Abstract
Background
The main neurobiological theories of the development of addiction, including tolerance, sensitization, incentive-sensitization, and allostasis have not been tested in longitudinal human alcohol response research. To address this issue, we conducted the first controlled prospective investigation of subjective and neuroendocrine responses to alcohol measured over a five year interval in at-risk young adult heavy drinkers and light drinker controls.
Methods
Participants were 156 individuals, 86 heavy drinkers (HD) and 70 light drinkers (LD), undergoing an initial oral alcohol challenge testing (0.8 g/kg alcohol vs. placebo) and an identical re-examination testing 5–6 years later. Alcohol use disorder (AUD) symptoms and drinking behaviors were assessed in the interim follow-up period.
Results
At reexamination, HD continued to exhibit higher sensitivity on alcohol’s stimulating and rewarding effects with lower sensitivity to sedative effects and cortisol reactivity, relative to LD. In HD with high AUD symptom trajectories over follow-up, heightened alcohol stimulation and reward persisted at reexamination. HD with low AUD symptoms showed reduced alcohol stimulation over time and lower reward throughout compared with the HD with high and intermediate AUD symptoms.
Conclusions
Results support the early stage phase of the allostasis model, with persistently heightened reward sensitivity and stimulation in heavy drinkers exhibiting AUD progression in early mid-adulthood. While there are multiple pathways to development of a disorder as complex as AUD, maintenance of alcohol stimulatory and rewarding effects may play an important role in the continuation and progression of alcohol addiction.
Keywords: alcohol response, subjective, stimulation, reward sensitivity, tolerance, heavy drinking progressing with AUD
INTRODUCTION
Alcohol use disorder (AUD) is associated with numerous consequences for the individual and society including psychological, occupational, and health consequences, as well as public safety harms and annual financial costs exceeding $223 billion in the US(1). Thus, identifying the mechanisms underlying the development and maintenance of AUD has become increasingly important for AUD prevention and treatment. Four leading neurobiological theories of the development of addiction include tolerance, sensitization, incentive-sensitization, and allostasis. These theories purport nervous system adaptations to repeated alcohol exposure underlie the progression of compulsive drinking and development of addiction but they lead to differential predictions about the nature of these responses over time. While these theories are crucial to our understanding of AUD, they are largely based on animal data and their predictions have not yet been directly tested in controlled longitudinal human studies. The present study provided the first comprehensive repeated evaluation of alcohol responses in at-risk drinkers to test these neurobiological theories of AUD progression.
The most longstanding theory of alcohol adaptation is chronic tolerance(2–7), i.e., the need for markedly increased amounts of alcohol to achieve a desired effect or experiencing markedly diminished effects with continued use of the same amount of alcohol. Tolerance, a diagnostic criteria for AUD from DSM-III (1980) to DSM-5 (2013)(8), implies that attenuation of subjective alcohol responses over time plays a key role in the development of addiction. In contrast, the sensitization theory asserts that greater stimulant effects over time underlie addictive processes(9), based on rodent data showing that stimulant-like and locomotor alcohol responses increase after repeated exposures(10, 11). These effects are particularly strong in selectively bred mouse lines (12–14) sensitized responses may also include adrenal hormones(15). The incentive-sensitization theory of addiction(16, 17) also emphasizes sensitization process, but specifies that repeated use of a drug produces neuroadapations that sensitize motivational reward to drugs and associated drug stimuli (i.e.., processes of “wanting”) distinct from the neurocircuitry mediating hedonic reward (liking) which may not sensitize over time. Finally, allostatic theory asserts heightened brain reward sensitivity and positive reinforcement characterize the early stages of addiction(18), but reward insensitivity and negative reinforcement underlie the later and more severe stages(18–20). Thus, while some researchers may not agree on the contributions of positive versus negative reinforcement factors underlying addiction(21, 22), there is consensus on the critical need for longitudinal controlled human alcohol response investigation. Human studies in this area have been limited to retrospective patient reports(23), post mortem brain tissue methods(24), or cross-sectional laboratory paradigms(2, 3, 25–29), none of which directly measure alcohol responses in the same individuals over time. The few published test-retest studies of alcohol responses have included only brief between-session intervals with a focus on measurement reliability(30, 31).
To address this issue, we conducted the Chicago Social Drinking Project (CSDP), a prospective alcohol response re-examination study. The CSDP examined 190 non-alcohol dependent young adult heavy and light drinkers who were primarily in their 20s (mean age 25.6 ± 3.2 SD years) at enrollment. Our previously published results showed that compared with light drinkers, heavy drinkers exhibited both higher alcohol sensitivity, in terms of subjective stimulation and reward (liking and wanting)(29), as well as lower sensitivity, in terms of subjective sedation(29), and salivary cortisol reactivity(29, 32). These findings were replicated in a second independent heavy drinker cohort using identical procedures(33). Further, in heavy drinkers, greater alcohol stimulation and reward and lower sedation predicted binge drinking escalations at two-year follow-up(29), with greater stimulation and reward predicting more AUD symptoms experienced through six years(34).
In the current phase of CSDP, participants were invited back between their fifth and sixth year of the study to participate in two re-examination laboratory sessions. The goal was to conduct empirical tests of the neurobiological theories of alcohol adaptations underlying the propensity to develop addiction. We examined whether the alcohol response differences observed at initial testing persisted or changed in heavy versus light drinkers, and whether the degree of change related to trajectories of AUD progression among the heavy drinkers. Tolerance theory would be supported if the heaviest drinkers over time showed an overall reduced alcohol response at re-examination compared with initial testing, whereas sensitization would be supported if the heaviest drinkers showed higher stimulant responses. The allostasis model’s early phase of addiction, which may most closely match a five-year interval in young adults, would be supported if alcohol reward sensitivity was maintained, and the later stage would be supported if reward sensitivity was diminished. Finally, increases over time in alcohol wanting would support the incentive-sensitization theory.
