Summary
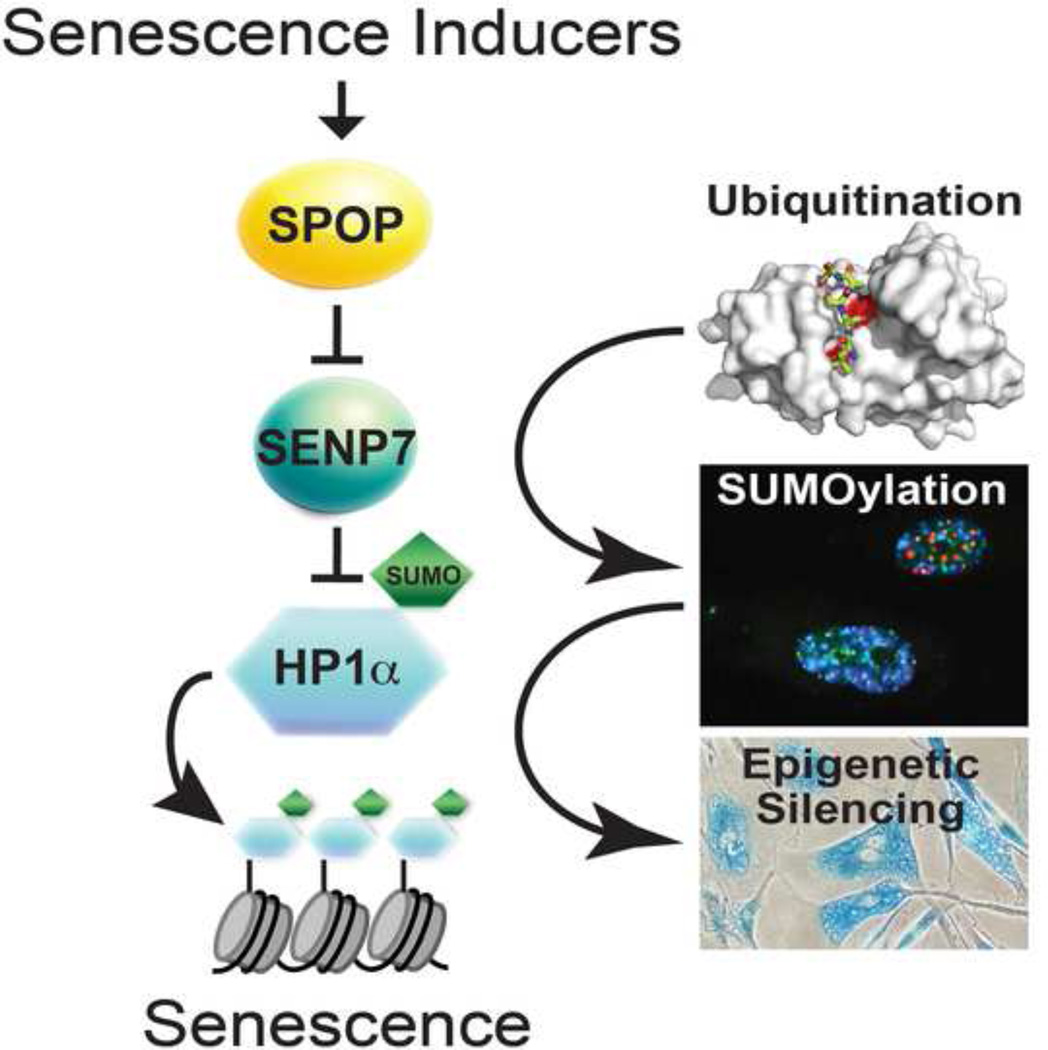
The SPOP gene, encoding an E3 ubiquitin ligase adaptor, is frequently mutated in a number of cancer types such as prostate. However, the mechanisms by which SPOP functions as a tumor suppressor remain poorly understood. Here we show that SPOP promotes senescence, an important tumor suppression mechanism, by targeting the SENP7 deSUMOylase for degradation. SPOP is upregulated during senescence. This correlates with ubiquitin-mediated degradation of SENP7, which promotes senescence by increasing HP1α sumoylation and the associated epigenetic gene silencing. Ectopic wild-type SPOP, but not its cancer-associated mutants, drives senescence. Conversely, SPOP knockdown overcomes senescence. These phenotypes correlate with ubiquitination and degradation of SENP7, and HP1α sumoylation, subcellular re-localization and its associated gene silencing. Furthermore, SENP7 is expressed at higher levels in prostate tumor specimens with SPOP mutation (n=13) compared to those with wild-type SPOP (n=80). In summary, SPOP acts as a tumor suppressor by promoting senescence through degrading SENP7.
Graphical abstract
Introduction
Speckle-type POZ protein (SPOP) is a bric-a-brac-tramtrack-broad/poxvirus and zinc finger (BTB/POZ) domain protein that functions as an adaptor for the E3 ubiquitin ligase Cullin 3. Recent genome-wide next generation sequencing studies have revealed that SPOP is frequently mutated in a number of cancer types such as prostate and endometrial (Barbieri et al., 2012; Berger et al., 2011; Le Gallo et al., 2012). These findings suggest that SPOP is a putative tumor suppressor. SPOP binds to its substrates via its N-terminal meprin and traf homology (MATH) domain (Zhuang et al., 2009), while it interacts with Cullin 3 through the BTB-domain at its C-terminus (Pintard et al., 2003; Xu et al., 2003). SPOP mutations observed in human cancers are clustered in its substrate binding MATH domain (Barbieri et al., 2012; Berger et al., 2011), suggesting that SPOP mutations may promote cancer via altering the function of its substrates. Indeed, SPOP mutations correlate with changes in the ubiquitin landscape in prostate cancer (Theurillat et al., 2014). Despite the fact that a number of SPOP substrates have been described (such as Ci/Gli, macroH2A, Daxx, SRC3, AR and DEK) (An et al., 2014; Hernandez-Munoz et al., 2005; Kwon et al., 2006; Li et al., 2014; Theurillat et al., 2014; Zhang et al., 2006), the mechanistic basis by which SPOP functions as a tumor suppressor remains poorly understood.
Cellular senescence is a state of stable cell growth arrest (Perez-Mancera et al., 2014). It is an important tumor suppression mechanism by halting the progression of cancer progenitor cells harbouring the initial oncogenic hits. Oncogenic signaling triggers senescence via mechanisms such as formation of senescence-associated heterochromatin foci (SAHF), which are specialized domains of facultative heterochromatin that contribute to senescence by helping silence proliferation-promoting genes (such as the E2F target genes) (Narita et al., 2003). Heterochromatin markers such as heterochromatin protein 1 (HP1) proteins are components of SAHF and are associated with the promoters of the proliferation-promoting genes in senescent cells (Narita et al., 2003). Activation of these signaling pathways cultivates the expression of markers of senescence such as an increased senescence-associated β-galactosidase (SA-β-gal) activity (Dimri et al., 1995).
Small ubiquitin-like modifiers (SUMO) is a dynamic post-translational protein modification that regulates the function and subcellular localization of its target proteins (Cubenas-Potts and Matunis, 2013). SUMO has been implicated in regulating senescence (Bischof et al., 2006; Li et al., 2006; Yates et al., 2008). SUMO is conjugated to its targets by SUMO-conjugating machinery, while removal of SUMO is performed by a class of enzymes called Sentrin/SUMO-specific proteases (SENP) through their isopeptidase activity (Mukhopadhyay and Dasso, 2007). Here we report that SPOP epigenetically promotes senescence by ubiquitin-mediated degradation of SENP7, which facilitates HP1α associated gene silencing via its sumoylation. Our studies indicate that SPOP acts a tumor suppressor by promoting cellular senescence.
