Abstract
Background
Due to multiple comorbidities, hemodialysis (HD) patients are prescribed many oral medications, including phosphate binders (PBs), often resulting in a high “pill burden”.
Methods
Using data from the international Dialysis Outcomes and Practice Patterns Study (DOPPS), we assessed associations between PB pill burden, patient-reported PB non-adherence, and levels of serum phosphorus (SPhos) and parathyroid hormone (PTH), using standard regression analyses. The study included data collected from 5,262 HD patients from dialysis units participating in the DOPPS in 12 countries.
Results
PB prescription ranged from a mean of 7.4 pills/day in the United States (US) to 3.9 pills/day in France. About half of the patients were prescribed at least 6 PB pills/day, and 13% were prescribed at least 12 PB pills/day. Overall, the proportion of patients who reported skipping PBs at least once in the past month was 45% overall, ranging from 33% in Belgium to 57% in the US. There was a trend toward greater PB non-adherence and a higher number of prescribed PB pills/day. Non-adherence to PB prescription was associated with high SPhos (>5.5 mg/dL) and PTH (> 600 pg/mL).
Conclusions
Adherence to PB is a challenge for many hemodialysis patients and may be related to the number of PB pills prescribed. Prescription of a simplified PB regimen could improve patient adherence and perhaps improve SPhos and PTH levels.
Keywords: adherence, mineral bone disorder, phosphate binders, pill burden
INTRODUCTION
Mortality remains high in end-stage renal disease (ESRD) patients, and cardiovascular (CV) disease is the major cause 1. Accumulated observational data support disordered mineral metabolism, including high serum phosphorus (SPhos) levels as risk factors for CV and overall mortality 2–6. These observations are supported by mechanistic studies showing adverse effects of high phosphorus on smooth muscle, vascular calcification, and endothelial function 7,8,9, thus providing strong supporting evidence for the role of high SPhos levels in the pathophysiology of CV events. Determinants of SPhos levels in hemodialysis (HD) patients include dietary intake, residual renal function, and clearance of phosphorus by dialysis as well as phosphate binders (PB) regimens. A causal link between lowering SPhos with PB and reducing CV risk has not been shown. Observational studies do show an association between PB prescription and better survival in incident and prevalent HD patients 10,11, and in facilities with a greater percentage of PB prescriptions 11. Bias by indication may contribute to these findings, as healthier patients tend to have better nutrition and higher phosphorus levels. However, based on observational data most HD patients with hyperphosphatemia are prescribed PBs, both with the goal of potentially reducing CV risk and bone disease 12.
Adherence to a prescribed medication regimen can be a challenge for HD patients 13. In addition to medications for often multiple comorbid conditions, prescription patterns of the most common PBs indicate that patients must take 1–4 PB pills 3–5 times daily. Previous studies have shown a high pill burden in HD patients 14,15, and that PBs account for approximately half the daily pill burden of dialysis patients. Both the higher total and higher PB pill burden have been associated with lower PB regimen adherence 16. Few studies have assessed whether HD patients actually take all of their medications, although studies in other populations have shown high levels of non-adherence to self-administered medication regimens 17. Non-adherence to PB treatment likely contributes to higher SPhos levels, in addition to expense.
Given the high pill burden in HD patients, we hypothesized that prescription of more PB pills would be associated with higher patient-reported PB non-adherence, and that non-adherence would be associated with higher SPhos and parathyroid hormone (PTH) levels.
METHODS
Data Source
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is an international prospective cohort study of in-center HD patients ≥18 years old. Patients are randomly selected from a representative sample of dialysis facilities in each country 18,19. This analysis included data from participants in DOPPS phase 4 (2009–2011) in Australia, Belgium, Canada, France, Germany, Italy, Japan, New Zealand, Spain, Sweden, the United Kingdom, and the United States (US). Demographics, comorbid conditions, laboratory values, and prescriptions were abstracted from medical records using uniform and standardized data collection tools in all countries.
Variables
Patient-reported PB non-adherence was assessed on the DOPPS self-administered patient questionnaire (PQ) for patients prescribed PBs. The PQ was typically completed shortly after study entry (median: 1.1 months, interquartile range [IQR]: 0.6 to 2.3 months, 95th percentile: 12.2 months). For the question “During the last month, how often did you skip taking your phosphate binders altogether?” response categories included: (1) 1–3 times, (2) 3–6 times, (3) 7–10 times, (4) more than 10 times, and (5) I took all the phosphate binders that my doctor prescribed. Categories 3 and 4 (≥ 7 times) were combined for analyses to avoid sparse data cells.
The number of prescribed PB pills/day at the time the patient completed the PQ was abstracted from medication records, and categorized into 5 groups: (1) 1–2, (2) 3–5, (3) 6–8, (4) 9–11, and (5) ≥ 12. Facility median PB pills/day was calculated among patients prescribed a PB and categorized into 3 groups: (1) 1–3, (2) 4–6, and (3) ≥ 7. PB type was classified into 5 mutually exclusive groups: (1) calcium-based only, (2) sevelamer only, (3) lanthanum only, (4) other PB type only, and (5) multiple PB types. The Phase 4 Medical Director Survey also requested the serum phosphorus facility target range for most patients in the facility.