METHODS AND MATERIALS
Design
The CSDP is a within-subject, double-blind, randomized-order study of responses to alcohol and placebo beverages in 190 young adult non-alcohol dependent drinkers. The study was approved by the University of Chicago Institutional Review Board. Initial laboratory testing was conducted March 2004-July 2006, and re-examination testing was conducted March 2009-October 2011. Participants returned for re-examination on average 63 months (±1.5 SD) after their initial assessment. Both testing phases included two 4½ hour individual sessions separated by at least 24 hours and conducted at the Clinical Addictions Research Laboratory at the University of Chicago. Participants completed measures before and after ingesting a blinded beverage that contained either 0.8 g/kg alcohol or placebo administered in random order at each phase.
Initial Testing Phase
Participants were recruited via local media and internet advertisements and word-of-mouth referrals. Initial inclusion criteria were: age 21–35 years, weight 110–210 pounds, good general health, not pregnant or lactating, no current or past major medical or Axis I psychiatric disorders including alcohol and substance dependence (other than nicotine), and no current use of any centrally-acting medications. The medical screening by the study nurse included a brief physical assessment, health history, vital signs, a blood draw to confirm normal liver enzyme levels (<2 SD of normal range), and a urine toxicology screen (cocaine, opiates, benzodiazepines, amphetamines, barbiturates, and PCP) and pregnancy test for women. A trained research assistant conducted the alcohol Quantity-Frequency Interview (QFI)(35) and the alcohol disorders module from the Structured Clinical Interview for DSM-IV (SCID), non-patient version (36). The participant also completed demographic measures, a two-generational biological family history (FH) tree for alcohol use disorders and the FH Research Diagnostic Criteria for drinking consequences(37), an alcohol Timeline Follow-back for past month drinking(38), the Alcohol Use Disorders Identification Test (AUDIT (39)), and the Drinker Inventory of Consequences (Dr-InC2R (40). Heavy drinkers (HD) were defined as weekly binge drinkers (consuming >5 drinks for men, >4 for women, per occasion 1–4 times weekly) with at least 10 but no more than 40 drinks consumed per week for at least the past two years. Light drinkers (LD) averaged consuming 1–5 drinks per week with no/rare binge episodes (<5 times/year). These criteria were based upon established guidelines(41–43) and were consistent with prior studies (44–50). Positive FH was defined as having at least one biological first-degree biological relative or two or more second-degree relatives with alcohol use disorders.
Laboratory Procedures
The testing sessions for both phases were conducted in the afternoon and commenced between 12 and 5pm. To reduce alcohol expectancy, the Alternative Substance Paradigm(51) was used: participants were informed that their allocated beverage might contain a stimulant, sedative, alcohol, or a placebo, or a combination of these substances. Upon arrival, the participant completed self-report measures and engaged in objective breath tests to confirm compliance with recent alcohol abstinence. Urine samples were collected prior to one session, chosen randomly, for toxicology in all participants, and prior to each session for women to verify non-pregnancy. Participants were interviewed to confirm compliance with 3-hour abstinence from food, caffeine, and smoking. Each participant then consumed a standard snack at 20% of daily kilocalorie needs per body weight (55% carbohydrates, 10% protein, and 35% fat)(52) followed by acquisition of baseline measures for approximately 15–20 minutes.
Starting at experimental time 0, the participant consumed his/her beverages presented in lidded, clear plastic cups in two equal portions with each portion consumed over 5 minutes separated by a 5 minute rest with the research assistant present(45, 46, 53). The average total beverage volume was 471 mL containing 190-proof ethanol (1% volume for placebo as a taste mask, 16% volume for alcohol beverage). The beverage was prepared with water, a flavored drinking mix, and a sucralose-based sugar substitute. Doses for women were 85% of those of men to adjust for sex differences in total body water(54, 55). Dependent measures and breathalyzer tests (Alco-Sensor IV, Intoximeter; St. Louis, MO) were repeated at 30, 60, 120, and 180 minutes. Other objective responses were obtained after the subjective measures and these results are presented elsewhere(53, 56). Breathalyzer readings were programmed to read 0.000 mg/dl, with actual values downloaded later. At the end of each session, after breath alcohol concentration (BrAC) was <40 mg/dl(57), the participant was transported to his/her lodging using a car service.
Alcohol Responses
The Biphasic Alcohol Effects Scale (BAES)(58) was used to assess subjective stimulation and sedation, and the Drug Effects Questionnaire (DEQ)(59), a 10cm visual analogue scale, was used to assess hedonic alcohol reward, like: “do you LIKE the effects you are feeling now?” (midpoint as neutral) and motivational reward, want more: “would you like MORE of what you consumed, right now?” The instructions asked for current mood state at each time point and did not reveal that alcohol was ingested (60). Saliva samples were provided by participants at each timepoint via a cotton Salivette® (Sarstedt AG & Company; Nümbrecht, Germany). Samples were stored at −20°C and later assayed for levels of the stress hormone cortisol by high sensitivity enzyme immunoassay at the University of Chicago CRC Core Laboratory. The inter- and intra-assay coefficients of variation at initial testing were 6.88% and 7.12%, respectively, and at re-examination were 6.60% and 7.99%, respectively. The main dependent analytic variables were: 1). stimulation and sedation: net change scores from the BAES; calculated by subtracting the pre-beverage rating from the 60 min post-beverage rating (peak BrAC) in the alcohol session minus the same change score from the placebo session; 2). like and want more: change scores for the DEQ calculated by subtracting the 60 min post-beverage rating in the alcohol session minus the placebo session, since the DEQ pertains to drug effects and so does not include a pre-beverage administration; and 3). cortisol: net change scores calculated by subtracting the pre-beverage from the last (180 min) post-beverage level minus the same change score from the placebo session. This later timepoint was used given the delay in time course for cortisol secretion(29, 45, 61).
