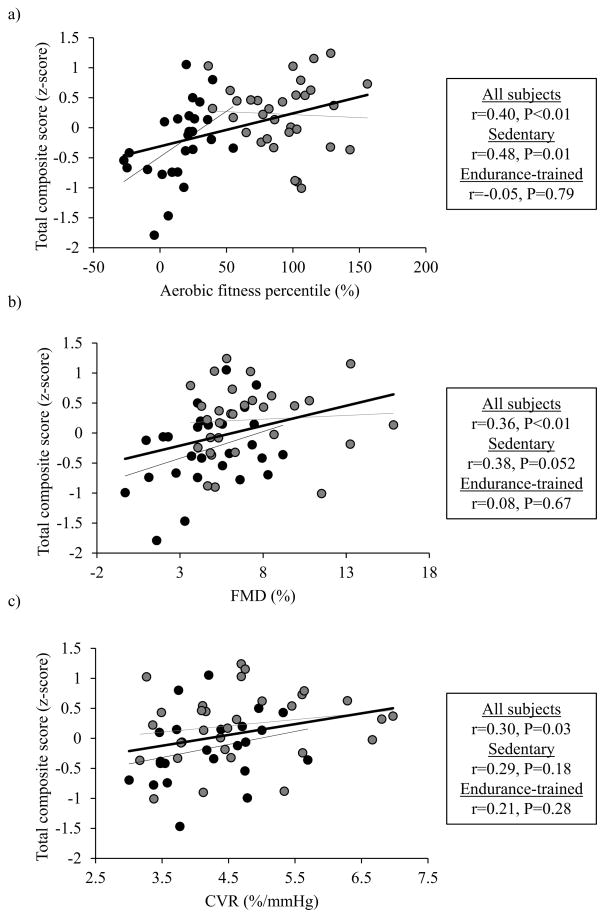
Next, we examined the dose-dependent association of cognitive performance with cardiorespiratory fitness and vascular reactivity within the sedentary and endurance-trained groups separately. Interestingly, higher total cognitive composite scores were correlated with greater cardiorespiratory fitness in the sedentary but not endurance-trained group (Figure 2a). In addition, brachial FMD also showed a trend of positive correlation with total cognitive composite scores in the sedentary but not endurance-trained group (Figure 2b). The CVR (HYPO-HYPER) was not correlated with the composite scores, when analyzed within each of the groups separately (Figure 2c).
Figure 2.
Associations of total cognitive composite scores with aerobic fitness percentile (Figure 2a), vascular endothelial function assessed by brachial artery flow-mediated dilation (FMD) (Figure 2b), and cerebrovascular reactivity (CVR) to changes in end-tidal CO2 from hypo- to hypercapnia (Figure 2c) in the sedentary (black circles) and endurance-trained (gray circles) middle-aged participants. Aerobic fitness percentile was calculated based on age- and sex-adjusted regression established by the American College of Sports Medicine. Thin black and gray lines represent linear regression for the sedentary and endurance-trained subjects respectively. Thick black lines represent linear regression for all subjects.
Discussion
The primary findings from the present study are as follows. First, endurance-trained middle-aged participants demonstrated better cognitive performance on memory, attention-executive function, and total composite scores and greater brachial FMD than the sedentary peers. Endurance-trained participants also showed a strong trend towards the higher levels of CVR (HYPO-HYPER) compared with the sedentary subjects. Second, brachial FMD and CVR (HYPO-HYPER) were positively correlated with better cognitive performance, especially memory and total composite scores. Finally, fitness-related group differences in cognitive function were abolished after controlling for the effects of cerebral and peripheral vascular reactivity measures. Collectively, these findings suggest that better cognitive performance in middle-aged endurance-trained athletes is mediated, at least in part, by their greater vascular function. To the best of our knowledge, this is the first study to demonstrate that higher levels of cerebral and peripheral vascular reactivity are associated with better cognitive performance in middle-aged endurance-trained adults.
Midlife vascular disease and risk factors are associated with higher incidence of cognitive impairment in late life (17). The human brain relies heavily on the blood circulation for its energy source. Thus, vascular dysregulation of cerebral blood flow may lead to hypoperfusion that in turn can elevate the risk of impaired action potential generation and protein synthesis, cerebral amyloid deposition, glutamatergic excitotoxicity, and infarct (16, 27, 38). Conversely, habitual aerobic exercise ameliorates vascular health, an effect which may further translate into improved cognitive performance (32, 42). To extend this area of research, we investigated whether higher level(s) of cerebral and/or peripheral vascular function in endurance-trained middle-aged adults is associated with better cognitive performance. Consistent with our hypothesis, endurance-trained participants demonstrated better cognitive performance and higher brachial artery endothelium-dependent vasodilation. There was also a strong trend towards greater CVR in the endurance-trained group. Moreover, greater levels of vascular reactivity in both peripheral and cerebral arteries were positively associated with cognitive performance, suggesting that the benefit of regular aerobic exercise on cognitive function is, at least in part, mediated by higher vascular function.
