Abstract
Astrocytes are specialized and the most abundant cell type in the central nervous system (CNS). They play important roles in the physiology of the brain. Astrocytes are also critically involved in many CNS disorders including focal ischemic stroke, the leading cause of brain injury and death in patients. One of the prominent pathological features of a focal ischemic stroke is reactive astrogliosis and glial scar formation. Reactive astrogliosis is accompanied with changes in morphology, proliferation and gene expression in the reactive astrocytes. This study provides an overview of the most recent advances in astrocytic Ca2+ signaling, spatial and temporal dynamics of the morphology and proliferation of reactive astrocytes as well as signaling pathways involved in the reactive astrogliosis after ischemic stroke based on results from experimental studies performed in various animal models. This review also discusses the therapeutic potential of reactive astrocytes in a focal ischemic stroke. As reactive astrocytes exhibit high plasticity, we suggest that modulation of local reactive astrocytes is a promising strategy for cell-based stroke therapy.
Keywords: Ischemic stroke, reactive astrocytes, glial scar, Ca2+ signaling, morphology, cell proliferation, stroke therapy
Introduction
Astrocytes are the most numerous glial cell type in the central nervous system (CNS). In a normal brain, there are two major types of astrocytes: Fibrous and protoplasmic astrocytes. They can be found in white matter such as the corpus callosum and in grey matter such as the cortex. Glial fibrillary acidic protein (GFAP) is primarily expressed in the thick main processes and has been considered as a ‘pan-astrocyte’ marker (Brenner, 2014), but its expression levels are higher in the fibrous astrocytes than in the protoplasmic astrocytes. Transcriptome analysis found that the Aldh1L1gene is most widely and homogenously expressed in the astrocytes, while immunostaining with an anti-Aldh1L1 antibody revealed that the Aldh1L1 protein is highly expressed in the astrocyte cell body and its extensive processes (Cahoy et al., 2008). Thus Aldh1L1 is now considered as a new ‘pan-astrocyte’ marker.
It has been long recognized that astrocytes play a critical role in the physiology and are very important for overall brain architecture as well as function. Astrocytes can maintain ionic homeostasis by acting as a potassium (K+) sink to (Djukic et al., 2007). Astrocytes can remove synaptically released glutamate by their glutamate transporters to avoid glutamate excitotoxicity (Huang et al., 2004; Bergles et al., 1999). Astrocytes also mediate Ca2+ signaling and intercellular waves through the stimulation of different G-protein coupled receptors (GPCRs) via phospholipase-C/inositol 1,4,5-triphosphate (PLC/IP3) pathway in vivo. These GPCRs include metabotropic glutamate receptors (mGluRs) (Ding et al., 2007; Fellin et al., 2004; Sun et al., 2013), P2Y receptors (Ding et al., 2009; Thrane et al., 2012; Sun et al., 2013; Wang et al., 2006; Nizar et al., 2013), GABAB receptors (GABABRs) (Ding et al., 2009; Meier et al., 2008), noradrenergic receptors (Bekar et al., 2008; Ding et al., 2013; Paukert et al., 2014 ). Due to the intimate physical contact with synapses, astrocytes are considered as a part of the ‘tripartite’ synapse where they can listen and talk to the synapse by regulating Ca2+ increase in response to neuronally released transmitters and by gliotransmitter release (Haydon, 2001). We have also known that astrocytes and the blood vessels have intimate anatomic relationship. This was further confirmed by studies using fluorescence imaging and electron microscopy showing that astrocyte endfeet almost completely cover the cerebral vascular surface (Simard et al., 2003; Petzold et al., 2008; Mathiisen et al., 2010; Kacem et al., 1998). Astrocytic Ca2+ signaling is involved in functional hyperemia from in vitro studies of brain slice preparations (Zonta et al., 2003; Mulligan and MacVicar, 2004; Gordon et al., 2008); however, its role in the regulation of cerebral blood flow (CBF) in vivo is controversial as suggested by results from studies using type 2 IP3 receptor knockout mice (Jego et al., 2014; Takata et al., 2013; Nizar et al., 2013; Bonder and McCarthy, 2014).