Interim Follow-up Phase
In the interval between initial testing and re-examination, participants completed annual telephone and secured internet follow-ups through 6 years(34), excluding year 3 to avoid participant burden. The follow-ups included similar measures as those at baseline screening, including the SCID DSM-IV AUD module(36), TLFB(38), QFI(35), AUDIT(39), and Dr-InC2R(40). The number of AUD criteria endorsed was the main dependent outcome variable assessed during follow-up to reflect the dimensional nature of these symptoms(62). The DSM-IV criteria are similar to those in DSM-5(8, 62) with the former including an item for alcohol-related legal problems that has since been dropped and excluding the DSM-5 item for craving that had not yet been developed.
At the 5-year follow-up, 187 individuals (98.4%) continued to participate, and telephone re-screening was conducted to determine eligibility for re-examination. Most participants (95%, 178/187) remained eligible: they were current drinkers with no major medical or psychiatric contraindications. From this eligible subject pool, 156 (88% of those eligible, 83% of the original sample) returned to participate in re-examination sessions. Participants undergoing re-examination did not differ from those who did not on major background characteristics or geographical location (all ps >.23). As needed, transportation (airfare, local transport, etc.) and lodging arrangements were provided by the study. Supplemental Figure S1 shows the CONSORT diagram.
Trajectory Subgroups in HD
Our prior report (King et al., 2014(34)) indicated that the HD and LD continued to differ significantly through follow-up on all measures of alcohol drinking behaviors and problems. Table 1 depicts demographic, health, and drinking comparisons between the groups, and Supplemental Table S1 depicts baseline characteristics of the groups. In brief, the LD largely continued with low-risk drinking. While they consumed alcohol more frequently over time, binge drinking and alcohol problems were rare, and they formed one low-risk trajectory group(34). In contrast, the HD comprised three AUD subgroups over time, derived by trajectory analysis which differentiated those with low, intermediate, and high symptoms (see Table 1).
Table 1.
Characteristics of Participants at Retesting and Over Follow-Up HD AUD subgroups
| Demographics & Health | LD n=70 |
HD n=86 |
Low n=26 |
Intermediaten=51 | High n=9 |
|---|---|---|---|---|---|
| Age (years) | 31.3 (0.4) | 30.3 (0.3) | 31.0 (0.6) | 30.0 (0.4) | 29.9 (1.2) |
| Education (years) | 18.0 (0.4) | 16.2 (0.2)*** | 15.9 (0.4) | 16.3 (0.3) | 16.0 (1.2) |
| Sex (male) | 49% | 57% | 50% | 61% | 56% |
| Race (Caucasian) | 67% | 83%* | 77% | 84% | 89% |
| Family History Positive (AUD) a | 34% | 44% | 35% | 51% | 67% |
| Age at First Drink (years) | 17.7 (0.3) | 15.0 (0.3)*** | 15.5 (0.4) | 14.8 (0.3) | 14.2 (0.8) |
| Beck Depression Inventory | 3.1 (0.4) | 4.3 (0.5) | 2.3 (0.6) | 4.7 (0.6) | 7.8 (1.7)**i |
| Spielberger State Anxiety (T-Score) | 47.7 (0.9) | 48.8 (0.8) | 45.9 (1.2) | 49.0 (1.1) | 56.4 (2.2) ***j |
| Marijuana Use (weekly or more) | 1% | 26%*** | 19% | 24% | 56%*k |
| Cigarette Use (weekly or more) | 1% | 37%*** | 19% | 28% | 78%*j |
| Stimulant Use (monthly or more)b | 0% | 5% | 4% | 2% | 22%* j |
| Major Axis I Disorder c | 6% | 12% | 4% | 12% | 33%*k |
| Substance Dependence d | 0% | 11%* | 0% | 12% | 33%*k |
| Nicotine Dependence c | 0% | 19%*** | 8% | 22% | 56%*j |
| AST (units/L)e | 20.8 (0.7) | 22.2 (1.2) | 23.7 (3.6) | 21.3 (0.9) | 23.1 (2.9) |
| ALT (units/L)e | 20.1 (1.8) | 21.6 (2.0) | 24.0 (5.8) | 20.2 (1.4) | 22.1 (4.9) |
|
| |||||
| Average Drinking Over Follow- Upf | |||||
|
| |||||
| Frequency (days/month) | 6.8 (0.5) | 12.1 (0.5)*** | 10.1 (1.0) | 12.5 (0.6) | 15.5 (2.4)*k |
| Quantity (drinks/drinking day) | 1.8 (0.1) | 5.0 (0.2)*** | 4.0 (0.3) | 5.1 (0.3) | 7.5 (0.8) ***i |
|
| |||||
| Maxima Drinking Over Follow- Upg | |||||
|
| |||||
| Max # drinks in one occasion | 4.6 (0.2) | 12.6 (0.6)*** | 10.7 (1.0) | 13.0 (0.6) | 18.6 (3.0) ***j |
| Binge Frequency (days/month) h | 1.1 (0.2) | 10.2 (0.5)*** | 7.5 (0.7) | 10.3 (0.5) | 17.2 (2.5) ***i |
| AUD symptom count | 0.6 (0.1) | 2.8 (0.2)*** | 0.9 (0.2) | 3.2 (0.2) | 6.4 (0.6) ***i |
| AUDIT total score | 5.3 (0.3) | 14.8 (0.7)*** | 10.2 (0.7) | 15.6 (0.8) | 23.8 (1.9) ***i |
| DrInC-2R total score | 7.2 (0.9) | 23.9 (1.4)*** | 14.8 (1.8) | 25.0 (1.5) | 44.0 (2.6) ***i |
Note. Data are Mean (SEM) or N (%).
p < .05,
p < .01,
p < .001
Data pertain to that obtained during or self-report of use patterns the year prior to re-examination, unless otherwise noted;
Family history positive defined as having one biological primary or three or more biological secondary relatives with an alcohol use disorder;
Stimulant use included both prescription and recreational drugs;
Ever meeting DSM-IV major Axis I diagnosis (depression, anxiety disorders, etc.) or nicotine dependence at any point during the follow-up period;
Seven of the nine substance dependent persons met criteria for cannabinoids, one for cocaine, and one for prescription opiates;
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) blood tests for liver functioning;
Drink based on standard definition of one drink = 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor, from average of past month TLFB interviews at 1-, 2, 4-, 5-, and 6-years;
Refer to maxima of TLFB or survey measures from 1-, 2-, 4-, 5-, and 6-year follow-up;
Binge defined as ≥5 drinks per occasion for males and ≥4 drinks for females.
