Abstract
Myelin is formed by specialized myelinating glia: oligodendrocytes and Schwann cells in the central and peripheral nervous systems, respectively. While there are distinct developmental aspects and regulatory pathways in these two cell types, myelination in both systems requires the transcriptional activator Sox10. Sox10 interacts with cell type-specific transcription factors at some loci to induce myelin gene expression, but it is largely unknown how Sox10 transcriptional networks globally compare between oligodendrocytes and Schwann cells. We used in vivo ChIP-Seq analysis of spinal cord and peripheral nerve (sciatic nerve) to identify unique and shared Sox10 binding sites and assess their correlation with active enhancers and transcriptional profiles in oligodendrocytes and Schwann cells. Sox10 binding sites overlap with active enhancers and critical cell type-specific regulators of myelination, such as Olig2 and Myrf in oligodendrocytes, and Egr2/Krox20 in Schwann cells. Sox10 sites also associate with genes critical for myelination in both oligodendrocytes and Schwann cells, and are found within super-enhancers previously defined in brain. In Schwann cells, Sox10 sites contain binding motifs of putative partners in the Sp/Klf, Tead, and nuclear receptor protein families. Specifically, siRNA analysis of nuclear receptors Nr2f1 and Nr2f2 revealed downregulation of myelin genes Mbp and Ndrg1 in primary Schwann cells. Our analysis highlights different mechanisms that establish cell type-specific genomic occupancy of Sox10, which reflects the unique characteristics of oligodendrocyte and Schwann cell differentiation.
Keywords: chromatin, Schwann, oligodendrocyte, transcription, enhancer, myelin
INTRODUCTION
Myelin is required to enable saltatory conduction of action potentials, and is synthesized by two different cell types—oligodendrocytes and Schwann cells—in the central and peripheral nervous system (CNS and PNS), respectively (reviewed in Nave and Trapp 2008; Quintes et al. 2010). While oligodendrocytes and Schwann cells share the ability to extend membranes and spirally ensheathe axons, these systems are developmentally and functionally independent (Nave 2010; Nave and Trapp 2008). At the cellular level, an individual oligodendrocyte can myelinate several axons, whereas a Schwann cell wraps a single axon. In addition, the response of oligodendrocytes and Schwann cells to nerve injury is also significantly different (Brosius Lutz and Barres 2014). Transcriptional regulation is also remarkably distinct, as relatively few transcription factors (e.g. Sox10 and Yy1) are known to be required for both CNS and PNS myelination (He et al. 2007; He et al. 2010; Mitew et al. 2013; Svaren and Meijer 2008; Weider et al. 2013).
Sox10 is required for terminal differentiation and maintenance of the oligodendrocyte lineage, and is required throughout Schwann cell development (Bremer et al. 2011; Britsch et al. 2001; Hornig et al. 2013; Stolt et al. 2002). While most myelin disorders affect either the CNS or PNS specifically, the importance of Sox10 regulation in both types of myelinating glia is demonstrated by dominant-negative Sox10 mutations in PCWH (peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease) (Inoue et al. 2004; Inoue et al. 2002). Consistent with the central role of Sox10 in both cell types, there are a number of genes expressed in both oligodendrocytes and Schwann cells (e.g. Mag, Mbp, Cnp, Plp1). In addition, the key role of Sox10 is also illustrated by stem cell and transdifferentiation approaches for glial differentiation, which have employed Sox10 as a driver of glial differentiation (Kim et al. 2014; Najm et al. 2013; Wang et al. 2014; Yang et al. 2013).
The ability of Sox10 to support differentiation in these two cell types is dependent upon interactions with factors that are expressed in either tissue (Emery 2013). For example, Sox10 interacts with Olig2 and Myrf transcription factors during oligodendrocyte development and coordinates the regulation of several genes involved in CNS myelination (Hornig et al. 2013; Mitew et al. 2013; Wissmuller et al. 2006). Similarly, peripheral nerve myelination requires the zinc finger transcription factor Egr2/Krox20 (Le et al. 2005a; Topilko et al. 1994), which interacts with Sox10 in the activation of several target genes (Bondurand et al. 2001; Denarier et al. 2005; Jones et al. 2012; LeBlanc et al. 2007; Srinivasan et al. 2012; Svaren and Meijer 2008). These cooperating transcription factors can be specific to either myelinating cell type, as Olig2 and Myrf are expressed in oligodendrocytes and Egr2 is expressed in Schwann cells. Since Sox10 can interact with a diverse array of other transcription factors (Wissmuller et al. 2006), coordinated interactions of Sox10 with other factors likely play a vital role in programming the genome of myelinating cells to fulfill their unique roles (Svaren and Meijer 2008; Weider et al. 2013).
Despite the fundamental importance of Sox10 in both types of myelinating glia, there is relatively little known about genome-wide mechanisms by which Sox10 coordinates transcription in the two cell types. To determine if the genomic distribution of Sox10 mediates its regulation of shared and cell type-specific gene networks, we profiled Sox10 binding sites during CNS and PNS myelination in vivo by ChIP-Seq. Our data indicate substantial differences in localization of Sox10 binding in the CNS and PNS, and suggest that cooperating transcription factors are involved in programming the distinct features of myelinating glia.
MATERIALS AND METHODS
ChIP-Seq
All animal experiments were performed according to protocols approved by the University of Wisconsin School of Veterinary Medicine Institutional Animal Care and Use Committee. Sox10 ChIP assays were performed on pooled spinal cords and sciatic nerve from postnatal day 15 (P15) Sprague-Dawley rats, and samples were prepped for sequencing (ChIP-Seq) as previously described (Srinivasan et al. 2012).
H3K27ac (Active Motif, 39133) ChIP assays in the PNS were done on pooled P15 rat sciatic nerve, and significant ChIP enrichment (>8-fold) was determined using Homer with default parameters to detect histone mark enrichment (Heinz et al. 2010). Egr2 ChIP-Seq (Srinivasan et al. 2012) reads were re-mapped to the Rn5 genome build using Bowtie (Langmead et al. 2009).
CNS ChIP-Seq data sets for Olig2, Myrf, H3K27ac, H3K4me3, and H3K9me3 (Bujalka et al. 2013; Yu et al. 2013) were converted to the rat genome build (Rn5) using the liftOver utility from the University of California-Santa Cruz genome browser (Karolchik et al. 2014; Yu et al. 2013). Data sets from respective experiments in oligodendrocyte precursor cells, immature oligodendrocytes, and mature oligodendrocytes were merged using the mergePeaks program in Homer (Heinz et al. 2010). Super-enhancer data sets from human brain tissue (Hnisz et al. 2013) were combined and then converted to the Rn5 genome build using liftOver (Bujalka et al. 2013).
Sox10 ChIP-Seq differential binding analysis
Bowtie alignment of the raw reads results in 27,244,645 and 23,813,129 reads for the two CNS replicates; and 34,047,253 and 4,796,289 reads for the PNS replicates (Langmead et al. 2009). Input data for CNS and PNS immunoprecipitations were sequenced in parallel, and yielded 27,437,979 and 87,735,088 reads for CNS; and 28,039,282 and 70,069,084 reads for PNS. ChIP enrichment detection was performed using the R package MOSAiCS (MOdel-based one and two Sample Analysis and inference for ChIP-Seq Data) with the “Input Only” model, controlling the false discovery rate (FDR) at 0.05 (Kuan et al. 2011). MOSAiCS implements a model-based approach where the background distribution for unbound regions take into account systematic biases such as mappability and GC content and the enrichment regions are described with a two component Negative Binomial mixture model. The differential binding sites between two conditions (CNS versus PNS) were detected using the R package DBChIP (Liang and Keles 2012), controlling the FDR at 0.05. DBChIP detected differentially occupied regions were further filtered by requiring the number of minimum base coverage of ChIP sample within enrichment window to be greater than 10 for CNS and 5 for PNS, so that the total numbers of differential binding sites are comparable between two conditions. The raw data files for the ChIP-Seq analysis have been deposited in NCBI Geo under accession numbers GSE64703 and GSE64971.
Bioinformatic Analysis
The R packages biomaRt and ChIPpeakAnno (Kasprzyk 2011; Zhu et al. 2010) were used to compare the extent of overlap between binding sites from ChIP-Seq data sets, to identify the genomic distribution of sites, and to annotate sites to respective gene lists. To perform gene ontology (GO) analyses we used David (Database for Annotation, Visualization, and Integrated Discovery) (Huang et al. 2009). We performed GO terms clustering using the highest stringency, and also identified enriched Kegg pathways with a p-value < 0.05. To identify enriched motifs in sequences of interest, we used the findMotifsGenome and findMotifs programs in Homer with different options to specify whether to identify enriched motifs over scrambled background sequences or over another particular data set (Heinz et al. 2010).
Highly expressed genes in oligodendrocytes and Schwann cells were identified from published array data on oligodendrocyte and sciatic nerve development, respectively (Cahoy et al. 2008; Le et al. 2005a). After updating the gene symbols based on the latest annotation (Mouse430_2 and Mouse430A_2 Release 34), probe signals were percentiled to identify the most highly expressed genes.
