Abstract
Glial activation is hypothesized to contribute directly to opioid withdrawal. This study investigated the dose-dependent effects of a glial cell-modulator, ibudilast, on withdrawal symptoms in opioid-dependent volunteers after abrupt discontinuation of morphine administration.
Methods
Non-treatment seeking heroin-dependent volunteers (N = 31) completed the inpatient, double-blind, placebo-controlled, within-subject and between-group study. Volunteers were maintained on morphine (30 mg, QID) for 14 days and placebo (0 mg, QID) for the last 7 days of the 3-week study. Volunteers also received placebo (0 mg, PO, BID) capsules on Days 1–7. On days 8–21, volunteers were randomized to receive ibudilast (20 or 40 mg, PO, BID) or placebo capsules. Subjective and clinical ratings of withdrawal symptoms were completed daily using daily using the Subjective Opioid Withdrawal Scale (SOWS) and Clinical Opioid Withdrawal Scale (COWS). Medication side effects were also monitored.
Results
Relative to the first two weeks, all groups exhibited withdrawal during the third week as assessed by the SOWS and COWS (p ≤ 0.0001). Although overall SOWS scores did not differ between groups, exploratory analyses pooling the two ibudilast groups demonstrated that they had lower ratings of withdrawal symptoms on SOWS items (‘Anxious,’ ‘Perspiring,’ ‘Restless,’ ‘Stomach Cramps’) during detoxification relative to the placebo group. Ibudilast was well tolerated; no serious adverse events occurred during the study.
Conclusion
Pharmacological modulation of glial activity with ibudilast decreased some subjective ratings of opioid withdrawal symptoms. These exploratory findings are the first to demonstrate the potential clinical utility of glial modulators for treating opioid withdrawal in humans.
Keywords: Detoxification, Glia, Morphine, Opioid, Subjective effects, Withdrawal
INTRODUCTION
Epidemiological reports have demonstrated that opioid dependence continues to be a significant public health concern, underscoring the need for various treatment options to prevent and treat opioid-related substance use disorders (SAMHSA, 2013). A contributing factor to the continued use of opioids is the characteristic withdrawal syndrome that develops after cessation of drug administration (Koob and Le Moal, 2001). Current effective pharmacotherapy modalities for treating opioid-related substance use disorders are primarily opioid agonist treatments such as methadone and buprenorphine, which decrease rates of relapse and ameliorate the withdrawal symptoms that hinder the treatment process (Stotts et al., 2009). While long-term agonist maintenance may be the most clinically appropriate treatment option for some patients (Bart, 2012), other options for treating opioid-related substance use disorders may require an agonist taper, as is the case when transitioning from a full agonist, like methadone, to a partial agonist maintenance medication, like buprenorphine. When transitioning from agonist to naltrexone maintenance, complete detoxification is required (Lobmaier et al., 2010). Under these circumstances, non-opioid adjunctive medications to help alleviate withdrawal are critical to ensure the comfort of the patient. However, the adverse effects of some adjunctive medications currently utilized to treat withdrawal symptoms limit their clinical utility. For example, adrenergic agonists, such as clonidine and lofexidine, produce negative hemodynamic effects (Gowing et al., 2003), and benzodiazepines have significant abuse liability risks (Lintzeris et al., 2009). The current study was designed to explore the therapeutic potential of Ibudilast, a glial cell-modulator, as a non-opioid adjunct medication to attenuate withdrawal symptoms during detoxification. The favorable safety profile of Ibudilast is evidenced by its clinical use for over 20 years to treat asthma and post-stroke dizziness in Japan with minimal side effects (Gibson et al., 2006; Rolan et al., 2009).
Preclinical studies have reliably demonstrated that opioid administration induces a neuroimmune response by increasing glial cell activity, resulting in production of a variety of immune factors including cytokines and chemokines (Beitner-Johnson et al., 1993; Walter, 1997; Cannon, 2000; Garrido et al., 2005). This proinflammatory response contributes to the development of opioid tolerance and dependence in preclinical investigations (Angst and Clark, 2006; Liu et al., 2011). In rodents, proinflammatory cytokine receptor antagonists attenuate behavioral signs of opioid withdrawal (Hutchinson et al., 2008; Hutchinson et al., 2009), verifying that the opioid-induced proinflammatory response contributes to the development of dependence. These findings strongly suggest that glial cell-inhibitors have clinical potential to treat opioid-related substance use disorders and also improve the therapeutic use of opioid analgesics by decreasing neurobiological substrates that contribute to the development of dependence.