The present study confirms and further adds important information to the literature. First, our endurance-trained middle-aged participants demonstrated greater levels of normocapnic MCA-BFV at rest than their sedentary peers. This is consistent with findings from Ainslie et al. demonstrating that regular aerobic exercise is associated with higher levels of MCA-BFV throughout adulthood (3). Thus, these findings suggest that regular aerobic exercise attenuates age-related reductions in cerebral blood flow. Second, higher brachial FMD in endurance-trained participants was associated with better cognitive performance. Endothelial function, as assessed by brachial FMD, is a well-established indicator of systemic endothelial function (10, 12). Thus, if this also holds true for the brain, regular aerobic exercise may improve, or at least preserve, the integrity of cerebrovascular endothelium or blood-brain barrier (BBB), which plays a pivotal role for the normal brain function and structure (discussed below) (2). Third, endurance-trained participants demonstrated a strong trend towards higher CVR which in turn was positively correlated with better cognitive performance. Prior research on middle-aged and older women has similarly demonstrated that greater VO2max is associated with lower mean arterial pressure and higher cerebrovascular conductance during the isocapnic resting conditions (11). In addition, these exercise-related adaptations in vascular function were correlated with better cognitive outcomes (11). In the present study, we did not find that mean arterial pressure and cerebrovascular conductance are correlated with cognitive function. The discrepancy in these observations may be attributed to differences in the subject population and/or the inclusion of both sexes. However, our results exhibiting the association between CVR (HYPO-HYPER) and cognitive performance indicates that cerebral blood flow response to a greater range of CO2 stimuli may have higher sensitivity for detecting differences in cognitive function. Fourth, Murrell et al. (37) and Ivey et al. (30) recently reported that a 3–6 month aerobic exercise training intervention improved CVR in healthy older subjects, as well as patients with stroke. Using an interventional study design, their findings strengthen our results but also emphasize a need of future studies investigating the impact of exercise-related improvements in cerebrovascular function on cognitive outcome. Fifth, endurance-trained athletes demonstrated elevated HDL-cholesterol levels which in turn were positively correlated with higher CVR. HDL-cholesterol increases with aerobic exercise training (6) and inhibits the formation of atherosclerosis (31). Consistent with our finding, Bakker et al. also demonstrated a positive association between HDL-cholesterol and CVR in the elderly (7). Therefore, these findings collectively suggest that exercise-related increase in HDL-cholesterol confers a favorable impact on cerebrovascular function and prevent the formation of intracranial atherosclerosis. Finally, when analyzing the sedentary and endurance-trained groups separately, we found a stronger correlation of cognitive performance with cardiorespiratory fitness and vascular endothelial function in the sedentary group. This suggests that sedentary subjects have greater benefits in their cognitive performance by improving cardiorespiratory fitness or vascular endothelial function than those who have already been participating in regular aerobic exercise.
The physiological mechanism underlying the associations among cardiorespiratory fitness, vascular reactivity, and cognitive function can only be speculated due to the cross-sectional nature of the present study. First, endothelium-dependent vasodilation plays an important role in modulating the extent of functional hyperemia (28). Given that 50–60% of total cerebrovascular resistance is controlled by the large cerebral arteries and pial arterioles, ascending or conducted vasodilation that propagates proximally to the remote feeding arterioles prevents the local hypoperfusion (20, 22, 29). Vasodilation of downstream vessels increases flow velocity in upstream branches and leads to the local release of endothelium-dependent vasodilators (36). Endothelium-dependent vasodilation, measured by brachial FMD, resembles the hemodynamic response to neuronal activation since both responses are elicited by increased shear stimulus. Indeed, brachial FMD has been correlated with the degree of functional hyperemia measured by blood-oxygen-level dependent (BOLD) using functional MRI, suggesting that a greater level of endothelium-dependent vasodilation in endurance-trained subjects facilitates blood flow to active neurons (26). Second, vascular endothelial function may reflect the integrity of BBB. The BBB is primarily composed of a single endothelial layer at the level of cerebral capillaries and plays a crucial role in controlling the molecular trafficking between the brain and blood (2). The disruption of BBB causes leakage of potentially hazardous molecules into the brain parenchyma and impairs the clearance of cerebral metabolites (e.g., amyloid-β) from the intracellular space (50). Third, CVR may assess global health of intra- and extra-parenchymal arterioles since CO2 reactivity measured at MCA is primarily mediated by vasodilation of the downstream arterioles (24). The vasodilation elicited by neuronal activation and hypercapnia takes place in the arterioles of similar size and may involve a common vasoactive substance (29). Indeed, a cyclooxygenase inhibitor (i.g., indomethacin) attenuates both the hemodynamic responses to neuronal activation and the changes in PaCO2 (8, 44).
