Abstract
This paper reviews Drosophila voltage-gated Na+ channel mutations encoded by the para (paralytic) gene and their contributions to seizure disorders in the fly. Numerous mutations cause seizure-sensitivity, for example, parabss1, with phenotypes that resemble human intractable epilepsy in some aspects. Seizure phenotypes are also seen with human GEFS+ spectrum mutations that have been knocked into the Drosophila para gene, paraGEFS+ and paraDS alleles. Other para mutations, paraST76 and paraJS act as seizure-suppressor mutations reverting seizure phenotypes in other mutants. Seizure-like phenotypes are observed from mutations and other conditions that cause a persistent Na+ current through either changes in mRNA splicing or protein structure.
Keywords: Drosophila, epilepsy, intractable epilepsy, sodium channel, seizure-suppressor genes
Introduction
Drosophila investigations have traditionally modeled fundamental features of basic biological science, particularly classical and molecular genetics, development, and neurobiology (Rubin and Lewis, 2000; Celniker et al., 2009; Rieder and Larschan, 2014). Recently, there has been increased interest in modeling human diseases in the fly with the goal of uncovering fundamental principles underlying the causes of human pathology. There is also the compelling prospect of novel therapeutics identified by the study of disease-causing genes followed by targeted drug development, or by establishing platforms for high-throughput drug screening. Drosophila models have been introduced for numerous disorders including Parkinson's disease, neurodegenerative disease, glioma, sleep disorders, and others (Reiter and Bier, 2002; Guo, 2012; Hirth, 2010; Read, 2011; Sehgal and Mignot, 2011; Freeman et al., 2013).
In this review, we examine Drosophila mutations that modify seizure-susceptibility as a model for genetic contributions to human epilepsy. This is the type of investigation for which Drosophila is well suited compared with some other animal models. For example, phenotypic characterization is difficult in C. elegans because of challenges in electrophysiological analysis. This makes is hard to exploit the superior mutant isolation, genetic capabilities, and genomics available for the worm. Mouse phenotypes are a more relevant model for human seizures, however the large number of genes presenting with epilepsy, potential genetic background complexities, and positional cloning requirements present difficulties with genetic analysis. Thus, the combination of advanced electrophysiology methods, mutant isolation, genetic analysis, and molecular biology available for Drosophila provide methodological advantages. Also, available are large collections of behavioral and neurological mutants, libraries of RNAi and transposon insertions. For example, there are extant collections of single gene mutations that cause seizure-sensitivity with phenotypes resembling those of idiopathic human epilepsies in some aspects. Also, a novel class called seizure-suppressor mutations revert seizure-phenotypes and may provide targets for anti-epileptic drugs.
Our focus in this review is on mutations of the Drosophila voltage-gated Na+ channel gene para (paralytic). Some Na+ channel mutations cause extreme seizure-sensitivity; others act as seizure-suppressors. Our appreciation is increasing for the important and complex role of Na+ channels in seizure-disorders. Na+ channel mutations of the human SCN1A gene are responsible for a surprisingly large number of epilepsies. Expression of seizure phenotypes is dependent on a number of mainly unidentified genetic and environmental factors. Several of these complexities have been approached in Drosophila, a tractable system because of available genetic, behavioral, and electrophysiological methodologies. In this review, we examine para to gain appreciation of its the complex contributions to generation of seizures. Although here we are restricting our consideration to Na+ channel epilepsies, Drosophila also provides a valuable model for other epilepsies such as progressive myoclonus epilepsy-ataxia syndrome due a mutation in the human homolog of the fly prickle gene whose product is involved in transcription repression (Bassuk et al., 2008; Tao et al., 2011). The fly can also model other seizure disorder pathologies due to ionic homeostasis via glial dysfunction (Rusan et al., 2014), mitochondrial and metabolic disruption (Royden et al., 1987; Zhang et al., 1999; Fergestad et al., 2006), or sleep deprivation-induced seizure activity (Lucey et al., 2015).
Human Na+ channel epilepsy
Human seizure disorders are a significant health concern due to the large number of affected individuals, the devastating ramifications of untreated seizure episodes, and limitations in AED (anti-epileptic drug) options. About 10% of the population will experience a seizure sometime during their lifetime, and 1% of people suffer the persistent, spontaneous seizures that define epilepsy (Shneker and Fountain, 2003). The epilepsies are a collection of about 40 different neurological disorders characterized by large numbers of neurons firing uncontrollably and synchronously, usually in a rhythmic pattern. Generalized seizures are marked by the widespread involvement of both brain hemispheres and include tonic-clonic (grand mal), absence (petit mal), and myoclonic disorders. Partial seizures are limited to localized brain regions, often the temporal lobe. Especially difficult are the intractable epilepsies. About 15 million people worldwide cannot adequately control their seizures with any available medication (Kwan and Brodie, 2000). Uncontrolled bouts of seizure, especially in infants and children, interfere with normal brain development leading to permanent deficits in cognitive capabilities.
Especially interesting are human epilepsies caused by mutations in the Na+ channel gene, especially SCN1A. Three SCN1A epilepsies are: GEFS+ (generalized epilepsy with febrile seizures plus) characterized by febrile seizures that persist beyond age 6y; SMEI (severe myoclonic epilepsy in infancy, Dravet's syndrome), an intractable epilepsy frequently resulting in convulsive status epilepticus; and ICEGTC (intractable childhood epilepsy with generalized tonic-clonic), an atypical SMEI that does not cause myoclonic seizures. Dravet's syndrome and ICEGTC are severe epilepsies that have been generally associated with non-heritable spontaneous mutations of Na+ channel genes, especially SCN1A.
GEFS+ has been thought of as a familial epilepsy syndrome (Parihar and Ganesh, 2013; Catterall, 2014; Goldberg-Stern et al., 2014). Gene discovery in GEFS+ families was an important milestone in epilepsy genetics. About 10% of GEFS+ families have SCN1A mutations, mainly missense mutations. GEFS+ is also associated with mutations in genes encoding a Na+ channel beta 1 subunit (SCN1B) and a GABAA receptor subunit (GABRG). GEFS+ families can show considerable variability of seizure phenotypic severity. For example, in a large Israeli family carrying a single SCN1A missense mutation (K1372E), phenotypes varied from Dravet's syndrome (1 family member) to GEFS+ (13 family members), with 3 family members identified as unaffected carriers (Goldberg-Stern et al., 2014). From this, and other examples of variabililty, GEFS+ is thought of as a continuum of disorders referred to as the GEFS+ spectrum.