Results
SPOP is upregulated during senescence
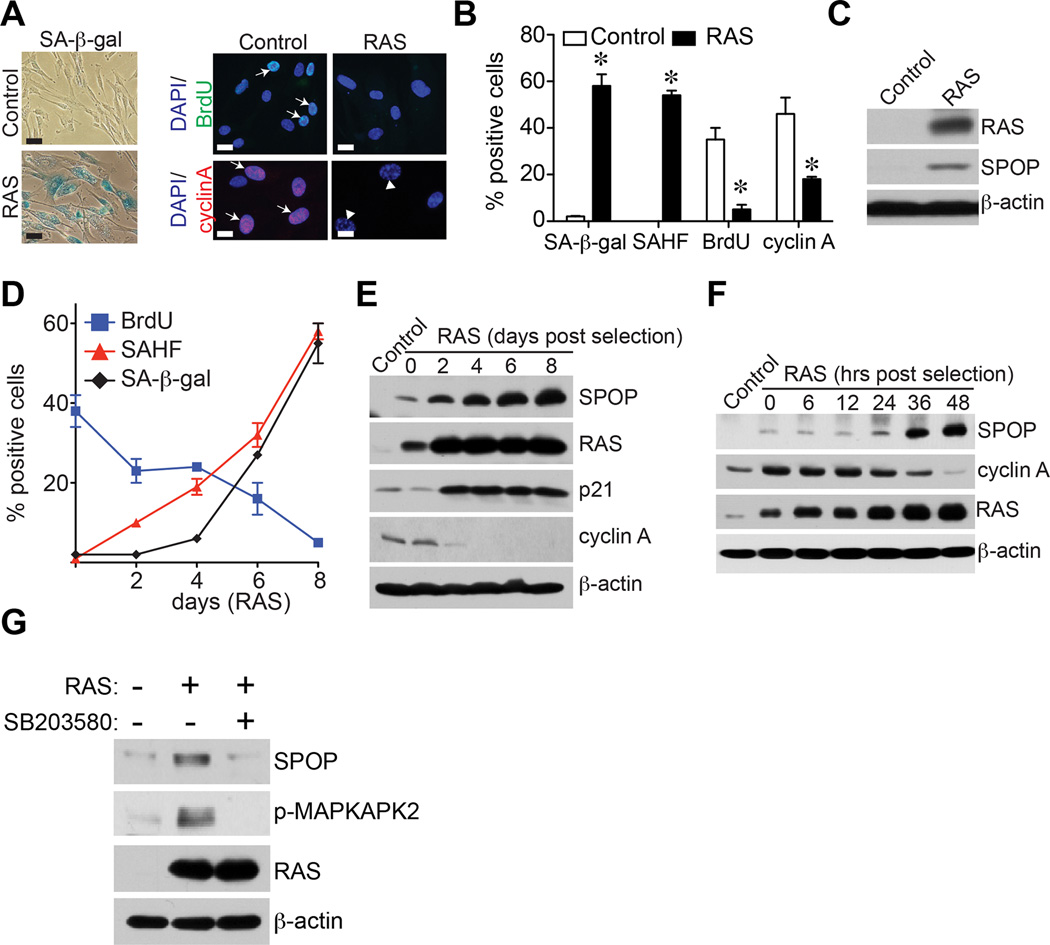
To determine whether SPOP is regulated during senescence, IMR90 primary human fibroblasts were induced to undergo senescence by oncogenic RAS, a well-established model for studying senescence in normal human cells in vitro (Figure S1A). The senescence status was confirmed by markers such as SA-β-gal activity and formation of SAHF (Figure 1A–B). Consistently, cell proliferation markers such as BrdU incorporation and cyclin A expression were decreased by RAS-infection (Figure 1A–B). Interestingly, SPOP was upregulated in senescent cells (Figure 1C). Next, we performed a detailed time-course study for SPOP upregulation and expression of markers of senescence (such as SA-β-gal activity, SAHF formation and upregulation of p21) and cell proliferation markers (including BrdU incorporation and cyclin A expression). Indeed, SPOP upregulation was accompanied by induction of markers of senescence and senescence-associated cell cycle exit (Figure 1D–F). SPOP upregulation was also observed during senescence induced by knockdown of the tumor suppressor PTEN or extended cell passaging, but not by DNA damage agent Doxorubicin (Figure S1B–J). Similar observation was also made in BJ primary human fibroblasts (Figure S1K–M). Together, we conclude that SPOP is upregulated during senescence.
Figure 1. SPOP is upregulated during senescence.
-
(A)IMR90 primary human fibroblasts were infected with retrovirus encoding control or RAS to induce senescence. Drug-selected cells were stained for SA-β-gal activity, labeled with BrdU for 30 minutes or stained for cyclin A expression at day 8. Arrows point to examples of positive cells for the indicated markers. Scale Bar = 10 µm
-
(B)Same as (A), but quantified for SA-β-gal staining, SAHF, BrdU and cyclin A positive cells in control and RAS-infected cells. A total of 200 cells were examined for each of the indicated groups. Error bars represent mean of three independent experiments with SD. * p<0.01.
-
(C)Same as (A), but examined for expression of SPOP, RAS and β-actin by immunoblotting.
-
(D)Time course for senescence markers such as SA-β-gal activity and SAHF formation and the cell proliferation marker BrdU incorporation in control and RAS-infected IMR90 cells. Mean of three independent experiments with SD.
-
(E–F)Same as (D), but examined for expression of SPOP, RAS, p21, cyclin A and β-actin by immunoblotting at the indicated time points.
-
(G)Same as (A), but the RAS-infected cells were treated with or without 5 µM p38 inhibitor SB203580 for 24 hours before harvesting cells for immunoblotting. The expression of SPOP, RAS and phospho-MAP kinase activated protein kinase 2 (p-MAPKAPK2), a direct target of p38 kinase, in the indicated cells was examined by immunoblotting. β-actin expression was used as a loading control.
We next determined the mechanism by which SPOP is upregulated during senescence. Notably, there was no increase in SPOP mRNA levels in senescent cells (Figure S1N). This suggests that SPOP upregulation occurs at the post-transcriptional level. Notably, there is evidence to suggest that p38 regulates SPOP protein stability (Bunce et al., 2008). Thus, we sought to determine whether SPOP upregulation is dependent upon p38 activity. Toward this goal, we suppressed the p38 activity using a small molecular inhibitor SB203580. Indeed, SB203580 treatment significantly decreased the levels of SPOP in treated cells (Figure 1G). Together, we conclude that SPOP is upregulated at the post-transcriptional level in a p38-dependent manner during senescence.
Ectopic expression of wild-type SPOP, but not its cancer-associated mutants, drives senescence
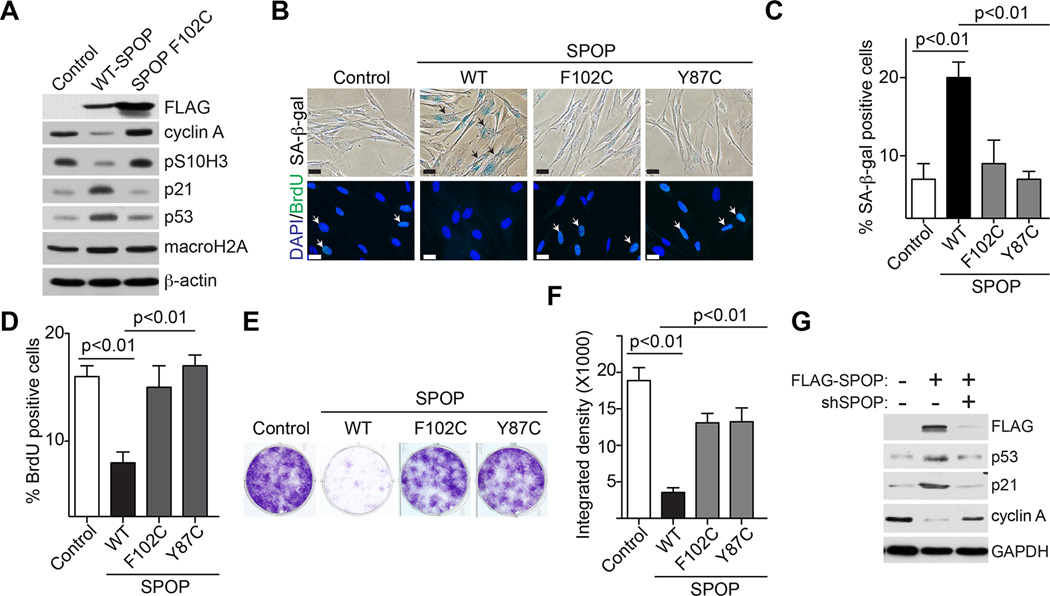
Since we observed an upregulation of SPOP during senescence (Figure 1), we determined whether ectopic expression of wild-type SPOP is sufficient to drive senescence. We ectopically expressed wild-type SPOP in IMR90 cells (Figure 2A). Indeed, ectopic SPOP expression induced expression of markers of senescence such as SA-β-gal activity and upregulation of p53 and p21, while it suppressed cell proliferation markers such as BrdU incorporation, serine 10 phosphorylated histone H3 (pS10H3) and cyclin A expression (Figure 2A–D). Notably, DNA damage markers such as γH2AX expression and foci formation were not activated during senescence induced by SPOP (Figure S2A–C). Expression of the known SPOP substrate macroH2A that is implicated in senescence (Zhang et al., 2005) was not affected by SPOP expression (Figure 2A). This suggests that senescence induced by SPOP is mediated by previously unidentified substrates. Consistently, SPOP induced an apparent cell growth inhibition as determined by focus formation (Figure 2E–F). The observed effects were specifically due to SPOP overexpression because knockdown of the ectopic SPOP suppressed the senescence phenotype in these cells (Figure 2G and data not shown). The p53 and pRB pathways play critical roles in senescence (Perez-Mancera et al., 2014). Consistently, knockdown of either p53 or pRB impaired senescence induced by SPOP (Figure S2D–G). We conclude that ectopic expression of wild-type SPOP induces senescence.
Figure 2. Ectopic expression of wild-type SPOP, but not cancer associated SPOP mutants, induces senescence and the associated cell growth arrest.
-
(A)IMR90 cells were infected with retrovirus encoding wild-type or the indicated cancer-associated mutant SPOP. Six days post drug-selection, the indicated cells were examined for expression of the indicated proteins by immunoblotting.
-
(B)Same as (A), but stained for SA-β-gal activity or examined for BrdU incorporation. Arrows point to examples of SA-β-gal and BrdU positive cells. Scale Bar = 10 µm
-
(C–D)Quantification of (B), 200 cells from each of the indicated groups were examined for expression of SA-β-gal activity (C) or BrdU positivity (D). Mean of three independents with SD.
-
(E–F)Same as (A), but 3000 of each of the indicated cells were seeded into 6-well plates for focus formation assays. Cells were fixed and stained with 0.05% crystal violet after growing for an additional 10 days (E). The integrated density was quantified using NIH ImageJ software (F).