Growing evidence indicates that astrocytes are heterogeneous in morphology, molecular expression and physiological function under normal conditions (Zhang and Barres, 2010; Matyash and Kettenmann, 2010). Morphologically, protoplasmic astrocytes and fibrous astrocytes are different. Protoplasmic astrocytes are complex (sponge like) and highly branched with numerous fine processes and their endfeet wrap around blood vessels, while fibrous astrocytes are less complex and have thicker and less branched processes (Wilhelmsson et al., 2006; Bushong et al., 2002). Numerous studies have found that different genes are expressed among different subsets of astrocytes in vivo (Zhang and Barres, 2010). GFAP expression is higher in the astrocytes in corpus callosum, but it is expressed in astrocytes in the cortex at lower levels (Xie et al., 2010). Electrophysiologically, astrocytes exhibit a different current-voltage relationship with one type of astrocytes, known as outward rectifying astrocytes, when compared to the other known as variably rectifying astrocytes (Zhou and Kimelberg, 2000). Astrocytes also exhibit different properties of Ca2+ signaling in vivo. Two-photon (2-P) in vivo Ca2+ imaging has shown that astrocytes in the cortical layer 1 (L1) nearly doubled the Ca2+ activity compared to the astrocytes in L2/3 in anaesthetized rats; moreover, Ca2+ signals in the processes in the same astrocyte were asynchronous in L1 while those in L2/3 were more synchronous (Takata and Hirase, 2008). The morphological, molecular and functional heterogeneity of astrocytes indicates a diversity among astrocytes and the complex physiological and pathological roles that astrocytes play in the CNS.
Astrocytes respond to different neurological diseases through a common phenomenon of GFAP upregulation, a process termed as reactive astrogliosis. Severe CNS injuries such as stroke, traumatic brain injury (TBI), and spinal cord injury (SCI), as well as neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease, and amyotrophic lateral sclerosis (ALS) all cause a massive up-regulation of GFAP. Therefore, GFAP is considered a reliable marker to characterize reactive astrocytes. However, given the different causes and onsets of diseases, the temporal and spatial changes of the reactive astrocytes are different. For example, in the AD brain, due to slow disease progression, the reactive astrocytes are more evenly distributed and do not form glial scars. While after ischemic stroke or SCI, reactive astrocytes in the peri-infarct region express higher GFAP and eventually form glial scar, which establishes both a physical and biochemical barrier that separates dead and vital tissues. Thus, the properties of reactive astrocytes in chronic neurodegenerative diseases are different from those seen in acute conditions like focal ischemia and SCI. Although similar phenomena, such as glial scar formation is observed in both focal ischemia and SCI, in experimental animal models of SCI, the injury occurs in the large area of white matter rather than in grey matter as seen in ischemic strokes. The functions and role of reactive astrocytes have been much more extensively studied in SCI than in the focal ischemic stroke (for reviews see Burda and Sofroniew (2014); Sofroniew (2009); Sofroniew and Vinters (2010); Silver and Miller (2004); Rolls et al. (2009)). Thus, this article will review the dynamic changes in astrocytic Ca2+ signaling, morphology and proliferation of reactive astrocytes. The article also examines the distribution of reactive astrocytes surrounding the ischemic core, i.e., in the penumbra, in experimental animal models of focal ischemic stroke. Discussion then focuses on the signaling pathways involved in reactive astrogliosis after focal ischemia followed by the therapeutic potential of reactive astrocytes in ischemic stroke. For extensive reviews of reactive astrocytes in various aspects in different neurological diseases, readers are advised to consult a few detailed reviews (Burda and Sofroniew, 2014; Sofroniew and Vinters, 2010; Escartin and Bonvento, 2008; Anderson et al., 2014).