Human Na+ channelopathies are ordinarily resistant to pharmacotherapy. For example, Dravet's syndrome is generally unresponsive to prescription of a single antiepileptic drug (AED), and seizures are exacerbated by certain AEDs that block Na+ channels such as, lamotrigine and carbamazepine (Guerrini et al., 1998; Thanh et al., 2002). Improved, albeit incomplete seizure control can occasionally occur with drug polytherapy, concurrent use of combinations of multiple AEDs: valproate, topiramate, stiripentol, clobazam, or bromides, possibly acting by different mechanisms of enhancing GABAergic inhibition (Chiron and Dulac, 2011; Stephen et al., 2012).
Voltage-gated Na+ channels
Molecules mediating electrical excitability in the fruit fly are generally similar to those in humans (Frank et al., 2013). Excitatory chemical synaptic transmission is through ligand-gated channels, mainly acetylcholine and glutamate receptors. Chemical synaptic inhibition is mainly through GABA receptors. Ca++ regulates transmitter release through voltage-gated presynaptic Ca++ channels. Ionic currents, carried by Na+ ions, flow into nerve cells and are responsible for activation of the action potential and its regenerative conduction along the axon. Na+ ions enter the cell through Na+ channels, integral membrane proteins with a number of unique functional properties: voltage sensitivity, responsible for opening channels; ion selectivity, responsible for filtering Na+ for conductance while selecting against other cationic species; and channel inactivation, responsible for time dependent closing of the channel.
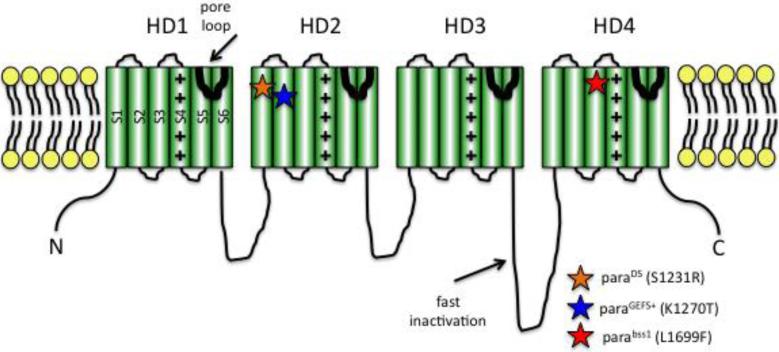
All voltage-gated Na+ channels, including those of human and fly, are generally similar in structure and function. A large Na+ channel alpha subunit polypeptide, is organized into four homologous domains (HD1-4) arranged around a central pore, conserved in all Na+ channels (Figure 1; Catterall, 2014; Littleton and Ganetzky, 2000). Each HD contains 6 transmembrane alpha-helical segments (S1-S6) connected by intracellular or extracellular loops of amino acid sequences. Numerous structure-function analyses have shown that a short alpha-helical loop connecting S5 and S6 of each HD dips into the membrane and their combined association forms the channel pore (Figure 1). Supporting this identification for the channel pore is that mutations in the S5-S6 loop region cause alterations in ion selectivity; and the tetrodotoxin pore blocker binds within the segment. Na+ channel activation occurs via the movement of positively-charged residues in the S4 segment. An intracellular loop connecting HD3 and 4 is responsible for fast inactivation of the channel. This loop is thought to fold into the intracellular mouth of the pore, thereby blocking conductance during fast inactivation.
Figure 1. Cartoon depiction of voltage-gated Na+ channel structure.
The channel contains four homology domains (HD1-4) organized around a water-filled pore. Each HD contains six membrane-spanning segments (S1-6). S4 contains positively-charged arginine and lysine residues and acts to sense voltage. An intracellular loop between HD3-4 is responsible for fast inactivation. The S5-6 linker forms the channel pore. The paraGEFS+ and paraDS mutations are located in S1 and S2 of HD2, respectively (orange star, blue star). As described in the text, corresponding mutations in SCN1A cause human epilepsies. The parabss1 mutation is located in S3 of HD4 (red star).
Human neurons and muscle express numerous functionally distinct voltage-gated Na+ channels, with diversity arising from the differential expression of 9 different alpha subunit genes. Five distinct Na+ channel alpha subunit polypeptides named Nav1.1 to Nav1.5 are each encoded by the genes SCN1A to SCN5A, respectively. Four other human alpha subunits named Nav1.6 to Nav1.9 are each encoded by the genes SCN8A to SCN11A, respectively (Goldin, 2001; Catterall et al., 2003; Catterall, 2014). Additional diversity arises from interaction of alpha subunits with one of four different beta subunits. Na+ channel diversity in Drosophila arises differently than for humans. The fly possesses a single Na+ channel alpha subunit encoding gene, para, with channel diversity arising from alternative splicing. The para gene is large, spanning about 60kb of genomic DNA (Loughney et al., 1989; Ramaswami and Tanouye, 1989 Thackeray, et al., 1995; Feng, et al., 1995; Warmke, et al.,1997).
There are 26 para exons: 13 are constitutive and 15 are alternatively spliced (Thackeray and Ganetzky, 1994; 1995; O'Dowd et al., 1995; Dong, 2007; Olson et al., 2008; Lin et al., 2009; Lin and Baines, 2014). Annotation for para describes 60 possible transcripts and 57 unique polypeptides. Eleven exons are spliced independently and result in changes in cytoplasmic loop segments. Electrophysiology of splice variants shows differences in Na+ channel kinetics. For example,splice variants that include the exon F show a hyperpolarizing shift in activation kinetics of the para channel that is expected to increase excitability for neurons containing these variants.
Two splice events are mutually exclusive: the inclusion of exon C or D, and of exon K or L. Para exons C and D encode a portion of the membrane spanning segments of S4 and S5 in HD2 and exons K and L encode the membrane spanning segments of S3 and S4 in HD3. The mutually exclusive exon pair K and L is particularly interesting because neurons containing exon L show a marked increase in the frequency of action potential firing and apparently contribute to seizure-sensitivity in several Drosophila neurological mutants (Lin et al., 2009; Lin and Baines, 2014). Na+ channels carrying para exon L show incomplete channel inactivation. This results in a longer-lasting residual Na+ current called a persistent current (INaP). It is this increased INaP in para exon L channels that appear to be responsible for the action potential frequency increase.