-
(G)IMR90 cells were infected with a control retrovirus or one encoding wild-type SPOP together with an shRNA that targets the open reading frame of SPOP or controls. The cells were examined for expression of the indicated proteins by immunoblotting 6 days post drug-selection.
We next determined whether SPOP mutants observed in prostate cancers are impaired in senescence induction. We ectopically expressed two MATH domain mutated SPOPs (F102C and Y87C) that are frequently observed in prostate cancers (Barbieri et al., 2012; Berger et al., 2011; Jones et al., 2014; Le Gallo et al., 2012). Compared with wild-type SPOP, the SPOP mutants were impaired in senescence induction as determined by a significant decrease in SA-β-gal activity and p53 and p21 expression (Figure 2A–C and Figure S2H). Consistently, there was an increase in cell proliferation markers such as BrdU incorporation and expression of pS10H3 and cyclin A in cells expressing SPOP mutants compared with wild-type SPOP (Figure 2A–D). Indeed, SPOP-induced growth inhibition was also significantly impaired by the cancer-associated mutants as determined by focus formation (Figure 2E–F). We conclude that wild-type SPOP drives senescence, while its cancer-associated mutants are impaired in senescence induction.
SPOP knockdown suppresses senescence
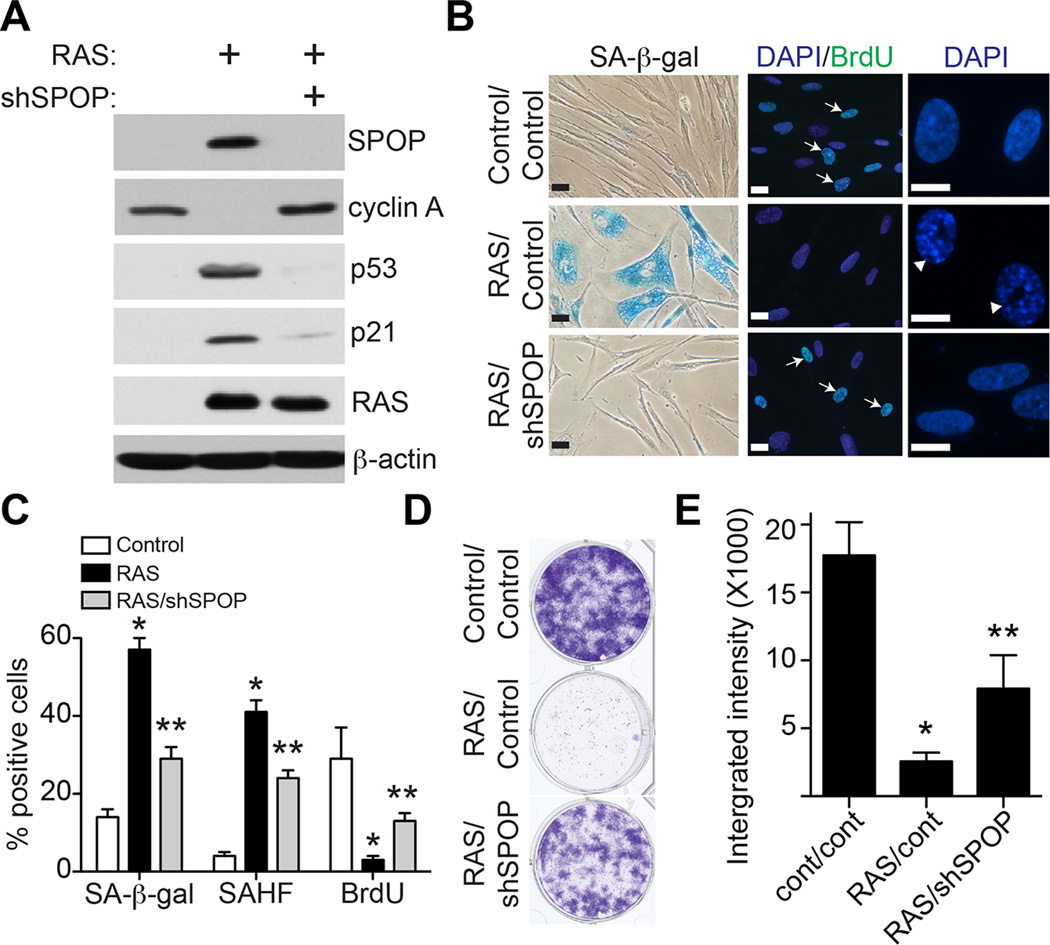
We next determined whether SPOP knockdown suppresses senescence. IMR90 cells were induced to undergo senescence with oncogenic RAS together with an shRNA to the human SPOP gene (shSPOP) or control. Indeed, SPOP knockdown suppressed the expression of senescence markers such as SA-β-gal activity, SAHF formation and upregulation of p53 and p21 (Figure 3A–C). In contrast, cell proliferation markers such as cyclin A expression and BrdU incorporation were significantly increased in shSPOP/RAS cells compared with RAS-expressing cells (Figure 3A–C). Consistently, shSPOP significantly rescued growth inhibition induced by oncogenic RAS as determined by focus formation (Figure 3D–E). We conclude that SPOP knockdown suppresses RAS-induced senescence.
Figure 3. SPOP knockdown suppresses senescence.
-
(A)IMR90 cells were infected with a retrovirus encoding oncogenic RAS together with a lentivirus encoding shSPOP or control. 8 days post drug-selection, the indicated cells were examined for expression of SPOP, cyclin A, p53, p21, RAS and β-actin by immunoblotting.
-
(B)Same as (A), but examined for SA-β-gal activity, BrdU incorporation or examined for SAHF formation by DAPI staining for the punctate pattern in cell nuclei. Arrows point to examples of cells positive for the indicated markers. Arrow heads indicate SAHF positive cells. Scale Bar = 10 µm
-
(C)Quantitation of (B). 200 cells from each of the indicated groups were examined for the expression of the indicated markers. Mean of three independent experiments with SD. * p<0.01 compared with controls and ** p<0.01 compared with RAS-infected only.
-
(D)Same as (A), 6 days post drug-selection, 3000 cells from the each of the indicated groups were seeded in 6-well plates for focus formation assays. Cells were fixed and stained with 0.05% crystal violet after growing for an additional 12 days.
-
(E)Quantification of (D). The integrated intensity of foci form by the indicated cells was quantified by using the NIH ImageJ software. Mean of three independent experiments with SD. * p<0.01 compared with controls and ** p<0.01 compared with RAS-infected only.
SENP7 is a SPOP substrate that is degraded during senescence
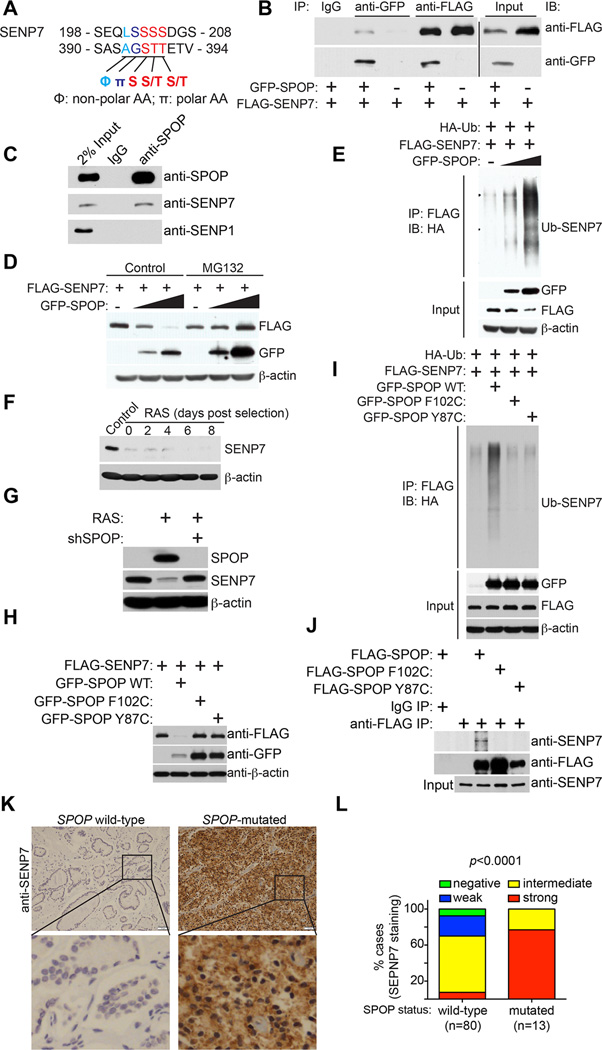
We next determined the mechanism by which SPOP regulates senescence. We performed an in silico analysis of proteins that are putative SPOP substrates based on the SPOP binding recognition motif Φ-π-S-S/T-S/T (Φ: non-polar residue and π: polar residue) (Zhuang et al., 2009). This analysis revealed that the deSUMOylase SENP7 contains two SPOP binding motifs (Figure 4A). These motifs are unique to SENP7 as the other six members of the SENPs (SENP1–3, SENP5–6 and SENP8) do not contain SPOP binding motifs (Figure S3A–B). In addition, SENP7 has two isoforms, namely SENP7L and SENP7S (Bawa-Khalfe et al., 2012). Notably, only SENP7L, but not SENP7S, contains the SPOP binding motif (Figure S3C). To validate this analysis, we performed co-immunoprecipitation (co-IP) analysis in 293T cells ectopically expressing GFP-SPOP and FLAG-SENP7. Indeed, ectopic SPOP and SENP7 co-immunoprecipitated with each other (Figure 4B). In addition, we treated senescent IMR90 cells, in which SPOP expression is induced, with the proteasome inhibitor MG132 to prevent potential degradation of SENP7 by the upregulated SPOP. Co-IP analysis revealed that endogenous SPOP co-immunoprecipitated with endogenous SENP7 (Figure 4C). As a negative control, there was no co-immunoprecipitation between SPOP and SENP1 (Figure 4C).