Ibudilast, a glial cell-modulator and relatively non-selective phosphodiesterase inhibitor, decreases neurobiological markers indicative of the opioid-induced proinflammatory response, and attenuates both antagonist-precipitated and deprivation-induced morphine withdrawal in rodents (Hutchinson et al., 2009). In addition to its clinical utility for asthma and post-stroke dizziness (Gibson et al., 2006; Rolan et al., 2009), Ibudilast is currently being explored as a potential treatment for neuropathic pain (Rolan et al., 2008), progressive multiple sclerosis (Barkhof et al., 2010; Johnson et al., 2014), and alcohol- and methamphetamine-use disorders in human volunteers (Cooper et al., 2012; Beardsley and Hauser, 2014; Ray et al., 2014). The current study sought to investigate the potential of ibudilast to reduce opioid-withdrawal symptoms after abrupt discontinuation of daily morphine administration in non-treatment-seeking heroin-dependent volunteers. With no ancillary medications administered to alleviate withdrawal symptoms, differences in subjective and clinical assessment of withdrawal symptoms between volunteers receiving placebo, low dose (20 mg, BID), and high dose (40 mg, BID) of ibudilast were compared. An additional aim of the study was to establish the safety and tolerability of ibudilast in the context of both opioid stabilization and abrupt discontinuation of opioid administration.
MATERIALS AND METHODS
Volunteers
Normal, healthy non-treatment seeking heroin users 21–45 years of age were recruited through local newspaper advertisements. Those who met inclusion/exclusion criteria after an initial telephone screen were invited to the laboratory for further screening. Prior to enrollment, volunteers gave written informed consent, received a psychiatric and medical evaluation, and provided detailed drug use and medical histories. Volunteers were accepted into the study if they were healthy, as determined by a physical examination (including electrocardiogram, and urine and blood chemistries), were opioid dependent, as determined by a naloxone challenge, and not physically dependent on any other substances aside from nicotine or caffeine. Volunteers seeking treatment for their opioid use and women who were pregnant or nursing were excluded from study participation. Volunteers were admitted into the study only after written informed consent to participate was given and eligibility criteria were verified. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki.
Drugs
Morphine sulfate solution (Cardinal Distribution Company, Syracuse, NY) was mixed in orange-flavored Gatorade with 1 ml peppermint oil floated on top to mask the taste of the drug. A total volume of 200 ml was administered at each dosing and was consumed within 5 min. We have used a similar procedure in other protocols to successfully mask the flavor of the beverage (Comer et al., 2010). Ibudilast (placebo, 20 mg, and 40 mg; obtained from MediciNova, Inc.) was administered in a size 00 opaque capsule with lactose filler prepared by the New York State Psychiatric Institute Research Pharmacy. These doses were chosen based upon the safety and tolerability of 80 mg/day ibudilast observed in diabetic neuropathic pain patients (Rolan et al., 2009).
Design and Procedures
This inpatient study consisted of three 1-week phases. Study Week 1 consisted of a morphine stabilization period during which all volunteers received morphine solution (30 mg, PO) four times per day (0800, 1400, 1900, and 2300 hr) and placebo ibudilast (0 mg, PO) two times a day at 0830 and 2030 hr. This week served to stabilize volunteers and standardize the level of opioid dependence before the detoxification phase. Study medication was introduced at the beginning of Study Week 2. Volunteers were randomly assigned to receive placebo, 20, or 40 mg ibudilast (PO) twice per day at 0830 and 2030 hr. All volunteers continued to receive morphine (30 mg, PO) four times per day (0800, 1400, 1900, and 2300 hr). This phase was implemented to ensure that steady-state ibudilast plasma levels were achieved prior to the morphine discontinuation phase (Rolan et al., 2008). Study Week 3 consisted of the morphine discontinuation phase when placebo (0 mg, PO) was substituted for morphine four times per day (0800, 1400, 1900, and 2300 hr). Volunteers continued to receive placebo, 20 mg, or 40 mg ibudilast twice per day during Study Week 3. During Week 3, ‘rescue medications’ including loperamide, ondansetron, Imodium, and clonazepam were available to help alleviate withdrawal symptoms only after a volunteer decided to discontinue study participation. Throughout the 21-day study, volunteer ratings and observer ratings of opioid withdrawal and physiological effects (pupil diameter, heart rate, blood pressure, respiratory rate, body temperature) were assessed twice a day at 1100 and 1600 hr.
On the 4th days of Study Weeks 1 and 2, volunteers participated in laboratory sessions designed to investigate the effects of ibudilast on the analgesic, subjective, and physiological effects of oxycodone (0, 25, and 50 mg PO). These findings will be discussed in a future publication. On these days, venous blood samples (15 cc each) were drawn and used to determine ibudilast plasma levels. Samples were drawn from an indwelling catheter into plastic vacuum tubes containing K2 EDTA at regular intervals before the morning dose of ibudilast (or placebo) and 9 additional samples were drawn at regular intervals during the 3-hour laboratory session. Samples were centrifuged (Vanguard V6500, Hamilton Bell Inc., Montvale, NJ) at 3000 rpm (approximately 1500 × g) for 10 minutes and plasma was transferred to polypropylene tubes and frozen (−20° C) until shipped with dry ice to the bioanalytical laboratory for analysis via high-performance liquid chromatography and tandem electrospray and positive ionization mass spectrometry with AV1040 (a structurally-related analog) as an internal standard (Rolan et al., 2008).