Outcome variables SPhos and PTH were collected as often as measured, up to monthly. SPhos was used from the same month the PQ was completed. Because PTH was measured less frequently, the most recent value (up to 3 months prior to PQ completion) was used. For the majority of the study period, intact PTH was the only assay available for clinical practice, and fewer than 10% of DOPPS facilities reported using bio-intact PTH assays. Data on comorbid conditions were collected at study baseline. Other adjustments – body mass index (BMI), serum albumin, creatinine, normalized protein catabolic rate (nPCR), hemoglobin, and single pool Kt/V – were updated with the most recent value. Self-reported data on marriage status, education, employment, living status, and patient-physician contact frequency were collected on the PQ and supplemented with baseline medical questionnaire (MQ) data if missing.
Among 10,843 patients who completed a PQ, 6,762 were prescribed a PB and responded to the PB non-adherence question. Patients were excluded if, at the time of PQ completion, they had no available laboratory values, they were not prescribed PBs, or they were on dialysis less than 90 days. 5,262 patients met the study inclusion criteria.
Statistical Analysis
Patient characteristics were summarized descriptively by (a) number of PB pills/day and (b) patient-reported PB non-adherence. Proportional odds models based on generalized estimating equations (GEE) were used to estimate the association between number of PB pills/day and patient-reported PB non-adherence (treated as a 4-category ordinal outcome variable). To account for clustering within facilities, an independent working correlation was assumed. The proportional odds assumption was verified using a score test. We also estimated the association between PB type and PB non-adherence in a similar proportional odds model.
Adjusted logistic regression models with SPhos > 5.5 mg/dL as the outcome were used to estimate the effects of both patient number of PB pills/day and facility median PB pills/day, with and without PTH adjustment. Models were based on GEE and assumed a compound symmetry covariance structure. Similar models were used to assess the association between PTH > 600 pg/mL and PB pills/day, with and without SPhos adjustment. Adjusted logistic regression was used to estimate the effects of PB non-adherence on both SPhos > 5.5 mg/dL and PTH > 600 pg/mL. Different cutpoints were used in sensitivity analyses: SPhos > 4.5 mg/dL and PTH > 300 pg/mL. Models were adjusted for country, age, sex, black race, BMI, dialysis vintage, 13 comorbidities (listed in Table 1), albumin, creatinine, nPCR, facility percent Kt/V <1.2, and facility percent catheter use.
Table 1A.
Demographic and clinical characteristics across categories of the number of phosphate binder pills prescribed per day
| Patient characteristic | Total number of Phosphate Binder pills per day | ||||
|---|---|---|---|---|---|
| 1–2 pills | 3–5 pills | 6–8 pills | 9–11 pills | ≥ 12 pills | |
| N patients | 692 (15%) | 1655 (36%) | 1033 (23%) | 597 (13%) | 592 (13%) |
| Age (years) | 65.0 ± 13.8 | 62.9 ± 14.5 | 61.0 ± 14.2 | 59.3 ± 14.4 | 58.2 ± 13.0 |
| Gender (% male) | 61% | 61% | 58% | 62% | 63% |
| Body mass index (kg/m2) | 25.2 ± 5.5 | 25.9 ± 6.1 | 26.7 ± 6.5 | 27.7 ± 7.2 | 27.2 ± 6.9 |
| Vintage (years) | 2.7 (1.0, 8.1) | 2.7 (0.9, 5.9) | 2.9 (1.1, 6.3) | 3.1 (1.3, 6.4) | 4.8 (2.5, 8.7) |
| Phosphorus (mg/dL) | 4.8 (4.0, 5.8) | 5.1 (4.2, 6.2) | 5.2 (4.3, 6.3) | 5.7 (4.7, 6.8) | 5.6 (4.6, 6.6) |
| Phosphorus >4.5 mg/dL (%) | 59% | 67% | 68% | 78% | 78% |
| Phosphorus >5.5 mg/dL (%) | 31% | 39% | 41% | 52% | 51% |
| PTH (pg/mL) | 192 (99, 348) | 230 (124, 388) | 236 (128, 411) | 262 (150, 458) | 228 (125, 434) |
| PTH >300 pg/mL (%) | 31% | 37% | 38% | 44% | 40% |
| PTH >600 pg/mL (%) | 10% | 12% | 13% | 16% | 15% |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 |
| Creatinine (mg/dL) | 8.5 ± 2.7 | 8.9 ± 2.8 | 9.2 ± 2.8 | 9.5 ± 2.9 | 10.5 ± 2.9 |
| Normalized PCR (g/kg/day) | 0.97 ± 0.24 | 1.01 ± 0.25 | 1.02 ± 0.24 | 0.99 ± 0.24 | 1.03 ± 0.23 |
| Hemoglobin (g/dL) | 11.4 ± 1.4 | 11.3 ± 1.3 | 11.4 ± 1.4 | 11.4 ± 1.4 | 11.3 ± 1.3 |
| Single Pool Kt/V | 1.52 ± 0.33 | 1.50 ± 0.31 | 1.49 ± 0.31 | 1.48 ± 0.29 | 1.48 ± 0.27 |
| Catheter Use (%) | 27% | 23% | 23% | 24% | 13% |
| Comorbid conditions (%) | |||||
| Coronary artery disease | 32% | 36% | 36% | 37% | 36% |
| Cancer (non-skin) | 17% | 15% | 12% | 13% | 9% |
| Other cardiovascular disease | 31% | 29% | 29% | 25% | 25% |
| Cerebrovascular disease | 14% | 15% | 13% | 12% | 10% |
| Coronary heart failure | 20% | 23% | 22% | 25% | 28% |
| Diabetes | 37% | 39% | 43% | 46% | 45% |
| Gastrointestinal bleeding | 4% | 5% | 5% | 4% | 5% |
| Hypertension | 82% | 85% | 85% | 85% | 83% |
| Lung disease | 12% | 11% | 12% | 13% | 10% |
| Neurologic disease (any) Dementia or Cognitive | 8% | 9% | 8% | 8% | 8% |