Figure 4. SENP7 is a SPOP binding substrate that is subjected to SPOP adaptor mediated degradation.
-
(A)SENP7 has two putative SPOP-binding consensus motifs.
-
(B)Ectopic SPOP and SENP7 interact with each other. 293T cells were transfected with the indicated GFP-SPOP and/or FLAG-SENP7 plasmids, and 16 hours post transfection, cells were treated with 20µM MG132 for 8 h and subjected to co-IP analysis using the indicated antibodies or IgG controls. The co-IP was analyzed by immunoblotting using anti-FLAG or anti-GFP antibodies.
-
(C)Endogenous SPOP interacts with SENP7 in RAS-infected IMR90 cells. IMR90 cells were infected with a retrovirus encoding oncogenic RAS to induce SPOP expression. Drug-selected cells were treated with 20 µM MG132 for 8 hours, and the cells were subjected to co-IP analysis using an anti-SPOP antibody or an isotype matched IgG control. The IP’d product was analyzed by immunoblotting using the indicated antibodies.
-
(D)SPOP degrades SENP7 expression in a proteasome-dependent manner. 293T cells were transfected with 100 ng FLAG-SENP7 together with 0, 500 or 1000 ng GFP-SPOP plasmids. 16 hours post transfection, cells were treated with control or 20 µM MG132 for 8 hours and subjected to immunoblotting analysis using the indicated antibodies.
-
(E)SPOP promotes polyubiquitination of SENP7. 293T cells were transfected with the indicated plasmids, and 16 hours post transfection, cells were treated with 20 µM MG132 for 8 hours and subjected to immunoprecipitation with an anti-FLAG antibody for SENP7. The ubiquitination level of SENP7 was analyzed by immunoblotting using an anti-HA antibody for ubiquitin.
-
(F)Time course analysis for SENP7 expression during senescence of IMR90 cells induced by oncogenic RAS. Expression of SENP7 and β-actin was examined at the indicated time points by immunoblotting.
-
(G)IMR90 cells were co-infected with a retrovirus encoding for oncogenic RAS together with a lentivirus encoding shSPOP or control. 8 days post drug-selection, cells were examined for expression of SPOP, SENP7 and β-actin by immunoblotting.
-
(H)Cancer-associated SPOP mutants lack the ability to degrade SENP7. 293T cells were transfected with FLAG-SENP7 together with GFP-tagged wild-type SPOP or the indicated SPOP mutants. 16 hours post-transfection, cells were subjected to immunoblotting analysis using the indicated antibodies.
-
(I)SPOP induced polyubiquitination of SENP7 is impaired by cancer associated SPOP mutants. 293T cells were transfected with the indicated plasmids, and 16 hours post-transfection, the indicated cells were treated with 20 µM MG132 for 8 hours and subjected to immunoprecipitation with an anti-FLAG antibody for SENP7. The ubiquitination level of SENP7 was analyzed by immunoblotting using an anti-HA antibody for ubiquitin.
-
(J)The cancer-associated SPOP mutants are impaired in SENP7 interaction. IMR90 cells were infected with a retrovirus encoding the indicated FLAG tagged wild-type or cancer-associated mutant SPOP or control. Drug-selected cells were treated with 20 µM MG132 for 8 hours, and the cells were subjected to co-IP analysis using an anti-FLAG antibody or an isotype matched IgG control. The IP’d product was analyzed by immunoblotting using the indicated antibodies.
-
(K)Examples of SENP7 immunohistochemical staining in SPOP-mutated or wild-type prostate tumor specimens. Scale bar = 50 µm.
-
(L)Quantification of SENP7 expression levels in 80 cases of SPOP wild-type and 13 cases of SPOP-mutated prostate tumor specimens.
We next determined whether SENP7 is subject to SPOP mediated degradation. We co-expressed SENP7 with a titrated amount of SPOP. Indeed, there was a SPOP dose-dependent decrease in SENP7 expression (Figure 4D), suggesting that SPOP promotes SENP7 degradation. The observed decease in SENP7 protein levels by SPOP was inhibited by the proteasome inhibitor MG132 (Figure 4D). This supports our hypothesis that SPOP promotes ubiquitin-mediated SENP7 degradation. To directly determine the effects of SPOP on SENP7 ubiquitination, we examined the ubiquitinated levels of SENP7 in cells expressing a HA-tagged ubiquitin treated with MG132 to prevent the proteasome dependent degradation of ubiquitinated SENP7. Indeed, there was a dose-dependent increase in ubiquitinated SENP7 in SPOP-expressing cells (Figure 4E).
Consistent with the hypothesis that SENP7 is a substrate for SPOP mediated degradation during senescence, we observed a decrease in SENP7 expression in senescent IMR90 cells (Figure 4F). And SPOP knockdown was sufficient to restore SENP7 expression in these cells (Figure 4G), further supporting the idea that SPOP mediates SENP7 degradation during senescence.
We next determined whether SPOP mutants observed in prostate cancer are impaired in their ability to degrade SENP7. Indeed, compared with wild-type SPOP that degrades SENP7 efficiently, cancer-associated SPOP mutants were impaired in SENP7 degradation (Figure 4H). Similar observations were also made in prostate cancer cell lines (Figure S4). Consistently, compared with wild-type SPOP, cancer-associated SPOP mutants were impaired in their ability to ubiquitinate SENP7 (Figure 4I). This correlated with an impaired interaction between the cancer-associated mutant SPOP and SENP7 (Figure 4J).
Finally, we determined the correlation of SENP7 protein with SPOP mutational status in prostate tumor specimens. We performed immunohistochemical staining of SENP7 in a series of 80 SPOP wild-type and 13 SPOP-mutated prostate tumor specimens (Figure 4K). SENP7 staining was scored as negative (0), weak (1), intermediate (2) or strong (3). Statistical analysis revealed that SENP7 is expressed at significantly (p<0.0001) higher levels in prostate tumors with SPOP mutations compared to those with wild-type SPOP (Figure 4L, and Tables S1 and S2). This further supports the notion that SENP7 is a substrate of SPOP mediated degradation.