Expressed genes were defined as those with detection values greater than 600, and were filtered to include genes present in both arrays (5,703 and 7,609 genes in the CNS and PNS, listed in Table S7). Highly expressed genes that are cell type-specific to either the oligodendrocyte or Schwann cell lineage were identified as the top 25 percent of probes for genes expressed in the CNS that were not expressed in the PNS (i.e. in the bottom 25% of the array), and vice versa, and were termed CNS-specific (104 total genes) and PNS-specific genes (383 total) (Table S8).
To identify a common gene set in myelinating glia, 5,172 genes expressed in both CNS and PNS (according to the above criteria) were filtered to 588 genes (myelinating glia genes) to include those previously shown to be >2-fold enriched in oligodendrocytes, compared to neurons and astrocytes in the CNS (Table S9) (Cahoy et al. 2008).
Cell line culture and siRNA treatment
Oli-Neu oligodendroglial cells were grown on poly-L-lysine-coated culture dishes in growth medium consisting of DMEM supplemented with 4.5 g/L glucose, L-glutamine, sodium pyruvate, 5% bovine growth serum, penicillin and streptomycin (Jung et al. 1995). Oli-Neu cells were transfected with 40nmol of siRNA targeting Sox10 (Ambion, s131239) or a negative siRNA control (Ambion, negative control #2 AM4613) with the Amaxa system (Lonza) using the Rat Neuron Nucleofection Solution.
Primary rat Schwann cells (RSCs) were grown on poly-L-lysine-coated culture dishes with DMEM supplemented with 4.5 g/L glucose, L-glutamine, sodium pyruvate, 5% bovine growth serum, penicillin, streptomycin, 0.2% bovine pituitary extract, and 2uM forskolin. RSCs were transfected with 75pmol siRNA targeting Nr2f1 (IDT, RNC.RNAI.N031130.12.3), Nr2f2 (IDT, RNC.RNAI.N080778.12.1), or a negative siRNA control (IDT, DS NC1) using the Lipofectamine RNAiMAX reagent (Invitrogen).
RNA was harvested at 48h after transfection and cDNAs were analyzed by RT-qPCR. Putative Sox10 target genes in Oli-Neu cells were identified using the Illumina MouseRef-8 v2.0 Expression BeadChIP Kit. Target genes were defined as genes expressed in Oli-Neu's (Detection Pval < 0.05) that were more than 2-fold reduced in cells treated with siRNA targeting Sox10 (listed in Table S10). Candidate Sox10- and Nr2f1/2-regulated target genes were tested by qRT-PCR, performed on at least 3 independent experiments, and p-values were obtained from the Student's two-tailed t test (p < 0.05 is considered to be statistically significant). Primers are listed in supplementary material Table S11.
Mouse lines and conditional ablation of Myrf in myelinating cells
Maintenance of the Myrf exon 8 loxP-flanked line (Emery et al. 2009) (MyrfFL/FL), and the Myrf induced conditional knock out line (icKO; MyrfFL/FL, PLP–CreERT) was described previously (Koenning et al. 2012). Briefly, the MyrfFL/FL line was genotyped using primers AGGAGTGTTGTGGGAAGTGG and CCCAGGCTGAAGATGGAATA. Mice positive for the PLP–CreERT transgene (Doerflinger et al. 2003) were identified with the primers GCGGTCTGGCAGTAAAAACTATC (forward) and GTGAAACAGCATTGCTGTCACTT (reverse), using the internal control primers CTAGGCCACAGAATTGAAAGATCT (forward) and GTAGGTGGAAATTCTAGCATCATCC (reverse). Mice were maintained on a mixed C57BL/6 × 129 background.
Ten week old control (MyrfFL/FL) and Myrf icKO mice were treated with 4-hydroxytamoxifen (4OHT; Sigma-Aldrich) as described (Koenning et al. 2012). Briefly, 4OHT was dissolved in 90% corn oil and 10% ethanol to 10 mg/ml. Mice were given a daily intraperitoneal injection of 1mg (100ul) for 5 consecutive days. Spinal cords were dissected seven days after the first injection. Mice were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 10 ml of PBS.
Myrf icKO ChIP-qPCR and RT-qPCR
ChIP-qPCR on control and Myrf icKO mouse spinal cords was carried out with the following antibodies: goat IgG (Santa Cruz Biotechnology, sc-2028), Sox10 (R&D, AF2864), Olig2 (Millipore, AB9610), H3 (Active Motif, 39163), and H3K27ac (Active Motif, 39133). ChIP-qPCR was performed on three independent experiments as described previously (Srinivasan et al. 2012). P-values were obtained from the Student's two-tailed t test to compare fold enrichment in control versus Myrf icKO spinal cords (p < 0.05 is considered to be statistically significant).
RT-qPCR on control and Myrf icKO mouse spinal cords was performed on four independent experiments as described previously (Koenning et al. 2012). P-values were obtained from the Student's two-tailed t test (p < 0.05 is considered to be statistically significant). Primers used for qPCR are listed in supplementary materials Table S11.
RESULTS
In vivo analysis of Sox10 binding patterns in CNS and PNS myelinating glial lineages
To compare genome-wide binding patterns between the CNS and PNS myelinating glial lineages, we profiled Sox10 binding in spinal cord to complement our previous analysis in the PNS (Srinivasan et al. 2012). We performed Sox10 ChIP-Seq analysis in postnatal day 15 (P15) rat spinal cord, a peak timepoint of active myelination (Jang et al. 2006; Jones et al. 2007; LeBlanc et al. 2006; LeBlanc et al. 2007; Mager et al. 2008). Within the CNS, Sox10 is exclusively expressed in oligodendrocytes (Stolt et al. 2002), including progenitor and myelinating cells (Dawson et al. 2003; Lang et al. 2013). In previous work, we established Sox10 binding patterns in Schwann cells using ChIP-Seq analysis in P15 rat sciatic nerve (Srinivasan et al. 2012). Sox10 expression is also restricted to Schwann cells in sciatic nerve (Britsch et al. 2001), which primarily contains myelinating Schwann cells (~80%) although nonmyelinating Schwann cells are also present (Zorick et al. 1996). Ultimately, our in vivo approach allows for differential analysis across a spectrum of oligodendrocyte and Schwann cell developmental stages.
Sox10 ChIP-Seq profiles in spinal cord were determined by using a similar approach as our previous analysis in sciatic nerve (Srinivasan et al. 2012). Sox10 ChIP DNA from P15 rat spinal cord was used to prepare libraries for Illumina sequencing. In parallel, the input sonicated DNA for each sample was sequenced to assess the distribution of reads. ChIP and input reads from both the spinal cord and sciatic nerve were mapped to the rat genome (Rn5) and analyzed using MOSAiCS (Kuan et al. 2011) in a two-sample manner to directly account for background measured by input reads. This method accounts for mappability and GC bias in sequencing data and provides good false discovery rate control. MOSAiCS identified 6,761 Sox10 binding sites in spinal cord and 13,255 sites in sciatic nerve, and these data sets were used to compare genome-wide binding sites of the same transcription factor in the CNS and PNS myelinating glial lineages.
Sox10 binds to putative active enhancers marked by H3K27ac
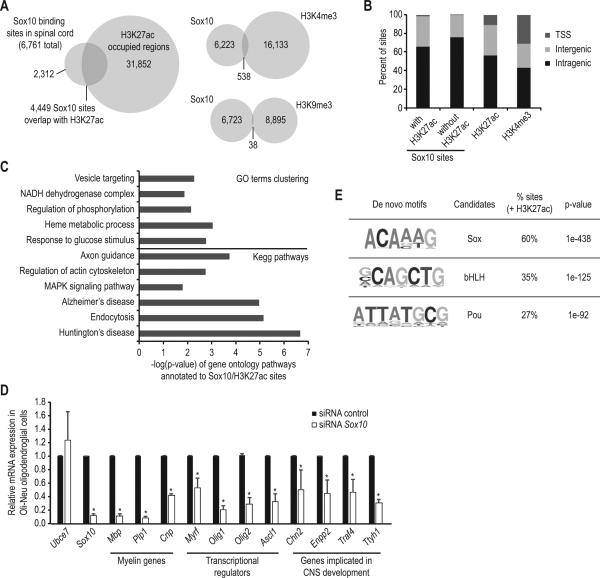
Since Sox10 is primarily described as a transcriptional activator, we hypothesized that Sox10 distribution would correlate with active enhancers. Recent work illustrated that actively engaged enhancers are commonly marked by acetylation of histone H3 lysine 27 (H3K27ac) (Creyghton et al. 2010; Rada-Iglesias et al. 2011). In addition, our recent studies identified substantial changes in distribution of H3K27ac after peripheral nerve injury, which correlated with altered gene expression patterns (Hung et al. 2015). Therefore, we first filtered CNS Sox10 binding patterns using recent data sets of H3K27ac and other histone marks in cultured rat oligodendrocytes (Yu et al. 2013). Of the 6,761 Sox10 binding sites in spinal cord, 66% of these overlapped with H3K27ac in oligodendrocytes, which indicates that most Sox10 sites correlate with active enhancers (Figure 1A). In contrast, there was relatively little overlap with a mark of active promoters, H3K4 trimethylation (H3K4me3). Sox10 also rarely co-occupies regions with H3K9 trimethylation (H3K9me3), a repressive histone mark. To further understand Sox10-mediated transcriptional mechanisms, we determined the genomic distribution of Sox10 with regard to gene bodies. Sox10 sites that also contain H3K27ac (Sox10/H3K27ac) are generally distal to the promoter region, as less than 2% are found at transcription start sites (TSSs) (Figure 1B). Previous studies also found that H3K27ac is found at promoters and distal enhancers in the genome (Creyghton et al. 2010; Rada-Iglesias et al. 2011). Therefore, Sox10 usually binds to enhancers marked by H3K27ac, and its binding sites are rarely found in proximal promoter regions, although important Sox10 binding sites in oligodendrocyte gene promoters have been identified (Li et al. 2007; Schlierf et al. 2006).