The para gene and its mutations
Befitting its central role in neuronal excitability, a large number of mutations have been identified for para with 117 reported alleles. The para mutations display a range of neurological phenotypes. Canonical para alleles were temperature sensitive (TS) paralytic such as parats1 and paraST76 (Suzuki, et al., 1971; Siddiqi and Benzer, 1976; Wu and Ganetzky, 1980). These are conditional mutations with flies displaying normal or nearly normal behavior at room (permissive) temperature, while undergoing reversible heat induced behavioral paralysis and loss of action potentials (restrictive temperature). Another TS para mutation, paracs, is an unusual cold-sensitive allele (Fahmy and Fahmy, 1960). The para gene is an essential gene and numerous lethal alleles are identified such as paraID30 and parahd5 (Ganetzky, 1984; Loughney et al., 1989). Some para alleles, such as parasbl1 and parasbl2, show broad olfactory defects altering the ability of these strains to respond to odorants in several chemical classes. TS paralysis is not observed for parasbl1 and parasbl2 (Lilly and Carlson, 1990). The Na+ channel is a target for DDT and pyrethroid insecticides. Several para alleles, such as parats1 and paraDN7 cause a target site resistance to these insecticides (Pittendrigh et al., 1997).
A number of para alleles have been found to directly affect seizure-susceptibility in the fly, especially interesting mutations forming a basis for modeling human epilepsy. Some para alleles cause flies to become seizure-sensitive such as parabss1, parabss2, paraGEFS+ and paraDS (Parker et al., 2011; Sun et al., 2012; Schutte, et al., 2014). Some para alleles such as paraST76, parats1 and paraJS1 act as second-site seizure suppressor mutations, reverting seizure-sensitive phenotypes of other mutations in double mutant combinations (Kuebler et al., 2001; Song and Tanouye, 2007). We discuss separately here the role of para mutations in Drosophila seizure studies and how they resemble human epilepsy in some aspects.
Seizure-like electrical activity in adult Drosophila
Susceptibility to seizures in adult flies can be evaluated by electrophysiology methods that evoke and record seizure-like electrical activity (Pavlidis et al., 1994; Pavlidis and Tanouye, 1995; Kuebler and Tanouye, 2000; Kuebler et al., 2001; Lee and Wu, 2002; 2006). These methods allow seizure-susceptibility to be quantified, especially valuable for comparing wild type and mutant flies, and other genetic and chemical manipulations (Kuebler and Tanouye, 2000; Kuebler et al., 2001; Song and Tanouye, 2006). Seizure-like activity is evoked by short wavetrains of high frequency electrical stimuli (HFS, 0.4ms pulses at 200Hz for 300ms) delivered to the brain at sufficient intensity and recorded from thoracic flight muscles. This seizure-like activity reflects motoneuron action potentials on a one-to-one basis. Seizure-like activity appears as uncontrolled, high-frequency (>150Hz) neuronal spiking. Seizure-like activity is extensive, spreading to at least 14 different muscles, containing 60 muscle fibers innervated by 58 motoneurons. Seizures are evoked in an all-or-nothing manner: HFS stimuli below a threshold voltage never elicit seizures; whereas just above threshold, seizures are always elicited. Seizure threshold is similar for all individuals of a given genotype. Thus, each genotype possesses a characteristic seizure-susceptibility that may be quantified by its threshold voltage. Normal seizure-susceptibility, the wild type range, is about 30 to 40V HFS (Canton Special=30.1, Oregon R=39.3V HFS; Kuebler et al., 2001).
Spontaneous seizure-like activity has been recorded from the neuromuscular junction of third instar larvae and the dorsal longitudinal flight muscle of adults. This is especially true for cacophony and shibire temperature-sensitive paralytic mutants that show spontaneous seizure-like activity at high temperatures (Rieckhof et al., 2003; Salkoff and Kelly, 1978). Seizure-sensitive mutants show nervous system excitability changes that have been observed in identified larval motoneurons by patch recording (Marley and Baines, 2011). These motoneurons receive excitatory input from cholinergic interneurons providing spontaneous rhythmic synaptic currents (SRCs). Under voltage clamp mutant motoneurons show that SRCs are increased in amplitude and duration. Under current clamp, mutant motoneurons show sustained depolarization and multiple action potentials (Marley and Baines, 2011). Similar recordings are used to record INaP in wild type and mutant motoneurons (Lin et al., 2012; Lin and Baines, 2014). Seizure-sensitive mutants have been examined electrophysiologically in adult antennal lobe inhibitory interneurons by current clamp and voltage clamp whole cell patch recordings (Sun et al., 2012; Schutte et al., 2014). These have been used to show changes in INaP and evoked neuronal firing properties in mutant compared to wild type antennal lobe neurons.
Seizure-sensitivity in parabss1 mutants
Mutants in one behavioral class, called the Bang-Sensitive (BS) paralytics, are consistently seizure-sensitive (Pavlidis and Tanouye, 1995; Kuebler et al., 2001). The BS class is 109 mutant alleles representing 25 genes and a variety of gene products, including the parabss1 Na+ channel mutation (Table 1). The parabss1 mutation causes severe seizure-sensitivity. This severity is based on a very low threshold (4.4 V HFS; Figure 2) for seizure-like activity evoked electrophysiologically, much lower than the threshold for wild type flies. The parabss1 mutation also has a severe BS phenotype following a mechanical shock defined as either a tap of the culture vial or brief vortex mixing (a “bang”; Figure 2). This BS stimulation causes an initial behavioral seizure lasting several seconds, and characterized by leg-shaking, abdominal muscle contractions, wing flapping and scissoring, and proboscis extensions. This is followed by behavioral paralysis: flies are immobile and unresponsive to mechanical stimulation, as described previously (Ganetzky and Wu, 1982). During this period, although the fly is mainly quiescent, resembling a tonic phase, the quiescence is broken up by multiple bouts of clonus-like activity. This is followed by recovery seizure resembling the initial seizure and clonus-like activity. The total paralytic recovery time for parabss1 recovery time is longer than for other BS mutants mainly because of the tonic-clonic activity; mean recovery time for parabss1 is ~240s.
Table 1.