Dependent Measures
The effects of ibudilast on opioid withdrawal severity were determined by the number of days until subjects chose to discontinue study participation, subjective ratings of opioid withdrawal symptoms measured by the SOWS, and clinician ratings of opioid withdrawal symptoms measured by the COWS. Physiological measurements obtained each day (weight change, blood pressure, respiration, body temperature, and heart rate) by the MHOWS were analyzed to determine both safety and tolerability of the study medication (Study Week 2) and effects of study medication on physiological signs of withdrawal (Study Week 3).
SOWS
The SOWS consisted of a 16-item questionnaire asking volunteers to rate on a scale of 0 (not at all) to 4 (extremely) the intensity of common autonomic, gastrointestinal, musculoskeletal, and mood symptoms of opioid withdrawal (Handelsman et al., 1987). The maximum summed score for this scale is 64 (Handelsman et al., 1987).
COWS
The COWS is an 11-item rating system administered by a trained observer that includes autonomic, gastrointestinal, musculoskeletal, and mood symptoms of opioid withdrawal and 3 physiological endpoints including blood pressure, pupil diameter and pulse rate (Wesson and Ling, 2003). Possible scores vary across items, ranging from 0 (minimum score; no indication of symptom) to 5 (maximum score). Scores of all items are summed to determine if withdrawal is mild (5–12), moderate (13–24), moderately severe (25–36), or severe (>36). The maximum summed score for this scale is 48 (Wesson and Ling, 2003).
MHOWS
Physiological endpoints to determine safety and tolerability of Ibudilast were assessed with the MHOWS. The MHOWS is an observer-administered scale that rates the presence or absence of opioid withdrawal symptoms and medication effects including physiological effects (pupil diameter, heart rate, blood pressure, respiration, body temperature, and weight; Jasinski, 1977).
Data Analysis
Differences between placebo and active medication groups in the numbers of days to require rescue medication for withdrawal during the morphine discontinuation phase were analyzed using the Kaplan-Meier Curves log-rank hypothesis test. Cox proportional hazards models were used to test the significant influence of covariates on the number of days of discontinued participation in the study. The covariates tested were: age, gender, ethnicity (African American, Caucasian, or Multiracial/Other), route of heroin administration (intranasal or intravenous), and dollars spent per week on heroin. Plasma ibudilast levels for the two active medication groups were also analyzed as a predictor of days to discontinue study participation. Weekly outcomes, the maximum SOWS and COWS scores for placebo and active medication groups, were analyzed using longitudinal mixed effect models with Poisson distribution with log link function combined with GEE methodology to estimate unstructured within-subject correlations. The main model included a two-way interaction between time and treatment (and corresponding main effects) that was used to estimate as precisely as possible the group difference between outcomes (maximum SOWS or COWS) at week 3. Additionally, each covariate (age, sex, ethnicity, dollar amount spent per day on heroin, and route of heroin administration) was included in the model separately to quantify its effect on the outcomes (maximum SOWS and COWS scores) over the study weeks. Differences were not observed among the three groups. Therefore, exploratory analyses were performed comparing the maximum scores for individual SOWS items between the two combined active groups (N = 20) and the placebo group (N = 10) during detoxification (3rd study week) using a nonparametric Mann-Whitney two-sample U test. Differences between groups in the peak ibudilast plasma levels during Study Week 2 were determined using a two-tailed t-test. Physiological endpoints (temperature, blood pressure, and heart rate) were analyzed using longitudinal mixed effect models with normal distribution combined with GEE methodology to estimate symmetric within-subject correlations. The models initially included the two-way interaction between time and treatment (and corresponding main effects). The interaction was omitted from the final models if its p-value > .05, and the final models then included only main effect of time and treatment condition. All results were considered statistically significant when corresponding p-values were smaller than level of significance 5%. Only data from volunteers who completed study participation were included in the analyses, these included participants who completed the 1st two weeks of the study (morphine stabilization and randomization to ibudilast dose).
RESULTS
Volunteers
Forty-five volunteers were enrolled in the study, and 31 completed the study (N = 10 for placebo and 40 mg BID ibudilast groups each, N = 11 for the 20 mg BID ibudilast group). The test medication was not detected in one volunteer randomized to the 20 mg BID ibudilast group; that volunteer’s data were not included in the withdrawal analyses (days to discontinue, SOWS, and COWS) but were included in reports for safety and tolerability. Of the 14 volunteers who discontinued study participation, 9 discontinued during the first study week for personal reasons, 4 dropped during the second study week for personal reasons, and 1 dropped from the study during the third study week for a family emergency. Data analyses were performed only for the participants who received at least one dose of placebo morphine during Week 3. Table 1 describes the volunteer population of study completers according to ibudilast dose group.
Table 1.