Figure 1. Sox10 binding sites in the spinal cord are enriched with H3K27ac, and are associated with genes implicated in myelination processes.
(A) Overlap analysis of Sox10 binding sites with different histone modifications in oligodendrocytes. (B) Genomic distribution of binding sites with regard to transcription start site (TSS), intergenic and intragenic regions. (C) Gene ontology (GO) terms clustering and Kegg pathway analysis on expressed genes within 50kb of Sox10/H3K27ac sites. (D) Relative mRNA expression levels in Oli-Neu oligodendroglial cells treated with siRNA targeting Sox10, as well as a non-targeting control siRNA. The mean of four independent experiments is shown (n=4, *p<0.05). (E) De novo motif analysis of Sox10/H3K27ac sites.
To reveal putative target genes of Sox10, Sox10/H3K27ac binding sites were annotated to the nearest expressed gene within 50kb. Expressed genes in oligodendrocyte development (CNS-expressed) were determined using a published microarray analysis (Cahoy et al. 2008). In total, 1,722 (out of 4,449) Sox10/H3K27ac sites are within 50kb of expressed genes, and we also found that 34% of CNS-expressed genes have a nearby Sox10 site colocalized with H3K27ac. Gene ontology analysis indicated that these putative target genes are associated with cell signaling and metabolic regulatory mechanisms (MAPK signaling, regulation of phosphorylation, response to glucose stimulus and NADH dehydrogenase complex) as well as with morphological processes that include regulation of actin cytoskeleton, axon guidance and endocytosis (Figure 1C).
To test if Sox10 is required for expression of putative target genes in oligodendrocytes, we employed siRNA targeting Sox10 in Oli-Neu oligodendroglial cells (Jung et al. 1995). Microarray analysis identified 160 candidate target genes that were downregulated at least 2-fold by Sox10 siRNA (listed in Table S10). Of these target genes, 45% were found within 50kb of a Sox10/H3K27ac site. We validated Sox10 regulation by qRT-PCR using four independent Oli-Neu samples treated with siRNA. While it is possible that some Sox10 targets may be obscured by potentially redundant regulation by Sox8 (Hornig et al. 2013; Kellerer et al. 2006; Stolt et al. 2004), this analysis confirmed regulation of known targets (i.e. Mbp, Plp1, Cnp, Myrf, Olig1, and Olig2) (Figure 1D), as well as novel target genes that are enriched in oligodendrocytes compared to astrocytes and neurons (Cahoy et al. 2008). Newly described Sox10 targets include the transcriptional regulator Ascl1 (Nakatani et al. 2013; Parras et al. 2007; Sugimori et al. 2007; Sugimori et al. 2008), as well as genes that required for CNS development (i.e. Chn2, Enpp2, Traf4, and Ttyh1) (Blaise et al. 2012; Kumada et al. 2010; Riccomagno et al. 2012; Wiernasz et al. 2014; Yuelling et al. 2012).
Sox10 binding sites are enriched for binding motifs of cooperating factors
Given that H3K27ac marks actively engaged enhancers (Creyghton et al. 2010; Rada-Iglesias et al. 2011) but not all Sox10 binding events, it is possible that certain combinations of transcription factor occupancy define active enhancers in oligodendrocytes. We hypothesized that Sox10/H3K27ac sites would be enriched in binding motifs for cooperating transcription factors that are not found in Sox10 sites that lack H3K27ac (also referred to here as “non-acetylated” sites). Therefore, we analyzed Sox10 sites, with and without H3K27ac, for enriched sequence motifs corresponding to transcription factor binding sites. De novo motif analyses revealed that Sox10/H3K27ac sites contain the Sox binding motif, as well as the binding motifs of bHLH and Pou domain proteins (Figure 1E). Non-acetylated Sox10 sites are enriched for the Sox binding motif (52%) and Jun (27%).
Sox10 binding sites overlap with binding of the bHLH factor, Olig2, as well as Myrf sites obtained by ChIP-Seq in cultured oligodendrocytes (Bujalka et al. 2013), so we next determined if the overlap is correlated with H3K27ac status. Sox10 sites overlap with Olig2 sites (Yu et al. 2013) regardless of H3K27ac status (Table S6). In contrast, Myrf binding sites overlap preferentially with Sox10/H3K27ac sites, compared to Sox10 sites lacking H3K27ac. Our results suggest that Sox10 and Olig2 bind together regardless of the H3K27ac status (i.e. both active and inactive enhancers), while colocalization of Sox10 and Myrf is associated with the H3K27ac mark of active enhancers.
While Sox10 binding patterns have been analyzed in sciatic nerve (Srinivasan et al. 2012), we performed a complementary analysis in this tissue using an updated Sox10 ChIP-Seq analysis. We also identified active enhancers in peripheral nerve using H3K27ac ChIP-Seq in P15 rat sciatic nerve. We identified 13,255 Sox10 binding sites in the sciatic nerve, 60% of which overlapped with H3K27ac sites (Sox10/H3K27ac) (Figure 2A). Conversely, approximately 21% of all active enhancers marked by H3K27ac are bound by Sox10. We hypothesized that H3K27ac enrichment might denote active Sox10-regulated enhancers, thus we tested if Sox10/H3K27ac sites, compared to non-acetylated sites, are preferentially associated with Sox10 target genes. We found that genes downregulated by siRNA targeting Sox10 in S16 rat Schwann cells (Srinivasan et al. 2012) were more likely to be within 50kb of a Sox10/H3K27ac binding site, compared to non-acetylated Sox10 sites (Figure 2B).
Figure 2. Sox10 binding sites in the sciatic nerve are enriched with H3K27ac.
(A) Overlap analysis of Sox10 binding sites with H3K27ac occupied regions in sciatic nerve. (B) Percent of genes within 50kb of a Sox10 binding site. Genes included were those misregulated (downregulated or upregulated) by siRNA Sox10 treatment in S16 Schwann cells. (C) Genomic distribution of binding sites with regard to transcription start site (TSS), intergenic and intragenic regions. (D) Gene ontology (GO) terms clustering and Kegg pathway analysis on expressed genes located within 50kb of Sox10/H3K27ac sites. (E) De novo motif analysis of Sox10/H3K27ac sites.
As in the CNS, Sox10/H3K27ac is also widely detected at more distal sites relative to the start sites of genes (Figure 2C). 4,889 Sox10/H3K27ac sites (62%) were within 50kb of an expressed gene, as determined by a microarray analysis of sciatic nerve development (Le et al. 2005a). We also found that 67% of expressed genes are within 50kb of Sox10/H3K27ac. These genes are significantly enriched in cell signaling/metabolic cascades (MAPK signaling, tricarboxylic acid cycle, and NADH dehydrogenase complex), morphological processes (focal adhesion), and disease (cancer and neurological disease) (Figure 2D).
Putative cooperating factors at Sox10/H3K27ac binding sites in the PNS were identified by de novo motif analyses, which revealed enrichment for the Sox binding motif, as well as the binding motif for Ets and Tead (transcriptional enhancer activation domain containing) proteins. The analysis also identified the motif for Sp/Klf zinc finger proteins (Figure 2E), which includes the binding motif of the Schwann cell-regulator Egr2 (Le et al. 2005a; Topilko et al. 1994). We previously found that 10% of Sox10 sites overlap with (or are proximal to) Egr2 binding sites (Srinivasan et al. 2012). In contrast to acetylated sites, non-acetylated Sox10 sites are enriched for the CTCF motif (13%).
Identification of unique Sox10 binding sites in oligodendrocytes and Schwann cells
To directly compare Sox10 binding patterns between sciatic nerve and spinal cord, we used DBChIP (Liang and Keles 2012). The core methodology of DBChIP relies on formally testing candidate binding regions for differential enrichment across multiple data sets. DBChIP provides a list of p-values quantifying evidence against equal enrichment and the corresponding fold-changes. Using this analysis we identified binding sites statistically unique to either spinal cord or sciatic nerve (Figure 3). To focus on actively engaged enhancers, we identified those sites enriched with H3K27ac (Sox10/H3K27ac): 3,042 unique Sox10/H3K27ac binding sites in the spinal cord (CNS-specific), and 3,104 unique sites in the sciatic nerve (PNS-specific). We also identified 1,016 Sox10/H3K27ac binding sites in common between the tissues. Our analysis identified substantially more unique Sox10/H3K27ac sites in each tissue compared to the smaller group of common sites.
Figure 3. Identification of unique and common Sox10/H3K27ac binding sites in spinal cord and sciatic nerve.
Schematic of our comparative analysis of Sox10 binding sites in spinal cord and sciatic nerve that revealed unique and common binding sites between the tissues. Unique sites were filtered to identify those that are statistically different using DBChIP, and both unique and shared sites were filtered to identify sites with H3K27ac enrichment.
