Abstract
Background
Multiple epidemiological studies from Europe and Asia have demonstrated increased cardiovascular risks associated with isolated elevation of home blood pressure (BP) or masked hypertension (MH). Previous studies have not addressed cardiovascular outcomes associated with MH and white coat hypertension (WCH) in the general population in the United States.
Objectives
The goal of this study was to determine hypertensive target organ damage and adverse cardiovascular outcomes associated with WCH (high clinic BP ≥140/90 mm Hg, normal home BP of <135/85 mm Hg), MH (high home BP ≥135/85 mm Hg, normal clinic BP <140/90 mm Hg), and sustained hypertension (SH, high home and clinic BP) in the Dallas Heart Study, a large, multiethnic probability-based population cohort.
Methods
We evaluated associations between WCH, MH, SH and aortic pulse wave velocity (APWV) by magnetic resonance imaging; urinary albumin to creatinine ratio (UACR); and cystatin C at study baseline. Then, associations between WCH and MH with incident cardiovascular outcomes (coronary heart disease, stroke, atrial fibrillation, heart failure, and cardiovascular death) over a median follow-up period of 9 years were assessed.
Results
The study cohort comprised 3,027 subjects (50% African Americans). The sample-weighted prevalence of WCH and MH were 3.3% and 17.8%, respectively. Both WCH and MH were independently associated with increased APWV, cystatin C, and UACR. Both WCH and MH were independently associated with higher cardiovascular events compared with the NT group, even after adjustment for traditional cardiovascular risk factors (adjusted HR: 2.09; 95% CI: 1.05 to 4.15 and adjusted HR: 2.03; 95% CI: 1.36 to 3.03, respectively).
Conclusions
In a multiethnic U.S. population, both WCH and MH were independently associated with increased aortic stiffness, renal injury, and incident cardiovascular events. Because MH is common and associated with an adverse cardiovascular profile, home BP monitoring should be routinely performed among U.S. adults.
Keywords: African Americans, Arterial Stiffness, Blood Pressure, Multiethnic, Outcome Assessment (Health Care)
Introduction
Home blood pressure (BP) monitoring has been endorsed by many hypertension guidelines as part of standard care to guide hypertension treatment, as it has been widely recognized that clinic BP may not accurately reflect out-of-office BP (1–3). The pattern of discordance between home versus clinic BP can be divided into 2 major categories; white coat hypertension (WCH; elevated office BP with normal ambulatory or home BP), or masked hypertension (MH; elevated ambulatory or home BP with normal office BP) (4). The cardiovascular prognosis of WCH is controversial. Although some studies have shown increased target organ damage and cardiovascular complications in WCH (5–7), others have demonstrated similar left ventricular mass (8) and prognosis when WCH was compared with a normotensive population (9). MH was shown to be associated with an increased risk of cardiovascular events in multiple populations in Europe and Asia (5,7,10–13). However, these studies included few individuals of African descent, the race/ethnic group with the greatest burdens of hypertension and hypertensive target organ damage. Furthermore, cardiovascular risks associated with WCH and MH differed, depending on the presence or absence of antihypertensive treatment (5,6). Among the treated population, MH is proposed to represent inadequately-treated hypertension, whereas patients with WCH are at risk of overtreatment due to persistently elevated office BP (14). However, the prognostic significance of treated and untreated MH and WCH has not been evaluated in the general population in the United States.
Accordingly, we determined the extent of target organ complications and cardiovascular prognosis associated with MH and WCH in participants of the Dallas Heart Study, a multiethnic probability-based population sample of Dallas County adults. The presence or absence of WCH and MH was determined by home and clinic BP measurements obtained in the same individuals using the same instruments and protocols. We also determined cardiovascular outcomes on the basis of the presence or absence of antihypertensive treatment prescribed to each participant.
Methods
Study Population
The Dallas Heart Study (DHS) is a multiethnic probability-based population sample of Dallas County, Texas residents ages 18 to 65 years, established in 2000, as previously described (15). This study was designed to oversample African Americans, with a resultant cohort that includes 54% African Americans and 49% women. The median age of our participants is 43 years. All participants in the DHS provided written informed consent, and the UT Southwestern Medical Center Institutional Review Board approved the study. The first DHS data collection (DHS-1) included an in-home visit (n = 6,101) to collect medical history, BP, and anthropometric measurements between years 2000 and 2002. During the in-home visit, 5 BP measurements were taken in the seated position using an automatic oscillometric device (Series #52,000, Welch Allyn, Inc., Arden, North Carolina). The surveyors verified treatment with antihypertensive medications and the type of antihypertensive treatment by drug, dose, and frequency. Of the initial 6,101 participants who were 30 to 65 years of age, 3,557 agreed to a second in-home visit with fasting phlebotomy and first-morning void urine samples collected, with serum cystatin C and urinary albumin to creatinine ratio (UACR) measured for the present study. Of these 3,557 participants, 3,027 completed a third study visit at UT Southwestern Medical Center, where clinic BP measurement was conducted in the same fashion as during the in-home visit, using the same oscillometric device. During the clinic visit, magnetic resonance imaging (MRI) was performed to assess aortic pulse wave velocity (APWV). All participants who completed 3 visits were invited to return for a follow-up study (Dallas Heart Study-2) between 2007 and 2009. Among these participants, 1,623 who were not on antihypertensive treatment at visit 1 returned for repeated BP measurement in DHS-2. During the DHS-2 visit, BP measurement was repeated in the clinic, using the same BP instruments and protocol (16).