Demographic and baseline characteristics of study volunteers.
| Group
|
|||
|---|---|---|---|
| PBO BID N = 10 |
20 mg BID N = 10 |
40 mg BID N = 10 |
|
|
| |||
| Age (years)a | 39.5 ± 4.5 | 38.2 ± 5.3 | 37.9 ± 4.3 |
| Ethnicity (B/W/M) b | 2/3/5 | 3/1/6 | 4/5/1 |
| Sex (M/F) b | 9/1 | 8/2 | 9/1 |
|
| |||
| Heroin Use | |||
| Years Regular Use a | 15.2 ± 6.2 | 12.8 ± 7.5 | 12.9 ± 6.5 |
| Preferred Route (IN/IV) b | 7/3 | 4/6 | 4/6 |
| $/Day a | 61.5 ± 13.1 | 47.0 ± 21.2 | 58.5 ± 36.6 |
Data are presented as means (± SD)
Data are presented via frequency. Ethnicity is indicated as Black (B), White (W), and Multiracial/Other (M), sex is indicated as male (M) and female (F), and preferred route of administration as intranasal (IN) or intravenous (IV).
Days to discontinue
The number of days to discontinue study participation did not differ among the placebo and active medications groups according to product-limit survival estimates (X22 = 0.05, p = .98). Also, plasma ibudilast levels did not predict study discontinuation (X12 = 0.15, p = .70); therefore, both active groups were combined for other measures and analysis of withdrawal (COWS and SOWS). Age, gender, ethnicity, route of heroin administration, and dollars spent per week on heroin were not significant predictors of retention.
Withdrawal measures
SOWS
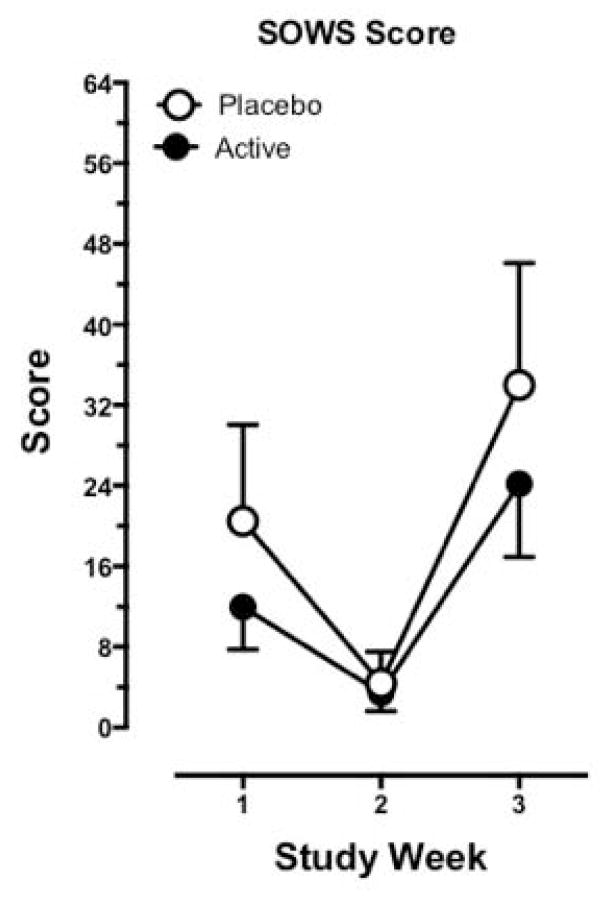
Figure 1 portrays SOWS scores as a function of study week and treatment group (active and placebo ibudilast). There were no significant differences between groups at 3rd study week detected (t28 = 1.43, p = .16). Holding dose and time constant, a significant effect of ethnicity (F2,26 = 3.98, p = .03) was revealed when added to the main model. On average, African Americans reported significantly lower SOWS scores than the Mixed group (t26 = −2.81, p = .009). Age, gender, route of heroin administration, and dollars spent per week on heroin were not found to be significant predictors of SOWS when added to the main model.
Figure 1.
Maximum cumulative SOWS scores (mean ± 95% confidence limits) for each study week (Week 1, morphine stabilization; Week 2, initiation of Ibudilast administration; Week 3, morphine discontinuation) according to treatment group (Active Group, N = 20; Placebo Group N = 10).