CNS-specific Sox10 binding sites represent unique regulatory mechanisms in oligodendrocytes
To determine if CNS-specific Sox10 binding sites are preferentially associated with oligodendrocyte-specific transcription factors, we performed de novo motif analyses, and also tested if CNS-specific sites (3,042 total sites, Table S1) were likely to overlap with Olig2 and Myrf sites. De novo motif analyses revealed that unique Sox10/H3K27ac sites are specifically enriched with the bHLH, Smad, and homeobox protein binding motifs (Figure 4A) compared to PNS-specific and common Sox10/H3K27ac sites. Consistent with the motif analysis, CNS-specific Sox10 sites overlap substantially with the bHLH factor, Olig2, as well as Myrf (Figure 4B) (Yu et al. 2013), which was noted previously when considering all Sox10 binding sites (Bujalka et al. 2013). 19% and 81% of unique Sox10/H3K27ac sites are co-occupied with Myrf and Olig2, respectively, while 16% of Myrf sites and 8% of Olig2 sites are co-occupied with unique Sox10/H3K27ac sites. In contrast, CNS-specific Sox10/H3K27ac sites generally do not overlap with Egr2 binding sites identified in sciatic nerve (Figure 4B). Therefore, the CNS-specific Sox10 sites are associated with motifs and binding patterns of oligodendrocyte-specific transcription factors.
Figure 4. Unique Sox10/H3K27ac binding sites in spinal cord are co-occupied with cell type-specific transcription factors.
(A) De novo motif analysis of unique CNS Sox10/H3K27ac sites (enrichment over sequences in unique PNS and common sites). (B) Overlap analysis of unique sites with ChIP-Seq data sets of oligodendrocyte-specific, and Schwann cell-specific master regulators (Olig2/Myrf, and Egr2, respectively). (C) Percent of cell type-specific genes that are within 50kb of a CNS-specific Sox10/H3K27ac site. (D) Schematic of transcription factors that regulate differentiation of oligodendrocyte precursors cells into myelinating oligodendrocytes. (E) Examples of unique Sox10/H3K27ac sites in the spinal cord that converge around loci of oligodendrocyte-specific master regulators (Olig1/2, Nkx2.2, Myrf, and Zfp488). Included are ChIP-Seq data sets from the PNS and CNS.
Given that there were many unique Sox10/H3K27ac binding sites, we hypothesized that they may regulate cell type-specific transcriptional mechanisms that help define molecular features of oligodendrocytes versus Schwann cells. Thus, we tested if unique Sox10/H3K27ac binding sites in the spinal cord are associated with genes expressed specifically in oligodendrocytes (oligodendrocyte-specific genes). Oligodendrocyte-specific and Schwann cell-specific genes were identified from microarray data sets in the CNS and PNS (Cahoy et al. 2008; Le et al. 2005b) (Table S8). Cell type-specific genes were obtained from the top 25% of expressed genes in one data set, that were not expressed in the other (i.e. were present in the bottom quartile). We observed that unique Sox10/H3K27ac binding sites in the spinal cord are often associated with oligodendrocyte-specific genes, although they are also associated with Schwann cell-specific genes to a lesser extent (Figure 4C). Interestingly, we identified several unique Sox10/H3K27ac sites near critical oligodendrocyte-specific transcription factor genes, which include Olig1, Olig2, Myrf, Nkx2.2, and Zfp488 (Figure 4D-E) consistent with previously described regulation by Sox10 (Hornig et al. 2013; Liu et al. 2007).
Myrf is not required for Sox10 and Olig2 localization and H3K27ac enrichment
One explanation for the unique distribution of Sox10 binding sites in spinal cord is that they are established in mature oligodendrocytes in concert with Myrf, which is induced in myelinating oligodendrocytes (Emery et al. 2009). To test this possibility, we determined if Myrf is required for establishing CNS-specific Sox10 binding sites by performing ChIP-qPCR assays with spinal cords of a conditional knockout of Myrf. Control (MyrfFL/FL) and induced conditional knock out (icKO; MyrfFL/FL, PLP–CreERT) mice were treated with 4-hydroxytamoxifen (4OHT) for 1 week at 10 weeks of age (Koenning et al. 2012) prior to ChIP analysis. We examined Sox10/Myrf binding at the Claudin-11 (Cldn11/Osp) gene, which encodes a tetraspan tight junction protein that constitutes about 7% of CNS myelin protein (Bronstein et al. 1996; Cahoy et al. 2008). A unique Sox10/H3K27ac binding site −3.3kb upstream of the Cldn11 locus in spinal cord (Figure S1A) overlaps with Myrf and Olig2 binding sites (Bujalka et al. 2013; Yu et al. 2013), but we found no significant changes between Sox10 binding in the control and Myrf icKO spinal cords (Figure 5B). Given that neither Olig2 nor Sox10 expression is reduced in the absence of Myrf (Bujalka et al. 2013; Emery et al. 2009), we tested if Olig2 binding was lost in the Myrf icKO. However, as seen with Sox10, Olig2 binding was not diminished in the Myrf icKO spinal cords (Figure 5C). Myrf is also not required for Sox10 and Olig2 binding at upstream enhancers that drive expression of the Sox10 locus (Figure S1B, 5B-C), as well as at sites in the Fatty acid 2-hydroxylase (Fa2h) and Myelin basic protein (Mbp) genes (Figure S1C-D, 5B-C), though these Sox10 binding sites are also found in the PNS.
Figure 5. Myrf is not required for Sox10 and Olig2 binding, and H3K27ac enrichment.
(A) Illustration of genomic regions assayed in ChIP-qPCR around Cldn11, Sox10, Fa2h, and Mbp loci. The * indicates Myrf binding sites in oligodendrocytes. (B-D) ChIP-qPCR analysis on spinal cords from control and Myrf icKO mice. Fold enrichment of (B) Sox10 and (C) Olig2 binding is relative to goat IgG, and fold enrichment of (D) H3K27ac is relative to total H3 levels. The mean of three independent experiments is shown (n=3, *p<0.05). The negative control site for ChIP-qPCR is a region +7.8kb from the Tekt3 gene, which is not expressed in oligodendrocytes, nor Schwann cells. (E) Relative mRNA expression in spinal cords from control and Myrf icKO mice. The mean of four independent experiments is shown (n=4, *p<0.05). Error bars represent mean ± s.d.
Myrf is preferentially associated with H3K27ac-enriched Sox10 binding sites (Table S6), whereas Olig2 overlaps with Sox10 regardless of H3K27ac status. Therefore, we also tested if Myrf is required to establish the H3K27ac chromatin mark. We found that the H3K27ac-enriched sites assayed at Cldn11, Fa2h and Mbp were unchanged in the icKO (Figure 5D). All three genes are reduced in the total knockout of Myrf (Emery et al. 2009), while only Fa2h and Mbp are downregulated in the Myrf icKO spinal cords (Figure 5E) (Koenning et al. 2012). Therefore, Myrf is not required for H3K27ac enrichment, although it may regulate H3K27ac at other sites that were not assayed.
PNS-specific Sox10 binding sites represent regulatory mechanisms unique to Schwann cells
Motif analysis of CNS-specific Sox10 binding sites successfully identified binding sites for Olig2 and Myrf factors. Therefore, we employed a similar approach to determine if Sox10 binding sites in sciatic nerve are preferentially associated with binding sites of specific transcription factors. In the PNS, 14% of unique Sox10/H3K27ac sites (3,104 total sites, Table S2) colocalize with Egr2 (Figure 6A). Importantly, unique Sox10/H3K27ac sites in the sciatic nerve overlap with only a few Myrf and Olig2 binding sites identified by ChIP-Seq in oligodendrocytes (Bujalka et al. 2013; Yu et al. 2013). De novo motif analyses of PNS-specific Sox10/H3K27ac sites reveal specific enrichment for Sp/Klf and Tead protein binding motifs compared to CNS-specific and common Sox10/H3K27ac sites (Figure 6B). Screening of known motifs revealed enrichment of Egr2 binding motifs in ~10% of PNS-specific Sox10 sites.
Figure 6. Unique Sox10/H3K27ac binding sites in the sciatic nerve are co-occupied with the Schwann cell-specific master regulator Egr2.
(A) Overlap analysis of unique sites with ChIP-Seq data sets of Schwann-specific, and oligodendrocyte-specific regulators (Egr2 and Olig2/Myrf, respectively). (B) Motif analysis of unique PNS Sox10/H3K27ac sites (enrichment over sequences in unique CNS and common sites). (C) Relative mRNA expression levels in primary rat Schwann cells treated with siRNA targeting Nr2f1 and/or Nr2f2, as well as a non-targeting control siRNA. The mean of three independent experiments is shown (n=3, *p<0.05). (D) Percent of cell type-specific genes that are within 50kb of a PNS-specific Sox10/H3K27ac site.