High>Intermediate>Low;
High>Intermediate=Low;
High>Low, High=Intermediate;
High=Intermediate>Low
Statistical Analysis
Demographic and drinking characteristics were compared across groups for each phase by Generalized Estimating Equations (GEE(63)). Pearson correlations were used to examine the association of each alcohol response (net change in stimulation, sedation, and cortisol; change in like, and want more) at initial and re-examination phases. These variables were examined in GEE models testing effects of group (HD, LD), phase, and their interactions. To further investigate the source of group differences, GEE analyses compared alcohol responses by phase among the three HD AUD trajectory subgroups. If the interaction was not significant, then this term was removed to examine the main effect of group or phase. Since FH is as a potential risk factor for development of alcohol problems(64), analyses were repeated including FH as a covariate with FH coded by 2 dummy variables: FH positive vs. negative, and FH not sure vs. negative.
RESULTS
BrAC Comparisons by Test Phase
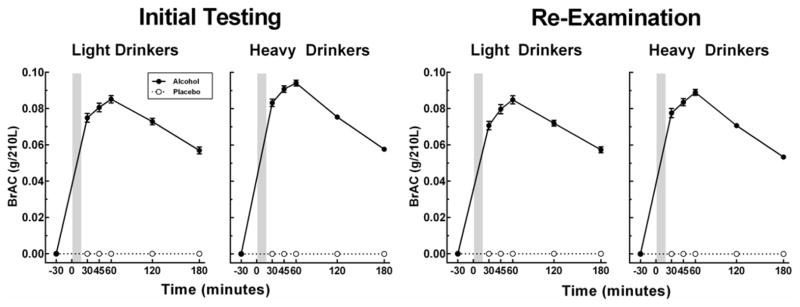
Figure 1 shows the BrAC curves at initial and re-examination testing for light and heavy drinkers. BrAC peak levels at 60 minutes were slightly higher at initial testing in the HD vs. LD (94 vs. 85 mg/dl, respectively, p<.001) but at re-examination there were no differences [89 vs. 85 mg/dl, p=.126]. All analyses controlled for peak BrAC levels at each testing phase.
Figure 1.
BrAC curve data as mean (SEM) for light and heavy drinkers at initial and re-examination testing.
Association of Alcohol Responses Over Time
Within-subject associations of each alcohol response between initial and re-examination phases were positive and significant [stimulation (r=.30, p<.001), liking (r=.26, p<.001), wanting (r= .30, p<.001), sedation (r=.42, p<.0001), and cortisol (r= .18, p<.05)].
Group Comparisons of Alcohol Responses
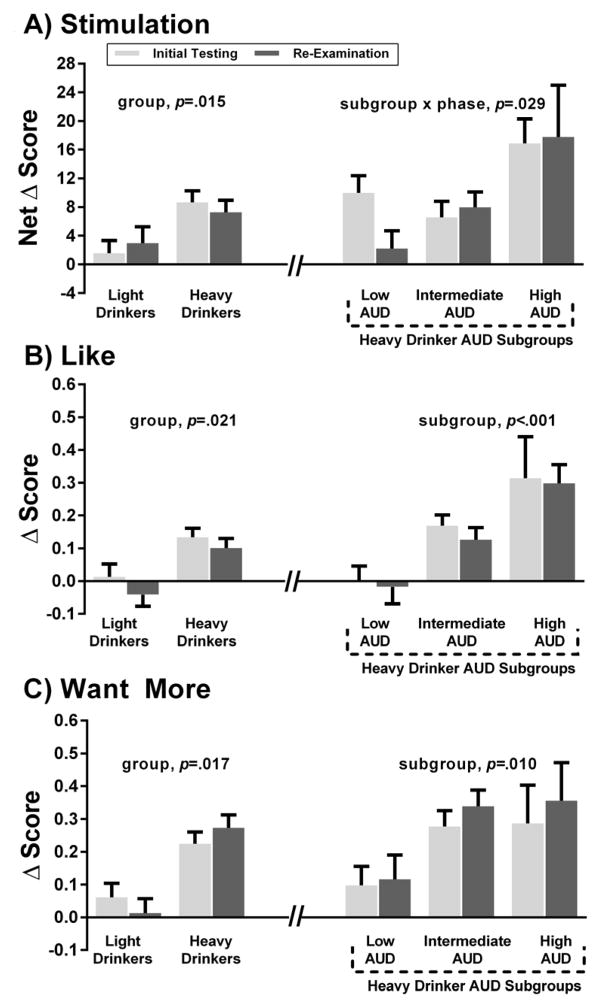
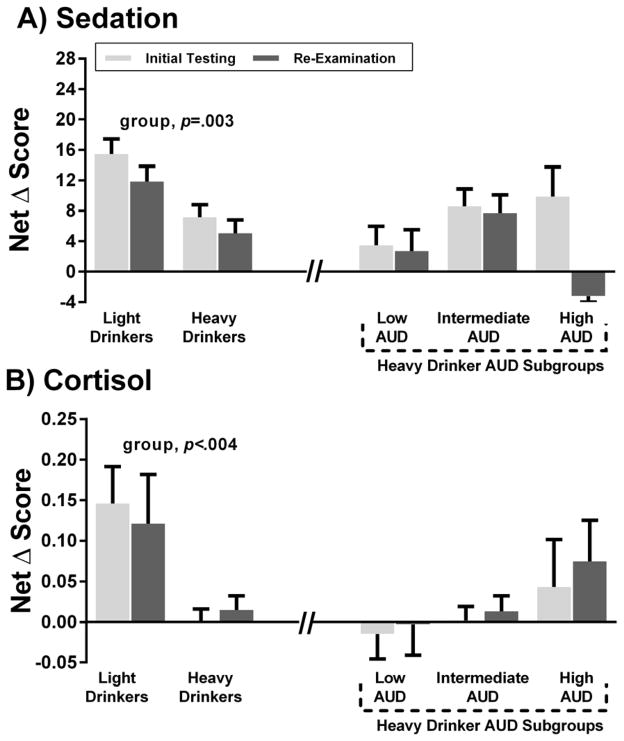
Detailed GEE results of the alcohol response analyses for HD and LD and among the three AUD subgroups within HD are summarized in Table 2. In brief, at both phases, relative to LD, HD exhibited higher sensitivity to alcohol stimulating and rewarding effects (Fig 2A–2C). HD also had lower sensitivity to sedation (Fig 3A) and cortisol response versus LD (Fig 3B). For the HD subgroups, there was a subgroup x phase interaction for stimulation such that the high AUD subgroup persisted with heightened stimulation at re-examination and the intermediate group persisted at intermediate levels, but the low AUD subgroup showed a reduction in stimulation over time. For alcohol reward, there were main effects of AUD subgroup: relative to the low AUD subgroup, the high and intermediate AUD subgroups had persistently higher alcohol liking and wanting over time than the low AUD subgroup. The relationships of stimulation, liking and wanting were positive for the high and intermediate AUD subgroups, suggesting that stimulation is pleasurable; these correlations were not significant in the low AUD subgroup (Supplemental Results). The AUD subgroups did not differ on alcohol sedation or cortisol response (Fig 3A, 3B). All findings remained after including age, sex, race, FH, as well as psychiatric disorders, substance and nicotine dependence as covariates in the same models.