| Impairment | 6% | 6% | 5% | 4% | 3% |
| Psychiatric disorder | 14% | 17% | 19% | 19% | 17% |
| Peripheral vascular disease | 30% | 26% | 26% | 25% | 23% |
| Recurrent cellulitis/gangrene | 10% | 9% | 11% | 12% | 12% |
| Physician contact frequency (%) | |||||
| 2 or 3 times per week | 51% | 43% | 39% | 34% | 32% |
| Weekly | 18% | 19% | 21% | 24% | 27% |
| Less than once per week | 31% | 38% | 40% | 41% | 41% |
| Skipped any HD sessions* | 3% | 3% | 4% | 5% | 5% |
| Shortened HD session by >30 mins* | 6% | 8% | 9% | 11% | 13% |
| Married (%) | 60% | 56% | 55% | 49% | 56% |
| At least high school eduction (%) | 56% | 59% | 60% | 63% | 65% |
| Employed (%) | 13% | 16% | 17% | 16% | 20% |
| Living status: alone (%) | 16% | 21% | 21% | 23% | 19% |
| Living status: with family (%) | 80% | 76% | 77% | 73% | 79% |
| Living status: nursing home (%) | 4% | 3% | 3% | 3% | 3% |
Note: N patients sum to 4,569 rather than 5,262 due to missing data on number of pills
Skipped or shortened any HD sessions in 30 days prior to DOPPS enrollment
Table shows Mean ± SD for variables with normal distributions, Median (IQR) for variables with skewed distributions, or %.
Missing covariate values were imputed multiply using the chained equation method 20 by IVEWARE 21. Results from five imputed data sets were combined for the final analysis using Rubin’s formula 22. The proportion of missing data was below 5% for all imputed covariates, with the exception of nPCR (19%), Kt/V (18%), number of pills/day (13%), PTH (11%), and BMI (6%). Sensitivity analyses were performed using 20 imputed datasets to reduce the between-dataset variability of the regression coefficients, and results were all qualitatively consistent. All analyses used SAS software, version 9.2 (SAS institute, Cary, NC). Study approval was obtained by a central institutional review board. Additional study approval and patient consent were obtained as required by national and local ethics committee regulations.
RESULTS
Number of phosphate binder pills prescribed per day
Proportions of patients prescribed only one type of PB were: (1) calcium-based: 37%, (2) sevelamer: 25%, (3) lanthanum: 7%, and (4) other PB type: 3%. Twenty-eight percent were prescribed any combination. Overall mean ± standard deviation (SD) number of PB pills/day was 6.0 ± 4.3 and ranged from 7.4 ± 4.7 in the US to 3.9 ± 2.7 in France (Figure 1). Mean ± SD number of PB pills/day was 8.4 ± 4.9 among patients with multiple types of PBs, 6.1 ± 4.4 among those on sevelamer only, 4.9 ± 3.1 among those on an “other” PB type, 4.7 ± 3.1 among those on calcium-based PBs only, and 3.1 ± 1.5 among those on lanthanum only. Table 1A shows the study population demographic and clinical characteristics across the five categories of PB pills prescribed per day. Patients prescribed more PB pills/day were younger, had a higher BMI, higher serum creatinine, higher phosphorus and PTH levels, and were more likely to have a high school education and be employed, but were less likely to have a catheter for access. Patients prescribed only 1–2 PB pills/day were more likely to see their physician 2–3 times/week than those prescribed ≥12 PB pills/day (51% vs. 32%). There was little variation in facility serum phosphorus targets across the 12 countries, as reported by the medical directors (data not shown). The mean lower target ranged from 2.59 (Sweden) to 3.55 (France) mg/dL; the mean upper target ranged from 4.96 (Australia-New Zealand) to 5.88 (Japan) mg/dL.
Figure 1.
Distribution of number of prescribed phosphate binder (PB) pills/day, by country, among N=4,569 patients (13% missing data). US=United States, JPN=Japan, ANZ=Australia/New Zealand, GER=Germany, SWE=Sweden, CAN=Canada, SPA=Spain, BEL=Belgium, ITA=Italy, UK=United Kingdom, FRA=France.
Patient-reported PB non-adherence
Patient-reported PB non-adherence, defined as skipping PBs more than 3 times in the past month, varied across countries. It was highest in the US (24%) and lowest in Belgium, Japan, Germany, and Spain (12% in each country) (Figure 2). Overall, 55% of patients reported taking all of their PB pills in the past month, 27% skipped 1–3 times, 10% skipped 3–6 times, and 8% skipped ≥7 times. Table 1B shows demographic and clinical characteristics across the four categories of patient-reported PB non-adherence. Patients who reported skipping PB pills more frequently were younger, had higher phosphorus and PTH levels, were more likely to have a psychiatric disorder, and were less likely to be married. Among patients who reported taking all their PBs, 46% saw their physician 2–3 times/week. Among patients who reported skipping PBs ≥7 times in the past month, only 29% saw their physician 2–3 times/week. Overall, patients prescribed sevelamer were less likely to be non-adherent than patients prescribed a Ca-based PB (data not shown).