Variable Definitions
Race/ethnicity was self-reported. Detailed methods for MRI in the DHS have been reported (17). The average of the third to fifth BP measured at home was used as the home BP and the average of the third to fifth BP measured at our medical center was used as the clinic BP. WCH was defined as normal home BP (<135/85 mm Hg) and elevated clinic BP (≥140/90 mm Hg). MH was defined as elevated home BP (≥135/85 mm Hg) and normal clinic BP (<140/90 mm Hg); sustained hypertension (SH) as elevation in both home BP (≥ 135/85 mm Hg) and clinic BP (≥140/90 mm Hg); and normotension (NT) as normal home and clinic BP. Presence of CKD was defined as estimated GFR <60 ml/min/1.73 m2 or elevated urinary albumin to creatinine ratio of at least 17 mg/g creatinine in women or 25 mg/g creatinine in men, as previously described (18). Each antihypertensive drug reported by DHS participants was categorized into the one of the following classes: angiotensin-converting enzyme (ACE) inhibitors; angiotensin II receptor blockers; beta-blockers; diuretics; aldosterone antagonists; calcium-channel blockers; alpha antagonists; central-acting antagonists; nitrates; and others. The number of classes of antihypertensive drugs is used as the number of drugs being taken by each participant.
Outcome Measures
Urine albumin and creatinine were measured in the first morning void urine sample, and the UACR was calculated in mg/g for each participant (18). Measurements of cystatin C were completed with a BNII nephelometer (Dade Behring, Inc., Deerfield, Illinois; now Siemens Healthcare Diagnostics, Inc.) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring, Inc.) (19). Aortic arch pulse wave velocity (APWV) was assessed using a breath-hold, velocity-encoded, phase-contrast gradient echo sequence acquired perpendicular to the course of the ascending aorta, using a 1.5 Tesla whole-body system (Intera, Philips Medical Systems, Best, Netherlands), as previously described (20). Mortality data were queried from the National Death Index (NDI) through December 2010. Cardiovascular death was defined by codes I00 to I99 of the International Statistical Classification of Diseases, 10th Revision. Two overlapping approaches were used to capture nonfatal cardiovascular (CV) events occurring after enrollment, as previously described (21). First, a detailed health survey regarding interval cardiovascular events was administered annually to study participants. Secondly, quarterly tracking was performed for hospital admissions using the Dallas-Fort Worth Hospital Council Data Initiative Database, a consortium of all acute-care hospitals in Dallas County, Texas. Primary clinical source documents were reviewed for all suspected nonfatal cardiovascular events and were independently adjudicated by an endpoint committee blinded to all study data. Adjudicated CV events included: unstable angina; myocardial infarction; coronary artery bypass grafting; percutaneous coronary intervention; stroke; transient ischemic attack; cerebrovascular revascularization; hospitalization for atrial fibrillation or heart failure; and cardiovascular death. Follow-up data for both fatal and nonfatal events were complete through December 31, 2010.
Statistical Analysis
Continuous variables are reported as median and interquartile range or mean with standard deviation, as appropriate, and categorical variables are presented as proportions. To account for sampling strategy and nonparticipation, sample weighting was used to determine the prevalence of WCH and MH in Dallas County and in subsets of the population, as previously described (22). For all other analyses evaluating associations within the DHS cohort, no sample weighting was used. Demographic and clinical variables, as well as cardiovascular risk factors, were compared using Spearman correlations, with adjustment for race and sex. All variables retained in the final model had a p value <0.05. The Kruskal-Wallis test was used to compare differences in APWV, UACR, and cystatin C between the WCH, MH, SH, and NT groups. The Wilcoxon rank-sum test was used for pairwise comparisons. Adjustment for multiple testing between WCH, MH, and SH versus control groups was not performed. Patients with missing data were excluded from the analysis.
Associations of WCH, MH, and SH with clinical endpoints were assessed by multivariable Cox proportional hazards regression. Subjects with a history of cardiovascular disease at baseline were excluded from the analysis (n = 227). Subjects without a clinical endpoint that were lost to follow-up for nonfatal events were censored at the time of last contact (n = 64, median follow up 5.0 years). Two multivariable models were assembled, with all variables retained in the final multivariable model with a p value <0.05, and included the following covariables: 1) age, race, sex, diabetes, smoking, body mass index (BMI), and total cholesterol level; 2) model 1 plus history of hypertension alone, Model 3: model 1 plus treatment for hypertension alone; Model 4: model 1 plus history of hypertension and treatment for hypertension. All p values are 2-sided and p < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina) and Prism version 6 (GraphPad, La Jolla, California).