A seeded motif analysis of PNS-specific sites revealed enrichment of a motif that belongs to a group of nuclear receptors, which includes Nr2f1/Coup TF I and Nr2f2/Coup TF II. Nr2f1 and Nr2f2 sites were shown to bind engaged enhancers in human neural crest cells (Rada-Iglesias et al. 2012). Since Schwann cells maintain expression of Nr2f1 and Nr2f2 throughout their development (Yamaguchi et al. 2004), we hypothesized that Nr2f1/2 may regulate key genes involved in peripheral nerve myelination. To test our hypothesis, we employed primary rat Schwann cells (RSCs) treated with siRNAs targeting Nr2f1 and/or Nr2f2. We identified critical myelin genes Mbp, Desert hedgehog (Dhh), and N-myc downstream regulated gene 1 (Ndrg1) as putative targets of Nr2f1/2 (Figure 6C). Interestingly, Ndrg1 is an alpha/beta hydrolase family member implicated in severe autosomal recessive demyelinating neuropathy CMT4D (King et al. 2011). The Ndrg1 locus contains a PNS Sox10 site enriched with Nr2f1/2 motifs (Figure S2A). Sox10 and Egr2 expression was not reduced by siRNAs targeting Nr2f1 and/or Nr2f2, but Egr2 expression appears to be modestly increased by siRNA targeting Nr2f2 alone (Figure 6C).
To further elucidate the role of unique PNS Sox10 binding sites, we tested if these sites are linked to cell type-specific gene expression in Schwann cells versus oligodendrocytes. We found that unique Sox10/H3K27ac binding sites in the sciatic nerve are often associated with Schwann cell-specific genes (Figure 6D). For example, unique Sox10/H3K27ac sites were found near the Schwann cell-specific genes Egr2 and Dhh (Figure S2B-C). While unique Sox10/H3K27ac sites are found near the Egr2 locus, there are Sox10/H3K27ac sites in the CNS and PNS that correspond to the previously described Myelinating Schwann cell Element (MSE) (Ghislain and Charnay 2006; Ghislain et al. 2002; Reiprich et al. 2010). In addition, the unique site identified within the Dhh locus was previously shown to be regulated by Sox10 and Egr2 (Jang et al. 2006; Küspert et al. 2012). Unique Sox10/H3K27ac sites also overlap with Egr2 at Pleckstrin homology domain-containing family G member 5 (Plekhg5), which is implicated in recessive intermediate CMT disease (Figure S2D) (Azzedine et al. 2013; Kim et al. 2013). As expected, relatively few Sox10/H3K27ac binding sites in the sciatic nerve are found near oligodendrocyte-specific genes.
Shared genes in myelinating glia are regulated by both cell type-specific and common Sox10/H3K27ac marked enhancers
Several myelin genes are expressed in both oligodendrocytes and Schwann cells (i.e. Plp1, Cnp, Mag, and Mbp), and there is evidence that these genes are regulated by Sox10 in both systems (Hornig et al. 2013; Li et al. 2007; Srinivasan et al. 2012; Stolt et al. 2002). Therefore, we had anticipated that Sox10 would utilize similar sites to regulate genes enriched during myelination in both cell types. A set of common genes (Myelinating glia genes) were defined as genes enriched in oligodendrocytes and Schwann cells, compared to astrocytes and neurons (Table S9) (Cahoy et al. 2008; Le et al. 2005a). In contrast to our expectation, we found that myelinating glia genes were often associated with unique Sox10/H3K27ac binding sites in the spinal cord and sciatic nerve. Using a set of genes induced in both cell types during myelination, we identified 179 nearby (<50kb; 38%) unique Sox10/H3K27ac sites in the spinal cord, and 185 (40%) nearby sites in the sciatic nerve. Therefore, even common myelin genes show distinct enhancer usage in the two cell types (examples shown in Figure 7A-B).
Figure 7. Unique sites can regulate a common set of genes expressed in oligodendrocytes and Schwann cells.
Examples of unique sites found around genes upregulated during myelination: (A) Amyloid beta (A4) precursor protein (App) and (B) Ubiquitin-like 3 (Ubl3). (C) Unique sites are detected around the Proteolipid protein 1 (Plp1) locus. The * indicates previously described elements regulated by Myrf in oligodendrocytes, and the elements termed wmN1 and wmN2 were previously found to drive expression in CNS and PNS tissue, respectively. Included in (A-C) are ChIP-Seq data sets from the PNS and CNS.
Our finding that shared genes between oligodendrocytes and Schwann cells may be regulated by unique Sox10/H3K27ac sites fits with previous transgenic studies of Plp1 and Mbp (Farhadi et al. 2003; Tuason et al. 2008). For example, Sox10 binds to distinct elements within the Plp1 locus in the CNS and PNS, which were previously characterized as CNS- or PNS-specific elements (termed wmN1 and wmN2, respectively) in transgenic assays (Figure 7C) (Tuason et al. 2008). While Olig2 binds wmN1, the major cell type-specific regulators of myelination (Myrf and Egr2) do not appear to bind wmN1/2. However, both Olig2 and Myrf colocalize with a CNS-specific Sox10/H3K27ac site far upstream of the Plp1 locus (Bujalka et al. 2013). Similarly, one PNS-specific Sox10 binding site corresponds to the previously described M4 element upstream of the Mbp gene (Farhadi et al. 2003), which drives expression of a reporter specifically in peripheral nerve (Figure S1D).
Relatively few Sox10/H3K27ac sites (1,016, Table S3) are shared between the CNS and PNS, yet shared sites are often associated with myelinating glia genes. We found that 22% of myelinating glia genes are found within 50kb of common Sox10/H3K27ac sites. Our analysis suggests that shared enhancers are partially responsible for the transcriptional program that drives myelination in oligodendrocytes and Schwann cells. Motif analysis of common sites revealed enrichment with the motifs of homeobox and nuclear receptor proteins, which were also enriched in unique CNS and PNS Sox10 sites. While Myrf, Olig2, and Egr2 binding motifs did not emerge from the motif analysis, common Sox10/H3K27ac sites were co-occupied with these factors more often than by chance (Table S6). Only 17 common sites contained all three cell type-specific transcription factors in their respective data sets, suggesting that it is a rare occurrence.
Sox10/H3K27ac binding sites in oligodendrocytes and Schwann cells are associated with super-enhancers in brain
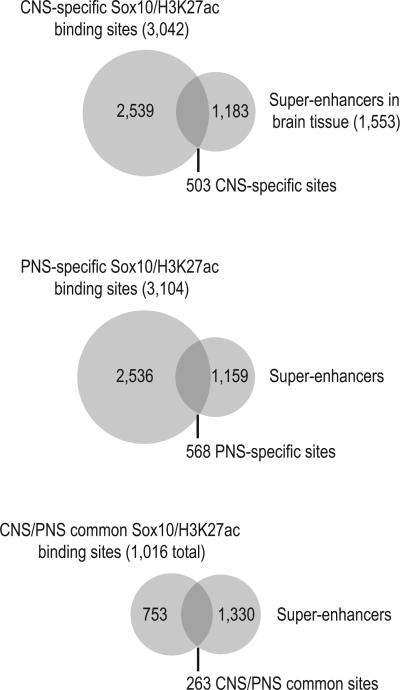
Given that Sox10 is a critical transcriptional activator that plays a major role in defining myelinating glial cells, we tested if Sox10/H3K27ac sites might be associated with “super-enhancers.” A recent study (Whyte et al. 2013) characterized super-enhancers as extended genomic regions that encompass several enhancers, Mediator coactivator binding, and H3K27 acetylation enrichment, and drive expression of genes that define cell identity. Sox10 was previously identified as a candidate regulator of super-enhancers in brain tissue (Hnisz et al. 2013) and interacts with Mediator in oligodendrocytes and Schwann cells (Vogl et al. 2013). Given that both unique and shared Sox10/H3K27ac binding sites may regulate shared myelination genes, we hypothesized that Sox10/H3K27ac binding sites are generally part of super-enhancers that define myelinating glial cells. We found that 17% and 18% of CNS- and PNS-specific Sox10/H3K27ac sites are found within super-enhancers in brain tissue, respectively (Figure 8). While relatively few Sox10/H3K27ac sites are shared between the CNS and PNS, 25% of these sites are localized within brain super-enhancers.
Figure 8. Unique and common Sox10/H3K27ac binding sites are associated with super-enhancers.
Overlap analysis of unique and common sites with super-enhancers previously identified in brain tissue.
Interestingly, super-enhancers bound by Sox10 are associated with genes upregulated during myelination in oligodendrocytes and Schwann cells (Table 1 & 2). One brain super-enhancer enriched with shared Sox10/H3K27ac coincides with several regulatory elements near the Sox10 locus (Antonellis et al. 2008; Wahlbuhl et al. 2012; Werner et al. 2007), as well as with Olig2/Myrf binding in the CNS and Egr2 in the PNS (Figure S1B). Another super-enhancer around the Fa2h locus is bound by Sox10/H3K27ac and respective master regulators Olig2/Myrf/Egr2 (Figure S1C).
Table 1.
Super-enhancers in brain tissue that contain common Sox10/H3K27ac binding sites and are associated with genes upregulated during CNS/PNS myelination.
| Chr | Start | Stop | Gene name | Gene Alias | Gene Description |
|---|---|---|---|---|---|
| chr7 | 120381030 | 120482265 | Sox10 | - | SRY (sex determining region Y)-box 10 |
| chr18 | 15686218 | 156903740 | Zfp191 | Znf24 | Zinc finger protein 191 |
| chr18 | 78438167 | 78510644 | Mbp | Mbps | Myelin basic protein |
| chr20 | 46161230 | 46190450 | Fyn | - | FYN oncogene related to SRC, FGR, YES |
| chr10 | 88275907 | 88322583 | Cnp | CNPF/Cnpl | 2′,3′-cyclic nucleotide 3′ phosphodiesterase |
| chr19 | 54340748 | 54407369 | Fa2h | Wdr59 | Fatty acid 2-hydroxylase |
| chrX | 29989018 | 30057477 | Gpm6b | M6b | Glycoprotein m6b |
| chrX | 30064170 | 30145920 | Gpm6b | M6b | Glycoprotein m6b |
Table 2.