Dynamics of reactive astrocytes in the penumbra after focal ischemia
Focal ischemic stroke, resulting from the blockage of cerebral blood vessels, leads to cell death and brain damage and causes human disability and death (Stapf and Mohr, 2002). After the onset of ischemia, astrocytes undergo numerous pathological alterations over time including rapid swelling (Nedergaard and Dirnagl, 2005; Barber and Demchuk, 2003; Swanson et al., 2004; Li et al., 2014) and enhanced Ca2+ signaling (Ding et al., 2009). Astrocytes can also become reactive following ischemia. The hallmark of reactive astrogliosis is the morphological changes and the increased expression levels of GFAP (Nedergaard and Dirnagl, 2005; Panickar and Norenberg, 2005). Reactive astrocytes eventual form glial scar in the penumbra that demarcates the ischemic core (infarction) from healthy tissue (Hayakawa et al., 2010; Barreto et al., 2011b; Shimada et al., 2011b; Bao et al., 2012; Li et al., 2014). In addition to reactive astrogliosis, focal ischemic stroke is also accompanied by other temporal and spatial dependent changes. As the clinical aim of stroke therapy is to salvage the penumbral cells, understanding the spatial and temporal changes of astrocytes at molecular and cellular levels will provide therapeutic strategy insights.
Ca2+ signaling in astrocytes after ischemia
A few in vitro and in vivo studies have demonstrated how ischemia induces enhanced Ca2+ signaling in different stages after ischemia onset (for extensive review see Ding (2013; 2014)). Using acute brain slice preparations and an oxygen-glucose deprivation (OGD) model, Duffy and MacVicar (1996) found that a short episode (5 min) of simultaneous hypoxia and hypoglycemia can induce an intracellular Ca2+ increase within an average of 7.5 min and it takes 2.5 min to reach a peak. After reoxygenation, astrocytic Ca2+ will remain elevated for a highly variable period of time ranging from several minutes to one hour. In the absence of extracellular Ca2+, astrocytic Ca2+ increase can still be observed with a relatively consistent duration. Electrophysiological recordings have shown that hypoxia and hypoglycemia also depolarize astrocytes (Duffy and MacVicar, 1996). The data from brain slice experiments suggest that astrocytes can mediate Ca2+ increase from the internal store release and influx of voltage-dependent Ca2+ influx. Alternatively, internal Na+ accumulation can lead to Ca2+ influx through reverse operation of the sodium-calcium exchanger (NCX). In another study using brain slices, OGD induced slow inward currents (SICs), which were mediated by extrasynaptic N-methyl-D-aspartate (NMDA) receptors in rat hippocampal CA1 pyramidal neurons (Dong et al., 2013). Moreover, dialysis of 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), an Ca2+ chelator, into the astrocytes reduced SIC frequency after OGD, implying that astrocytic Ca2+ activity stimulate extrasynaptic NMDA receptors. Using Ca2+ imaging, it was found that astrocytes are quiescent in spontaneous Ca2+ activity in brain slices under normal conditions, but astrocytes exhibits frequent Ca2+ elevations during OGD. Surprisingly, within the 10 min of OGD more than 60% of astrocytes have exhibited Ca2+ elevations. Moreover, a majority of astrocytes displayed more than two Ca2+ transients. To further demonstrate the importance of astrocytic Ca2+ activity in ischemia, slice preparations from type 2 IP3R knockout mice were used. Astrocytes exhibited rare Ca2+ transients during OGD and low frequency SICs in CA1 neurons in IP3R2 knockout mice as compared with wild type (WT) mice.
Using a photothrombosis (PT)-induced ischemia model and in vivo two-photon (2-P) imaging, Ding et al. (2009) found that astrocytes exhibit enhanced Ca2+ signaling characterized as intercellular Ca2+ waves starting ~20 min after PT. Both amplitude and frequency of astrocytic Ca2+ signal were significantly increased compared to relative quiescent Ca2+ signaling prior to ischemia. In addition, the magnitude of a Ca2+ signal was smaller in the penumbral region than the magnitude of a Ca2+ signal in the ischemic core. An important feature of the Ca2+ signals was that most of them started and returned to the basal level at the same time among the astrocytes in the imaging field, i.e., they exhibited a high degree of synchrony. Pharmacological study has suggested that glutamate and GABA released into the extracellular space following PT stimulated intercellular waves among astrocytes. The results represent the first direct evidence of an astrocytic Ca2+ response after ischemia in an in vivo setting. Acute increase in astrocytic Ca2+ signals have also been observed after a single vessel occlusion by PT (Zheng et al., 2013). Acute response of astrocytes with enhanced Ca2+ signaling thus may reflect the initial step in reactivation of astrocytes after ischemia.