Results
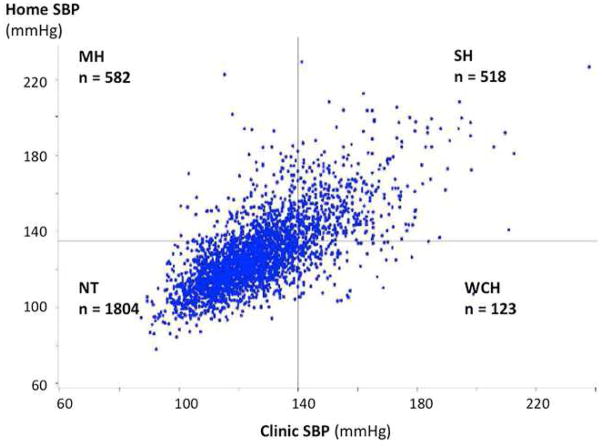
Baseline characteristics of all participants are shown in Table 1, stratified by hypertension category. Baseline characteristics of participants stratified by the presence or absence of antihypertensive medication treatment are shown in Online Table S1. The relationship between home and clinic systolic BP is shown in Figure 1. The sample weight-adjusted prevalence of WCH, MH, and SH were 3.3%, 17.8%, and 12%, respectively. In untreated participants, the prevalence was 2.2%, 13.6%, and 7.8%, respectively. Participants with WCH, MH, and SH were more likely to be black, older, obese, on antihypertensive medications, and to have prevalent hypertension, diabetes mellitus, hyperlipidemia, chronic kidney disease, and/or cardiovascular diseases (Table 1).
Table 1.
Baseline Characteristics
| Variables | NT (n = 1,804) | WCH (n = 123) | MH (n = 582) | SH (n = 518) | p Value * |
|---|---|---|---|---|---|
| Age, yrs† | 40 (34–48) | 49 (42–55) | 47 (41–54) | 50 (42–56) | <0.0001 |
| Men, % | 44.0 | 42.3 | 46.7 | 45.8 | 0.59 |
| Blacks, % | 41.7 | 63.4 | 55.8 | 68.9 | <0.0001 |
| Caucasians, % | 34.8 | 24.4 | 30.8 | 21.0 | <0.0001 |
| Hispanics, % | 21.1 | 12.2 | 12.4 | 8.1 | <0.0001 |
| HTN, % | 9.4 | 32.5 | 62.4 | 90.7 | <0.0001 |
| Anti-HTN meds, % | 12.8 | 35.0 | 29.6 | 39.1 | <0.0001 |
| Number of anti- HTN meds among treated‡ | 1.02 ± 0.84 | 1.19 ± 0.69 | 1.33 ± 0.74 | 1.37 ± 0.72 | <0.01 |
| DM, % | 6.7 | 21.1 | 17.2 | 21.2 | <0.0001 |
| BMI, kg/m2† | 28 (25–33) | 31 (27–38) | 31 (27–36) | 32 (27–36) | <0.0001 |
| Smokers, % | 26.2 | 26.8 | 29.6 | 32.4 | 0.038 |
| HL, % | 10.8 | 18.7 | 17.2 | 16.6 | <0.0001 |
| CKD, % | 4.8 | 13.3 | 9.6 | 19.3 | <0.0001 |
| CVD, % | 4.9 | 12.2 | 9.8 | 12.9 | <0.0001 |
| SBPH mm Hg‡ | 117 ± 10 | 123 ± 8 | 141 ± 12 | 154 ± 17 | <0.0001 |
| DBPH mm Hg‡ | 74 ± 7 | 76 ± 6 | 87 ± 7 | 92 ± 9 | <0.0001 |
| SBPC mm Hg‡ | 118 ± 10 | 146 ± 11 | 127 ± 8 | 153 ± 14 | <0.0001 |
| DBPC mm Hg‡ | 74 ± 7 | 88 ± 7 | 79 ± 7 | 91 ± 8 | <0.0001 |
p value comparing all 4 groups
Median (interquartile range)
Mean ± SD
BMI = body mass index; CKD = chronic kidney disease; CVD = cardiovascular disease; DBPc = clinic diastolic blood pressure; DBPH = home diastolic blood pressure; DM = diabetes; HL = hyperlipidemia; HTN = hypertension; MH = masked hypertension; NT = normotension; SBPC = clinic systolic blood pressure; SBPH = home systolic blood pressure; SH = sustained hypertension; WCH = white coat hypertension.
Figure 1. Relationship Between Home And Office Systolic Blood Pressure.
The sample weighted prevalence of MH and WCH are 17.8% and 3.3% respectively. MH = masked hypertension; WCH = white coat hypertension.