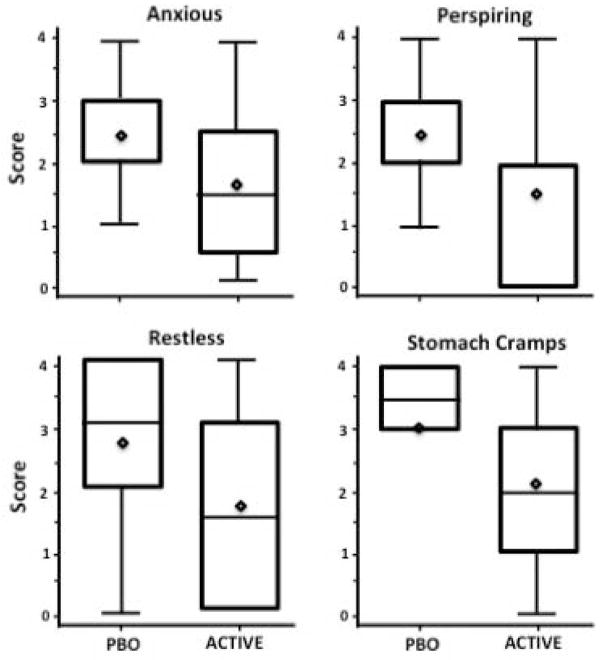
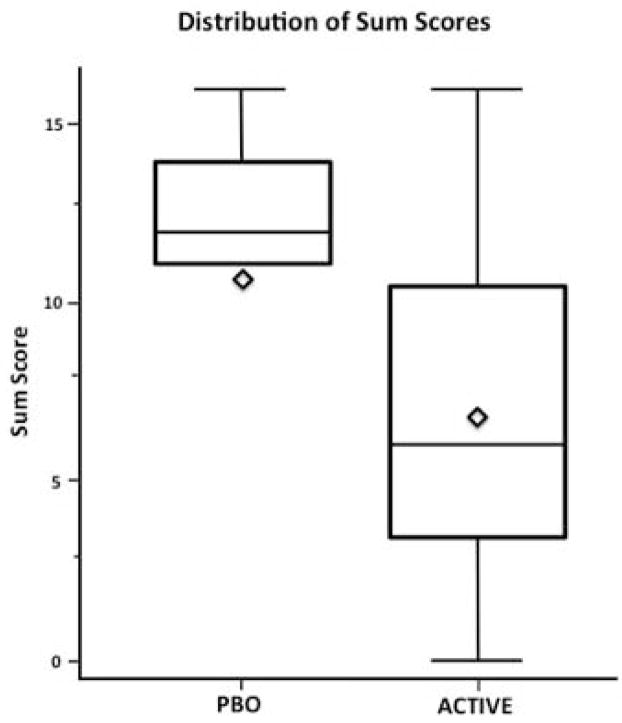
Figure 2 portrays distribution of maximum scores for individual SOWS items describing subjective ratings of physiological symptoms during the 3rd study week according to treatment group. The maximum of 4 most differentiating scores between treatment groups combined was significantly higher in the placebo group relative to the combined active groups (Figure 3; U = 201, p = .04).
Figure 2.
Distribution plot for individual SOWS items during Study Week 3 for each treatment group. Maximum score for each item is 4 (Active Group, N = 20; Placebo Group N = 10).
Figure 3.
Distribution plot for sum of individual SOWS items (Anxious, Perspiring, Restless, and Stomach Cramps) during Study Week 3 between Active Groups (N = 20) vs Placebo Group (N = 10).
COWS
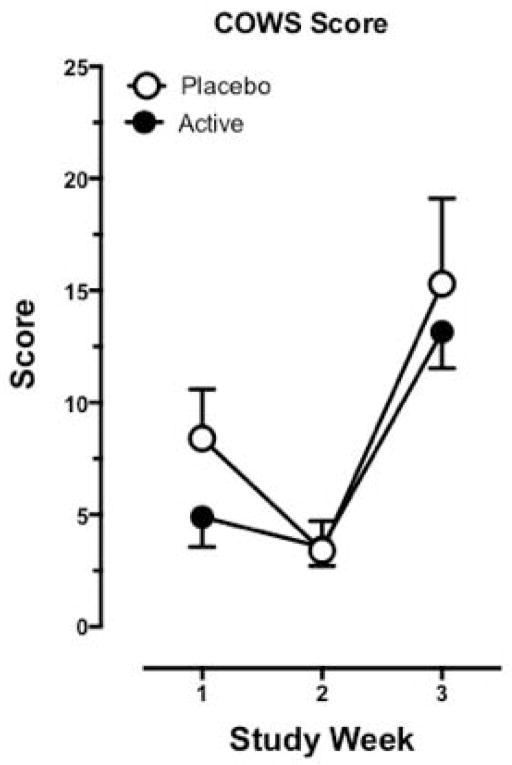
According to observer ratings of opioid withdrawal as measured using the COWS, all treatment groups exhibited greater opioid withdrawal symptoms during the 3rd study week when morphine was discontinued abruptly relative to the 1st and 2nd study weeks, as portrayed by Figure 3. There were no significant differences detected between groups at the 3rd study weeks (t28 = 1.22, p = .23). Holding dose and time constant, there was a significant effect of ethnicity (F2,26 = 7.00, p = .004): On average, lower COWS scores were observed in the African American group relative to the Mixed group (t26 = −2.88, p = .008). and than the Caucasians (t26 = −3.66, p = .001). Furthermore, intravenous heroin users were shown to have significantly higher COWS scores relative to intranasal users (t27 = −3.80, p = .001). Age, gender, and dollars spent per week on heroin were not significant predictors of COWS.