In addition to the acute changes in astrocytic Ca2+ signaling ~20 min after PT-induced ischemia, astrocytic Ca2+ signaling also changed in the chronic phase following ischemia. Winship et al. studied the neuronal and astrocytic Ca2+ signaling and functional rewiring in somatosensory neurons in the ipsilateral hemisphere after a stroke (Winship and Murphy, 2008). Astrocytic Ca2+ responses were examined in anesthetized mice to study Ca2+ responses in penumbra after two weeks, one month, and two months following PT. Their study observed a significant increase in the prevalence of responses to preferred limb stimulation in the somatosensory cortex. The selectivity of astrocyte responses was also significantly different between regions of overlap between contralateral hindlimb- and contralateral forelimb-evoked intrinsic optical signals and regions without intrinsic optical signals overlap. These results indicate that neuron–astrocyte communication is preserved, or even enhanced in the penumbra. The altered response of the astrocytic Ca2+ signaling to limbic stimulation in contralateral hemisphere in chronic phase after ischemia suggests the change of astrocytic Ca2+ plasticity after ischemia, which may contribute to brain remodeling during the recovery process. Further elucidation of the mechanisms of astrocytic Ca2+ signaling in acute and chronic phases can provide insights for potential therapeutic strategies for improved brain recovery after stroke.
Morphological change of reactive astrocytes and formation of glial scar after ischemia
It has been known that there is little expression of GFAP in the cortex of normal mice (also see previous studies (Zhang et al., 2010; Li et al., 2013; Li et al., 2014) (Figure 1A:A1). Astrocytes undergo dramatic changes in morphology associated with GFAP upregulation over time after an ischemic insult. Thus, GFAP expression has been used to inspect the morphological changes during astrogliosis following ischemia. Using endotelin-1 collagenase type IV-S injections, Mestriner et al. (2015) conducted a detailed study on morphology of reactive astrocytes in the penumbra 30 days after ischemic and hemorrhagic stroke in rats. The following parameters were analyzed based on GFAP staining in the regions of the sensorimotor cortex and dorsolateral striatum: 1) the GFAP immunohistochemistry intensity by measuring the optical density; 2) astrocyte morphology and polarization by measuring the the number (i.e., ramification) and length of primary processes of reactive astrocytes; 3) the density of GFAP+ astrocytes. As expected, optical density and GFAP+ astrocyte density significantly increased after stroke; but more importantly, the ramification and length of primary processes also increased compared with the astrocytes in a sham control group. No differences were found in these measurements based on ischemic and hemorrhagic stroke animals, but a difference was observed among different regions in the brain, indicating a regional heterogeneity in the morphology of reactive astrocytes. However, in this study, the morphological changes during early stages were not studied which could be very important in disease progression. Wagner et al. studied the morphology of reactive astrocytes in rats at day 4 after middle cerebral artery occlusion (MCAo) (Wagner et al., 2012). Based on the results from GFAP immunostaining, the process volume, process diameter, process length and branching levels in reactive astrocytes in the penumbral region were increased compared with the astrocytes in contralateral hemisphere and in the remote regions away from the ischemic core. In addition, the process volume and diameter in the peri-infarct region were larger than those in the remote regions. However, the mean process length of astrocytes in the contralateral hemisphere was longer than reactive astrocytes in the penumbra and the remote region, and the mean process length of reactive astrocytes in the remote region was slightly but significantly longer than these in the penumbra. On the other hand, the mean process branching was similar in the penumbra and in the remote region. The data suggest that reactive astrocytes become hypertrophic rather than increasing the length of their process at relatively acute time points of the injury.