Associations between WCH/MH and subclinical target organ damage
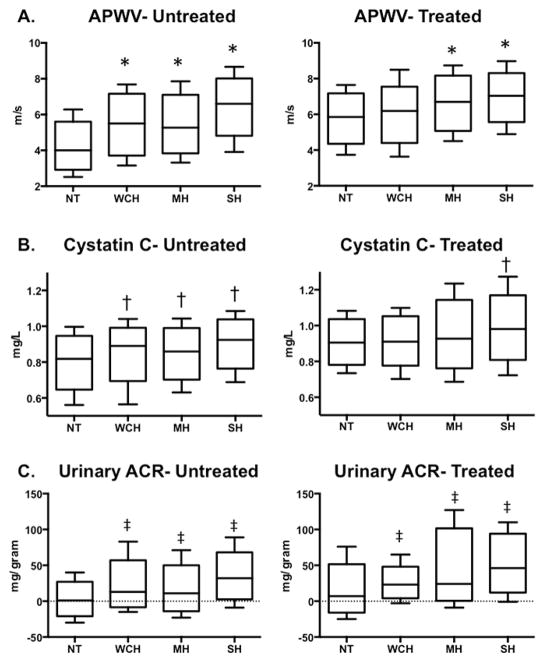
APWV and cystatin C levels were significantly higher in both the WCH and MH groups compared with the NT participants (5.53 ± 1.44, 5.39 ± 1.40 vs. 4.56 ± 1.33 m/s, respectively; and 0.88 ± 0.13 vs. 0.87 ± 0.15 vs. 0.82 ± 0.14 mg/l, respectively, both p < 0.01). Median UACR was also higher in the WCH and MH groups compared with the NT group (16, IQR: −1 to 31 vs. 13, IQR: 0 to 34 vs. 2, IQR −1 to 15 mg/g creatinine, respectively, p < 0.01). When the analyses were stratified according to the presence or absence of antihypertensive treatment, untreated WCH and SH subjects had significantly higher APWV compared with NT subjects, whereas the group with untreated MH was not significantly different from the NT group after multivariable adjustment (Figure 2A). In contrast, among the subgroup treated with antihypertensive medication, WCH, MH, and SH were all associated with higher APWV than observed in the NT group. WCH and MH were associated with higher levels of cystatin C compared with the NT group in the untreated, but not in the treated subgroups after multivariable adjustment, whereas SH was associated with increased cystatin C levels, regardless of treatment status (Figure 2B). WCH, MH, and SH were also associated with higher UACR compared with the NT group, regardless of treatment status (Figure 2C). The number of antihypertensive drugs prescribed in the WCH group was similar to number prescribed to the MH and NT groups (1.19 ± 0.69 vs. 1.33 ± 0.74 vs. 1.02 ± 0.84, respectively, p = NS vs. treated NT). The number of antihypertensive drugs prescribed in the SH group (1.37 ± 0.72) was also not different from the MH group (1.33 ± 0.74, p = NS), but higher then in the treated NT group (p < 0.01, Table 1).
Figure 2. Comparison of Markers of Subclinical Vascular and Renal Damage Among Participants with WCH, MH, SH, and the Control Group.
(A) APWV (62 WCH, 341 MH, 254 SH, and 1,347 NT in the untreated [control] group; 27 WCH, 109 MH, 128 SH, 145 NT among the treated group). (B) Cystatin C Level (69 WCH, 381 MH, 276 SH, and 1,443 NT in the untreated group; 30 WCH, 118 MH, 144 SH, and 167 NT in the treated group). (C) UACR (73 WCH, 389 MH, 292 SH, and 1,516 NT in the untreated group; 31 WCH, 126 MH, 153 SH, and 175 in the treated group). APWV = aortic pulse wave velocity; BMI = body mass index; BP = blood pressure; NT = normotension; SH = sustained hypertension; UACR = urinary albumin to creatinine ratio. Other abbreviations as in Figure 1.
*p < 0.05 compared with NT after adjustment for age, sex, race, BMI, smoking, and diabetes.