Table 2.
GEE analysis summary of alcohol responses in Light (LD) and Heavy Drinkers (HD) by testing phase and HD AUD subgroups by testing phase
| Alcohol Responses | Group (LD vs. HD) | Phase | Group x Phase | Post Estimation Comparisons a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | se | p | β | se | p | β | se | p | ||
|
| ||||||||||
| Stimulation | 6.19 | 2.54 | 0.015 | 1.41 | 2.15 | 0.512 | −1.67 | 2.92 | 0.567 | HD > LD |
| Like | 10.86 | 4.69 | 0.021 | −5.37 | 4.29 | 0.211 | 2.76 | 5.80 | 0.634 | HD > LD |
| Want More | 13.82 | 5.80 | 0.017 | −4.86 | 5.17 | 0.348 | 11.09 | 7.00 | 0.113 | HD > LD |
| Sedation | −7.86 | 2.63 | 0.003 | −3.63 | 2.11 | 0.086 | 1.28 | 2.86 | 0.655 | HD < LD |
| Cortisol | −0.15 | 0.05 | 0.004 | −0.03 | 0.05 | 0.579 | 0.05 | 0.06 | 0.467 | HD < LD |
| Alcohol Responses | HD AUD Subgroup | Phase | AUD Subgroup x Phase | Post Estimation Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | se | p | β | se | p | β | se | p | ||
|
| ||||||||||
| Stimulation | 1.10 | 2.49 | 0.659 | −11.65 | 5.39 | 0.031 | 6.20 | 2.84 | 0.029 | Low AUD: Initial > Re-Exam Phase b |
| Like | 16.59 | 4.45 | <.001 | −2.48 | 10.27 | 0.809 | −0.80 | 5.40 | 0.883 | High > Intermediate > Low c |
| Want More | 12.60 | 4.89 | 0.010d | 6.34 | 4.48 | 0.157 | 3.46 | 7.24 | 0.633 | High = Intermediate > Low c |
| Sedation | 4.28 | 2.77 | 0.123e | 5.23 | 5.72 | 0.360 | −4.35 | 3.01 | 0.148 | N/A |
| Cortisol | 0.02 | 0.03 | 0.342e | 0.005 | 0.07 | 0.940 | 0.06 | 0.03 | 0.854 | N/A |
Note: Results from GEE analyses [coefficient (β), standard error (se), and p value] for alcohol response measures for group (HD vs. LD) or AUD subgroup (the HD AUD Subgroups), phase (initial, reexamination), and their interaction(s). Alcohol responses were either net change (stimulation, sedation, and cortisol) or change (like, want more) scores from placebo for each phase.
Group (HD vs. LD) comparisons;
Group x Phase comparison;
Subgroup (High, Intermediate, Low AUD) comparisons;
The main subgroup effect was significant after the interaction term was removed;
The main effects remained insignificant in the model when the interaction terms were removed.
Figure 2.
Figure 2A–C. Alcohol stimulation, liking and wanting at initial and re-examination phases.
Data are shown for light (n=70) and heavy drinker groups (n=86), as well the heavy drinker AUD trajectory subgroups, including low AUD (n=26), intermediate AUD (n=51), and high AUD (n=9). Fig 2A is the net change score (alcohol session peak BrAC minus baseline change score minus the same change score for the placebo session) for the BAES stimulation; three outliers (<3 SD below mean) were removed in this analysis. Fig 2B and 2C are the DEQ like and want more change scores (alcohol session minus placebo session), respectively. GEE results are depicted for group and group x phase effects, see Table 2 for post-estimation testing results.
Figure 3.
Figure 3A–B. Alcohol sedation and cortisol response at initial and re-examination phases.
Data are shown for light (n=70) and heavy drinker groups (n=86), as well the heavy drinker AUD trajectory subgroups, including low AUD (n=26), intermediate AUD (n=51), and high AUD (n=9). Fig 3A is the net change score (alcohol session peak BrAC minus baseline change score minus the same change score for the placebo session) for the BAES sedation and Fig 3B is the net change score (alcohol session 180 minutes minus baseline change score minus the same change score for the placebo session) for salivary cortisol levels; three outliers (two > 3 SD above mean, one <3 SD below mean) were removed in this analysis. GEE results are depicted for group effects.