Interestingly, we found higher levels of end-tidal CO2 in endurance-trained subjects as compared with the sedentary group. This finding was unexpected given that previous studies of aerobic exercise training and CVR did not show such results (46). Although it is difficult to speculate the underling mechanism, reductions in end-tidal CO2 have been reported from patients with heart failure and are also correlated with lower cardiac performance (35). Thus, higher levels of end-tidal CO2 in our endurance-trained subjects may reflect their superior cardiac function. In addition, intermittent exposures to ventilatory stimulus during routine aerobic exercise training may desensitize the chemoreceptor sensitivity to PaCO2 and subsequently increase end-tidal CO2 in the endurance-trained group (34). Finally, sedentary participants may have ventilatory insufficiency at the level of lungs which can contribute to the reductions of end-tidal CO2 at rest (48). Importantly, the between-group differences in end-tidal CO2 are likely to have a small impact on the calculated CVR because its absolute changes from the baseline to hypo- or hypercapnia (or even from hypo- to hypercapnia) were very similar within each group.
The strength and novelty of the present study include the concurrent measurements of both cerebral and peripheral vascular reactivity, the inclusion of middle age as the primary target cohort, and the use of neurocognitive assessments with established reliability and validity. However, limitations of the present study also need to be underscored. First, endothelium-dependent vasodilation was measured from the brachial artery and may pose a limitation of site-specificity to the translation of this information to cerebral arteries. However, brachial endothelium-dependent vasodilation has been reported to reflect the systemic vascular endothelial function and may indicate the condition of cerebrovascular endothelium (10, 12). Second, TCD-based assessment of cerebral blood flow assumes the constant cross-sectional area of insonated artery during measurements; hence, changes in BFV of the artery are proportional to changes in blood flow at the downstream vascular beds. Of note, previous studies of TCD reported no significant changes in the MCA diameter during mild hypercapnia (24). Third, the cross-sectional study design does not address a cause-and-effect relationship. This limitation may also pertain to statistical analysis performed in the current study. Inclusion of additional covariates in the model may have introduced more noise into the regression models and attenuated the cognitive findings. Prospective studies will be necessary to fully assess the causal mechanisms underlying fitness-related improvements in cognitive function. Such causal relationship can be revealed only by prospective studies. Fourth, a relatively small sample size in the present study limits the generalizability of our findings. Finally, future studies should incorporate vascular neuroimaging markers (e.g., BOLD response to changes in PaCO2 and cognitive task) in order to elucidate the regional specificity of the effect of aerobic exercise training on cerebrovascular function (33). In the previous study of Alzheimer’s disease patients, BOLD-based CVR was attenuated in the forebrain regions (49). Currently, it remains unknown whether habitual aerobic exercise in middle age can enhance CVR in similar brain areas and preserve cognitive function.
Conclusions
Endurance-trained middle-aged participants demonstrated better cognitive performance on memory, attention-executive function, and total cognitive composite scores than the sedentary subjects. Better cognitive function in endurance-trained participants was associated with greater cerebrovascular CO2 reactivity and vascular endothelial function, as assessed by brachial FMD. The fitness-related group differences in cognitive performance were abolished when the effects of cerebral and peripheral vascular function were covaried. These findings collectively suggest that better cognitive performance in endurance-trained participants is mediated, at least in part, by enhanced cerebral and peripheral vascular functions.
Acknowledgments
The results of the present study do not constitute endorsement by American College of Sports Medicine. This study was supported in part by grants from the American Federation for Aging Research (8A0024, A.P.H.), the National Institute of Neurological Disorders and Stroke (R01NS75565, A.P.H.) and the National Institute on Aging (F31AG040890, M.M.G.). We thank Mohammed Alkatan, Mandeep Dhindsa, and Tariq Thannoun for technical assistance.
Footnotes
Disclosure: None.