SENP7 binds to SPOP via its first SPOP binding motif, and the ubiquitination of SENP7 by SPOP depends upon its interaction with SPOP
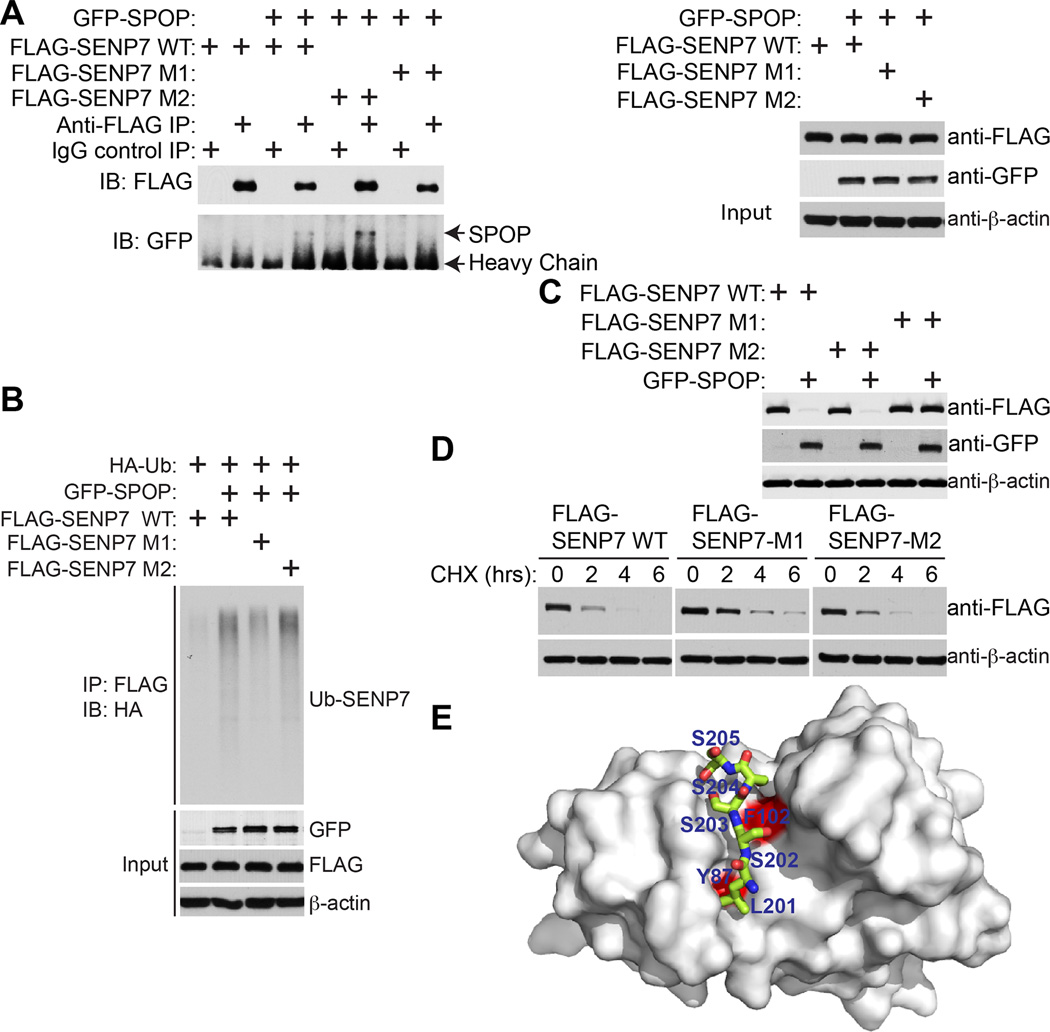
Since there are two SPOP binding motifs on SENP7 (Figure 4A), we determined whether the binding between SENP7 and SPOP requires one or both binding motifs. Accordingly, we mutated the two SPOP binding motifs in SENP7 individually and ectopically expressed the FLAG-tagged wild-type, binding site 1 mutated (M1) or binding site 2 mutated (M2) SENP7 together with a GFP-tagged SPOP. Co-IP analysis revealed that both wild-type and SENP7 M2 co-immunoprecipitated with SPOP, while the SENP7 M1 mutant was impaired in its interaction with SPOP (Figure 5A). Indeed, ubiquitin analysis showed that both wild-type and M2 mutant SENP7 were ubiquitinated by ectopic SPOP at comparable levels, while the SENP7 M1 mutant was impaired in its ubiquitination by SPOP (Figure 5B). Consistently, the expression levels of both wild-type and SENP7 M2 were reduced by SPOP, while the degradation of SENP7 M1 was impaired (Figure 5C). Finally, to directly determine the stability of SENP7 wild-type and mutants, we treated cells with the gene translation inhibitor cycloheximide (CHX) and chased the levels of wild-type or mutant SENP7 levels over 6 hours. Compared with wild-type SENP7 and SENP7 M2, which have comparable degradation rates, there was an increase in protein stability of the SENP7 M1 mutant (Figure 5D). Further, structure modeling indicated that the SPOP binding motif SENP7 peptide fits nicely into the binding pocket of the SPOP MATH domain (Figure 5E). We conclude that the first SPOP binding motif on SENP7 is required for its binding, ubiquitination and degradation by SPOP.
Figure 5. Degradation of SENP7 depends upon its interaction with SPOP via the first SPOP binding motif in SENP7.
-
(A)The first SPOP binding motif in SENP7 is required for its binding to SPOP. 293T cells were transfected with the indicated plasmids expressing GFP-SPOP, FLAG-SENP7 wild-type (WT), first SPOP binding motif mutated SENP7 (SENP7 M1) that SENP7 aa-201-205 LSSSS was mutated into LAAAS, or second SPOP binding motif deleted SENP7 (SENP7 M2) that deleted SENP7 aa 393–397 AGSTT (393–397). 16 hours post-transfection, cells were treated with 20 µM MG132 for 8 hours and subjected to co-IP analysis using the indicated antibodies.
-
(B)The first SPOP binding motif in SENP7 is required for its ubiquitination by SPOP. 293T cells were transfected with indicated plasmids, and 16 hours post-transfection, cells were treated with 20 µM MG132 and subjected to immunoprecipitation with an anti-FLAG antibody for SENP7. The ubiquitination level of SENP7 was analyzed by immunoblotting using an anti-HA antibody for ubiquitin.
-
(C)The first SPOP binding motif in SENP7 is required for its degradation by SPOP. 293T cells were transfected with the indicated plasmids. 16 hours post-transfection, cells were subjected to immunoblotting analysis using the indicated antibodies.
-
(D)Protein abundance of SENP7 wild-type and the indicated mutants determined by cycloheximide chase assay. Equal amounts of the indicated SENP7 wild-type or mutants were transfected into 293T cells, and cells were treated with 50 µg/ml cycloheximide (CHX) 16 hours post-transfection. Expression of transfected wild-type or the indicated SENP7 mutants was examined by immunoblotting at the indicated time points after CHX treatment.
-
(E)The first SPOP binding motif of SENP7 peptide (aa 201–205 LSSSS, shown in green sticks) bound to SPOP substrate binding MATH domain (shown in grey surface representation) is modeled based on two published x-ray crystal structures of substrate bound to the SPOP MATH domain (RCSB ID 3HQM and 3HQL).
Downregulation of SENP7 promotes senescence, which correlates with an increase in HP1α sumoylation and its subcellular re-localization and the associated gene silencing
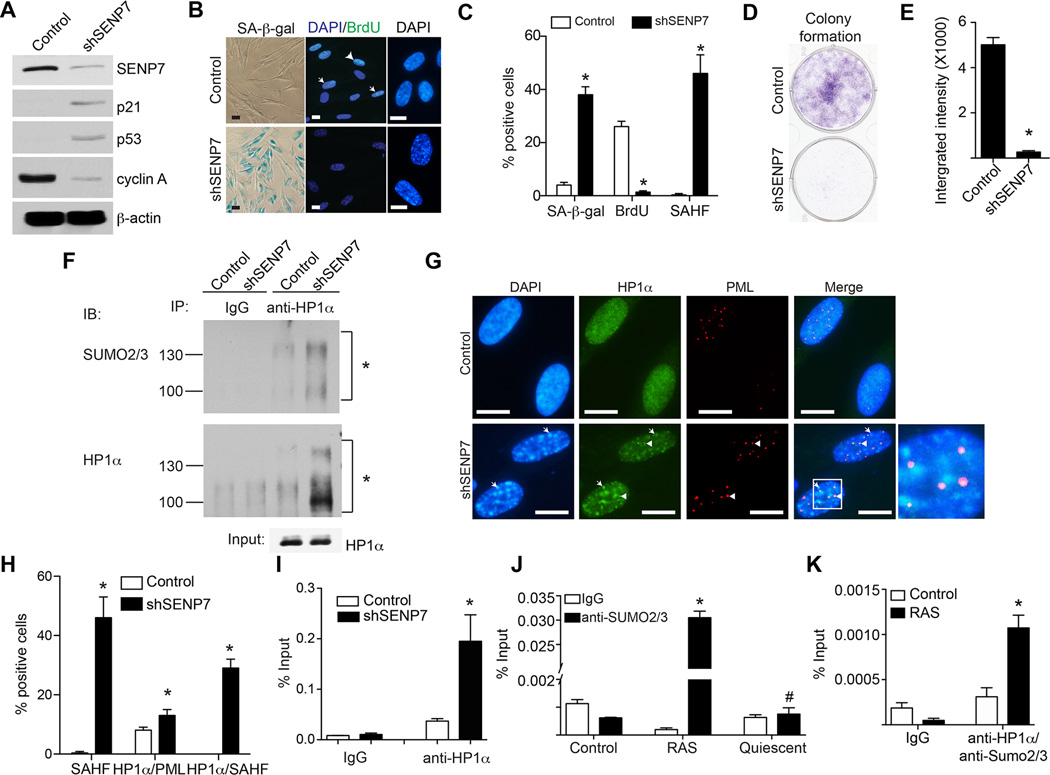
We next determined whether SENP7 downregulation drives senescence of primary IMR90 cells. We knocked down SENP7 expression using an shSENP7 in IMR90 cells. Indeed, SENP7 knockdown induced the expression of markers of senescence such as SA-β-gal activity, SAHF formation and upregulation of p21 and p53 (Figure 6A–C). Consistently, cell proliferation markers such as cyclin A expression and BrdU incorporation were suppressed by SENP7 knockdown (Figure 6A–C). Likewise, cell growth was inhibited by SENP7 knockdown as determined by focus formation (Figure 6D–E). Similar observations were also made in prostate cancer cells (Figure S5). Knockdown of either p53 or pRB impaired senescence induced by SENP7 knockdown as evidenced by a decrease in the SA-β-gal positive cells (Figure S5F–H). Further, ectopic SENP7 suppressed senescence induced by SPOP overexpression as indicated by a decrease in the SA-β-gal positive cells (Figure S5I–K). We conclude that SENP7 downregulation promotes senescence.
Figure 6. SENP7 knockdown induces senescence, which correlates with an increase in HP1α sumoylation and its associated gene silencing.
-
(A)IMR90 cells were infected with a lentivirus encoding shSENP7 or control. Day 8 after drug-selection, the expression of SENP7, p21, p53 and cyclin A was examined by immunoblotting. β-actin expression was used a loading control.
-
(B)Same as (A), but stained for SA-β-gal activity, examined for BrdU incorporation or stained with DAPI to visualize SAHF. Arrows point to examples of BrdU positive cells. Scale Bar = 10 µm
-
(C)Quantification of (B). 200 cells from each of the indicated groups were examined for SA-β-gal activity, BrdU or SAHF positive cells. Mean of three independent experiments with SD. * p<0.01.
