Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a rare form of ALK-negative ALCL, usually presenting as an accumulation of seroma fluid between the implant itself and the surrounding fibrous capsule.1 It was first described in 1997.2 From 1997 to 2012, the United States Food and Drug Administration reported a total of 60 registered instances of BIA-ALCL with a mean interval to lymphoma diagnosis of 10.9 years.3-5 It is estimated that 1 of 500,000 women receiving breast implants will develop BIA-ALCL.5, 6 Recent studies have suggested that patients can be managed conservatively, by drainage of the fluid and removal of the implant and capsule, if no invasion of the capsule or breast tissue is identified. 3, 7 However, some patients manifest invasion of the capsule, and involvement of breast, lymph nodes, or more rarely distant sites. The risk factors for progression are not fully delineated.
We report a unique case of BIA-ALCL arising in a patient with Li-Fraumeni syndrome and germline mutations in TP53. The case is notable for the short interval between the placement of the implants and the development of ALCL, which was only 3 years. Additionally, the patient had a good outcome, despite the underlying high genetic risk factors. After capsulectomy and implant removal, the patient had no recurrence of BIA-ALCL with clinical follow up of 7 years.
The patient first presented at age 31 with intraductal carcinoma of the left breast. She was treated with lumpectomy, radiation therapy, followed by six cycles of adjuvant chemotherapy (Cyclophosphamide, Methotrexate, and 5-Fluorouracil). Four years later the patient presented with intraductal carcinoma of the contralateral breast, treated with lumpectomy, external radiation therapy, and brachytherapy with interstitial radium implants. Prophylactic bilateral mastectomies were performed one year later, followed by breast reconstruction.
Over the subsequent years the patient was diagnosed with adrenocortical carcinoma, initially treated with left adrenalectomy, nephrectomy and splenectomy. She subsequently developed metastatic adrenocortical carcinoma to liver and lung, treated with subtotal hepatic resection and left thoracotomy to remove pulmonary nodules. A subsequent liver recurrence was treated with mitotane, adriamycin, vincristine, and etoposide (14 cycles), became negative on PET scan, and never recurred. Other neoplasms diagnosed during her disease course included malignant fibrous histiocytoma, basal cell carcinoma, carcinoma of the ampulla of vater and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). In the latter, interphase FISH studies of peripheral blood identified deletions in 13q14.3 and TP53, with no changes in ATM or trisomy 12. She was followed with a diagnosis of CLL/SLL without therapy and had stable disease.
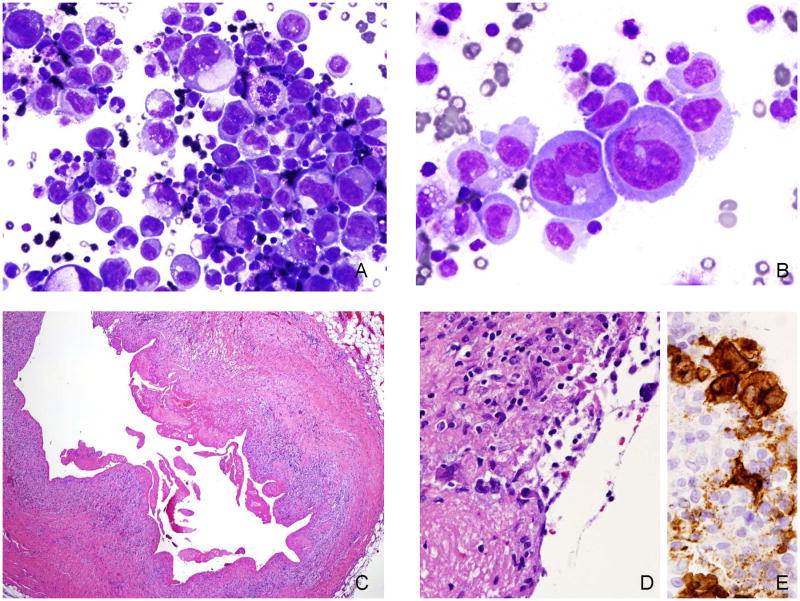
Twenty-one years after her original diagnosis of intraductal carcinoma, and three years after receiving bilateral breast implants, the patient noted asymmetric swelling of the right breast. A fine needle aspiration of a breast seroma was performed, leading to the diagnosis of BIA-ALCL. The fluid contained numerous atypical large lymphoid cells with markedly pleomorphic nuclei with prominent nucleoli, frequent bi and multinucleation and moderate to abundant amounts of basophilic sometimes vacuolated cytoplasm (Figure 1A, 1B). There were a few admixed macrophages and small lymphocytes in the background. Immunocytochemical studies showed that the atypical cells were positive for LCA, CD30, CD3 and p53. They were negative for pancytokeratin, CK7, CAM5.2, Melan A, MART-1, inhibin, synaptophysin, calrectinin, CD163, HHV8 and ALK-1. Flow cytometric analysis revealed positivity for CD2, CD25, CD30, CD4, CD45 (bright), CD7 (partial), CD38 (partial) and CD26. The cells were negative for CD3 (surface), CD5, CD27 and CD19. PCR analysis showed no evidence of significant clonal rearrangement for the T cell receptor gamma-chain gene. pancytokeratin, CK7, CAM5.2, Melan A, MART-1, inhibin, synaptophysin, calrectinin, CD163, HHV8 and ALK-1. Flow cytometric analysis revealed positivity for CD2, CD25, CD30, CD4, CD45 (bright), CD7 (partial), CD38 (partial) and CD26. The cells were negative for CD3 (surface), CD5, CD27 and CD19. PCR analysis showed no evidence of significant clonal rearrangement for the T cell receptor gamma-chain gene.