Super-enhancers associated with unique Sox10/H3K27ac sites, as well as with genes upregulated during CNS/PNS myelination.
| Chr | Start | Stop | Gene name | Gene Alias | Gene Description |
|---|---|---|---|---|---|
| chrX | 107368378 | 107396778 | Plp1 | Plp | Proteolipid protein 1 |
| chr1 | 90513116 | 90518434 | Mag | - | Myelin-associated glycoprotein |
| chr3 | 127092187 | 127144220 | Mal | - | Mal, T-cell differentiation protein |
| chr11 | 28194989 | 28274080 | App | Abeta | Amyloid beta (A4) precursor protein |
Our analysis highlights several genes of which we have a limited understanding of transcriptional regulation. For instance, we also detected a super-enhancer that contains a common Sox10/H3K27ac site +5.3kb from the transcription factor 4 (Tcf4; also known as E2-2, not Tcf7l2) promoter, which overlaps with Olig2 (CNS) and Egr2 (PNS) (Figure S3). Tcf4 is a bHLH transcription factor that is expressed in both oligodendrocytes and Schwann cells, as well as in other cell types. Common variants in Tcf4 are linked to Hirschsprung disease and schizophrenia (Jiang et al. 2011; Quednow et al. 2014), and rare mutations cause Pitt-Hopkins syndrome, an autism-spectrum/neurodevelopmental disorder (Forrest et al. 2014; Sweatt 2013). Tcf4 is also downregulated in a CMT1a rat model (Vigo et al. 2005).
DISCUSSION
Our analysis provides the first comparative genome-wide view of transcriptional mechanisms between oligodendrocytes and Schwann cells by coupling genome-wide Sox10 binding and chromatin marks. Sox10 is an ideal candidate for a comparative analysis because it is required for myelin development and maintenance (Bremer et al. 2011; Britsch et al. 2001; Hornig et al. 2013; Kuhlbrodt et al. 1998; Stolt et al. 2002). While there is a core of shared Sox10 binding sites, there is a greater number of Sox10 binding sites that are significantly unique to either myelinating cell type.
Unique Sox10-bound enhancer sequences are enriched with very different motif profiles, some of which belong to established cell type-specific master regulators (Olig2/Myrf in the CNS, and Egr2 in the PNS). Motif analysis also revealed that unique CNS Sox10 sites are enriched with Smad and homeobox proteins, both of which are protein families that regulate oligodendrocyte development (Dutta et al. 2014; Palazuelos et al. 2014; Qi et al. 2001; Weng et al. 2012). Interestingly, unique sites in the PNS are enriched with the motif of Tead proteins, which interact with YAP/TAZ coactivators in the Hippo signaling pathway (Chen et al. 2010). While the role Teads play in Schwann cell development is not yet clear, they were recently implicated in neuregulin stimulation pathways (Serrano et al. 2013), which are critical for Schwann cell differentiation and myelination (Newbern and Birchmeier 2010). In addition, both unique PNS sites and common sites are enriched for nuclear receptor motifs. Null Nr2f1 mice exhibit a delay in oligodendrocyte differentiation (Yamaguchi et al. 2004), but lack a PNS phenotype perhaps due to compensation by Nr2f2. Accordingly, our siRNA data show that downregulation of both Nr2f1 and Nr2f2 is required to downregulate specific genes in cultured primary Schwann cells. Given our limited understanding of Tead proteins and nuclear receptors in myelination, analysis of these transcription factors could provide insight into glial development.
The origin of myelinating glia in vertebrates has been a subject of considerable speculation. The distinct developmental origins of oligodendrocytes and Schwann cells in the neural tube and neural crest, respectively, suggest that these two cell types arose independently from some type of ensheathing glia (Appel 2013; Zalc et al. 2008). Our data show that, despite the shared dependence on Sox10, Sox10 binding sites are significantly different between the two cell types. Indeed, many shared genes expressed in myelinating glia are regulated by different enhancers in the PNS and CNS, suggesting independent enhancer elements have evolved in these two cell lineages to regulate common genes. However, there is a core of shared Sox10 binding sites, and the active enhancers near the Sox10 locus itself are also largely shared (Figure S1B), suggesting some common mechanisms underlying induction of Sox10 binding and its activation of enhancers.
Because coordinated binding of multiple factors drive tissue-specific enhancers (Arvey et al. 2012; Gertz et al. 2013; Stefflova et al. 2013), these data sets help to elucidate how CNS- and PNS-specific enhancers are regulated by different external/environmental contexts. The combinatorial binding events identified by overlap and motif analysis are largely consistent with previous bioinformatics analysis of cis-regulatory modules in myelinating glia (Fulton et al. 2011), and may reflect physical interaction of Sox10 with other transcription factors such as Olig2, Myrf and Egr2 (Hornig et al. 2013; Jones et al. 2007; LeBlanc et al. 2007; Weider et al. 2013; Wissmuller et al. 2006). However, collaborative enhancer activation does not always require physical interaction between two or more factors (Kaplan et al. 2011; Mirny 2010; Reiprich et al. 2010), and coordinate binding of two or more factors would be expected to more efficiently recruit coactivators such as CBP/p300 and the Mediator complex. Therefore, the apparent diversity of CNS- and PNS-specific Sox10-bound enhancers may be a function of several integrating regulatory events, which include sequence-specific binding by cooperating transcription factors, and availability of binding partners/co-factors (Kamachi and Kondoh 2013).
One limitation of this analysis is that it does not differentiate Sox10 binding patterns in oligodendrocytes vs. their progenitors, nor in nonmyelinating vs. myelinating Schwann cells. Nonetheless, in vivo ChIP-Seq retains the nuclear impacts of axonal signals that are required for terminal differentiation of oligodendrocytes and Schwann cells. Interestingly, our analysis was able to detect binding of Sox10 to the Gjb1 (also known as Connexin 32) gene, which is involved in gap junction formation in mature oligodendrocytes (Wasseff and Scherer 2011), whereas the promoter region was not positive for activating histone modifications in ChIP-Seq analysis of oligodendrocytes differentiated in vitro (Yu et al. 2013).
Our analysis can also be used to identify disease mechanisms resulting from noncoding regulatory mutations. Substantial progress has been made in identifying genetic causes of CNS and PNS myelin disorders, such as Pelizaeus-Merzbacher and Charcot-Marie-Tooth disease (Nave and Trapp 2008; Scherer and Wrabetz 2008). Interestingly, noncoding mutations that are disease-causing (or disease-modifying) have been identified in Sox10 binding sites near the Connexin 32/GJB1, Connexin 43/GJC2, and Myelin Protein Zero genes (Antonellis et al. 2010; Bondurand et al. 2001; Gotoh et al. 2014; Houlden et al. 2004; Meyer et al. 2011; Osaka et al. 2010; Schlierf et al. 2006). Finally, understanding the coordination of glial differentiation by Sox10 and other factors will also elucidate stem cell approaches for glial development, which have employed Sox10 as a driver of glial differentiation (Najm et al. 2013; Wang et al. 2014; Yang et al. 2013).
Supplementary Material
Main Points.
ChIP-Seq analysis of Sox10 binding in vivo was used to identify shared and specific binding sites in oligodendrocytes and Schwann cells.
Cell type-specific binding sites are enriched with motifs of the distinct transcription factors involved in Schwann cell and oligodendrocyte development.
Schwann cell Sox10 binding sites are enriched with nuclear receptor binding sites and Nr2f1/Nr2f2 are required for expression of Mbp and Ndrg1 in primary Schwann cells.
ACKNOWLEDGMENTS
We thank Marie Adams at the UW Biotechnology Center for performance and preliminary analysis of Illumina sequencing, and Xiao-yu Liu and the Bioinformatics Resource Center for bioinformatic assistance.
This work was supported by a grant from National Institutes of Health: NS075269 to JS and P30 core grant HD03352. We affirm that we have no conflict of interest.