-
(D)Same as (A), but examined for focus formation. 3000 of the indicated cells were seeded in 6-well plates, and cells were fixed and stained with 0.05% crystal violet after growing for an additional 12 days.
-
(E)Quantitation of (D). The integrated intensity of foci formed by the indicated cells was measured using the NIH ImageJ software. Mean of three independent experiments with SD. * p<0.001.
-
(F)Same as (A), but examined for HP1α sumoylation. The indicated cells were subjected to immunoprecipitation with an anti-HP1α antibody or an isotype matched IgG control using buffers with NEM, an inhibitor of SUMO protease (please see material and methods for details). The immunoprecipitated samples were subjected to immunoblotting using an anti-HP1α or anti-SUMO2/3 antibody to detect the sumoylation of HP1α. Asterisks indicated sumoylated HP1α.
-
(G)SENP7 knockdown promotes SAHF formation and HP1α’s localization into SAHF. Same as (A), but stained for HP1α, PML bodies and DAPI to visualize SAHF. Arrows point to the co-localized HP1α and SAHF. Arrowheads indicate the co-localized HP1α and PML bodies. Scale Bar = 10 µm
-
(H)Quantification of (F). 100 cells from each of the indicated groups were examined for SAHF formation, co-localization of HP1α with PML, co-localization of HP1α with SAHF. Mean of three independent experiments with SD. * p<0.05 compared with controls.
-
(I)SENP7 knockdown enhances HP1α’s association with the CCNA2 gene promoter. Same as (A), but the cells were subjected to chromatin immunoprecipitation (ChIP) analysis using an anti-HP1α antibody or an isotype matched IgG control for the CCNA2 promoter. * p<0.01 compared with controls.
-
(J)The enhanced association of Sumo2/3 with the CCNA2 gene promoter in cells undergoing senescence but not quiescence. IMR90 cells were infected with RAS for 48 hours to induce senescence or made quiescent by contacting inhibition and serum starvation (0.1% serum). The indicated cells were subjected to ChIP analysis using an anti-SUMO2/3 antibody or an isotype matched IgG control for the CCNA2 gene promoter. Mean of three independent experiments with SD. * p<0.01 and # p>0.05 compared with controls.
-
(K)Same as (J), but control or RAS-infected cells (48 hours post infection) were subjected to sequential ChIP analysis using an anti-HP1α antibody followed by an anti-SUM2/3 antibody for the CCNA2 gene promoter. Mean of three independent experiments with SD. * p<0.01 compared with controls.
We next determined the mechanism by which SENP7 downregulation promotes senescence. It has previously been demonstrated that SENP7 binds to HP1α and regulates HP1ot’s localization to pericentromeric heterochromatin via its sumoylation (Maison et al., 2012). SENP7 is a SUMO2/3 chain removal deSUMOylase (Shen et al., 2009). Since SENP7 knockdown induces SAHF formation (Figure 6B–C), we examined the effects of SENP7 knockdown on SUMO2/3 modified HP1α levels. Indeed, compared with controls, there was an increase in SUMO2/3 modified HP1α in SENP7 knockdown cells (Figure 6F). Sumoylation is known to regulate target protein subcellular localization including their PML body localization (Cubenas-Potts and Matunis, 2013). In addition, localization of HP1α into PML bodies is known to facilitate its deposition into SAHF (Zhang et al., 2005). Thus, we examined the changes in distribution of HP1α in SENP7 knockdown cells compared with controls. We observed a significant increase in the localization of HP1α into SAHF and a significant but mild increase of 1.5-fold in its localization into PML bodies (Figure 6G–H), thus, arguing that HP1α-sumoylation is only weakly participating to PML localization. This suggests that SENP7 downregulation promotes SAHF formation by increasing HP1α-sumoylation to facilitate its deposition into SAHF. Consistently, both SPOP ectopic expression and SENP7 knockdown significantly suppressed the E2F reporter activity (Figure S5L). Finally, we sought to determine whether this correlates with the recruitment of HP1α onto the promoters of proliferation-promoting genes such as the E2F target gene CCNA2 (encoding for cyclin A), whose expression is suppressed by SENP7 knockdown (Figure 6A) and is a known target of HP1α and SAHF mediated gene silencing (Narita et al., 2003). We performed chromatin immunoprecipitation (ChIP) analysis for the CCNA2 gene promoter using antibodies to HP1α or an IgG control. Indeed, there was a significant increase in the association of HP1α with the CCNA2 gene promoter in SENP7 knockdown cells compared with controls (Figure 6I). Notably, ChIP analysis revealed that there was a significant increase in the association of SUMO2/3 with the CCNA2 promoter during senescence (Figure 6J). However, there is no enrichment of SUMO2/3 in the CCNA2 promoter in quiescent cells (Figure 6J). Consistently, sequential ChIP using an anti- HP1α followed by an anti-SUMO2/3 antibody showed a significant enrichment of SUMO2/3-HP1α in the CCNA2 promoter, but not in the negative control ACTB promoter, compared with controls (Figure 6K and Figure S5M). In contrast, there is no enrichment of SUMO2/3-HP1α in the CCNA2 promoter in quiescent cells (Figure S5N). We conclude that SENP7 knockdown promotes senescence, which correlates with an increase in HP1α sumoylation, subcellular re-localization to SAHF and its-associated silencing of certain proliferation-promoting genes.
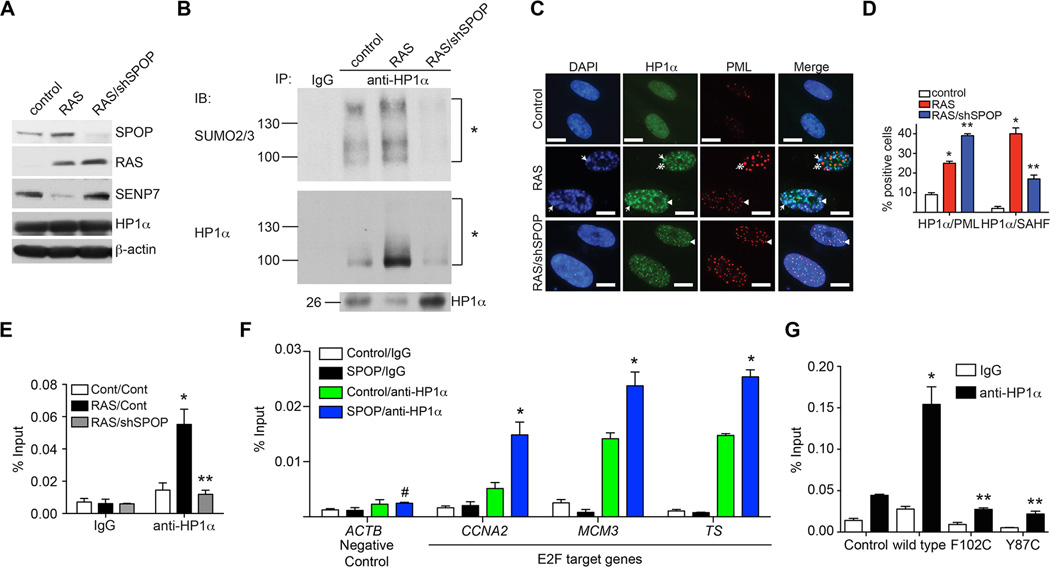
The effects of SPOP on senescence correlate with changes in HP1α sumoylation, HP1α’s SAHF localization and its associated gene silencing
SPOP knockdown suppressed senescence (Figure 3), which correlated with an increase in its substrate SENP7 expression (Figure 7A). Thus, we determined the effects of SPOP knockdown on HP1α sumoylation, subcellular localization and its association with the CCNA2 gene promoter. Compared with controls, there was an increase in sumoylated HP1α in RAS-infected cells, which correlated with its SAHF localization and its recruitment on the promoter of the CCNA2 gene (Figure 7B–E). SPOP knockdown caused a decrease in the levels of sumoylated HP1α in senescent cells (Figure 7B). This correlated with a decrease in the localization of HP1α to SAHF and its association with the CCNA2 gene promoter (Figure 7C–E). SPOP knockdown increased HP1α’s localization into PML bodies (Figure 7C–D). Since SPOP knockdown decreased HP1α sumoylation due to an increase in SENP7 (Figure 7A–B), this suggests that HP1α sumoylation is important for translocation of HP1α from PML bodies to SAHF (Zhang et al., 2005). Notably, HP1α’s association with the promoters of the E2F target genes such as CCNA2, MCM3 and Thymidylate Synthase (TS), but not with a negative control ACTB gene promoter, was enhanced by wild-type SPOP (Figure 7F). Compared with wild-type SPOP, cancer-associated SPOP mutants were impaired in recruitment of HP1α to the CCNA2 gene promoter (Figure 7G). This correlated with the ability of wild-type but not mutant SPOP in degrading SENP7 and inducing senescence (Figures 2 and 5). Together, these data support a model whereby upregulation of SPOP promotes senescence by degrading SENP7 deSUMOylase, which in turn leads to an increase in the physical presence of sumoylated HP1α at certain proliferation promoting genes concomitant with their silencing.
Figure 7. The effects of SPOP on senescence correlate with HP1α sumoylation and its associated gene silencing.
-
(A)IMR90 cells were infected with a retrovirus encoding oncogenic RAS together with a lentivirus encoding shSPOP or control. 8 days post drug-selection, the expression of SPOP, RAS, SENP7, HP1α and β-actin was examined by immunoblotting in the indicated cells.