Finally, analyses were repeated for stimulating and rewarding alcohol responses at rising and declining BrAC limbs, i.e., 30 and 120 minutes net change scores, for each phase (Supplemental Figure S2, Supplemental Tables S2 and S3). Results showed that the magnitude of responses and the differences between LD and HD and across AUD subgroups were largely similar during the rising limb as they were during peak BrAC at 60 minutes. At the declining limb, stimulating and rewarding responses were lower in magnitude than at the earlier time points but heightened responses remained in HD, particularly for wanting in the high AUD subgroup.
DISCUSSION
This study was the first longitudinal examination of alcohol responses measured under controlled conditions in heavy binge drinkers varying in their progression of AUD through early- to mid-adulthood. In this phase of the CSDP, we report that the heightened alcohol stimulation and reward and lower sedation and cortisol response observed in heavy versus light drinkers at initial testing (29) remained at reexamination testing 5–6 years later. Specifically, among heavy drinkers with increasing symptoms of AUD, at re-examination at a peak BrAC near 90 mg/dl, heightened stimulating and rewarding alcohol effects with lower sedative and neuroendocrine effects persisted. In contrast, heavy drinkers with few emerging symptoms of AUD (and markedly less binge drinking) showed reduced alcohol stimulation at reexamination with persistent lower alcohol reward than in heavy drinkers with either intermediate or high AUD symptoms. Light drinkers largely continued low-risk drinking and rare AUD symptoms over time with an overall protective alcohol response “footprint”: persistently low sensitivity to alcohol stimulation and reward and high sensitivity to alcohol sedative and stress hormone effects. All these aforementioned effects remained after controlling for covariates and other risk factors, such as FH, and group differences were largely apparent on the rising and declining limbs as well as at peak BrAC.
In terms of the neurobiological theories of the development of addiction through alcohol response adaptation, the findings in those progressing with alcohol problems provide initial support for the early stage of addiction in the allostasis model with heightened stimulation and reward sensitivity that did not diminish over time. Koob and colleagues have coined the later stage of addiction as “the dark side of addiction”(65) because it is characterized by development of reward insensitivity and drinking behaviors related to negative reinforcement. This type of alcohol adaptation was not observed in heavy drinkers progressing with AUD, and the majority of persons in this subgroup either maintaining heightened stimulation or increasing further at re-examination, with only 2 of 9 high AUD individuals showing a lessening of response. This could be due to several reasons: a 5-year re-examination interval in humans may not be sufficiently long to show later-stage changes in reward sensitivity, allostasis may only occur only in the most extreme alcoholics with significant withdrawal not selected for the sample due to ethical constraints, or the allostasis phenomena modeled in animal studies may not translate to the development of human AUD.
Importantly, there was little support for tolerance in terms of a comprehensive subjective phenomenon(8) in the progression of AUD, as “markedly diminished effects at the same amount of alcohol” was not evident in heavy drinkers as a group, or in the subgroup showing the high AUD trajectory over time. Rather, initially heavy drinkers with fewer AUD symptoms over time showed tolerance in alcohol stimulation at re-testing. Whether this change was the cause or result of their less intensive bingeing over time, or an associated feature unrelated to their drinking course, remains to be determined. Nonetheless, if tolerance were the key mechanism in the progression of AUD, reduced levels of stimulation in those with greater AUD symptoms in the interim would have been observed.
Finally, the results did not support sensitization theory, as heavy drinkers with progressive AUD subjects maintained but did not further increase their already heightened sensitivity to stimulation. Motivational reward (wanting) was persistently elevated in heavy drinkers with both intermediate and high AUD symptoms. Thus, incentive sensitization, i.e., an increase in alcohol’s incentive salience (wanting), was not evident over the interval tested, and hedonic reward (liking) also remained elevated in heavy drinkers with high AUD. Putting these data together, it is possible that neither tolerance nor sensitization processes were observed because they may have taken place earlier in the drinking history of these young adults, or responses approached ceiling or floor effects for what can be observed in the controlled laboratory environment. It is also possible that behavioral and objective biomarkers in animal models (locomotion, tail flick, conditioned incentive procedures, etc.) do not directly translate to subjective responses and the progression of human AUD. While objective biomarkers in animal models are well-developed, human subjective responses and clinical phenomena are crucial to our understanding of the processes affecting addiction propensity.
The findings inform our conceptualization of alcohol responses during the processes of continued excessive and harmful drinking leading to AUD. Previous studies relying on proxy measures of brain-behavior relationships to alcohol response(2, 3, 23–29) have not been able to address within-person changes or stabilization in alcohol response over time. The current study’s focus on evaluating alcohol responses longitudinally, in combination with new developments in molecular and cellular studies of the basis of addiction(66), may help identify important substrates for addictive processes leading to and sustaining alcohol use disorder. The findings may have relevance to medication development, as altering euphoric and pleasurable effects of alcohol would be important targets for novel treatments and are hypothesized to underlie in part the efficacy of opioid receptor antagonists, including naltrexone and nalmefene(67–76). Moreover, innovative early intervention and psychoeducation efforts(77, 78) may be further refined to target information on higher sensitivity to stimulating and rewarding alcohol effects to prevent longer-term hazardous dependent drinking.
Study strengths included a placebo-controlled prospective design with 624 individual laboratory sessions with alcohol and placebo conditions, excellent follow-up retention, and inclusion of a comparison low-risk group to account for general drift and temporal effects. Limitations included that the reexamination interval may not have been sufficient to observe the extent of neuroadaptative responses to alcohol(18) and beverages were consumed over short interval to capture direct alcohol effects and minimize variability but this may not translate to typical drinking situations. Further, trajectory analyses resulted in unequal subgroup sample sizes and participants under age 21 were not enrolled due to legal restrictions of alcohol administration in the U.S. precluding study of alcohol responses in earlier developmental periods. The results support prior work showing high alcohol-induced stimulation, reward, and craving in heavy drinkers or alcoholics(33, 79, 80) as well as low alcohol-induced sedation and cortisol in other at-risk drinkers(81). However, heavy drinkers have also shown reduced alcohol-related stimulation and ventral striatal brain activation relative to social drinkers(82). Significant methodological differences across studies, i.e., sample size, subject characteristics, alcohol dose, route of administration, and degree of naturalism to usual contexts(16, 83), hamper direct comparisons to discern the source of this discrepancy. Thus, while the present study includes one of the largest samples to date and the only investigation of alcohol responses over time, replication will be a necessary next step to assure confidence in the main findings.