Figure 2.
Distribution of patient-reported PB non-adherence, defined as how often the patient skipped taking their PB medication in the past month, by country, among N=5,262 patients. BEL=Belgium, JPN=Japan, GER=Germany, SPA=Spain, ITA=Italy, SWE=Sweden, FRA=France, CAN=Canada, ANZ=Australia/New Zealand, UK=United Kingdom, US=United States.
Table 1B.
Demographic and clinical characteristics across categories of patient-reported non-adherence to phosphate binder prescription
| Patient characteristic | All patients | Number of times skipped PBs in past month | |||
|---|---|---|---|---|---|
| Took them all | 1–3 times | 3–6 times | ≥ 7 times | ||
| N patients | 5,262 | 2913 (55%) | 1417 (27%) | 516 (10%) | 416 (8%) |
| Age (years) | 61.8 ± 14.2 | 63.8 ± 13.3 | 60.4 ± 14.8 | 58.5 ± 14.8 | 56.7 ± 15.5 |
| Gender (% male) | 61% | 60% | 61% | 59% | 62% |
| Body mass index (kg/m2) | 26.1 ± 6.4 | 25.7 ± 6.2 | 26.4 ± 6.5 | 26.8 ± 6.7 | 27.3 ± 7.0 |
| Vintage (years) | 3.2 (1.2, 7.1) | 3.3 (1.1, 7.5) | 2.9 (1.2, 6.5) | 3.6 (1.3, 6.9) | 2.8 (1.0, 6.6) |
| Phosphorus (mg/dL) | 5.2 (4.3, 6.3) | 5.1 (4.2, 6.0) | 5.3 (4.4, 6.4) | 5.6 (4.6, 6.8) | 5.7 (4.8, 7.1) |
| Phosphorus >4.5 mg/dL (%) | 69% | 65% | 70% | 76% | 79% |
| Phosphorus >5.5 mg/dL (%) | 41% | 36% | 44% | 52% | 55% |
| PTH (pg/mL) | 224 (117, 398) | 209 (106, 357) | 227 (130, 412) | 275 (149, 480) | 281 (144, 504) |
| PTH >300 pg/mL (%) | 37% | 33% | 38% | 45% | 48% |
| PTH >600 pg/mL (%) | 12% | 10% | 14% | 17% | 19% |
| Albumin (g/dL) | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 |
| Creatinine (mg/dL) | 9.2 ± 2.9 | 9.3 ± 3.0 | 9.3 ± 2.9 | 9.2 ± 2.9 | 9.0 ± 2.8 |
| Normalized PCR (g/kg/day) | 1.00 ± 0.24 | 1.01 ± 0.23 | 0.99 ± 0.24 | 1.00 ± 0.26 | 1.01 ± 0.26 |
| Hemoglobin (g/dL) | 11.3 ± 1.3 | 11.3 ± 1.3 | 11.4 ± 1.3 | 11.3 ± 1.5 | 11.3 ± 1.4 |
| Single Pool Kt/V | 1.50 ± 0.30 | 1.50 ± 0.30 | 1.49 ± 0.31 | 1.53 ± 0.29 | 1.47 ± 0.32 |
| Catheter Use (%) | 22% | 22% | 21% | 22% | 24% |
| Comorbid conditions (%) | |||||
| Coronary artery disease | 36% | 36% | 34% | 36% | 38% |
| Cancer (non-skin) | 13% | 14% | 13% | 13% | 12% |
| Other cardiovascular disease | 28% | 30% | 27% | 22% | 26% |
| Cerebrovascular disease | 13% | 14% | 15% | 10% | 11% |
| Coronary heart failure | 23% | 22% | 24% | 21% | 28% |
| Diabetes | 41% | 39% | 42% | 43% | 48% |
| Gastrointestinal bleeding | 5% | 5% | 5% | 6% | 3% |
| Hypertension | 84% | 84% | 83% | 86% | 86% |
| Lung disease | 12% | 11% | 10% | 12% | 16% |
| Neurologic disease (any) Dementia or Cognitive | 8% | 9% | 8% | 7% | 9% |
| Impairment | 5% | 5% | 5% | 3% | 5% |
| Psychiatric disorder | 17% | 15% | 16% | 21% | 25% |
| Peripheral vascular disease | 26% | 26% | 26% | 24% | 28% |
| Recurrent cellulitis/gangrene | 10% | 9% | 10% | 10% | 15% |
| Physician contact frequency (%) | |||||
| 2 or 3 times per week | 41% | 46% | 37% | 32% | 29% |
| Weekly | 21% | 20% | 23% | 22% | 24% |
| Less than once per week | 38% | 34% | 40% | 47% | 48% |
| Skipped any HD sessions* | 4% | 2% | 4% | 5% | 9% |
| Shortened HD session by >30 mins* | 9% | 7% | 10% | 14% | 17% |
| Married (%) | 56% | 60% | 54% | 52% | 45% |
| At least high school eduction (%) | 61% | 59% | 61% | 63% | 67% |
| Employed (%) | 17% | 15% | 18% | 21% | 20% |
| Living status: alone (%) | 20% | 18% | 22% | 22% | 23% |
| Living status: with family (%) | 77% | 78% | 77% | 77% | 75% |
| Living status: nursing home (%) | 3% | 4% | 2% | 1% | 3% |
Skipped or shortened any HD sessions in 30 days prior to DOPPS enrollment
Table shows Mean ± SD for variables with normal distributions, Median (IQR) for variables with skewed distributions, or %.