REFERENCES
- Antonellis A, Dennis MY, Burzynski G, Huynh J, Maduro V, Hodonsky CJ, Khajavi M, Szigeti K, Mukkamala S, Bessling SL. A rare myelin protein zero (MPZ) variant alters enhancer activity in vitro and in vivo. PLoS One. 2010;5:e14346. doi: 10.1371/journal.pone.0014346. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonellis A, Huynh JL, Lee-Lin SQ, Vinton RM, Renaud G, Loftus SK, Elliot G, Wolfsberg TG, Green ED, McCallion AS. Identification of neural crest and glial enhancers at the mouse Sox10 locus through transgenesis in zebrafish. PLoS Genet. 2008;4:e1000174. doi: 10.1371/journal.pgen.1000174. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B. Nonmammalian Vertebrate Glia. In: Kettenman H, Ransom BR, editors. Neuroglia. Oxford University Press; New York, NY: 2013. pp. 24–31. [Google Scholar]
- Arvey A, Agius P, Noble WS, Leslie C. Sequence and chromatin determinants of cell-type-specific transcription factor binding. Genome Res. 2012;22:1723–34. doi: 10.1101/gr.127712.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzedine H, Zavadakova P, Planté-Bordeneuve V, Vaz Pato M, Pinto N, Bartesaghi L, Zenker J, Poirot O, Bernard-Marissal N, Arnaud Gouttenoire E. PLEKHG5 deficiency leads to an intermediate form of autosomal-recessive Charcot-Marie-Tooth disease. Hum Mol Genet. 2013;22:4224–32. doi: 10.1093/hmg/ddt274. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise S, Kneib M, Rousseau A, Gambino F, Chenard MP, Messadeq N, Muckenstrum M, Alpy F, Tomasetto C, Humeau Y. In vivo evidence that TRAF4 is required for central nervous system myelin homeostasis. PLoS One. 2012;7:e30917. doi: 10.1371/journal.pone.0030917. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N, Girard M, Pingault V, Lemort N, Dubourg O, Goossens M. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10. Hum Mol Genet. 2001;10:2783–95. doi: 10.1093/hmg/10.24.2783. [DOI] [PubMed] [Google Scholar]
- Bremer M, Fröb F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022–32. doi: 10.1002/glia.21173. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Kozak CA, Chen XN, Wu S, Danciger M, Korenberg JR, Farber DB. Chromosomal localization of murine and human oligodendrocyte-specific protein genes. Genomics. 1996;34:255–7. doi: 10.1006/geno.1996.0278. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A, Barres BA. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell. 2014;28:7–17. doi: 10.1016/j.devcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, Mitew S, Hill AF, Lu QR, Wegner M. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 2013;11:e1001625. doi: 10.1371/journal.pbio.1001625. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Loh PG, Song H. Structural and functional insights into the TEAD-YAP complex in the Hippo signaling pathway. Protein Cell. 2010;1:1073–83. doi: 10.1007/s13238-010-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–6. doi: 10.1073/pnas.1016071107. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–88. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Denarier E, Forghani R, Farhadi HF, Dib S, Dionne N, Friedman HC, Lepage P, Hudson TJ, Drouin R, Peterson A. Functional organization of a Schwann cell enhancer. J Neurosci. 2005;25:11210–7. doi: 10.1523/JNEUROSCI.2596-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Dutta DJ, Zameer A, Mariani JN, Zhang J, Asp L, Huynh J, Mahase S, Laitman BM, Argaw AT, Mitiku N. Combinatorial actions of Tgfβ and Activin ligands promote oligodendrocyte development and CNS myelination. Development. 2014;141:2414–28. doi: 10.1242/dev.106492. others. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Emery B. Playing the field: Sox10 recruits different partners to drive central and peripheral myelination. PLoS Genet. 2013;9:e1003918. doi: 10.1371/journal.pgen.1003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–85. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi HF, Lepage P, Forghani R, Friedman HC, Orfali W, Jasmin L, Miller W, Hudson TJ, Peterson AC. A combinatorial network of evolutionarily conserved myelin basic protein regulatory sequences confers distinct glial-specific phenotypes. J Neurosci. 2003;23:10214–23. doi: 10.1523/JNEUROSCI.23-32-10214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest MP, Hill MJ, Quantock AJ, Martin-Rendon E, Blake DJ. The emerging roles of TCF4 in disease and development. Trends Mol Med. 2014 doi: 10.1016/j.molmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Fulton DL, Denarier E, Friedman HC, Wasserman WW, Peterson AC. Towards resolving the transcription factor network controlling myelin gene expression. Nucleic Acids Res. 2011;39:7974–91. doi: 10.1093/nar/gkr326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz J, Savic D, Varley KE, Partridge EC, Safi A, Jain P, Cooper GM, Reddy TE, Crawford GE, Myers RM. Distinct properties of cell-type-specific and shared transcription factor binding sites. Mol Cell. 2013;52:25–36. doi: 10.1016/j.molcel.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Charnay P. Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006;7:52–8. doi: 10.1038/sj.embor.7400573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M. Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development. 2002;129:155–66. doi: 10.1242/dev.129.1.155. [DOI] [PubMed] [Google Scholar]
- Gotoh L, Inoue K, Helman G, Mora S, Maski K, Soul JS, Bloom M, Evans SH, Goto Y, Caldovic L. GJC2 promoter mutations causing Pelizaeus-Merzbacher-like disease. Mol Genet Metab. 2014;111:393–8. doi: 10.1016/j.ymgme.2013.12.001. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–30. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, Svaren J, Casaccia P. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci. 2010;13:1472–80. doi: 10.1038/nn.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–89. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–47. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig J, Fröb F, Vogl MR, Hermans-Borgmeyer I, Tamm ER, Wegner M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013;9:e1003907. doi: 10.1371/journal.pgen.1003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Girard M, Cockerell C, Ingram D, Wood NW, Goossens M, Walker RW, Reilly MM. Connexin 32 promoter P2 mutations: a mechanism of peripheral nerve dysfunction. Ann Neurol. 2004;56:730–4. doi: 10.1002/ana.20267. [DOI] [PubMed] [Google Scholar]
- Huang dW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Inoue K, Khajavi M, Ohyama T, Hirabayashi S, Wilson J, Reggin JD, Mancias P, Butler IJ, Wilkinson MF, Wegner M. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet. 2004;36:361–9. doi: 10.1038/ng1322. others. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shilo K, Boerkoel CF, Crowe C, Sawady J, Lupski JR, Agamanolis DP. Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg-Hirschsprung disease: phenotypes linked by SOX10 mutation. Ann Neurol. 2002;52:836–42. doi: 10.1002/ana.10404. [DOI] [PubMed] [Google Scholar]
- Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. Journal of Neurochemistry. 2006;98:1678–1687. doi: 10.1111/j.1471-4159.2006.04069.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Ho YY, Hao L, Nichols Berrios C, Chakravarti A. Copy number variants in candidate genes are genetic modifiers of Hirschsprung disease. PLoS One. 2011;6:e21219. doi: 10.1371/journal.pone.0021219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Brewer MH, Srinivasan R, Krueger C, Sun G, Charney KN, Keles S, Antonellis A, Svaren J. Distal enhancers upstream of the Charcot-Marie-Tooth type 1A disease gene PMP22. Human Molecular Genetics. 2012;21:1581–91. doi: 10.1093/hmg/ddr595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Jang SW, Mager GM, Chang L-W, Srinivasan R, Gokey NG, Ward RM, Nagarajan R, Svaren J. Interactions of Sox10 and Egr2 in myelin gene regulation. Neuron Glia Biology. 2007;3:377–387. PMC2605513. doi: 10.1017/S1740925X08000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Krämer E, Grzenkowski M, Tang K, Blakemore W, Aguzzi A, Khazaie K, Chlichlia K, von Blankenfeld G, Kettenmann H. Lines of murine oligodendroglial precursor cells immortalized by an activated neu tyrosine kinase show distinct degrees of interaction with axons in vitro and in vivo. Eur J Neurosci. 1995;7:1245–65. doi: 10.1111/j.1460-9568.1995.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140:4129–44. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- Kaplan T, Li XY, Sabo PJ, Thomas S, Stamatoyannopoulos JA, Biggin MD, Eisen MB. Quantitative models of the mechanisms that control genome-wide patterns of transcription factor binding during early Drosophila development. PLoS Genet. 2011;7:e1001290. doi: 10.1371/journal.pgen.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolchik D, Barber GP, Casper J, Clawson H, Cline MS, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 2014;42:D764–70. doi: 10.1093/nar/gkt1168. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzyk A. BioMart: driving a paradigm change in biological data management. Database (Oxford) 2011;2011:bar049. doi: 10.1093/database/bar049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerer S, Schreiner S, Stolt CC, Scholz S, Bosl MR, Wegner M. Replacement of the Sox10 transcription factor by Sox8 reveals incomplete functional equivalence. Development. 2006;133:2875–86. doi: 10.1242/dev.02477. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hong YB, Park JM, Choi YR, Kim YJ, Yoon BR, Koo H, Yoo JH, Kim SB, Park M. Mutations in the PLEKHG5 gene is relevant with autosomal recessive intermediate Charcot-Marie-Tooth disease. Orphanet J Rare Dis. 2013;8:104. doi: 10.1186/1750-1172-8-104. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Lim H, Li Z, Oh Y, Kovlyagina I, Choi IY, Dong X, Lee G. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell. 2014;15:497–506. doi: 10.1016/j.stem.2014.07.013. [DOI] [PubMed] [Google Scholar]
- King RH, Chandler D, Lopaticki S, Huang D, Blake J, Muddle JR, Kilpatrick T, Nourallah M, Miyata T, Okuda T. Ndrg1 in development and maintenance of the myelin sheath. Neurobiol Dis. 2011;42:368–80. doi: 10.1016/j.nbd.2011.01.030. others. [DOI] [PubMed] [Google Scholar]
- Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, Emery B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32:12528–42. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan PF, Chung D, Pan G, Thomson JA, Stewart R, Keles S. A Statistical Framework for the Analysis of ChIP-Seq Data. Journal of the American Statistical Association. 2011:891–903. doi: 10.1198/jasa.2011.ap09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Yamanaka Y, Kitano A, Shibata M, Awaya T, Kato T, Okawa K, Abe T, Oshima N, Nakahata T. Ttyh1, a Ca(2+)-binding protein localized to the endoplasmic reticulum, is required for early embryonic development. Dev Dyn. 2010;239:2233–45. doi: 10.1002/dvdy.22348. others. [DOI] [PubMed] [Google Scholar]