Ibudilast plasma levels
Peak ibudilast plasma levels on the 4th day of drug administration were significantly higher in the 40 mg BID ibudilast group relative to the 20 mg BID ibudilast group (53.4 ± 24.3 ng/ml vs. 27.9 ± 8.1 ng/ml, respectively; t18 = 3.16, p = .006); ibudilast was not detected in plasma from the placebo group.
Safety and Tolerability
Adverse Events
No serious adverse events occurred during the study. Table 2 depicts adverse events according to treatment group occurring during the 2nd study week, during the morphine stabilization phase. Insomnia was the most prevalent symptom and was exhibited in all three groups. Overall, adverse events were mild and not clinically significant; differences between treatment groups in frequency of adverse events were not observed.
Table 2.
Adverse events during the 2nd study week for each treatment group.
| Group | |||
|---|---|---|---|
| Adverse Event | PBO BID (N = 10) |
20 mg BID (N = 11) |
40 mg BID (N = 10) |
| Agitation | 1 (10%) | ||
| Anorexia | 1 (9.1%) | 2 (20%) | |
| Anxiety | 1 (10%) | 2 (20%) | |
| Backache | 1 (9.1%) | ||
| Chills | 1 (10%) | ||
| Constipation | 1 (10%) | 1 (9.1%) | 1 (10%) |
| Ear Infection | 1 (10%) | ||
| Fatigue | 1 (9.1%) | 1 (10%) | |
| GI Upset | 2 (20%) | 1 (9.1%) | 1 (10%) |
| Headache | 1 (10%) | ||
| Heartburn | 1 (10%) | ||
| Hot Flashes | 1 (10%) | ||
| Irritable | 1 (10%) | 1 (10%) | |
| Insomnia | 2 (20%) | 2 (18.2%) | 3 (30%) |
| Itchiness | 1 (10%) | 1 (9.1%) | |
| Leg Cramps | 1 (10%) | ||
| Muscle Aches | 1 (10%) | ||
| Nausea | 2 (20%) | 1 (10%) | |
| Runny Nose | 1 (10%) | ||
| Sore Throat | 1 (10%) | ||
| Sweating | 1 (10%) | 1 (10%) | |
| Tremors | 2 (20%) | ||
| Vomiting | 1 (10%) | ||
Adverse effects of ibudilast are reported for week 2, when administration of study medication was initiated. During this period, volunteers were stabilized on morphine and therefore not experiencing opioid withdrawal symptoms.
Physiological Measures
Physiological outcomes (temperature, blood pressure, and heart rate) did not differ over time between the treatment arms; the corresponding two-way interactions between time and treatment were not significant and were omitted from the final model. In the main effects models, the physiological outcomes did not significantly differ between groups. Diastolic blood pressure and heart rate was significantly increasing at Study Week 3 compared to weeks 1 and 2 for all the groups (F2, 58 = 3.36, p = .04; F2, 58 = 43.87, p < .0001, respectively).
DISCUSSION
The current study was designed to assess the safety and tolerability of ibudilast under conditions of morphine maintenance and abrupt discontinuation, and to obtain preliminary information regarding its ability to alleviate withdrawal symptoms during detoxification. In the current population, the high (40 mg BID) and low (20 mg (BID) ibudilast dose conditions were well tolerated and not associated with serious adverse events. Additionally, ibudilast administration was associated with a distinct decrease in several somatic signs of opioid withdrawal when morphine was discontinued. Although differences were not observed between the placebo and active ibudilast groups in the amount of time that elapsed before rescue medications were requested, exploratory analyses revealed an attenuation of subjective ratings of withdrawal observed with ibudilast administration suggesting that the test medication may be effective in decreasing the severity of certain withdrawal symptoms. The safety and tolerability of the ibudilast doses used in the current study were established and provided critical information for the feasibility of future investigations to assess the potential effectiveness of higher doses (Johnson et al., 2014). Additionally, these studies lay the groundwork for determining if the effects of ibudilast extend beyond minimizing withdrawal symptoms to include attenuation of the abuse liability of opioids and the development of tolerance to their effects, endpoints that are important when considering the therapeutic use of opioid analgesics.