Number of PB pills prescribed per day and patient-reported PB non-adherence
Figure 3 shows the association between number of prescribed PB pills/day and PB non-adherence. The cumulative odds ratio (OR) of skipping PB pills more often (i.e., being in a higher category) was 1.05 (95% confidence interval [CI]: 1.01–1.10) per 3 more PB pills/day adjusted for country, and 1.03 (95% CI: 0.99–1.08) per 3 more PB pills/day after further case-mix adjustment. There was a trend towards better adherence for patients prescribed only 1–2 PB pills/day versus ≥3 PB pills/day; cumulative OR=0.86 (95% CI: 0.72–1.03).
Figure 3.
Proportional odds logistic regression models used to estimate the association between number of PB pills/day and PB non-adherence (treated as a 4-category ordinal outcome variable). Both models adjusted for country and accounted for facility clustering effects using Generalized Estimating Equations (GEE). Case-mix adjustment: age, sex, black race, body mass index, vintage, 13 comorbidities, albumin, creatinine, nPCR, facility percent Kt/V < 1.2, and facility percent catheter use. Trend was assessed by including number of PB pills/day as a continuous variable (p for trend = 0.01 adjusted for country and 0.15 after case-mix adjustment).
Number of PB pills prescribed per day and control of SPhos and PTH levels
Patients prescribed a higher number of PB pills/day were more likely to have SPhos > 5.5 mg/dL (Figure 4, Panel 1), consistent with the indication for PB prescription and/or possible non-adherence to the higher pill burden regimen. Treating PB pills/day as a continuous variable to assess the trend, OR (95% CI) of SPhos > 5.5 mg/dL was 1.09 (1.03–1.15) per 3 more PB pills/day. On the other hand, facilities with a higher median number of prescribed PB pills/day were less likely to have patients with SPhos > 5.5 mg/dL, probably reflecting the therapeutic effect of more aggressive PB prescription. The OR (95% CI) of SPhos > 5.5 mg/dL was 0.86 (0.76–0.97) per 3 PB pills/day higher facility median. Results were qualitatively consistent after adjustment for PTH and also when using a cutpoint of 4.5 mg/dL. Patients with a higher number of prescribed PB pills/day were more likely to have PTH > 600 pg/mL, but this relationship was attenuated after adjustment for SPhos (Figure 4, Panel 2). Facility median prescribed PB pills/day was not strongly associated with PTH > 600 pg/mL. In sensitivity analyses using a cutpoint of 300 pg/mL, PB pills/day was similarly weakly associated with elevated PTH.
Figure 4.
Logistic regression models with SPhos > 5.5 mg/dL and PTH > 600 pg/mL as the outcome variables used to estimate the effects of (a) patient number of PB pills/day and (b) facility median number of PB pills/day. All models adjusted for country and accounted for facility clustering effects using Generalized Estimating Equations. Case-mix adjustment: age, sex, black race, body mass index, vintage, 13 comorbidities, albumin, creatinine, nPCR, facility percent Kt/V < 1.2, and facility percent catheter use. For models with SPhos > 5.5 mg/dL as the outcome, effects were estimated with and without adjustment for PTH. For models with PTH > 600 pg/mL as the outcome, effects were estimated with and without adjustment for SPhos.
Patient-reported PB non-adherence and SPhos and PTH levels
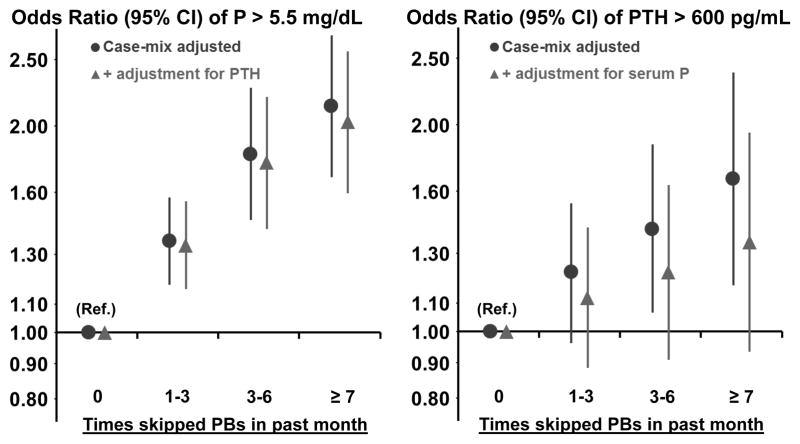
Patients who skipped PBs more often were more likely to have high SPhos in a model adjusted for case-mix but not for PB pills/day (Figure 5). Compared to patients who took all of their PB medications, patients who skipped PBs ≥ 7 times in the past month were much more likely to have a SPhos > 5.5 mg/dL (OR=2.14, 95% CI: 1.68–2.72). Even patients who only skipped 1–3 times in the past month were more likely to have SPhos > 5.5 mg/dL (OR=1.36, 95% CI: 1.18–1.57). The association was attenuated only slightly by adjustment for PTH. Results were consistent within each DOPPS region: North America, Europe/Australia-New Zealand, and Japan (data not shown, p for region interaction = 0.7). Patients on hemodiafiltration (within Europe only) and with longer dialysis session length were less likely to have a high SPhos, though a sensitivity analysis additionally adjusting for these two variables only minimally attenuated the association between PB adherence and SPhos. Further adjustment for total alkaline phosphatase did not impact model results. It must also be noted that the association between HDF, longer treatment time, and lower S Phos was observed within this subset of the DOPPS sample restricted to patients who both responded to the self-administered patient questionnaire and were prescribed a PB; the association may differ when analyzing the complete DOPPS cohort.