Some seizure-sensitive mutants: gene products and seizure thresholds (HF V).
| Seizure-sensitive Mutant | Seizure Threshold | Gene Product |
|---|---|---|
| bang senseless (parabss1, parabss2) | 3.2 ,3.7V | Na channel (Parker et al., 2011) |
| paraGEFS+ | unknown | Na channel (Sun et al., 2012) |
| paraDS | unknown | Na channel (Schutte et al., 2014) |
| easily shocked (eas) | 3.4V | ethanolamine kinase (Pavlidis et al., 1994)) |
| slamdance (sda) | 6.7V | aminopeptidase N (Zhang et al., 2002) |
| bang sensitive (bas1,bas2) | 7.6, 3.8V | unknown product |
| technical knockout (tko) | 9.9V | ribosomal protein S12 (Royden et al., 1987) |
| jitterbug (jbug) | 10.5V | unknown product |
| couch potato (cpo) | 11.1V | RNA-binding protein (Glasscock and Tanouye, 2005) |
| kazachoc (kcc) | 17.0V | K+ Cl− cotransporter (Hekmat-Scafe, et al., 2006) |
| knockdown (kdn) | 20.2V | citrate synthase (Fergestad et al., 2006) |
| stress-sensitive (sesB) | unknown | adenine translocase (Zhang et al., 1999) |
| rock-n-roll (rnr) | unknown | unknown product |
| prickle (pk) | unknown | planar cell polarity (Tao et al., 2011) |
| wild type (CS) | 30.1V | N/A (Kuebler et al., 2001) |
Seizure threshold is voltage of high-frequency stimulation (V HFS). For comparison the seizure threshold is 30.1 V HFS for Canton-Special wild-type flies (Kuebler et al., 2001).
Figure 2. Drosophila parabss1 mutant behavior and electrophysiology seizure-like phenotypes.
(A) Cartoon depicting stereotypic behavioral phenotype of parabss1 flies subjected to a mechanical shock (10s vortex = BANG): initial seizure-like behavior, followed by complete paralysis, then a tonic-clonic-like period that is unique to parabss1 and not evident in other BS mutant genotypes. In the figure, one clonus-like event is depicted, but the number can vary, as can the duration of the period, as described in the text. The tonic-clonic-like period is followed by a recovery seizure and the fly then recovers. Not depicted is a quiescent period of variable duration often observed between the recovery seizure and recovery, as well as the refractory period during which flies are resistant to further seizures that occurs immediately following recovery. (B) For parabss1/Y hemizygous males, recovery time from behavioral paralysis is substantially longer than for parabss1/+ heterozygous females or for another BS mutant,easPC80/Y. (C) Electrical recording from a dorsal longitudinal muscle (DLM) fiber showing seizure-like neuronal activity and synaptic failure in a parabss1/Y animal subjected to a 4V high frequency electrical stimulus (HFS), as well as single-pulse stimuli to trigger the giant fiber (GF) circuit, allowing assessment of synaptic function (giant fiber response, GFR). Following an initial seizure (IS) is a period of synaptic failure within which GF stimulation fails to evoke a DLM response (SF1-5), unlike that seen prior to the seizure. As depicted, during the period of synaptic failure, spontaneous secondary seizures are observed (SS1-4). Although in this trace four secondary seizures are observed, the number is variable. A final recovery seizure (RS) is observed, and shortly thereafter, GF system transmission is restored (response recovery, RR). Calibration bar is shown below: 2mV, 10s. (D) For easPC80/Y, HFS stimulation causes an initial seizure, synaptic failure, and recovery seizure, however no secondary seizures are observed. Calibration: 2mV, 10s. (E) At higher sweep speed, depicted are initial seizure, secondary seizures and recovery seizure from the recording in (B) Calibration: 2mV, 1.5s. (F) At higher sweep speed, depicted are normal GF response (GFR), synaptic failures (SF) and response recovery (RR) from the recording in (B) Calibration 2mV, 10ms. (Figure reprinted from Parker et al., 2011).
The mutation causing parabss1 is a mis-sense substitution for a single amino acid residue (L1699F) in HD4 (Parker et al., 2011). This is a conserved leu residue within the S3 membrane-spanning segment that may be part of the voltage sensor driving Na+ channel inactivation (Alabi et al., 2007; Bosmans et al., 2008; Catterall et al., 2008). Electrophysiology experiments showed changes in inactivation consistent with this interpretation. The L1699F mutation causes a shift in fast inactivation voltage to more positive potentials compared to wild type, In Xenopus oocytes expressing para cDNA (Parker et al., 2011). In parabss1 neurons, altered Na+ channel inactivation, appears responsible for the great overall hyperexcitability observed in the mutant. The parabss1 mutant is the most severely seizure-sensitive mutant thus far identified in Drosophila (Parker et al., 2011). It has the lowest threshold to evoked seizure-like activity recorded electrophysiologically. It displays the longest and most penetrant BS paralytic behavior. The phenotypes of parabss1 are the most difficult to ameliorate by antiepileptic drug or seizure-suppressor mutation. It has been presented as a Drosophila model for human intractable epilepsy (Parker et al., 2011).
GEFS+ spectrum mutations knocked into the para channel cause seizure-like neurological phenotypes in the fly
Mutations of the human SCN1A Na+ channel gene are responsible for GEFS+ spectrum epilepsies (Parihar and Ganesh, 2013; Catterall, 2014; Goldberg-Stern et al., 2014). Several of these have been knocked into the para gene and examined for phenotypes (Sun et al 2012; Schutte et al 2014). The mutations examined thus far give rise to temperature-dependent seizure-like activity. The SCN1A mutation (K1270T) is an amino acid substitution in transmembrane segment S2 of HD3, and is responsible for GEFS+ epilepsy (Abou-Khalil et al 2001). The paraGEFS+ mutation is a K→T knock-in at the corresponding para location (Sun et al., 2012).
Electrophysiology recordings suggest an interesting, novel mechanism for temperature-induced seizure-sensitivity in paraGEFS+ flies (Sun et al., 2014). In GABAergic inhibitory interneurons, but apparently not in cholinergic excitatory neurons, temperature increase causes a reduction in burst firing frequency and then a cessation of firing. It is proposed that this results in an overall loss of inhibition leading to seizure activity at high temperature (Sun et al., 2014). The paraGEFS+ lesion appears to result in an abnormality in INaP shifting deactivation threshold to more negative voltages at high temperatures. This leads to sustained depolarizations in the GABAergic neurons. Interestingly, the sustained depolarization does not lead to greater inhibition, rather decreased inhibition due to the loss of firing. The result is semidominant temperature-induced seizure-like activity.It was suggested that a normal temperature-dependent shift in Na+ current deactivation is exacerbated by the mutations; in humans, this may contribute to febrile seizures in GEFS+ and perhaps normal individuals (Sun et al., 2012).