-
(B)Same as (A), but examined for HP1α sumoylation. The indicated cells were subjected to immunoprecipitation with an anti-HP1α antibody or an isotype matched IgG control. The IP’d samples were subjected to immunoblotting using an anti-HP1α or anti-SUMO2/3 antibody to examined sumoylated HP1α levels. Asterisks indicate sumoylated HP1α.
-
(C)SPOP knockdown suppresses HP1a’s SAHF localization. Same as (A), but stained for HP1α, PML bodies and DAPI to visualize SAHF. Arrows point to examples of colocalized HP1α and SAHF. Arrowheads indicate colocalized HP1α and PML bodies. The asterisk illustrates an example of a PML body with no HP1α. Scale Bar = 10 µm
-
(D)The quantification of (C). 200 cells from each of the indicated groups were examined for SAHF formation, co-localization of HP1α with PML and co-localization of HP1α with SAHF. Mean of three independent experiments with SD. * p<0.01 controls vs. RAS-infected cells and ** p<0.01 RAS-infected vs. RAS/shSPOP-expressing cells.
-
(E)Same as (A), but examined for HP1α’s association with the CCNA2 gene promoter by ChIP analysis. The indicated cells were subjected to ChIP analysis using an anti-HP1α antibody or an isotype matched IgG control. Mean of three independent experiments with SD. * p<0.01 controls vs. RAS-infected cells and ** p<0.01 RAS-infected vs. RAS/shSPOP-expressing cells.
-
(F)IMR90 cells expressing wild-type SPOP or control were subjected to ChIP analysis using an anti-HP1α antibody or an isotype matched IgG control. The immunoprecipitated DNA was subjected to qPCR analysis using primers that amplify the indicated promoters of E2F target genes such as CCNA2, MCM3 and TS, while the ACTB gene promoter was used a negative control for analysis. Mean of three independent experiments with SD. * p<0.05.
-
(G)SPOP wild-type but not its cancer-associated mutants enhances the interaction between HP1α and the CCNA2 gene promoter. Same as (F), but for the indicated cells subjected to ChIP analysis using an anti-HP1α antibody or an isotype matched IgG control for the CCNA2 gene promoter. Mean of three independent experiments with SD. * p<0.01 compared with controls and ** p<0.01 compared with wild type SPOP expressing cells.
Discussion
Our data support the hypothesis that SPOP functions as a tumor suppressor by promoting cellular senescence. This is consistent with the findings from recent genome-wide deep sequencing studies that SPOP is mutated a number of cancer types (Barbieri et al., 2012; Berger et al., 2011; Le Gallo et al., 2012). Counterintuitively, SPOP is also overexpressed in other cancer types such as clear cell renal cell carcinoma (Liu et al., 2009), where it acts as a tumorigenic hub (Li et al., 2014). The molecular basis for the observed functional differences in different cancer types remains unclear. It is possible that overcoming SPOP-induced senescence promotes tumorigenesis in cancers where SPOP is overexpressed. In this context, SPOP’s role in cancer is akin to that of oncogenes, where oncogene activation induces senescence, while bypass of senescence induced by oncogenes contributes to cancer progression. In cancers with SPOP mutations, cells harbouring SPOP mutations are impaired in the senescence tumor suppression mechanism. This contributes to the development of SPOP mutated cancers. Thus, SPOP’s role in cancer is likely context and tumor type dependent.
Our findings demonstrate that SPOP promotes senescence, which correlates with HP1α-associated gene silencing. This is due to its ability to degrade SENP7 deSUMOylase, a SPOP substrate identified in the current study. Consequently, SENP7 downregulation leads to an increase in sumoylation levels of HP1α. This has previously been linked to pericentromeric localization of HP1α (Maison et al., 2012). In addition, SENP7 has been shown to promote chromatin relaxation for homologous recombination DNA repair (Garvin et al., 2013), which is consistent with the notion that its degradation promotes heterochromatin formation. The ability of SPOP wild-type and its cancer-associated mutants to induce senescence correlates with HP1α-associated silencing of certain proliferation-promoting genes. Together, these data support the notion that epigenetic gene silencing contributes to senescence induced by SPOP.
SENP7 knockdown induces formation of microscopically evident SAHF in addition to an increase in HP1α sumoylation and its localization to proliferation-promoting genes such as CCNA2 (Figure 6). Despite the fact that we observed an enhanced interaction between HP1α and the CCNA2 gene promoter, we did not observe formation of microscopically evident SAHF in SPOP overexpressing cells (data not shown). This is likely due to the fact that SENP7 acts downstream of SPOP, thus driving a more pronounced phenotype as reflected by visible SAHF formation. Regardless, both SPOP overexpression and SENP7 knockdown decreases cyclin A expression, which correlates with HP1α’s recruitment to the CCNA2 gene promoter. Consistently, it has recently been demonstrated that SENP7 loss induces senescence of breast cancer cells, where SENP7 was first linked to HP1α sumoylation-mediated silencing of E2F target genes (Bawa-Khalfe et al., 2012). These findings support the hypothesis that SPOP and SENP7 function in the same pathway at the molecular level.
SPOP is mutated in a number of cancer types and, most notably, in prostate cancer (up to 13%) (Barbieri et al., 2012; Berger et al., 2011). Primary prostate tumors with SPOP mutations typically lack other genetic changes such as PTEN and PIK3CA alterations, ETS-fusions or TP53 mutations (Barbieri et al., 2012). Thus, SPOP mutations represent a distinct subtype of prostate cancer. Our findings show that SENP7 knockdown drives senescence of prostate cancer cell lines (Supplementary Fig. 5). Silencing of proliferation-promoting E2F target genes induces senescence regardless of p53 and pRB status (Maehara et al., 2005; Narita et al., 2003). Our data indicate that SENP7 knockdown is sufficient to directly silence E2F target genes (such as CCNA2), which correlates with gene promoter recruitment of HP1α (Figure 6). There are no established cancer cell lines that carry mutations in SPOP (based on cBioportal analysis), which prevented us from testing the effects of SENP7 knockdown in SPOP-mutated cells. Regardless, our results suggest that SENP7 inhibition may be an intervention strategy for SPOP mutated cancers. In addition, since SENP7 knockdown induces senescence of SPOP wild-type prostate cancer cell lines (Figure S5), this suggests that SENP7 inhibition may synergize with other targeted therapies in prostate cancer. Finally, these findings also suggest that patients harbouring SPOP mutation might be less sensitive to treatments that are known to induce senescence.
In summary, we showed that SPOP promotes cellular senescence, an important tumor suppression mechanism, by degrading SENP7 deSUMOylase, a SPOP binding substrate. This correlates with HP1α-associated epigenetic gene silencing during senescence through a relay of ubiquitination and sumoylation post-transcriptional modifications. These findings establish that SPOP functions as a tumor suppressor in the context of cellular senescence and the associated cell growth arrest.
Experimental Procedures
Chromatin immunoprecipitation (ChIP) and ubiquitinated protein analysis
Chromatin immunoprecipitation was performed as previously described (Tu et al., 2013) using rabbit anti-HP1α antibody (Novus), anti-SUMO2/3 antibody (Abcam) or an isotype-matched IgG control. Immunoprecipitated DNA was analyzed using SYBR Green quantitative PCR (SABiosciences) against the indicated gene promoters using primers as detailed in Supplmental information.
For ubiquitinated protein analysis, cells were treated with 20 µM MG132, a protease inhibitor, for 8 hours to prevent degradation of the ubiquitinated proteins prior to harvest. For co-immunoprecipitation (co-IP), cells were harvested with cold buffer A [10mM HEPES-KOH (pH 8.0), 10mM KCl, 1.5mM MgCl2, 0.34M sucrose, 10% Glycerol, 0.5% TritonX-100, pH7.5] supplemented with Complete Protease inhibitor cocktail (Roche) and 1mM PMSF for 5 min on ice and centrifuged at 3000rpm at 4 °C for 5 min to collect the nucleus pellets. The pellets were then resuspended in lysis buffer (50mM Tris-HCl pH8.0, 1mM EDTA, 0.5% NP-40, 300mM NaCl, 10 mM NaF, protease inhibitor cocktail, 1 mM PMSF) and rotated at 4 °C for 20 min. The lysate was then centrifuged at 12,000g at 4 °C for 10 min, and the supernatant was used for co-IP. The supernatant was incubated with anti-SPOP, anti-GFP, anti-FLAG antibody or an IgG control for 3 hours at 4 °C and subsequently with Protein G Dynabeads (Life Technologies) for 1 hour. The beads were washed with NETN buffer 3 times, boiled in Laemmli sample buffer and subjected to immunoblotting.
Structure modeling
A model of the SPOP MATH domain bound to the first SPOP binding motif of SENP7 peptide (aa 201–205, LSSSS) was generated using the x-ray crystal structure of SPOP-substrate complexes RCSB ID 3HQM and 3HQL. The two crystal structures were overlaid in Coot (Emsley et al., 2010) and the peptide residues were mutated to the corresponding LSSSS peptide using sequence alignment as a guide. The figure was prepared in pymol (The PyMOL Molecular Graphics System, Version 1.5.0.5 Schrödinger, LLC.).