Figure 1.
A, B. Cytological preparation of the seroma fluid shows numerous large pleomorphic lymphoid cells with deeply basophilic cytoplasm. Some cells contain cytoplasmic vacuoles. Scattered small lymphocytes and nuclear debris are present in the background. C. Following removal of the implants, the capsule was examined and shows a marked fibrinous exudate. D. The surface of the capsule contains a few atypical cells, better highlighted with the CD30 stain [E].
Following the diagnosis of ALCL, the implant and surrounding capsule were removed. Sections of the capsule around the implant showed granulation tissue (Figure 1C) with rare CD30 positive large atypical lymphoid cells (Figure 1D, 1E) but no evidence of invasion. The atypical cells were additionally positive for CD3, EMA, and TIA-1 but negative for ALK, CD4 and CD8. The contralateral implant also was removed but showed no evidence of lymphoma.
This case illustrates that even in this very high-risk patient, BIA-ALCL was treated successfully with conservative management, consisting of implant removal but no further chemotherapy or radiation. In the ensuing seven years she had no evidence of recurrence of lymphoma. An unusual feature was the relatively short interval between placement of the implants and development of lymphoma, much less than the median of 10 years.3, 8 We believe this is likely attributable to the underlying Li-Fraumeni syndrome. However, it is difficult to entirely exclude prior radiation and chemotherapy as contributing factors, although these have not been implicated in epidemiological studies of BIA-ALCL.3, 8 The basis for the very indolent clinical behavior of BIA-ALCL is unknown. Studies of cell lines have suggested that the tumor cells are dependent on the cytokine milieu resulting from the inflammatory reaction to the implant, or secreted by the tumor cells themselves. Lechner et al. provided evidence for an autocrine loop, acting through the JAK/STAT pathway.9 The indolent behavior of a neoplastic process in a confined space is reminiscent of EBV-positive diffuse large B-cell lymphomas arising in a background of chronic inflammation.10 These aggressive appearing B-cell lymphomas also arise after years of chronic inflammation, e.g. chronic pyothorax, hydrocele, but have a low risk of systemic spread.
Footnotes
Author contributions: Drs. Lee and Jaffe wrote the manuscript, provided diagnostic information, and immunophenotypic and molecular data. Dr. Filie participated in the diagnosis. Dr. Arthur provided cytogenetic data. Dr. Fojo provided clinical information and managed the patient during her clinical course.
Ethics: The authors affirm that: (i) informed, written consent has been obtained;
(ii) studies have been performed according to the Declaration of Helsinki;
(iii) This work was performed in the context of the following clinical trial: ClinicalTrials.gov Identifier: NCT00071058
Initiated and approved by the National Cancer Institute, Institutional Review Board October 9, 2003
Antonio Fojo, MD, NCI, NIH, Principle Investigator
National Cancer Institute, Institutional Review Board Bethesda, Maryland, USA
References
- 1.Thompson PA, Lade S, Webster H, Ryan G, Prince HM. Effusion-associated anaplastic large cell lymphoma of the breast: Time for it to be defined as a distinct clinico-pathological entity. Haematologica. 2010;95:1977–1979. doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plastic and reconstructive surgery. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 3.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: Long-term follow-up of 60 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and drug administration. Anaplastic large cell lymphoma (ALCL) in women with breast implants: Preliminary FDA findings and analyses. 2011 http://www.Fda.Gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/breastimplants/ucm239996.Htm.
- 5.U.S. Food and drug administration. FDA medical device safety communication: Reports of anaplastic large cell lymphoma (ALCL) in women with breast implants. 2011 http://www.Fda.Gov/medicaldevices/safety/alertsandnotices/ucm240000.Htm.
- 6.Kenkel JM. Discussion: Anaplastic large cell lymphoma and breast implants: A systematic review. Plastic and reconstructive surgery. 2011;127:2151–2153. doi: 10.1097/PRS.0b013e318219c741. [DOI] [PubMed] [Google Scholar]
- 7.Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: An indolent t-cell lymphoproliferative disorder. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21:455–463. doi: 10.1038/modpathol.3801024. [DOI] [PubMed] [Google Scholar]
- 8.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. Jama. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 9.Lechner MG, Megiel C, Church CH, et al. Survival signals and targets for therapy in breast implant-associated ALK--anaplastic large cell lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4549–4559. doi: 10.1158/1078-0432.CCR-12-0101. [DOI] [PubMed] [Google Scholar]
- 10.Loong F, Chan AC, Ho BC, et al. Diffuse large b-cell lymphoma associated with chronic inflammation as an incidental finding and new clinical scenarios. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:493–501. doi: 10.1038/modpathol.2009.168. [DOI] [PubMed] [Google Scholar]