The SCN1A mutation (S1231R) is an amino acid substitution in the S1 segment of HD3, and is responsible for Dravet's syndrome, an intractable epilepsy (Fujiwara et al 2003). The paraDS mutation is a S→R knock-in at the corresponding para location (Schutte et al 2014). Flies carrying the paraDS mutation show semidominant seizure-like behaviors induced at high temperature. Electrophysiology recordings from GABAergic inhibitory interneurons in the brains of adult paraDS flies indicate a reduction in transient Na+ current (INaT) that is exacerbated by increased temperature. There is also a change in INaP: deactivation of the persistent current occurs at more positive voltages. These changes in Na+ current result in lowered action potential firing frequencies in inhibitory interneurons, and this overall reduced inhibition appears to account for seizure-sensitivity (Schutte et al 2014)
Abnormal persistent Na+ current contributes to seizure-sensitivity in other Drosophila mutants
Baines and co-workers have shown there are changes in para expression that exacerbate the severity of seizure phenotypes in several BS mutants (Marley and Baines 2011; Lin et al 2012; Lin and Baines 2014). BS mutants eas (easily shocked) and sda (slamdance) show behavioral and electrophysiological seizure-like phenotypes that are nearly as severe as parabss1 (Kuebler and Tanouye, 2000). Analyses of eas and sda larval neurons show an increase in INaP and that this increase contributes importantly to neuronal hyperexcitability (Marley and Baines 2011; Lin et al 2012; Lin and Baines 2014). The abnormal INaP appears to be due to alteration of para alternative exon usage: exon L is favored over exon K. In eas and sda mutants, 100% of para transcripts express exon L, while 0% express exon K; ordinarily, in wild type larvae about 25% of transcripts contain exon K. Exons L and K are a pair of mutually exclusive alternate exons forming the HD3 voltage sensor (S4 segment) with the presence of exon L responsible for INaP.
These findings are surprising because eas and sda have not previously been implicated in Na+ channel function, regulation, or splicing. For example, the eas gene encodes ethanolamine kinase, and the mutation is a deletion causing a truncated product (Pavlidis et al., 1994). Also, the eas phenotype may be rescued by expressing the wild type product in adult flies (Kroll and Tanouye 2013). Similarly, the sda gene encodes the transmembrane ectoenzyme aminopeptidase N, catalyzing the removal of amino acids from the N-terminal of a variety of small peptide substrates (Zhang et al., 2001). A novel mechanism has been proposed for the interaction between BS mutations and exon L splicing (Lin and Baines 2014). It is suggested that defects in BS mutants cause increased synaptic excitation, favoring the inclusion of exon L, which, in turn, further increases neuronal excitability. This creates a self-reinforcing cycle leading to hyperexcitability promoting the incidence of seizure in these BS mutants (Lin and Baines 2014).
Alleles of para, and also mlenapts are seizure-suppressors for other BS mutants
Many anti-epileptic drugs such as phenytoin, carbamazepine, and lamotrigine target voltage-gated Na+ channels. The apparent mechanism of action for these drugs is to reduce neuronal excitability through Na+ channel loss-of-function (Kuo 1998; Kuo et al. 1997). This suggests that loss-of-function para mutations could affect seizure-susceptibility by a similar loss-of-function mechanism. This is the case for several para alleles, especially paraST76 and paraJS that act as seizure-suppressor mutations. Another mutation that also suppresses seizures is mlenapts (maleless no-action potential temperature-sensitive), a Na+ channel regulatory mutation that also causes para loss-of-function (Wu et al., 1978; Reenan et al., 2000).
The canonical para mutants were loss-of-function temperature-sensitive paralytic mutants. These mutants, such as paraST76, undergo immediate and reversible behavioral paralysis with a loss of neuronal action potentials when the temperature is increased from 25°→29°C (Suzuki et al 1971; Siddiqi and Benzer 1976; Wu and Ganetzky, 1980; Loughney et al., 1989; Ramaswami and Tanouye, 1989). Electrophysiology shows that the paraST76 mutant is a seizure-resistant mutant with a seizure threshold of 65.06±7.2V HFS, about 2X that of wild type (Kuebler et al., 2001). The mle gene encodes an RNA helicase-like protein (Kernan et al. 1991; Lee and Hurwitz 1993). The no-action potential temperature-sensitive allele (mlenapts) is a gain-of-function mutation that also causes temperature sensitive behavioral paralysis by a reduction in adult brain voltage-gated Na+ channels as assessed by tetrodotoxin-binding studies; Na+ channel structure is apparently unaltered by the mutation (Jackson et al. 1984; Kauvar 1982). This results in a loss of action potentials and behavioral paralysis at elevated temperature (37°C, Wu et al., 1978). Electrophysiology shows that the mlenapts mutant is also a seizure-resistant mutant with a seizure threshold of 72.2±7.3V HFS, about 3X greater than wild type (Kuebler et al., 2001).
The paraST76 and mlenapts mutations help define a novel class of genes called seizure-suppressors from double mutant combinations between with BS mutants (Kuebler et al., 2001). Genetic suppressors are modifying mutations that yield individuals phenotypically more like the wild type, i.e., the mutant phenotype is suppressed. Thus, seizure-suppressors are mutations that revert or partially revert the seizure-sensitive phenotype of BS mutants, but can be genetically separated, by recombination, from the mutation that they suppress. For example, the paraST76;sda double mutant has a seizure threshold of 38.9 ± 8.0 V HFS indicating seizure-suppression to the wild type range (Kuebler et al 2001). The mlenapts mutation also suppresses sda with a seizure thresholds of 89±10.2 V HFS, substantially higher than the wild type range (Kuebler et al 2001).
Seizure-suppression is also observed for paraJS, identified in a genetic screen specifically for seizure-suppression (Song and Tanouye, 2007). This mutation is due to a transposon insertion in the para 3’ untranslated region which reduces transcription by about 0.55, and is shown to suppress BS mutants eas and sda. For example, paraJS reverts sda BS behavior and electrophysiology nearly to wild type values (Song and Tanouye, 2007). The phenotypes of eas are also suppressed significantly. Unlike other Na+ channel suppressor mutations, paraJS as a single mutation causes no other neurological phenotypes; it is not a temperature-sensitive paralytic and it is not seizure-resistant. The only apparent phenotype for paraJS is suppression of BS mutants.
Modifying seizure-sensitivity in an intractable epilepsy model: gish suppresses seizures in parabss1 heterozygote flies
Human GEFS+ spectrum seizure disorders can express a variety of phenotypes, ranging from unaffected carriers to GEFS+ presentations to Dravet's syndrome within a single lineage (Parihar and Ganesh, 2013; Catterall, 2014; Goldberg-Stern et al., 2014). The currently favored explanation for this range is genetic background variation within different individuals of the lineage: genetic enhancers contribute to more extreme phenotypes; suppressors ameliorate phenotypes. A recent genetic screen identified enhancers and suppressors for parabss1, the Drosophila mutation that most resembles intractable epilepsy in some aspects (Howlett et al., 2013). One seizure-enhancer mutation identified is in a gene named chn (charlatan) which normally encodes an Neuron-Restrictive Silencer Factor/RE1-Silencing Transcription factor transcriptional repressor of neuronal-specific genes. Mutations of chn increase the time of paralysis for parabss1 without enhancing other aspects of seizure-sensitivity. In particular, seizure threshold in electrophysiology analysis does not appear to be reduced.