Other non-opioidergic pharmacotherapies have been investigated for their therapeutic potential during detoxification, and have shown promise in decreasing withdrawal symptoms. Specifically, clinical studies investigating the alpha-2 adrenergic agonists lofexidine (Yu et al., 2008) and clonidine (Gold et al., 1978) reported that these agents specifically decreased somatic and autonomic withdrawal symptoms during opioid detoxification compared to placebo, which was similar to those symptoms attenuated in the current study including irritability and nervousness (Gold et al., 1979; Gold et al., 1980; Jasinski et al., 1985). However, the clinical utility of these types of medications is limited due to their effects on blood pressure and cognitive performance (Schroeder et al., 2007), adverse effects that are not associated with ibudilast administration. Furthermore, a recent meta-analysis comparing opioid agonist treatment (buprenorphine and methadone) to alpha-2 adrenergic agonists for detoxification suggests that agonist treatments are superior, with higher rates of study retention in groups randomized to receive the opioids relative to the adrenergic agonists (Meader, 2010). Although these findings may suggest that the therapeutic potential of ibudilast may be similarly limited, the distinct mechanisms by which ibudilast and the adrenergic agents decrease withdrawal have implications regarding differences in their therapeutic potential. Alpha-2 adrenergic agonists target adrenergic hyperactivity, proposed to be a primary contributing factor to physiological and behavioral opioid withdrawal symptoms (Gold et al., 1981), a mechanism that likely mediates these medications’ adverse hemodynamic and cognitive effects, which limits their clinical utility. As a glial-cell modulator, ibudilast prevents and reverses opioid-induced glial cell activation. By affecting the neuroadaptive and behavioral changes that are associated with the proinflammatory response to repeated opioid exposure (Hutchinson et al., 2009; Cooper et al., 2012), ibudilast has the potential to impact a broader range of negative neurobiological and behavioral consequences of repeated opioid exposure. For instance, preclinical studies have demonstrated that glial cell-modulators, including ibudilast, disrupt the conditioned rewarding effects of opioids in rodents (Johnston et al., 2004; Narita et al., 2006; Hutchinson et al., 2009) and decrease the magnitude of dopamine release in the nucleus accumbens in response to morphine administration (Bland et al., 2009). Though the effects of glial-cell modulators on opioid self-administration have yet to be determined, the neurobiological and behavioral evidence suggests that this class of drugs would also attenuate the reinforcing effects of opioids, unlike adrenergic agents that do not decrease opioid self-administration in laboratory animals (Negus and Rice, 2009). As such, the therapeutic potential of glial cell-modulators is not restricted to ameliorating withdrawal symptoms, but includes the ability to decrease effects that are thought to be associated with the abuse liability of these drugs.
Though the findings from this preliminary investigation are promising, a larger sample size is required to provide adequate power to establish significant differences in withdrawal symptoms as a function of medication condition. The study was also not designed to directly investigate the potential of ibudilast to decrease the reinforcing effects of opioids. This behavioral endpoint is a critical avenue for future investigations in determining the potential for the test medication to decrease relapse to drug use in newly abstinent patients and to determine ibudilast’s protective effects in preventing the potential progression from therapeutic use of prescription opioid analgesics to non-medical use and abuse. Future lines of investigation should establish the effects of glial-cell modulators on abuse-related endpoints including subjective ratings of opioid effects, craving in response to drug cues, drug self-administration, and relapse to drug use under conditions of abstinence.
CONCLUSION
Ibudilast represents a novel pharmaco-therapeutic approach to prevent and attenuate the neurobiological adaptations that contribute to opioid abuse and dependence. In the current study, ibudilast was well tolerated with no adverse effects exceeding those of placebo in an opioid-dependent population. The current findings suggest ibudilast’s potential to decrease some withdrawal symptoms after abrupt discontinuation of morphine administration. Further studies investigating a wider dose range, with a larger sample size, and additional behavioral endpoints will clarify the clinical efficacy of ibudilast for treating opioid use-related disorders.
Figure 4.
Maximum cumulative COWS scores (mean ± 95% confidence limits) for each study week (Week 1, morphine stabilization; Week 2, initiation of Ibudilast administration; Week 3, morphine discontinuation) according to treatment group (Active Group, N = 20; Placebo Group N = 10).
Acknowledgments
FUNDING: The authors gratefully acknowledge the National Institute on Drug Abuse for providing funding for this study (DA09236 and DA027755) and Medicinova for providing study medication and supplemental financial support. NIDA and Medicinova had no role in study design or in the decision to submit the paper for publication. They also had no role in the collection, analysis and interpretation of data in the writing of this report. KWJ was an employee of Medicinova, the developer of ibudilast, during the study period but has not been an employee for over 1 year. SKV consults for Grunenthal, USA and Reckitt Benckiser.
The authors also acknowledge and appreciate the assistance of Janet Murray, RN, Claudia Tindall, RN, Greta Bielczyc, BS, and Joseph Lazar, MD.
Footnotes
DISCLOSURE: ZDC, MP, AG, MAS, JM, DM, JDJ, PAS, and SDC have no other competing financial interests in relation to the work described.
AUTHORS CONTRIBUTION: ZDC, KWJ, and SDC were responsible for study concept and design, ZDC, SDC, SKV, JDJ, PAS were responsible for study management and data collection, MP and AG were responsible for statistical analyses, MAS, JM, and DM provided medical coverage. We are grateful to Dr. Richard W. Foltin for his assistance in conducting this study.