In summary, alcohol responses were examined in a unique longitudinal framework in heavy drinkers and light drinker controls. Alcohol response differences between these groups observed at initial testing remained 5–6 years later, with greater alcohol stimulating and rewarding effects and lower sedation and cortisol excretion in heavy drinkers. The leading theories of addiction based on animal models were tested in this direct repeated alcohol challenge investigation. Empirical evidence was provided for the early stage of addiction in the allostasis theory with continued heightened alcohol stimulation and reward sensitivity in heavy drinkers with increasing AUD symptoms during the transition of young to early middle adulthood. These sustained pleasurable effects may increase the drive for excessive drinking(34) despite mounting consequences and alcohol problems. At the same time, heavy drinkers with low AUD symptoms indicative of less harmful drinking over time exhibited reduced alcohol stimulation at re-examination while heavy drinkers with intermediate AUD symptoms were largely intermediate on their alcohol responses, i.e., in between the low and high AUD groups in alcohol sensitivity. The combination of changes observed supports alcohol response phenotype as an important factor in the development and continuation of excessive drinking and may inform future prevention and intervention approaches. While there are multiple pathways to development of a disorder as complex as AUD, maintenance of alcohol stimulatory and rewarding effects and lower sedative and neuroendocrine responses should be further examined as potential important pathways underlying development and progression of alcohol addiction processes.
Supplementary Material
Acknowledgments
This research was supported by grant R01-AA013746 (AK) from the National Institute on Alcohol Abuse and Alcoholism for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review and approval of the manuscript; and decision to submit the manuscript for publication, the Alcoholic Beverage Medical Research Foundation (DC) for the management, analysis, and interpretation of the data, the Indiana Alcohol Research Center P60 AA07611-27 (SO) and K05AA014223 and the New York State Psychiatric Institute (DH) for the interpretation of the data; and preparation, review and approval of the manuscript, the University of Chicago Comprehensive Cancer Center (#P30-CA14599) for the design and conduct of the study and review, or approval of the manuscript, the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research (#UL1 RR024999) for the collection and analysis of data. Appreciation is extended to Royce Lee, MD for medical supervision and oversight, Ima Garcia for medical assistance, Lia Smith for technical assistance, and Katherine Foster, Constantine Trela, and Michael Palmeri for data collection and database management assistance.
Footnotes
DISCLOSURES
Dr. King has provided consultation to Lundbeck and the U.S. Food and Drug Administration.
Dr. Cao has provided consultation to the U.S. Food and Drug Administration.
All other authors report no biomedical financial interests or potential conflicts of interest.
Clinical Trials Name: Chicago Social Drinking Project
URL: http://clinicaltrials.gov/ct2/show/NCT00961792
Registration #: NCT00961792
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Corbin WR, Scott C, Leeman RF, Fucito LM, Toll BA, O’Malley SS. Early subjective response and acquired tolerance as predictors of alcohol use and related problems in a clinical sample. Alcohol Clin Exp Res. 2013;37:490–497. doi: 10.1111/j.1530-0277.2012.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morean ME, Corbin WR. Subjective alcohol effects and drinking behavior: the relative influence of early response and acquired tolerance. Addict Behav. 2008;33:1306–1313. doi: 10.1016/j.addbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Schuckit MA, Smith TL, Hesselbrock V, Bucholz KK, Bierut L, Edenberg H, et al. Clinical implications of tolerance to alcohol in nondependent young drinkers. Am J Drug Alcohol Abuse. 2008;34:133–149. doi: 10.1080/00952990701877003. [DOI] [PubMed] [Google Scholar]
- 5.Matson LM, Kasten CR, Boehm SL, 2nd, Grahame NJ. Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res. 2014;38:267–274. doi: 10.1111/acer.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabakoff B, Hoffman PL. Tolerance and the etiology of alcoholism: hypothesis and mechanism. Alcohol Clin Exp Res. 1988;12:184–186. doi: 10.1111/j.1530-0277.1988.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay DS, Woods SC. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol Rev. 1997;104:170–193. doi: 10.1037/0033-295x.104.1.170. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed) 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 9.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 10.Hunt WA, Lands WE. A role for behavioral sensitization in uncontrolled ethanol intake. Alcohol. 1992;9:327–328. doi: 10.1016/0741-8329(92)90075-l. [DOI] [PubMed] [Google Scholar]
- 11.Fish EW, DeBold JF, Miczek KA. Repeated alcohol: behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology (Berl) 2002;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- 12.Crabbe JC, Young ER, Deutsch CM, Tam BR, Kosobud A. Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav. 1987;27:577–581. doi: 10.1016/0091-3057(87)90371-6. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- 14.Masur J, Oliveira de Souza ML, Zwicker AP. The excitatory effect of ethanol: absence in rats, no tolerance and increased sensitivity in mice. Pharmacol Biochem Behav. 1986;24:1225–1228. doi: 10.1016/0091-3057(86)90175-9. [DOI] [PubMed] [Google Scholar]
- 15.Phillips TJ, Burkhart-Kasch S, Terdal ES, Crabbe JC. Response to selection for ethanol-induced locomotor activation: genetic analyses and selection response characterization. Psychopharmacology (Berl) 1991;103:557–566. doi: 10.1007/BF02244259. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 17.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 21.Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, et al. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostafin BD, Marlatt GA, Troop-Gordon W. Testing the incentive-sensitization theory with at-risk drinkers: wanting, liking, and alcohol consumption. Psychol Addict Behav. 2010;24:157–162. doi: 10.1037/a0017897. [DOI] [PubMed] [Google Scholar]