Figure 5.
Logistic regression models with SPhos > 5.5 mg/dL and PTH > 600 pg/mL as the outcome variables used to estimate the effects of non-adherence to PB prescription, defined as how often the patient skipped taking their PB medication in the past month. All models adjusted for country and accounted for facility clustering effects using Generalized Estimating Equations. Case-mix adjustment: age, sex, black race, body mass index, vintage, 13 comorbidities, albumin, creatinine, nPCR, facility percent Kt/V < 1.2, and facility percent catheter use. For models with SPhos > 5.5 mg/dL as the outcome, effects were estimated with and without adjustment for PTH. For models with PTH > 600 pg/mL as the outcome, effects were estimated with and without adjustment for SPhos. Test for trend for SPhos: p<0.0001 adjusting for case-mix and p<0.0001 after additional adjustment for PTH. Test for trend for PTH: p=0.001 adjusting for case-mix and p=0.06 after additional adjustment for SPhos.
Patients who skipped PBs more often were also more likely to have high PTH levels (Figure 5). The estimated effects were attenuated greatly, by nearly one half, after adjustment for SPhos; patients who skipped PBs ≥ 7 times in the past month were more likely to have a PTH > 600 pg/mL (OR=1.35, 95% CI: 0.93–1.95) compared to patients who took all their PBs. Results were qualitatively consistent when using a cutpoint of 300 pg/mL.
DISCUSSION
Our study is the first to report high prescribed PB pill burden and high patient-reported PB non-adherence in an international cohort of HD patients. It is notable that in the US, fewer than half of the patients reported taking all their PBs in the month prior to the survey. Even in Japan, a country where remarkable adherence to treatment regimens has been demonstrated 23, 35% of the patients reported skipping PBs at least once in the month prior to the survey.
Reasons for PB non-adherence may include overall treatment non-adherence, lower socioeconomic status, or lack of understanding of likely benefits of lower SPhos 24,25,26. Another possible reason is a high pill burden. Consistent with other observational studies, we show a trend towards an association (unadjusted p=0.01, adjusted p=0.15) between higher PB pill burden and patient-reported PB non-adherence 16,27. Our results are also consistent with findings from a recent paper by Wang et al that estimated adherence using the medication possession ratio (MPR), defined as the proportion of time a patient had sufficient medication to have taken it as prescribed 28. Wang et al, in a sample of patients limited to the US, showed an association between lower MPR and higher pill burden. It is notable that the Wang et al study and our study, using different definitions of adherence, both found associations between lower adherence and higher prescribed pill burden. At least one other study, a randomized trial, showed improved PB adherence and equivalent phosphorus control with fewer prescribed pills 29. Simpler, more achievable PB regimens could lead to better adherence and perhaps lower phosphorus and PTH levels. For patients with high SPhos, it may be more effective to first assess what pills patients are actually taking and make efforts to reduce the pill number, before increasing the PB prescription. Assessment of other causes of a high SPhos such as high dietary intake, inadequate dialysis treatment time, and increased bone resorption may be particularly relevant for some patients. If inadequately addressed, these factors may lead to prescription of additional PBs, an increased PB burden, and thus may perhaps contribute to non-adherence. Other strategies include patient education, increasing prescribed PB strength, addressing financial barriers, and perhaps even addressing mental health issues such as depression. The trend toward improved PB adherence with greater physician-contact time could be related to selection bias, as physicians may spend more time with patients who follow the treatment plan. However, the trend toward improved PB adherence with greater physician-contact time could also be because the greater contact time gives physicians and other staff more chance to review a PB regimen, emphasize the importance of adherence, address patient-specific barriers to adherence, and tailor the prescription to an individual patient.
Our study shows a trend toward an association between higher number of PB pills prescribed and SPhos > 5.5 mg/dL and serum PTH > 600 pg/mL, cutpoints chosen based on common clinical practice guidelines at the time of the study. Because patients with higher SPhos are likely to be prescribed more PBs, even with adjustment, SPhos levels may be higher in patients using more binders. A facility-level approach was applied to reduce this potential treatment by indication bias. The facility-level analysis should lessen bias in the estimate of the causal association between number of prescribed PB pills and SPhos, by capturing facility tendency to prescribe more or fewer PBs rather than patient-level indications for PB dosing. This approach can reduce confounding due to unmeasured or poorly specified patient-level variables. In the facility-level analysis, higher facility median prescribed PB pills was associated with lower SPhos, suggesting that PB adherence may be related to dialysis unit practices. Responsibility for medication adherence may sometimes be placed entirely on the patient, but dialysis facilities have an important role in patient education and consistent medication review and reconciliation. Focused education and implementation of standardized nutrition guidelines by renal dieticians may increase patient and staff knowledge, as well as improve nutrition status and reduce SPhos 30,31.