†p < 0.01 compared with NT after adjustment for age, sex, race, diabetes, self-reported hypertension, home systolic BP, estimated glomerular filtration rate, high-sensitivity C-reactive protein, and allometric height
‡p < 0.05 compared with NT after adjustment for age, sex, race, diabetes, self-report hypertension, home systolic BP, estimated glomerular filtration rate, high sensitivity C-reactive protein, and allometric height
Associations Between WCH/ MH and Cardiovascular Outcomes
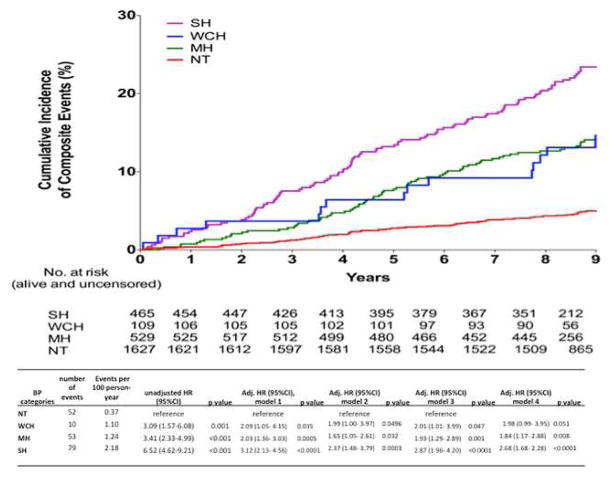
Over a median follow-up period of 9.4 years (IQR: 9.0 to 9.8), 47 cardiovascular deaths and 194 composite cardiovascular events were recorded. Hazard ratios (HRs) for composite cardiovascular events in participants with WCH, MH, and SH are presented in Table 2 and Kaplan–Meier survival curves are presented in Figure 3. In analyses adjusting for age, sex, and race/ethnicity, BMI, diabetes mellitus, smoking, and total cholesterol levels (model 1), WCH was independently associated with higher cardiovascular risk compared with the NT group (adjusted HR 2.09 [95% CI: 1.05 to 4.15]). The association of WCH with cardiovascular events remained significant in the model after adjustment for both traditional cardiovascular risk factors and antihypertensive medication treatment (Model 3, Table 2). After adjustment for both self-reported history of hypertension and prevalent antihypertensive treatment in Model 4, the association of WCH with composite cardiovascular events trended toward significance (p = 0.051).
Table 2.
Adjusted HRs for Composite Cardiovascular Events Associated With Each Hypertension Classification Compared With Participants With Normal BP
| BP Categories | Number of Events | Events per 100 Person- Yrs | Unadjusted HR (95% CI) | p Value | Adjusted HR (95% CI) Model 1 | p Value |
|---|---|---|---|---|---|---|
| NT | 52 | 0.37 | reference | reference | ||
| WCH | 10 | 1.10 | 3.09 (1.57–6.08) | 0.001 | 2.09(1.05–4.15) | 0.035 |
| MH | 53 | 1.24 | 3.41 (2.33–4.99) | <0.001 | 2.03(1.36–3.03) | 0.0005 |
| SH | 79 | 2.18 | 6.52 (4.62–9.21) | <0.001 | 3.12(2.13–4.56) | <0.0001 |
| BP Categories | Adjusted HR (95% CI) Model 2 | p Value | Adjusted HR (95% CI) Model 3 | p Value | Adjusted HR (95% CI) Model 4 | p Value |
|---|---|---|---|---|---|---|
| NT | reference | reference | reference | |||
| WCH | 1.99 (1.00–3.97) | 0.0496 | 2.01 (1.01– 3.99) | 0.047 | 1.98 (0.99– 3.95) | 0.051 |
| MH | 1.65 (1.05–2.61) | 0.032 | 1.93 (1.29– 2.89) | 0.001 | 1.84 (1.17– 2.88) | 0.008 |
| SH | 2.37 (1.48–3.79) | 0.0003 | 2.87 (1.96– 4.20) | <0.0001 | 2.68 (1.68– 2.28) | <0.0001 |
Model 1: age, sex, race, BMI, diabetes, smoking, serum cholesterol level
Model 2: model 1 + hypertension
Model 3: model 1 + treatment for hypertension
Model 4: model 1 + hypertension + treatment for hypertension
BP = blood pressure; CI = confidence interval; HR = hazard ration. Other abbreviations as in Table 1.
Figure 3. Kaplan-Meier Curves for the Cumulative Incidence of Composite Cardiovascular Events Among the NT, WCH, MH, and SH Groups.
There was a significant difference amongst the 4 groups over a median follow-up of 9.5 years. Abbreviations as in Figures 1 and 2.
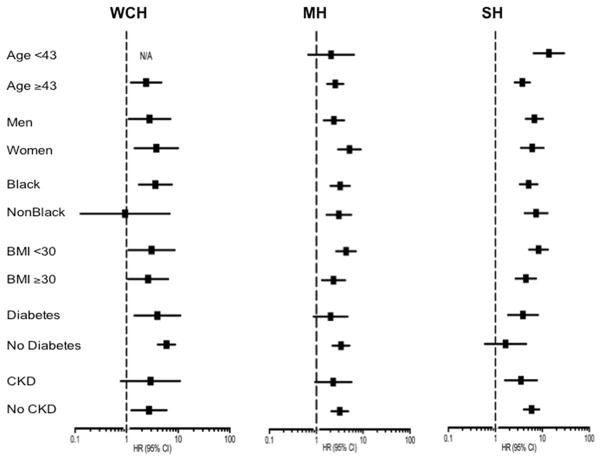
Similarly, MH was associated with increased cardiovascular risk compared with the NT group after adjustment of traditional risk factors (adjusted HR: 2.03 [95% CI: 1.36 to 3.03]), and in all models that accounted for both self-reported history of hypertension and antihypertensive treatment (Table 2). Associations of WCH and MH with composite CV events were consistent in subgroups (all p-interaction >0.1) (Figure 4).
Figure 4. Crude Hazard Ratios for Composite Cardiovascular Events Associated With WCH, MH, and SH in Selected Subgroups.