References
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Hulst HE, Drulovic J, Uitdehaag BM, Matsuda K, Landin R. MN166-001 Investigators. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74:1033–40. doi: 10.1212/WNL.0b013e3181d7d651. [DOI] [PubMed] [Google Scholar]
- Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis. 2012;31:207–25. doi: 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hauser KF. Glial modulators as potential treatment of psychostimulant abuse. Adv Pharmacol. 2014;69:1–69. doi: 10.1016/B978-0-12-420118-7.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–73. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2013 doi: 10.1111/adb.12106. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun. 2009;23:492–7. doi: 10.1016/j.bbi.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG. Inflammatory Cytokines in Nonpathological States. News Physiol Sci. 2000;15:298–303. doi: 10.1152/physiologyonline.2000.15.6.298. [DOI] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci. 2010;107:11313–8. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 2010;109:130–138. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, Evans SM, Kowalczyk WJ, Saccone PA, Comer SD. Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav Pharmacol. 2012;23:271–9. doi: 10.1097/FBP.0b013e3283536d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido E, Pérez-García C, Alguacil LF, Díez-Fernández C. The alpha2-adrenoceptor antagonist yohimbine reduces glial fibrillary acidic protein upregulation induced by chronic morphine administration. Neurosci Lett. 2005;383:141–4. doi: 10.1016/j.neulet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978;2:599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. Noradrenergic hyperactivity in opiate withdrawal supported by clonidine reversal of opiate withdrawal. Am J Psychiatry. 1979;136:100–2. doi: 10.1176/ajp.136.1.100. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AC, Sweeney DR, Kleber HD. Efficacy of clonidine in opiate withdrawal: a study of thirty patients. Drug Alcohol Depend. 1980;6:201–208. doi: 10.1016/0376-8716(80)90323-3. [DOI] [PubMed] [Google Scholar]
- Gold MS, Pottash AC, Sweeney DR, Extein I, Annitto WJ. Opiate detoxification with lofexidine. Drug Alcohol Depend. 1981;8:307–15. doi: 10.1016/0376-8716(81)90040-5. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White J. Alpha2 adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2003:CD002024. doi: 10.1002/14651858.CD002024. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–89. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–50. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Martin WR, editor. Handbook of Experimental Pharmacology. Vol. 45. Springer-Verlag; Berlin: 1977. pp. 197–258. [Google Scholar]
- Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42:1063–6. doi: 10.1001/archpsyc.1985.01790340041006. [DOI] [PubMed] [Google Scholar]
- Johnson KW, Matsuda K, Iwaki Y. Ibudilast for the treatment of drug addiction and other neurological conditions. Clinical Investigation. 2014;4:1–11. [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lintzeriz N, Nielson S. Benzodiazepines, methadone and buprenorphine: interactions and clinical management. Am J Addict. 2010;19:59–72. doi: 10.1111/j.1521-0391.2009.00007.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR. Naloxone-precipitated morphine withdrawal behavior and brain IL-1β expression: comparison of different mouse strains. Brain Behav Immun. 2011;25:1223–32. doi: 10.1016/j.bbi.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction – a clinical perspective. Eur J Clin Pharm. 2010;66:537–45. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Courtin C, Roques BP, Noble F. Cytoskeletal genes regulation by chronic morphine treatment in rat striatum. Neuropsychopharmacol. 2004;29:2208–15. doi: 10.1038/sj.npp.1300513. [DOI] [PubMed] [Google Scholar]
- Meader N. A comparison of methadone, buprenorphine and alpha(2) adrenergic agonists for opioid detoxification: a mixed treatment comparison meta-analysis. Drug Alcohol Depend. 2010;108:110–4. doi: 10.1016/j.drugalcdep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacol. 2006;31:2476–88. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacol. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Gibbons JA, He L, Chang E, Jones D, Gross MI, Davidson JB, Sanftner LM, Johnson KW. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol. 2008;66:792–801. doi: 10.1111/j.1365-2125.2008.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- Schroeder JR, Schmittner J, Bleiberg J, Epstein DH, Krantz MJ, Preston KL. Hemodynamic and cognitive effects of lofexidine and methadone coadministration: a pilot study. Pharmacotherapy. 2007;27:1111–9. doi: 10.1592/phco.27.8.1111. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–40. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey of Substance Abuse Treatment Services: Data on Substance Abuse Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. BHSIS Series S-66, HHS Publication No. (SMA) 14-4809. [Google Scholar]
- Walter MR. Structural biology of cytokines, their receptors, and signaling complexes: implications for the immune and neuroendocrine circuit. Chem Immunol. 1997;69:76–98. doi: 10.1159/000058654. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–9. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Yu E, Miotto K, Akerele E, Montgomery A, Elkashef A, Walsh R, Montoya I, Fischman MW, Collins J, McSherry F, Boardman K, Davies DK, O’Brien CP, Ling W, Kleber H, Herman B. A Phase 3 placebo-controlled, double-blind, multi-site trial of the alpha-2-adrenergic agonist, lofexidine, for opioid withdrawal. Drug Alcohol Depend. 2008;97:158–68. doi: 10.1016/j.drugalcdep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]