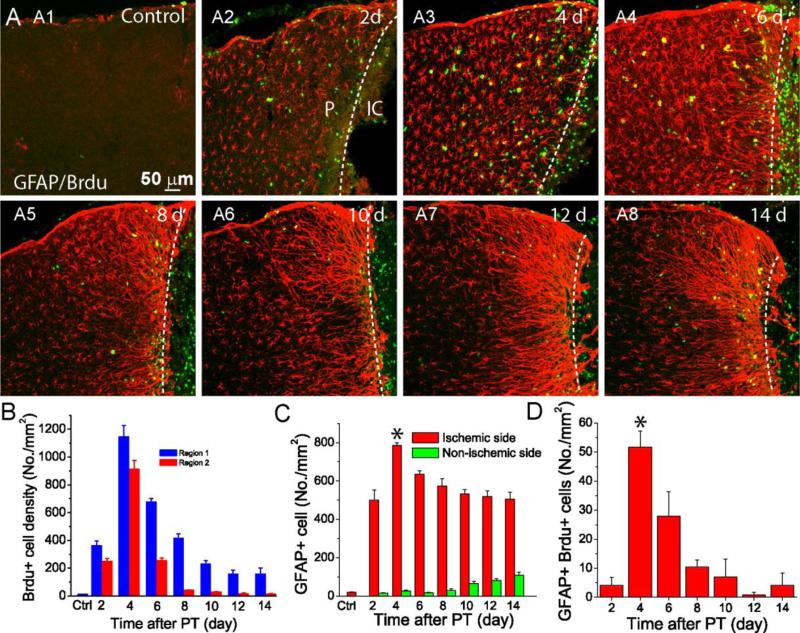
Figure 1. Time course of astrocyte proliferation and morphological changes after stroke.
A) Confocal images of GFAP and Brdu expression in the penumbra from mice at indicated times after PT. B) Brdu+ cell density presented as cell number per mm2 in Region 1(R1) and Region (R2) with an area of 200 μm×200 μm in the L2/3 of the cortex. R1 is located 0–200 μm from the edge of ischemic core, and R2 is located 200–400 μm from the edge of ischemic core (see the left panel of A2). C-D) The density of GFAP+ (C) and GFAP+Brdu+ (D) stained cells in the penumbra. The cells were counted in the penumbral region of 200 μm×400 μm in L2/3 of the cortex located 0-400 μm from the edge of ischemic core. Dash lines separate ischemic core (IC) and penumbra (P). Data were adapted from Li et al., 2014b.
Since reactive astrogliosis after ischemia is highly dependent on time and location, it is necessary to quantitatively examine the changes with a high temporal resolution. Li et al. (2014) presented a high temporal resolution study on the dynamic changes of reactive astrocytes in the cortex using a PT-induced mouse ischemia model. GFAP expression was examined in mouse brains at days 2, 4, 6, 8, 10, 12, and 14 post PT using confocal microscopy. A significant increase of GFAP expression levels was observed at day 2 post PT (Figure 1A:A2). Astrocytes exhibited a stellate morphology and hypertrophy with highly upregulated GFAP expression up to day 4 after PT (Figure 1A:A3). From day 6 after PT, reactive astrocytes became densely packed and changes to a stream-like structure with their elongated processes directing towards the ischemic core, i.e., a feature of polarization of reactive astrocyte and astroglial scar formation (Figure 1A:A4). In addition, reactive astrocytes became less hypertrophic after day 6 post. After day 10, reactive astrocytes in the penumbra remained similar but with longer processes as compared to days 6-8, indicating the maturation of glial scar (Figure 1A:A5-A8). An significant increase in GFAP expression was also observed in the regions further away from the scar border but with similar morphology to the astrocytes under normal conditions. Thus, the morphology of GFAP+ astrocytes in the penumbra progressed through dynamic modifications over time with dramatic changes occurring between days 2-4 after PT.
In healthy mouse brains, astrocytes exhibit non-overlapping domains (Bushong et al., 2002). However, in an epileptic brain, it has been shown that reactive astrocytes interdigitate, i.e., they exhibit overlapping domains between the neighboring astrocytes (Oberheim et al., 2008). However, in an electrically induced mouse lesion model, reactive astrocytes in the cortex exhibit minimal interdigitation and are similar to those in the cortex of control animals (Wilhelmsson et al., 2006). Thus, it is likely that the severity of brain injury determines the degree of overlapping between reactive astrocytes. Although there is no report to indicate that reactive astrocytes interdigitate or remain non-overlapping domains after stroke, it is likely that they tend to interdigitate as stroke causes severe reactive gliosis and cell proliferation. Whether reactive astrocytes interdigitate after focal ischemia, and if they do, whether the degree of interdigitation is temporal and spatial dependent, are worth further investigation.