The remarkably high patient-reported PB non-adherence suggests cost savings potential. For 2010 in the US, 4 of the most costly 6 oral medications for Part D-enrolled dialysis patients were PBs, with an estimated one year total cost of $448,170,874 32. Inclusion of oral medications in the Medicare Part B ESRD bundled payment system has been delayed until 2024 33. Monthly SPhos measurements are required in the US, and will be included in the Quality Incentive Program (QIP) starting in 2015 34. In the future, these reimbursement changes may provide financial incentive for efficient PB use. In the 2010 DOPPS Annual Report, 85% of US patients were prescribed PBs 35. Our analysis suggests that the number of PB pills the patient is prescribed, and that are paid for when filled, may be higher than the number of PB pills the patient actually takes. In response to high SPhos, providers may increase PBs, instead of assessing what pills patients are actually taking. Providers may also automatically refill expired PB prescriptions, instead of confirming adherence with the patient. Aligning prescriptions with actual patient behavior could provide net cost savings.
Patient characteristics and comorbid conditions were evenly distributed across groups of pills/day (Table 1A), and times PBs were skipped (Table 1B), with a few notable exceptions. Patients with skipped or shortened treatment sessions were also likely to report a higher number of skipped PB times in the past month. Higher vintage was associated with higher number of PB pills prescribed, consistent with the natural history of secondary hyperparathyroidism in ESRD patients 36, but was not associated with a higher number of skipped PB pills per month. Higher vintage patients were more compliant with a more complicated PB regimen than lower vintage patients. Our study does not assess whether higher vintage leads to better understanding of phosphorus control. However, in theory, education by nephrologists, renal dieticians, and nurses could improve a dialysis patient’s knowledge base over time, and thus improve PB adherence. More adherent patients may also simply live longer, whether because of better phosphorus control or because better phosphorus control is an indicator of better general adherence.
One of the strengths of this study is that it includes detailed information on socioeconomic factors that have been associated with adherence, such as education, living status, and employment. The most notable finding was that a high percentage of patients who were married or living with family reported that they took all their PBs in the month prior to the survey. This is consistent with prior work showing better outcomes in married patients, and may confirm the importance of family support in helping patients adhere to a treatment regimen 37.
This study has limitations. It is an observational study, so we cannot rule out bias by unmeasured confounders. The study design is cross-sectional, so inference of cause and effect between variables is limited, though analysis of facility-level variables can help reduce this limitation. In addition, cause and effect between different variables related to metabolic bone disease may be difficult to precisely describe using observational models, and the possibility for over-adjustment exists. We thus present models in Figures 4 and 5 with and without potential over-adjustment for SPhos and PTH.
There are also limitations to the data that were collected in this multinational, population-based study. There may be variability in PTH levels, depending on how the sample was collected, and depending on the assay used. It is likely that bias from type of assay is minimal, because fewer than 10% of DOPPS facilities reported using bio-intact PTH assays, and for most of the study period intact PTH (iPTH) was the only clinical assay in use. In addition, the bio-intact assay yields lower values than the intact PTH assay, leading to a decrease in the strength of the associations reported between non-adherence and higher PTH levels 38. It is also notable that actual pill counts are beyond the scope of the DOPPS dataset, and so we cannot verify patient self-reported data on the number of times they skipped their binders in the month prior to the survey. We cannot determine whether patients under- or over-reported their adherence. Our study also does not specifically collect information about patients’ diets, and so we cannot estimate the influence of actual phosphorus intake on the associations shown.
In summary, our results provide evidence that PB pill burden and adherence vary by country, and show a trend toward greater non-adherence with a higher PB pill burden. In addition to patient education and dietary advice, simplified and streamlined PB regimens could potentially offer opportunities for improvements in patient outcomes and cost savings.
Acknowledgments
Funding
The DOPPS program would not be possible without the generous financial support and strong commitment to independent scientific research by our principal study sponsors: Amgen (founding sponsor, since 1996), Kyowa Hakko Kirin (since 1999, in Japan), AbbVie Inc. (since 2009), Sanofi Renal (since 2009), Baxter Healthcare (since 2011), and Vifor Fresenius Medical Care Renal Pharma, Ltd (since 2011). Additional support is also provided for specific projects and countries by a number of generous organizations. The dopps.org website lists full details. Support from DOPPS sponsors is provided without restrictions on publications. The DOPPS is coordinated by Arbor Research Collaborative for Health, Ann Arbor, MI U.S.A.
F Tentori, A Karaboyas, BA Bieber, Y Li, RL Pisoni, and BM Robinson, are employees of Arbor Research Collaborative for Health.
F. Tentori is supported in part by NIDDK grant 1K01DK087762-01A1; she has received honoraria from Amgen, Dialysis Clinic Inc., and Renal Research Institute.
The authors wish to thank Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, for providing editorial assistance for this manuscript.
The results presented in this paper have not been published previously in whole or part, except in abstract form.
Footnotes
Conflicts of Interest
the coauthors have no conflicts of interest to declare.