None of the interactions were statistically significant.
To determine if the presence of WCH and MH is associated with progression to hypertension, which may influence cardiovascular outcomes, we assessed the association of WCH and MH with incident hypertension in the DHS-2. Because only clinic BP was obtained in DHS-2, we determined the incidence of hypertension by the presence of antihypertensive drug treatment. We found that higher proportions of participants with WCH and MH developed incident hypertension requiring prescription of antihypertensive medications in DHS-2 compared with the NT group after a median follow-up of 7 years (56% vs. 45% vs. 14%, p < 0.001, Online Figure 1).
Discussion
The major findings of our study are 3-fold. First, MH was common in our cohort, occurring in 18% of participants overall and in 14% of those not receiving antihypertensive treatment, comprising >50% of the prevalent hypertension cases in the cohort. Secondly, both WCH and MH were associated with markers of target organ damage, including increased aortic stiffness and renal damage, as evidenced by albuminuria and higher cystatin C levels. Thirdly, both WCH and MH were associated with increased cardiovascular events in a multiethnic, probability-based population sample of Dallas county adults.
Our study demonstrated a strikingly high prevalence of masked hypertension, exceeding the combined prevalence of each of the other 2 classifications of white coat hypertension and sustained hypertension. In analyses from the Coronary Artery Risk Development in Young Adults (CARDIA) Study, the investigators reported that the prevalence of masked hypertension was only 2.1% to 4.4%, whereas the prevalence of white coat hypertension was 3.3% to 3.9% (23). However, participants in the CARDIA study were more than a decade younger than our cohort and the sample size was limited to a subset of 281 subjects. In contrast, a recently published article from the Jackson Heart Study (JHS), which is limited to African Americans, reported 25.9% prevalence of MH and 7.5% prevalence of WCH (24). The difference in ethnic composition and the use of 24-h ambulatory BP monitoring in JHS may explain difference in the study results. Nevertheless, our data are quite consistent with a large international registry of ambulatory BP monitoring, in which the prevalence of MH among individuals between 40 and 50 years old was between 12% and 28%, and the reported prevalence of WCH was between 3.6% and 4.1% (25).
Previous studies have demonstrated inconsistent results in regards to cardiovascular outcomes associated with WCH. Results from a published meta-analysis conducted by Pierdomenica et al. showed that the cardiovascular prognosis of WCH is not significantly different from that of the normotensive group among an untreated population (9). Similarly, results of analyses from IDACO (International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes) (6) and Syst-Eur (Systolic Hypertension in Europe) (26) showed that WCH was not associated with an increase in cardiovascular events in the treated group. In contrast, results from a meta-analysis by Stergiou et al. (5) showed increased risk for CV events in untreated WCH. Explanations underlying the difference in study results are unknown, but may be related to the method of BP monitoring or to the nature of the population studied. The analyses from IDACO used ambulatory BP monitoring to define the presence of WCH and the results were derived from older individuals (>60 years of age) with isolated systolic hypertension, with uncertain generalizability to a broader age range or spectrum of BP abnormalities. In contrast, the meta-analysis from Stergiou et al. (5) was derived from home BP monitoring from 5 populations in Japan, Finland, Greece, and Uruguay. Our study represents the largest U.S. study to date that has evaluated CV outcomes associated with WCH, as determined by home and clinic BP measurements.
Underlying factors that result in differential BP levels between the in-home versus the clinical setting may influence outcomes of WCH and MH. WCH is proposed to result from stress-induced activation of the sympathetic nervous system during encounters with health care providers, (27) whereas MH is potentially induced by mental stress at home, excessive consumption of alcohol, caffeine consumption, and cigarette smoking, among other potential confounders (14). Furthermore, MH while on drug treatment is proposed to reflect a shift of phenotype from sustained hypertension to inadequately-treated hypertension (14). In contrast, WCH is thought to lead to overtreatment of hypertension because of persistently-elevated clinic BP (14). However, in our study, the number of antihypertensive drugs prescribed in the WCH group was similar to the group with controlled hypertension, both at home and in the clinic. Because average home BP in the treated WCH group (Online Table S1b) was similar to the treated NT group, our study did not suggest overtreatment of hypertension in the WCH group. Similarly, the number of antihypertensive drugs prescribed in MH was not different from the SH group, whereas both clinic and home BP were lower in the MH than in the SH group (Online Table S1b), which argues against undertreatment of hypertension in MH. Nevertheless, our data extend previous observations by demonstrating increased cardiovascular risk of MH and WCH, independent of cardiovascular risk factors and antihypertensive drug treatment.