References
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20(1):45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of Disease. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Cotter JD, George KP, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005–10. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1473–95. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 5.American_College_of_Sports_Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Baltimore, Md: Lippincott Williams & Wilkins; 2006. p. 79. [Google Scholar]
- 6.Assmann G, Gotto AM. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109(23 suppl 1):III-8–III-14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 7.Bakker S, de Leeuw F-E, Koudstaal P, Hofman A, Breteler M. Cerebral CO2 reactivity, cholesterol, and high-density lipoprotein cholesterol in the elderly. Neurology. 2000;54(4):987–9. doi: 10.1212/wnl.54.4.987. [DOI] [PubMed] [Google Scholar]
- 8.Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. Journal of Applied Physiology. 2012;112(11):1884–90. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17(5):913–5. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 10.Bode-Boger SM, Boger RH, Schroder EP, Frolich JC. Exercise increases systemic nitric oxide production in men. Journal of Cardiovascular Risk. 1994;1(2):173–8. [PubMed] [Google Scholar]
- 11.Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of Aging. 2010;31(12):2047–57. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. Journal of the American College of Cardiology. 1994;24(6):1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 13.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults a meta-analytic study. Psychological science. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 15.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med sci sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 16.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3(3):184–90. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 17.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–8. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dede DS, Yavuz B, Yavuz BB, et al. Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? Journal of the American Geriatrics Society. 2007;55(10):1613–7. doi: 10.1111/j.1532-5415.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 19.Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. Journal of consulting and clinical psychology. 1988;56(1):123. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66(1):8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiological Reviews. 1998;78(1):53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(51):22290–5. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32(5):737–41. discussion 41–2. [PubMed] [Google Scholar]
- 25.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences Journal Canadien des Sciences Appliquees Au Sport. 1985;10(3):141–6. [PubMed] [Google Scholar]
- 26.Gonzales MM, Tarumi T, Tanaka H, et al. Functional imaging of working memory and peripheral endothelial function in middle-aged adults. Brain Cogn. 2010;73(2):146–51. doi: 10.1016/j.bandc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Annals of Neurology. 1994;36(4):557–65. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 29.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. Journal of Neurophysiology. 1997;78(2):651–9. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 30.Ivey FM, Ryan AS, Hafer-Macko CE, Macko RF. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke. 2011;42(7):1994–2000. doi: 10.1161/STROKEAHA.110.607879. [DOI] [PubMed] [Google Scholar]
- 31.Kodama S, Tanaka S, Saito K, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Archives of internal medicine. 2007;167(10):999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 32.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 33.Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated-BOLD fMRI. Neuroimage. 2007;35(1):175–84. doi: 10.1016/j.neuroimage.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahler DA, Moritz ED, Loke J. Ventilatory responses at rest and during exercise in marathon runners. Journal of Applied Physiology. 1982;52(2):388–92. doi: 10.1152/jappl.1982.52.2.388. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto A, Itoh H, Eto Y, et al. End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. Journal of the American College of Cardiology. 2000;36(1):242–9. doi: 10.1016/s0735-1097(00)00702-6. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44(2):134–9. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 37.Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: Effect of age and 12-week exercise training. Age (Dordr) 2012;35(3):905–20. doi: 10.1007/s11357-012-9414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto Y, Yamamoto T, Kalaria RN, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012;123(3):381–94. doi: 10.1007/s00401-011-0925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okazaki K, Iwasaki K-i, Prasad A, et al. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. Journal of Applied Physiology. 2005;99(3):1041–9. doi: 10.1152/japplphysiol.00085.2005. [DOI] [PubMed] [Google Scholar]
- 40.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–6. [Google Scholar]
- 41.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–38. [PubMed] [Google Scholar]
- 42.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587(Pt 23):5541–9. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37(4):1010–5. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 44.St Lawrence KS, Ye FQ, Lewis BK, Weinberger DR, Frank JA, McLaughlin AC. Effects of indomethacin on cerebral blood flow at rest and during hypercapnia: an arterial spin tagging study in humans. Journal of Magnetic Resonance Imaging. 2002;15(6):628–35. doi: 10.1002/jmri.10111. [DOI] [PubMed] [Google Scholar]
- 45.Tarumi T, Gonzales MM, Fallow B, et al. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. Journal of hypertension. 2013;31(12):2400–9. doi: 10.1097/HJH.0b013e328364decc. [DOI] [PubMed] [Google Scholar]
- 46.Thomas BP, Yezhuvath US, Tseng BY, et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. Journal of Magnetic Resonance Imaging. 2013;38(5):1177–83. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wechsler D. WAIS-III administration and scoring manual. 3. San Antonio, TX: The Psychological Corporation; 1997. p. 496. [Google Scholar]
- 48.Yasunobu Y, Oudiz RJ, Sun X-G, Hansen JE, Wasserman K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. CHEST Journal. 2005;127(5):1637–46. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 49.Yezhuvath US, Uh J, Cheng Y, et al. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiology of aging. 2012;33(1):75–82. doi: 10.1016/j.neurobiolaging.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]