Among the epilepsies, especially troublesome are the intractable epilepsies: about 15 million epilepsy sufferers worldwide to not respond to any currently available medications (Picot et al., 2008; Laxer et al., 2014). Surprisingly, little is understood about differences that might be responsible for causing intractable epilepsy compared to more benign seizure disorders, and there are few promising treatments. The parabss1 mutants resemble intractable epilepsy in some aspects. They are the most seizure-sensitive flies that have been identified with the lowest threshold to evoked seizures, the longest paralytic recovery time marked by prominent tonic-clonic-like activity (Parker et al., 2011). The seizure-sensitivity of parabss1 has heretofore resisted all attempts to modify by suppressor mutation, or by drug (6 human antiepileptic drugs and anticonvulsants). Recently, however a parabss1 seizure-suppressor named gish (gilgamesh) has been identified (Howlett et al., 2013). Perhaps gish or a suppressor like it may lead to promising new drug discovery for intractable epilepsy. The normal gish gene encodes casein kinase and has been implicated in the wingless signaling pathway; casein kinase has not previously been implicated in epilepsy or neuronal excitability. Interestingly, seizure-suppression by gish was found to be specific for parabss1/+ heterozygotes: suppression was not observed with sda, eas, or parabss1 hemizygotes and homozygotes.
Conclusion
There is a growing appreciation for the complexities of Na+ channel contributions to human seizure disorders. These include especially the large number of Na+ channel mutations that present with epilepsy and the variable penetrance of seizure phenotypes. Many of these complexities may be approached experimentally in the fruit fly because it is a tractable system with powerful genetic and molecular genetic methodologies, behavioral and electrophysiological assays for seizure-susceptibility, and collections of neurological mutants available for testing. In this review, we describe recent and historical studies contributing to seizure disorders in the fly. Our focus has been especially on the para gene and Na+ channel interactions. There is, however a rich literature dealing with additional aspects of seizure susceptibility not covered here including neuron-glial interactions (Rusan et al., 2014) and spatial-temporal requirements for seizure expression (Kroll and Tanouye, 2013), for example.
Table 2.
List of seizure-suppressor mutants and their gene products.
| Seizure-suppressor Mutant | Gene Product |
|---|---|
| paralyzed (paraST76) | Na channel (Kuebler et al., 2001) |
| paralyzed (paraJS1) | Na channel (Song and Tanouye, 2007) |
| male lethal (mlenapts) | Na channel regulator (Kuebler et al., 2001) |
| shakingB (shakB2) | gap junction channel (Song and Tanouye, 2006) |
| shakingB (shakBJS) | gap junction channel (Song et al., 2007) |
| Shaker (ShKS133) | K channel (Kuebler et al., 2001) |
| escargot (esEP684 + 4 alleles) | Zn-finger transcription factor (Hekmat-Scafe et al., 2005) |
| meiosis-P26 (mei-P26EG16, mei-P261) | ring finger B-box coiled-coil-NHL protein (Glasscock et al., 2005) |
| topoisomerase I (top1JS + 3 alleles) | DNA topoisomerase type I (Song et al., 2007) |
| kazal-domain protein-1 (kdp1) | Kazal-type serine protease inhibitor (Hekmat-Scafe et al., 2005) |
| kazal-domain protein-2 (kdp2) | Kazal-type serine protease inhibitor (Hekmat-Scafe et al., 2005 |
| suppressor of eas7 (su(eas7)) | unknown (Glasscock et al., 2005) |
| suppressor of eas13 (su(eas13)) | unknown (Glasscock et al., 2005) |
| gilgamesh (gish04895 | casein kinase (Howlett et al., 2013) |
Acknowledgments
This study was supported by awards from the McKnight Foundation and the NIH (NS31231) to M.A.T. We thank the members of the Tanouye lab for helpful discussions throughout the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Khalil B, Ge Q, Desai R, Ryther R, Bazyk A, Bailey R, Haines JL, Sutcliffe JS, George AL., Jr. Partial and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurol. 2001;57:2265–2272. doi: 10.1212/wnl.57.12.2265. [DOI] [PubMed] [Google Scholar]
- Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–376. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk AG, Wallace RH, Buhr A, Buller AR, Afawi Z, Shimojo M, Miyata S, Chen S, Gonzalez-Alegre P, Griesbach HL, et al. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am. J. Hum. Genet. 2008;83:572–581. doi: 10.1016/j.ajhg.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmans F, Martin-Eauclair MF, Swartz KJ. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456:202–209. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu. Rev. Pharmacol. Toxicol. 2014;54:317–338. doi: 10.1146/annurev-pharmtox-011112-140232. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology, XXXIX Compendium of voltage-gated ion channels: sodium channels. Pharmacol. Rev. 2003;55:575–578. doi: 10.1124/pr.55.4.7. [DOI] [PubMed] [Google Scholar]
- Celniker SE, Dillon LAL, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, MacAlpine DM, Micklem G, Piano F, Snyder M, Stein L, White KP, Waterston RH, modENCODE Consortium Unlocking the secrets of the genome. Nature. 2009;459:927–930. doi: 10.1038/459927a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Dulac O. The pharmacologic treatment of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):72–75. doi: 10.1111/j.1528-1167.2011.03007.x. [DOI] [PubMed] [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invert. Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy OG, Fahmy MJ. Cytogenetic analysis of the action of carcinogens and tumor inhibitors in Drosophila melanogaster. Genetics. 1960;45:419–-438. doi: 10.1093/genetics/45.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Fergestad T, Bostwick B, Ganetzky B. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics. 2006;173:1357–1364. doi: 10.1534/genetics.106.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Wang X, Collins CA, Rodal AA, Yuan Q, Verstreken P, Dickman DK. New approaches for studying synaptic development, function, and plasticity using Drosophila as a model system. J. Neurosci. 2013;45:17560–17568. doi: 10.1523/JNEUROSCI.3261-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AA, Syed S, Sanyal S. Modeling the genetic basis for human sleep disorders in Drosophila. Commun. Integr. Biol. 2013;6(1):e22733. doi: 10.4161/cib.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Sugawara T, Mazaki-Miyazaki E, Takahashi Y, Fukushima K, Watanabe M, Hara K, Morikawa T, Yagi K, Yamakawa K, Inoue Y. Mutations of sodium channel alpha subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain. 2003;126:531–546. doi: 10.1093/brain/awg053. [DOI] [PubMed] [Google Scholar]