- 26.Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: a test of the incentive-sensitisation theory. Psychopharmacology (Berl) 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- 27.Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics. Alcohol Clin Exp Res. 1991;15:399–405. doi: 10.1111/j.1530-0277.1991.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 28.Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 29.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mundt JC, Perrine MW, Searles JS. Individual differences in alcohol responsivity: physiological, psychomotor and subjective response domains. J Stud Alcohol. 1997;58:130–140. doi: 10.15288/jsa.1997.58.130. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JR, Nagoshi CT. One-month repeatability of alcohol metabolism, sensitivity and acute tolerance. J Stud Alcohol. 1987;48:437–442. doi: 10.15288/jsa.1987.48.437. [DOI] [PubMed] [Google Scholar]
- 32.King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Roche DJ, Palmeri MD, King AC. Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res. 2014;38:844–852. doi: 10.1111/acer.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahalan D, Cisin IH, Crossley HM. Studies RCoA, editor. Monograph. New Brunswick, N. J: Rutgers Center of Alcohol Studies; 1969. American drinking practices: A national study of drinking behavior and patterns. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 37.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 38.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 39.Babor TF, De La Fuente JR, Saunders J, Grant M. WHO Publication No 924. World Health Organization; Geneva, Switzerland: 1992. The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. [Google Scholar]
- 40.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC) An instrument for assessing adverse consequences of alcohol abuse. Rockville, MD: NIAAA; 1995. [Google Scholar]
- 41.Dawson DA. US low-risk drinking guidelines: an examination of four alternatives. Alcohol Clin Exp Res. 2000;24:1820–1829. [PubMed] [Google Scholar]
- 42.Substance Abuse and Mental Health Services Administration. National survey on drug use and health. Bethesda, MD: Office of Applied Studies; 2005. [Google Scholar]
- 43.National Institute on Alcohol and Alcoholism. NIH Publication No 05–3769. Bethesda, MD: National Institutes of Health; 2005. Helping patients who drink too much: A clinician’s guide. [Google Scholar]
- 44.Esterlis I, Cosgrove KP, Petrakis IL, McKee SA, Bois F, Krantzler E, et al. SPECT imaging of nicotinic acetylcholine receptors in nonsmoking heavy alcohol drinking individuals. Drug Alcohol Depend. 2010;108:146–150. doi: 10.1016/j.drugalcdep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- 46.King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- 47.McKee SA, Harrison EL, Shi J. Alcohol expectancy increases positive responses to cigarettes in young, escalating smokers. Psychopharmacology (Berl) 2010;210:355–364. doi: 10.1007/s00213-010-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009;33:2154–2161. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCaul ME, Wand GS, Rohde C, Lee SM. Serum 6-beta-naltrexol levels are related to alcohol responses in heavy drinkers. Alcohol Clin Exp Res. 2000;24:1385–1391. [PubMed] [Google Scholar]
- 50.Wiers RW, van de Luitgaarden J, van den Wildenberg E, Smulders FT. Challenging implicit and explicit alcohol-related cognitions in young heavy drinkers. Addiction. 2005;100:806–819. doi: 10.1111/j.1360-0443.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 51.Conrad M, McNamara P, King A. Alternative substance paradigm: effectiveness of beverage blinding and effects on acute alcohol responses. Exp Clin Psychopharmacol. 2012;20:382–389. doi: 10.1037/a0029261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 53.Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend. 2007;91:10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 55.Sutker PB, Tabakoff B, Goist KC, Jr, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18(Suppl 1):349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- 56.Roche DJ, King AC. Alcohol impairment of saccadic and smooth pursuit eye movements: impact of risk factors for alcohol dependence. Psychopharmacology (Berl) 2010;212:33–44. doi: 10.1007/s00213-010-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institute on Alcohol and Alcoholism . National Advisory Council on Alcohol Abuse and Alcoholism- Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. 2005 Available on the National Institute on Alcohol Abuse and Alcoholism website ( http://wwwniaaanihgov/) under ‘About NIAAA—Advisory Council—Research Guidelines and Resources’: http://wwwniaaanihgov/Resources/ResearchResources/job22htm.
- 58.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 59.Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rueger SY, McNamara PJ, King AC. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES) Alcohol Clin Exp Res. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 62.Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 64.Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- 65.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 66.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 67.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 68.Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- 69.Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- 70.Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry. 1999;56:719–724. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- 71.Swift RM. Effect of naltrexone on human alcohol consumption. J Clin Psychiatry. 1995;56(Suppl 7):24–29. [PubMed] [Google Scholar]
- 72.Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- 73.King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- 74.Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- 75.Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- 76.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 77.Schuckit MA, Kalmijn JA, Smith TL, Saunders G, Fromme K. Structuring a college alcohol prevention program on the low level of response to alcohol model: a pilot study. Alcohol Clin Exp Res. 2012;36:1244–1252. doi: 10.1111/j.1530-0277.2011.01723.x. [DOI] [PubMed] [Google Scholar]
- 78.Fridberg DJ, ACK Integrating alcohol challenge feedback in a brief intervention for young adult heavy drinker-smokers: a pilot study. Alcoholism: Clinical and Experimental Research. 2014;38(Supplement 1) [Google Scholar]
- 79.Ray LA, Bujarski S, Mackillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid (OPRM1) gene and alcohol severity. Alcohol Clin Exp Res. 2013;37(Suppl 1):E116–124. doi: 10.1111/j.1530-0277.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas SE, Drobes DJ, Voronin K, Anton RF. Following alcohol consumption, nontreatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. J Stud Alcohol. 2004;65:330–335. doi: 10.15288/jsa.2004.65.330. [DOI] [PubMed] [Google Scholar]
- 81.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 82.Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leyton M, Vezina P. Striatal ups and downs: their roles in vulnerability to addictions in humans. Neurosci Biobehav Rev. 2013;37:1999–2014. doi: 10.1016/j.neubiorev.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.