- Küspert M, Weider M, Müller J, Hermans-Borgmeyer I, Meijer D, Wegner M. Desert hedgehog links transcription factor Sox10 to perineurial development. J Neurosci. 2012;32:5472–80. doi: 10.1523/JNEUROSCI.5759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Maeda Y, Bannerman P, Xu J, Horiuchi M, Pleasure D, Guo F. Adenomatous polyposis coli regulates oligodendroglial development. J Neurosci. 2013;33:3113–30. doi: 10.1523/JNEUROSCI.3467-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005a;102:2596–601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J. Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci. 2005b;8:932–40. doi: 10.1038/nn1490. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Jang SW, Ward RM, Wrabetz L, Svaren J. Direct Regulation of Myelin Protein Zero Expression by the Egr2 Transactivator. J Biol Chem. 2006;281:5453–5460. doi: 10.1074/jbc.M512159200. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Ward RM, Svaren J. Neuropathy-associated Egr2 mutants disrupt cooperative activation of myelin protein zero by Egr2 and Sox10. Mol Cell Biol. 2007;27:3521–9. doi: 10.1128/MCB.01689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lu Y, Smith HK, Richardson WD. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J Neurosci. 2007;27:14375–82. doi: 10.1523/JNEUROSCI.4456-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Keles S. Detecting differential binding of transcription factors with ChIP-seq. Bioinformatics. 2012;28:121–2. doi: 10.1093/bioinformatics/btr605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hu X, Cai J, Liu B, Peng X, Wegner M, Qiu M. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev Biol. 2007;302:683–93. doi: 10.1016/j.ydbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Mager GM, Ward RM, Srinivasan R, Jang SW, Wrabetz L, Svaren J. Active gene repression by the EGR2/NAB complex during peripheral nerve myelination. J Biol Chem. 2008;283:18187–18197. doi: 10.1074/jbc.M803330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Kurian MA, Morgan NV, McNeill A, Pasha S, Tee L, Younis R, Norman A, van der Knaap MS, Wassmer E. Promoter mutation is a common variant in GJC2-associated Pelizaeus-Merzbacher-like disease. Mol Genet Metab. 2011;104:637–43. doi: 10.1016/j.ymgme.2011.08.032. others. [DOI] [PubMed] [Google Scholar]
- Mirny LA. Nucleosome-mediated cooperativity between transcription factors. Proc Natl Acad Sci U S A. 2010;107:22534–9. doi: 10.1073/pnas.0913805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31:426–33. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H, Martin E, Hassani H, Clavairoly A, Maire CL, Viadieu A, Kerninon C, Delmasure A, Frah M, Weber M. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J Neurosci. 2013;33:9752–68. doi: 10.1523/JNEUROSCI.0805-13.2013. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–52. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–61. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21:922–8. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka H, Hamanoue H, Yamamoto R, Nezu A, Sasaki M, Saitsu H, Kurosawa K, Shimbo H, Matsumoto N, Inoue K. Disrupted SOX10 regulation of GJC2 transcription causes Pelizaeus-Merzbacher-like disease. Ann Neurol. 2010;68:250–4. doi: 10.1002/ana.22022. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Klingener M, Aguirre A. TGFβ signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci. 2014;34:7917–30. doi: 10.1523/JNEUROSCI.0363-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27:4233–42. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–33. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Brzózka MM, Rossner MJ. Transcription factor 4 (TCF4) and schizophrenia: integrating the animal and the human perspective. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-013-1553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintes S, Goebbels S, Saher G, Schwab MH, Nave KA. Neuron-glia signaling and the protection of axon function by Schwann cells. J Peripher Nerv Syst. 2010;15:10–6. doi: 10.1111/j.1529-8027.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T, Wysocka J. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell. 2012;11:633–48. doi: 10.1016/j.stem.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–83. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiprich S, Kriesch J, Schreiner S, Wegner M. Activation of Krox20 gene expression by Sox10 in myelinating Schwann cells. J Neurochem. 2010;112:744–54. doi: 10.1111/j.1471-4159.2009.06498.x. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Hurtado A, Wang H, Macopson JG, Griner EM, Betz A, Brose N, Kazanietz MG, Kolodkin AL. The RacGAP β2-Chimaerin selectively mediates axonal pruning in the hippocampus. Cell. 2012;149:1594–606. doi: 10.1016/j.cell.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–89. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlierf B, Werner T, Glaser G, Wegner M. Expression of Connexin47 in Oligodendrocytes is Regulated by the Sox10 Transcription Factor. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.05.072. [DOI] [PubMed] [Google Scholar]
- Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Sun G, Keles S, Jones EA, Jang SW, Krueger C, Moran JJ, Svaren J. Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve. Nucleic Acids Res. 2012;40:6449–6460. doi: 10.1093/nar/gks313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefflova K, Thybert D, Wilson MD, Streeter I, Aleksic J, Karagianni P, Brazma A, Adams DJ, Talianidis I, Marioni JC. Cooperativity and rapid evolution of cobound transcription factors in closely related mammals. Cell. 2013;154:530–40. doi: 10.1016/j.cell.2013.07.007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131:2349–58. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–70. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Bertrand N, Parras CM, Guillemot F, Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007;134:1617–29. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Parras CM, Nakatani H, Lebel M, Guillemot F, Nakafuku M. Ascl1 is required for oligodendrocyte development in the spinal cord. Development. 2008;135:1271–81. doi: 10.1242/dev.015370. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–51. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Pitt-Hopkins Syndrome: intellectual disability due to loss of TCF4-regulated gene transcription. Exp Mol Med. 2013;45:e21. doi: 10.1038/emm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Tuason MC, Rastikerdar A, Kuhlmann T, Goujet-Zalc C, Zalc B, Dib S, Friedman H, Peterson A. Separate proteolipid protein/DM20 enhancers serve different lineages and stages of development. J Neurosci. 2008;28:6895–903. doi: 10.1523/JNEUROSCI.4579-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo T, Nobbio L, Hummelen PV, Abbruzzese M, Mancardi G, Verpoorten N, Verhoeven K, Sereda MW, Nave KA, Timmerman V. Experimental Charcot-Marie-Tooth type 1A: a cDNA microarrays analysis. Mol Cell Neurosci. 2005;28:703–14. doi: 10.1016/j.mcn.2004.11.016. others. [DOI] [PubMed] [Google Scholar]
- Vogl MR, Reiprich S, Küspert M, Kosian T, Schrewe H, Nave KA, Wegner M. Sox10 Cooperates with the Mediator Subunit 12 during Terminal Differentiation of Myelinating Glia. J Neurosci. 2013;33:6679–90. doi: 10.1523/JNEUROSCI.5178-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlbuhl M, Reiprich S, Vogl MR, Bösl MR, Wegner M. Transcription factor Sox10 orchestrates activity of a neural crest-specific enhancer in the vicinity of its gene. Nucleic Acids Res. 2012;40:88–101. doi: 10.1093/nar/gkr734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pol SU, Haberman AK, Wang C, O'Bara MA, Sim FJ. Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1408295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasseff SK, Scherer SS. Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiol Dis. 2011;42:506–13. doi: 10.1016/j.nbd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weider M, Reiprich S, Wegner M. Sox appeal - Sox10 attracts epigenetic and transcriptional regulators in myelinating glia. Biol Chem. 2013;394:1583–93. doi: 10.1515/hsz-2013-0146. [DOI] [PubMed] [Google Scholar]
- Weng Q, Chen Y, Wang H, Xu X, Yang B, He Q, Shou W, Higashi Y, van den Berghe V, Seuntjens E. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron. 2012;73:713–28. doi: 10.1016/j.neuron.2011.12.021. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Hammer A, Wahlbuhl M, Bosl MR, Wegner M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucleic Acids Res. 2007;35:6526–38. doi: 10.1093/nar/gkm727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–19. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernasz E, Kaliszewska A, Brutkowski W, Bednarczyk J, Gorniak M, Kaza B, Lukasiuk K. Ttyh1 Protein is Expressed in Glia In Vitro and Shows Elevated Expression in Activated Astrocytes Following Status Epilepticus. Neurochem Res. 2014;39:2516–26. doi: 10.1007/s11064-014-1455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmuller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006;34:1735–44. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Zhou C, Lin SC, Durand B, Tsai SY, Tsai MJ. The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes. Dev Biol. 2004;266:238–51. doi: 10.1016/j.ydbio.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31:434–9. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, Liu L, Liu W, Wu LM, Mao M. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell. 2013;152:248–61. doi: 10.1016/j.cell.2012.12.006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuelling LW, Waggener CT, Afshari FS, Lister JA, Fuss B. Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain. Glia. 2012;60:1605–18. doi: 10.1002/glia.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalc B, Goujet D, Colman D. The origin of the myelination program in vertebrates. Curr Biol. 2008;18:R511–2. doi: 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Gazin C, Lawson ND, Pagès H, Lin SM, Lapointe DS, Green MR. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics. 2010;11:237. doi: 10.1186/1471-2105-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The Transcription Factors SCIP and Krox-20 Mark Distinct Stages and Cell Fates in Schwann Cell Differentiation. Mol Cell Neurosci. 1996;8:129–45. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.