Prostate tumor specimens and immunohistochemistry (IHC)
Prostate tumor specimens were obtained from Shanghai Changhai Hospital. Use of these specimens was approved by the Institute Review Board of Shanghai Changhai Hospital. SPOP mutation status in prostate tumors was determined as previously described (Blattner et al., 2014) based on an initial pre-PCR amplification step to enrich the SPOP exons 6 and 7 followed by a high-resolution melting screen and Sanger sequencing. Paraformaldehyde fixed paraffin embedded tumor samples were deparaffinized, rehydrated, and subjected to heat-mediated antigen retrieval. For IHC analysis, we used UltraSensitive™ S-P(Rabbit) IHC Kit (KIT-9706, Fuzhou Maixin Biotech) following the manufacturer’s instructions with minor modification. Briefly, the sections were incubated with 3% H2O2 for 15 mins at room temperature to quench endogenous peroxidase activity. After incubating in normal goat serum for 1 hour, sections were treated with primary antibody at 4°C overnight. IHC analysis of tumor samples was performed using primary antibodies against SENP7 (dilution 1:200; Novus Biologicals; catalog number: NB100-92106). The sections were then washed 3 times in PBS and treated for 30 mins with biotinylated goat-anti-rabbit IgG secondary antibodies (Fuzhou Maixin Biotech). After washing three times in PBS, sections were incubated with streptavidin-conjugated HRP (Fuzhou Maixin Biotech). After washing three times in PBS for 5 mins each, specific detection were developed with 3′3-diaminobenzidine (DAB-2031, Fuzhou Maixin Biotech). Images were taken by using an Olympus camera and matched software.
Statistical analysis
Graphpad Prism Version 5.0 was used to perform for statistical analyses. The student’s t test was used to determine p values of raw data. p value< 0.05 was considered as significant.
Supplementary Material
Acknowledgements
We thank Drs. Dario Altieri and Maureen Murphy for critical comments. This work was supported by NIH/NCI grants (R01CA160331 and R01CA163377 to R.Z.) and a DOD award (OC140632P1 to R.Z.). B.G.B is supported by a NIH/NCI grant (K99CA194318). K.M.A is supported by NIH/NCI grants (K99CA194309 and T32CA9171-35). J.Y. is supported by National Natural Science Foundation (81172009). Support of Core Facilities used in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
H.Z. and R.Z. designed experiments. H.Z., B.G.B, K.M.A. and Z.T. conducted experiments and analyzed the data. S.R., Y.Z. and Y.S. conducted experiments and analyzed the data related to Figure 5F–G and Table S1. E.S. contributed to Figure 5E. Y.J. contributed key reagents. H.Z. and R.Z. wrote the manuscript.
References
- Aird KM, Zhang G, Li H, Tu Z, Bitler BG, Garipov A, Wu H, Wei Z, Wagner SN, Herlyn M, et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell reports. 2013;3:1252–1265. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Wang C, Deng Y, Yu L, Huang H. Destruction of Full-Length Androgen Receptor by Wild-Type SPOP, but Not Prostate-Cancer-Associated Mutants. Cell reports. 2014;6:657–669. doi: 10.1016/j.celrep.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa-Khalfe T, Lu LS, Zuo Y, Huang C, Dere R, Lin FM, Yeh ET. Differential expression of SUMO-specific protease 7 variants regulates epithelial-mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17466–17471. doi: 10.1073/pnas.1209378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof O, Schwamborn K, Martin N, Werner A, Sustmann C, Grosschedl R, Dejean A. The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Molecular cell. 2006;22:783–794. doi: 10.1016/j.molcel.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Blattner M, Lee DJ, O’Reilly C, Park K, Macdonald TY, Khani F, Turner KR, Chiu YL, Wild PJ, Dolgalev I, et al. SPOP Mutations in Prostate Cancer across Demographically Diverse Patient Cohorts. Neoplasia. 2014;16:14–20. doi: 10.1593/neo.131704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce MW, Boronenkov IV, Anderson RA. Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. The Journal of biological chemistry. 2008;283:8678–8686. doi: 10.1074/jbc.M710222200. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cubenas-Potts C, Matunis MJ. SUMO: a multifaceted modifier of chromatin structure and function. Developmental cell. 2013;24:1–12. doi: 10.1016/j.devcel.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta crystallographica Section D, Biological crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin AJ, Densham RM, Blair-Reid SA, Pratt KM, Stone HR, Weekes D, Lawrence KJ, Morris JR. The deSUMOylase SENP7 promotes chromatin relaxation for homologous recombination DNA repair. EMBO reports. 2013;14:975–983. doi: 10.1038/embor.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Munoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, Nusinow DA, Panning B, Marahrens Y, van Lohuizen M. Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7635–7640. doi: 10.1073/pnas.0408918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Stransky N, McCord CL, Cerami E, Lagowski J, Kelly D, Angiuoli SV, Sausen M, Kann L, Shukla M, et al. Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nature communications. 2014;5:5006. doi: 10.1038/ncomms6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol JH, Baek SH, Chiba T, Tanaka K, Bang OS, et al. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. The Journal of biological chemistry. 2006;281:12664–12672. doi: 10.1074/jbc.M600204200. [DOI] [PubMed] [Google Scholar]
- Le Gallo M, O’Hara AJ, Rudd ML, Urick ME, Hansen NF, O’Neil NJ, Price JC, Zhang S, England BM, Godwin AK, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nature genetics. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ci W, Karmakar S, Chen K, Dhar R, Fan Z, Guo Z, Zhang J, Ke Y, Wang L, et al. SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer. Cancer cell. 2014;25:455–468. doi: 10.1016/j.ccr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Santockyte R, Shen RF, Tekle E, Wang G, Yang DC, Chock PB. Expression of SUMO-2/3 induced senescence through p53- and pRB-mediated pathways. The Journal of biological chemistry. 2006;281:36221–36227. doi: 10.1074/jbc.M608236200. [DOI] [PubMed] [Google Scholar]
- Liu J, Ghanim M, Xue L, Brown CD, Iossifov I, Angeletti C, Hua S, Negre N, Ludwig M, Stricker T, et al. Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science. 2009;323:1218–1222. doi: 10.1126/science.1157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara K, Yamakoshi K, Ohtani N, Kubo Y, Takahashi A, Arase S, Jones N, Hara E. Reduction of total E2F/DP activity induces senescence-like cell cycle arrest in cancer cells lacking functional pRB and p53. The Journal of cell biology. 2005;168:553–560. doi: 10.1083/jcb.200411093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C, Romeo K, Bailly D, Dubarry M, Quivy JP, Almouzni G. The SUMO protease SENP7 is a critical component to ensure HP1 enrichment at pericentric heterochromatin. Nature structural & molecular biology. 2012;19:458–460. doi: 10.1038/nsmb.2244. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends in biochemical sciences. 2007;32:286–295. doi: 10.1016/j.tibs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nature reviews Cancer. 2014;14:547–558. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- Pintard L, Willis JH, Willems A, Johnson JL, Srayko M, Kurz T, Glaser S, Mains PE, Tyers M, Bowerman B, et al. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature. 2003;425:311–316. doi: 10.1038/nature01959. [DOI] [PubMed] [Google Scholar]
- Shen LN, Geoffroy MC, Jaffray EG, Hay RT. Characterization of SENP7, a SUMO-2/3-specific isopeptidase. The Biochemical journal. 2009;421:223–230. doi: 10.1042/BJ20090246. [DOI] [PubMed] [Google Scholar]
- Theurillat JP, Udeshi ND, Errington WJ, Svinkina T, Baca SC, Pop M, Wild PJ, Blattner M, Groner AC, Rubin MA, et al. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science. 2014;346:85–89. doi: 10.1126/science.1250255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, Yen TJ, Zhang R. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Developmental cell. 2011;21:1077–1091. doi: 10.1016/j.devcel.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Zhuang X, Yao YG, Zhang R. BRG1 is required for formation of senescence-associated heterochromatin foci induced by oncogenic RAS or BRCA1 loss. Molecular and cellular biology. 2013;33:1819–1829. doi: 10.1128/MCB.01744-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D, Rafalska-Metcalf IU, Ivanov AV, Corsinotti A, Peng H, Lee SC, Trono D, Janicki SM, Rauscher FJ., 3rd The ATM substrate KAP1 controls DNA repair in heterochromatin: regulation by HP1 proteins and serine 473/824 phosphorylation. Molecular cancer research : MCR. 2012;10:401–414. doi: 10.1158/1541-7786.MCR-11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wei Y, Reboul J, Vaglio P, Shin TH, Vidal M, Elledge SJ, Harper JW. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425:316–321. doi: 10.1038/nature01985. [DOI] [PubMed] [Google Scholar]
- Yates KE, Korbel GA, Shtutman M, Roninson IB, DiMaio D. Repression of the SUMO-specific protease Senp1 induces p53-dependent premature senescence in normal human fibroblasts. Aging cell. 2008;7:609–621. doi: 10.1111/j.1474-9726.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, Jiang J. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Developmental cell. 2006;10:719–729. doi: 10.1016/j.devcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, et al. Formation of MacroH2A–containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Developmental cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Zhuang M, Calabrese MF, Liu J, Waddell MB, Nourse A, Hammel M, Miller DJ, Walden H, Duda DM, Seyedin SN, et al. Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Molecular cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.