References
- 1.Goodkin DA, Young EW, Kurokawa K, Prutz K, Levin N. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis. 2004;44(S2):S16–S21. doi: 10.1053/j.ajkd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Lowrie EG, Lew N. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 3.Kalpakian MA, Mehrotra R. Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dialysis. 2007;20(2):139–143. doi: 10.1111/j.1525-139X.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 5.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 6.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Giachelli CM. Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrol. 2003;14:S300–S304. doi: 10.1097/01.asn.0000081663.52165.66. [DOI] [PubMed] [Google Scholar]
- 8.Jono S, McKee MD, Murray, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 9.Di Marco GS, Konig M, Stock C, et al. High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int. 2013;83:213–222. doi: 10.1038/ki.2012.300. [DOI] [PubMed] [Google Scholar]
- 10.Isakova T, Gutierrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20(2):388–96. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes A, Tong L, Thumma J, et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Evaluation of possible confounding by nutritional status. Am J Kidney Dis. 2012;60:90–101. doi: 10.1053/j.ajkd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 13.Curtin RB, Svarstad BL, Keller TH. Hemodialysis patients’ noncompliance with oral medications. ANNA J. 1999;26:307–16. [PubMed] [Google Scholar]
- 14.The USRDS Dialysis Morbidity and Mortality Study: Wave 2. United States Renal Data System. Am J Kidney Dis. 1997;30:S67–S85. [PubMed] [Google Scholar]
- 15.Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: Comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842–8. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 16.Chiu Y, Teitelbaum I, Misra M, Marie de Leon E, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingersol KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of the literature. J Behav Med. 2008;31:213–224. doi: 10.1007/s10865-007-9147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study: an international hemodialysis study. Kidney Int. 2000;57:S74–S81. [Google Scholar]
- 19.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(Suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.IVEware: Imputation and Variance Estimation Software by: Trivellore E. Raghunathan, Peter W. Solenberger, John Van Hoewyk.
- 22.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 23.Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: Associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard R, Berger W, Bailie GR, Eisele G. Knowledge of hemodialysis and CAPD patients about their prescribed medicines. Clin Nephrol. 1990;34:173–178. [PubMed] [Google Scholar]
- 25.Chater AM, Parham R, Riley S, Hutchison AJ, Horne R. Profiling patient attitudes to phosphate binding medication: a route to personalizing treatment and adherence support. Psychol Health. 2014;29:1407–20. doi: 10.1080/08870446.2014.942663. [DOI] [PubMed] [Google Scholar]
- 26.Schlebusch L, Levin A. Psychotherapeutic management of good and poor compliance in haemodialysis. S Afr Med J. 1982;16:92–4. [PubMed] [Google Scholar]
- 27.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant. doi: 10.1093/ndt/gft280. Epub ahead of print 2013 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer D, Cline K, Plone MA, Dillon M, Burke SK, Blair AT. Results of a randomized crossover study comparing once-daily then thrice-daily sevelamer dosing. Am J Kidney Dis. 2006;48:437–444. doi: 10.1053/j.ajkd.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Toussaint ND, Pedagogos E, Beavis J, Becker GJ, Polkinghorne KR, Kerr PG. Improving CKD-MBD management in haemodialysis patients: barrier analysis for implementing better practice. Nephrol Dial Transplant. 2011 Apr;26(4):1319–26. doi: 10.1093/ndt/gfq602. [DOI] [PubMed] [Google Scholar]
- 31.Campbell KL, Ash S, Zabel R, McFarlane C, Juffs P, Bauer JD. Implementation of standardized nutrition guidelines by renal dietitians is associated with improved nutrition status. J Ren Nutr. 2009 Mar;19(2):136–44. doi: 10.1053/j.jrn.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 32.USRDS. Atlas of CKD and ESRD. [Accessed March 19, 2013];volume 2, chapter 6 - prescription drug coverage. 2012 :280. Available at http://www.usrds.org/atlas.aspx.
- 33.Feldman RL, Desmarais MP, Muller JS. Orals in the Bundle: A Policy Framework. Clin J Am Soc Nephrol. 2013;8(6):1043–7. doi: 10.2215/CJN.11621112. [DOI] [PubMed] [Google Scholar]
- 34.Department of Health and Human Services. Centers for Medicare & Medicaid Services. 42 CFR Parts 413 and 417. Medicare Program; End-Stage Renal Disease Prospective Payment System, Quality Incentive Program, and Bad Debt Reductions for All Medicare Providers; Final Rule. Federal Register. 2012 Nov 9;77(218) [PubMed] [Google Scholar]
- 35. [Accessed April 8, 2013];2010 DOPPS Annual Report. Available for download at: http://www.dopps.org/annualreport/
- 36.Mark SJ. Hyperparathyroid and Hypoparathyroid Disorders. NEJM. 2000;343(25):1863–1875. doi: 10.1056/NEJM200012213432508. [DOI] [PubMed] [Google Scholar]
- 37.King KB, Reis HT. Marriage and long-term survival after coronary artery bypass grafting. Health Psychol. 2012;31(1):55–62. doi: 10.1037/a0025061. [DOI] [PubMed] [Google Scholar]
- 38.Tentori F, Wang M, Bieber B, Karaboyas A, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. CJASN. 2014 Dec 16; doi: 10.2215/CJN.12941213. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]