- Ganetzky B. Genetic studies of membrane excitability in Drosophila: lethal interaction between two temperature-sensitive paralytic mutations. Genetics. 1984;108:897–911. doi: 10.1093/genetics/108.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Singhania A, Tanouye MA. The mei-p26 gene encodes an RBCC-NHL protein that regulates seizure susceptibility in Drosophila. Genetics. 2005;170:1677–1689. doi: 10.1534/genetics.105.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Tanouye MA. Drosophila couch potato mutants exhibit complex neurological abnormalities including epilepsy phenotypes. Genetics. 2005;169:2137–2149. doi: 10.1534/genetics.104.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg-Stern H, Aharoni S, Afawi Z, Bennet O, Appenzeller S, Pendziwiat M, Kuhlenbaumer G, Basel-Vanagaite L, Shuper A, Korczyn AD, Helbig I. Broad phenotypic heterogeneity due to a novel SCN1A mutation in a family with genetic epilepsy with febrile seizures plus. J. Child Neurol. 2014;29:221–226. doi: 10.1177/0883073813509016. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu. Rev. Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39:508–512. doi: 10.1111/j.1528-1157.1998.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Guo M. Drosophila as a model to study mitochondrial dysfunction in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2012;2:a009944. doi: 10.1101/cshperspect.a009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Dang KN, Tanouye MA. Seizure suppression by gain-of-function escargot mutations. Genetics. 2005;169:1477–1493. doi: 10.1534/genetics.104.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Lundy MY, Ranga R, Tanouye MA. Mutations in the K+/Cl− co-transporter gene kazachoc (kcc) increase seizure susceptibility in Drosophila. J. Neurosci. 2006;26:8943–8954. doi: 10.1523/JNEUROSCI.4998-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth F. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol. Disord. Drug Targets. 2010;9:504–523. doi: 10.2174/187152710791556104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett IC, Rusan ZM, Parker L, Tanouye MA. Drosophila as a model for intractable epilepsy: gilgamesh suppresses seizures in para(bss1) heterozygote flies. Genetics G3 (Bethesda) 2013;3:1399–1407. doi: 10.1534/g3.113.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson FR, Wilson SD, Strichartz GR, Hall LM. Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster. Nature. 1984;308:189–191. doi: 10.1038/308189a0. [DOI] [PubMed] [Google Scholar]
- Kauvar LM. Reduced [3H]-tetrodotoxin binding in the napts paralytic mutant of Drosophila. Mol. Gen. Genet. 1982;187:172–173. doi: 10.1007/BF00384402. [DOI] [PubMed] [Google Scholar]
- Kernan MJ, Kuroda MI, Kreber R, Baker BS, Ganetzky B. napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell. 1991;66:949–959. doi: 10.1016/0092-8674(91)90440-a. [DOI] [PubMed] [Google Scholar]
- Kroll JR, Tanouye MA. Rescue of easily shocked mutant seizure sensitivity in Drosophila adults. J. Comp. Neurol. 2013;521:3500–3507. doi: 10.1002/cne.23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J. Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- Kuebler D, Zhang HG, Ren X, Tanouye MA. Genetic suppression of seizure susceptibility in Drosophila. J. Neurophysiol. 2001;86:1211–1225. doi: 10.1152/jn.2001.86.3.1211. [DOI] [PubMed] [Google Scholar]
- Kuebler D, Tanouye MA. The anticonvulsant sodium valproate reduces seizure-susceptibility in mutant Drosophila. Brain Res. 2002;958:36–42. doi: 10.1016/s0006-8993(02)03431-5. [DOI] [PubMed] [Google Scholar]
- Kuo CC. A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Molec. Pharmacol. 1998;54:712–721. [PubMed] [Google Scholar]
- Kuo CC, Chen RS, Lu L, Chen RC. Carbamazepine inhibition of neuronal Na+ currents: quantitative distinction from phenytoin and possible therapeutic implications. Molec. Pharmacol. 1997;51:1077–1083. doi: 10.1124/mol.51.6.1077. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, Resnick T, Benbadis SR. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70. doi: 10.1016/j.yebeh.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Lee CG, Hurwitz J. Human RNA helicase A is homologous to the maleless protein of Drosophila. J. Biol. Chem. 1993;268:16822–16830. [PubMed] [Google Scholar]
- Lee J, Wu CF. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J. Neurosci. 2002;22:11065–11079. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wu CF. Genetic modifications of seizure susceptibility and expression by altered excitability in Drosophila Na(+) and K(+) channel mutants. J. Neurophysiol. 2006;96:2465–78. doi: 10.1152/jn.00499.2006. [DOI] [PubMed] [Google Scholar]
- Lilly M, Carlson J. smellblind: a gene required for Drosophila olfaction. Genetics. 1990;124:293–302. doi: 10.1093/genetics/124.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Baines RA. Regulation of membrane excitability: a convergence on voltage-gated sodium conductance. Molec. Neurobiol. 2014 doi: 10.1007/s12035-014-8674-0. 10.1007/s12035-014-8674-0/fulltext.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Gunay C, Marley R, Prinz AA, Baines RA. Activity-dependent alternative splicing increases persistent sodium current and promotes seizure. J. Neurosci. 2012;32:7267–7277. doi: 10.1523/JNEUROSCI.6042-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Wright DE, Muraro NI, Baines RA. Alternative splicing in the voltage-gated sodium channel DmNav regulates activation, inactivation, and persistent current. J. Neurophysiol. 2009;102:1994–2006. doi: 10.1152/jn.00613.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Loughney K, Kreber R, Ganetzky B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell. 1989;58:1143–1154. doi: 10.1016/0092-8674(89)90512-6. [DOI] [PubMed] [Google Scholar]
- Lucey BP, Leahy A, Rosas R, Shaw PJ. A new model to study sleep deprivation-induced seizure. SLEEP. 2015;38:777–785. doi: 10.5665/sleep.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley R, Baines RA. Increased persistent Na+ current contributes to seizure in the slamdance bang-sensitive Drosophila mutant. J. Neurophysiol. 2011;106:18–29. doi: 10.1152/jn.00808.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd DK, Gee JR, Smith MA. Sodium current density correlates with expression of specific alternatively spliced sodium channel mRNAs in single neurons. J. Neurosci. 1995;15:4005–4012. doi: 10.1523/JNEUROSCI.15-05-04005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson RO, Liu Z, Nomura Y, Song W, Dong K. Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochem. Molec. Biol. 2008;38:604–610. doi: 10.1016/j.ibmb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar R, Ganesh S. The SCN1A gene variants and epileptic encephalopathies. J. Hum. Genet. 2013;58:573–580. doi: 10.1038/jhg.2013.77. [DOI] [PubMed] [Google Scholar]
- Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the Para sodium channel gene that leads to seizures. Genetics. 2011;187:523–534. doi: 10.1534/genetics.110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Ramaswami M, Tanouye MA. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Tanouye MA. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J. Neurosci. 1995;15:5810–5819. doi: 10.1523/JNEUROSCI.15-08-05810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M-C, Baldy-Moulinier M, Daures J-P, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: A population-based study in a Western European country. Epilepsia. 2008;49:1230–1238. doi: 10.1111/j.1528-1167.2008.01579.x. [DOI] [PubMed] [Google Scholar]
- Pittendrigh B, Reenan R, ffrench-Constant RH, Ganetzky B. Point mutations in the Drosophila sodium channel gene para associated with resistance to DDT and pyrethroid insecticides. Molec. Gen. Genet. 1997;356:602–610. doi: 10.1007/s004380050608. [DOI] [PubMed] [Google Scholar]
- Ramaswami M, Tanouye MA. Two sodium channel genes in Drosophila: implications for channel diversity. Proc. Natl. Acad. Sci. USA. 1989;86:2079–2082. doi: 10.1073/pnas.86.6.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read RD. Drosophila melanogaster as a model system for human brain cancers. Glia. 2011;59:1364–1376. doi: 10.1002/glia.21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenan RA, Hanrahan CJ, Ganetzky B. The mle(napts) RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron. 2000;25:139–49. doi: 10.1016/s0896-6273(00)80878-8. [DOI] [PubMed] [Google Scholar]
- Reiter LT, Bier E. Using Drosophila melanogaster to uncover human disease gene function and potential drug target proteins. Expert Opin. Ther. Targets. 2002;6:387–399. doi: 10.1517/14728222.6.3.387. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J. Biol. Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Rieder LE, Larschan EN. Wisdom from the fly. Trends Genet. 2014;11:479–481. doi: 10.1016/j.tig.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royden CS, Pirrotta V, Jan LY. The tko locus, site of a behavioral mutation in D. melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell. 1987;51:165–173. doi: 10.1016/0092-8674(87)90144-9. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Lewis EB. A brief history of Drosophila's contributions to genome research. Science. 2000;287:2216–2218. doi: 10.1126/science.287.5461.2216. [DOI] [PubMed] [Google Scholar]
- Rusan ZM, Kingsford OA, Tanouye MA. Modeling glial contributions to seizures and epileptogenesis: cation-chloride cotransporters in Drosophila melanogaster. PLoS ONE. 2014;9:e101117. doi: 10.1371/journal.pone.0101117. doi:10.1371/journal.pone.0101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Kelly L. Temperature-induced seizure and frequency dependent neuromuscular block in a ts mutant of Drosophila. Nature. 1978;273:156–158. doi: 10.1038/273156a0. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneker BF, Fountain NB. Epilepsy. Dis. Mon. 2003;49:426–478. doi: 10.1016/s0011-5029(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Schutte RJ, Schutte SS, Algara J, Barragan EV, Gilligan J, Staber C, Savva YA, Smith MA, Reenan R, O'Dowd DK. Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J. Neurophysiol. 2014;112:903–912. doi: 10.1152/jn.00135.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1976;73:3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Hu J, Tanouye MA. Seizure suppression by top1 mutations in Drosophila. J. Neurosci. 2007;27:2927–2937. doi: 10.1523/JNEUROSCI.3944-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Tanouye MA. Seizure suppression by shakB2, a gap junction mutation in Drosophila. J. Neurophysiol. 2006;95:627–635. doi: 10.1152/jn.01059.2004. [DOI] [PubMed] [Google Scholar]
- Song J, Tanouye MA. A role for para sodium channel gene 3' UTR in the modification of Drosophila seizure susceptibility. Devel. Neurobiol. 2007;67:1944–1956. doi: 10.1002/dneu.20519. [DOI] [PubMed] [Google Scholar]
- Stephen LJ, Forsyth M, Kelly K, Brodie MJ. Antiepileptic drug combinations—have newer agents altered clinical outcomes? Epilepsy Res. 2012;98:194–198. doi: 10.1016/j.eplepsyres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O'Dowd DK, Reenan R. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J. Neurosci. 2012;32:14145–14155. doi: 10.1523/JNEUROSCI.2932-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D, Grigliatti T, Williamson R. Temperature-sensitive mutations in Drosophila melanogaster, VII. A mutation (parats) causing reversible adult paralysis. Proc. Natl. Acad. Sci. USA. 1971;68:890–893. doi: 10.1073/pnas.68.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Manak JR, Sowers L, Mei X, Kiyonari H, Abe T, et al. Mutations in Prickle orthologs cause seizures in flies, mice, and humans. Am. J. Hum. Genet. 2011;88:138–149. doi: 10.1016/j.ajhg.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray JR, Ganetzky B. Developmentally regulated alternative splicing generates a complex array of Drosophila para sodium channel isoforms. J. Neurosci. 1994;14:2569–2578. doi: 10.1523/JNEUROSCI.14-05-02569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray JR, Ganetzky B. Conserved alternative splicing patterns and splicing signals in the Drosophila sodium channel gene para. Genetics. 1995;141:203–214. doi: 10.1093/genetics/141.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh TN, Chiron C, Dellatolas G, Rey E, Pons G, Vincent J, Dulac O. Long-term efficacy and tolerance of stiripentaol in severe myoclonic epilepsy of infancy (Dravet's syndrome). Arch. Pediatr. 2002;9:1120–1127. doi: 10.1016/s0929-693x(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan RAG, Wang P, Qian S, Arena, Cohen CJ. Functional expression of Drosophila para sodium channels: modulation by the membrane protein tipE and toxin pharmacology. J. Gen. Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B. Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature. 1980;286:814–816. doi: 10.1038/286814a0. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Jan LY, Jan YN. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc. Natl. Acad. Sci. USA. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye MA. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002;162:1283–1299. doi: 10.1093/genetics/162.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Roote J, Brogna S, Davis AW, Barbash DA, Nash D, Ashburner M. Stress sensitive B encodes an adenine nucleotide translocase in Drosophila melanogaster. Genetics. 1999;153:891–903. doi: 10.1093/genetics/153.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]