Our study is limited by a relatively small number of participants with WCH. Although cardiovascular risk associated with WCH in the nonblack participants appears to be lower than in blacks in Figure 4, we do not have adequate statistical power to examine an interaction between race and the impact of WCH on cardiovascular outcomes, given a relatively low total number of events in nonblacks (63 composite events). The median time between the home visit and clinic visit in the MH group was 2.30 months (IQR: 1.08 to 7.79 months), and we cannot exclude the possibility that treatment of hypertension between the home and clinic visits may have led to normalization of office BP and overdiagnosis of masked hypertension. However, previous studies have shown a very small percentage of participants in the community-based BP screening program received a new diagnosis of hypertension or treatment of hypertension after BP screening, unless additional medical or behavioral interventions were implemented (28–31). The rates of progression to sustained hypertension among individuals with WCH and MH are also unknown because home BP was not measured during the follow-up study in the Dallas Heart Study (DHS-2). However, higher proportions of participants with WCH and MH developed incident hypertension requiring prescription with antihypertensive medications in DHS-2 compared with the NT group, which may explain the increased risk of cardiovascular events in WCH and MH. Home BP was obtained by surveyors, rather than by self-measurement, which may not truly reflect home BP. The use of local lay, ethnically-congruent field staff should minimize the alerting reaction during home BP measurement, which was further reduced by averaging the last 3 of 5 BP measurements. The dosage of antihypertensive drugs in each class is also unknown. The number of BP medications was estimated from the number of drug classes, rather than by direct assessment. However, it is unlikely that more than 1 drug per class was prescribed to each participant. Lastly, statistical analysis in this study was not adjusted for multiple testing between WCH, MH, and SH versus control groups. However, our analysis was performed on the basis of a separate, pre-stated hypothesis suggested by prior published evidence. Because we did not stray into nonhypothesis-driven exploration, we believe this is an acceptable approach.
Conclusions
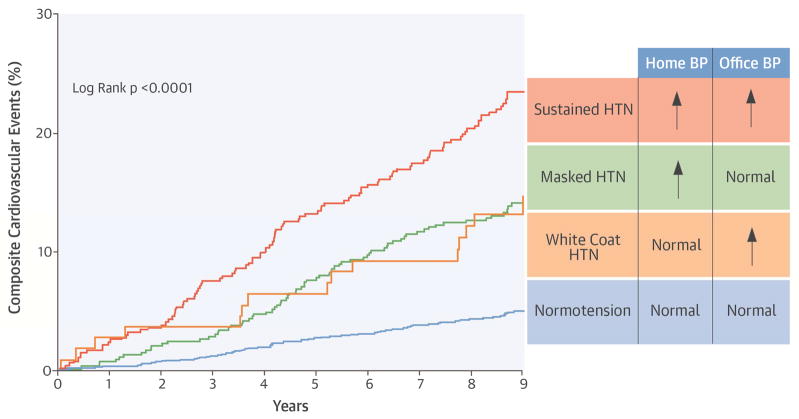
Our study provides the first direct evidence of increased target organ damage and increased long-term risk for cardiovascular complications associated with WCH and MH in a multiethnic population in the United States (Central Illustration). Given the high prevalence of MH in our cohort, which could have been missed with office BP monitoring alone, the present study provides support for routine use of home BP monitoring in U.S. adults with and without antihypertensive drug treatment.
Central Illustration. Composite Cardiovascular Events Associated With Masked Hypertension and White Coat Hypertension.
Kaplan-Meier curves for the cumulative incidence of composite cardiovascular events among the NT, WCH, MH, and SH groups. BP = blood pressure; HTN = hypertension; MH = masked hypertension; NT = normotension; SH = sustained hypertension; WCH = white coat hypertension.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Patients with normal blood pressure (BP) readings in clinic but elevated levels at home (masked hypertension) and those whose BP is elevated in the medical office setting but normal at home (white coat hypertension) are at elevated risk of target organ damage and adverse cardiovascular events in middle-age. Masked hypertension is more common than both sustained and white coat hypertension.
Competency in Patient Care
Home BP monitoring should be routinely obtained in adults with established or suspected hypertension.
Translational Outlook
More studies are needed to determine if antihypertensive drug treatment of masked or white coat hypertension improves cardiovascular outcomes and to identify optimum treatment regimens for patients with each type of hypertension.
Acknowledgments
Funding: Dr. Vongpatanasin is supported by the UT Southwestern O’Brien Kidney Center and the Kaplan Chair in Hypertension Research. Dr. Victor is supported by the National Center for Advancing Translational Sciences UCLA CTSI (UL1TR000124), the Lincy Foundation, and the Burns and Allen Chair in Cardiology Research. The Dallas Heart Study was funded by the Donald W. Reynolds Foundation and was partially supported award by Award #UL1TR001105 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Abbreviations
- APWV
aortic pulse wave velocity
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- MH
masked hypertension
- NT
normotension
- SH
sustained hypertension
- UACR
urinary albumin to creatinine ratio
- WCH
white coat hypertension
Footnotes
Disclosures: All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037–114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 2.National Clinical Guideline Centre (UK) Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. London, UK: Royal College of Physicians; 2011. [PubMed] [Google Scholar]
- 3.Parati G, Stergiou GS, Asmar R, et al. ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension Practice Guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–85. doi: 10.1038/jhh.2010.54. [DOI] [PubMed] [Google Scholar]
- 4.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 5.Stergiou GS, Asayama K, Thijs L, et al. International Database on HOme blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675–82. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 6.Franklin SS, Thijs L, Hansen TW, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Significance of white-coat hypertension in older persons with isolated systolic hypertension: a meta-analysis using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes population. Hypertension. 2012;59:564–71. doi: 10.1161/HYPERTENSIONAHA.111.180653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancia G, Bombelli M, Brambilla G, et al. Long-term prognostic value of white coat hypertension: an insight from diagnostic use of both ambulatory and home blood pressure measurements. Hypertension. 2013;62:168–74. doi: 10.1161/HYPERTENSIONAHA.111.00690. [DOI] [PubMed] [Google Scholar]
- 8.Björklund K, Lind L, Vessby B, et al. Different metabolic predictors of white-coat and sustained hypertension over a 20-year follow-up period: a population-based study of elderly men. Circulation. 2002;106:63–8. doi: 10.1161/01.cir.0000019737.87850.5a. [DOI] [PubMed] [Google Scholar]
- 9.Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52–8. doi: 10.1038/ajh.2010.203. [DOI] [PubMed] [Google Scholar]
- 10.Niiranen TJ, Asayama K, Thijs L, et al. IDHOCO Investigators. Optimal number of days for home blood pressure measurement. Am J Hypertens. 2015;28:595–603. doi: 10.1093/ajh/hpu216. [DOI] [PubMed] [Google Scholar]
- 11.Niiranen TJ, Mäki J, Puukka P, et al. Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension. 2014;64:281–6. doi: 10.1161/HYPERTENSIONAHA.114.03292. [DOI] [PubMed] [Google Scholar]
- 12.Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of "masked hypertension" detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–9. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 13.Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–5. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 14.Franklin SS, O’Brien E, Thijs L, et al. Masked hypertension: a phenomenon of measurement. Hypertension. 2015;65:16–20. doi: 10.1161/HYPERTENSIONAHA.114.04522. [DOI] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, et al. Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Chandra A, Neeland IJ, Berry JD, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64:997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 17.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer H, Toto R, Peshock R, et al. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–13. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 19.Patel PC, Ayers CR, Murphy SA, et al. Association of cystatin C with left ventricular structure and function: the Dallas Heart Study. Circ Heart Fail. 2009;2:98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]
- 20.Maroules CD, Khera A, Ayers C, et al. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson. 2014;16:33. doi: 10.1186/1532-429X-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maroules CD, Rosero E, Ayers C, et al. Abdominal aortic atherosclerosis at MR imaging is associated with cardiovascular events: the Dallas Heart Study. Radiology. 2013;269:84–91. doi: 10.1148/radiol.13122707. [DOI] [PubMed] [Google Scholar]
- 22.Jain T, Peshock R, McGuire DK, et al. Dallas Heart Study Investigators. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas heart study. J Am Coll Cardiol. 2004;44:1011–7. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 23.Muntner P, Lewis CE, Diaz KM, et al. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2015;28:640–8. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz KM, Veerabhadrappa P, Brown MD, et al. Prevalence, determinants, and clinical significance of masked hypertension in a population-based sample of African Americans: the Jackson Heart Study. Am J Hypertens. 2015;28:900–8. doi: 10.1093/ajh/hpu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conen D, Aeschbacher S, Thijs L, et al. Age-specific differences between conventional and ambulatory daytime blood pressure values. Hypertension. 2014;64:1073–9. doi: 10.1161/HYPERTENSIONAHA.114.03957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagard RH, Staessen JA, Thijs L, et al. Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Response to antihypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Circulation. 2000;102:1139–44. doi: 10.1161/01.cir.102.10.1139. [DOI] [PubMed] [Google Scholar]
- 27.Grassi G, Seravalle G, Buzzi S, et al. Muscle and skin sympathetic nerve traffic during physician and nurse blood pressure measurement. J Hypertens. 2013;31:1131–5. doi: 10.1097/HJH.0b013e3283605c71. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton W, Round A, Goodchild R, et al. Do community based self-reading sphygmomanometers improve detection of hypertension? A feasibility study. J Public Health Med. 2003;25:125–30. doi: 10.1093/pubmed/fdg027. [DOI] [PubMed] [Google Scholar]
- 29.Westerman RF, Tesselaar HJ, Donker AJ. Screening for hypertension by volunteers in a middle-class community. J Hum Hypertens. 1990;4:330–3. [PubMed] [Google Scholar]
- 30.Fleming S, Atherton H, McCartney D, et al. Self-screening and non-physician screening for hypertension in communities: a systematic review. Am J Hypertens. 2015 Mar 23; doi: 10.1093/ajh/hpv029. [E-pub ahead of print], http://dx.doi.org/0.1093/ajh/hpv029. [DOI] [PMC free article] [PubMed]
- 31.Victor RG, Ravenell JE, Freeman A, et al. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med. 2011;171:342–50. doi: 10.1001/archinternmed.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.