Reactive astrocyte proliferation after ischemia
Cell proliferation including reactive astrocyte proliferation in response to ischemic stroke has been well documented using PT-induced focal ischemia and MCAo models (Nowicka et al., 2008; Barreto et al., 2013; Haupt et al., 2007; Li et al., 2013; Shen et al., 2012; Stoll et al., 1998; Schroeter et al., 2002). Bromodeoxyuridine (Brdu) labeling and immunostaining can be used to assess the spatial and temporal changes in cellular proliferation (Barreto et al., 2011; Zamanian et al., 2012; Haupt et al., 2007; Panickar and Norenberg, 2005; Nowicka et al., 2008; Li et al., 2013). To precisely study the rate of proliferation of astrocytes and microglia at different times after ischemia, Li et al. (2014) developed a ‘time-block’ protocol to label proliferating cells in the penumbra at different times after ischemia. Mice were administered with Brdu at days 1, 3, 4, 5, 9, 11, and 13 post PT for two consecutive days and transcardially perfused after one day from the last injection. In normal mice, there were almost no Brdu+ cells in the cortex. The density of Brdu+ cells exhibited a temporal and spatial dependent manner (Li et al., 2014). Brdu+ cells significantly increased from day 1 to day 2 post PT and reached their peak during days 3 and 4 post PT; then, they declined over time and persisted until day 14 (Figure 1A-B). The region closest to the ischemic core had a higher density of Brdu+ cells than those regions further away from the ischemic core. These data demonstrated that the rate of proliferating cells generated in the penumbra is highly dependent on the location and time. Nevertheless, Brdu+ cell estimated from this labeling protocol may underestimate the total number of proliferating cells since a single daily injection of Brdu may not label all the proliferating cells (Wanner et al., 2013).
Although it is well known that the common feature of reactive astrocytes is increased GFAP expression levels, whether reactive astrogliosis is merely through the upregulation of GFAP or through cell proliferation is still not clear. The proliferation of reactive astrocytes can be studied using GFAP and Brdu double staining. In the study from Li et al. (2014), they showed that Brdu+ cells and GFAP+ astrocytes dramatically increased from day 2 post PT and reached peak at day 4; however, overall, the GFAP+Brdu+ proliferating astrocytes only accounted for a small percentage of total Brdu+ cells, which reached a peak value of about 6% from days 3 to 4 post PT and then declined sharply (Figure 1D). Based on the labeling protocol, the GFAP+Brdu+/Brdu+ ratio at each time point represents the relative rate of the generation of reactive astrocytes. On the other hand, the GFAP+Brdu+/GFAP+ ratio also reached the highest level within days 3 to 4 post PT. These data demonstrated that focal ischemic stroke increase the population of proliferating reactive astrocytes in a highly temporal dependent manner. The results indicate that glial scar is formed largely from the existing astrocytes through the upregulation of GFAP, rather than from newly generated astrocytes through proliferation. Although proliferation rate reduces dramatically after 8 days following PT, the morphology of reactive astrocytes maintains straight processes pointing to ischemic core for a prolonged time based on the GFAP expression, suggesting an irreversible change of reactive astrocytes in the penumbra (Haupt et al., 2007; Li et al., 2014). This phenomenon also suggests that the expression of certain genes cannot be reversed during the recovery from focal ischemic injury and glial scar formation causes substantial tissue remodeling and permanent and persistent structural changes in the penumbra following ischemia.
Different responses of astrocytes to ischemia
In addition to the heterogeneity in astrocyte morphology, even in the same anatomical location of the brain such as within the cortex (Benesova et al., 2009; Matyash and Kettenmann, 2010), the astrocytes respond to ischemic stroke in different manners. As mentioned before, it is well know that protoplasmic astrocytes are different from fibrous astrocytes in morphology and GFAP expression. Their sensitivity to ischemia is also different. Lukaszevicz et al. (2002) studied the reactivity and fate of protoplasmic and fibrous astrocytes after permanent MCAo in mice. Loss of GFAP was observed within minutes in the ischemic core. The fibrous astrocytes exhibited a transient and limited hypertrophy without discernible cell death as compared to protoplasmic astrocytes. Their results demonstrated that protoplasmic astrocytes have a higher sensitivity to ischemia than the fibrous cortical astrocytes, with a rapid demise of the protoplasmic astrocytes. In another study using imaging of GFP+ optic nerve astrocytes and hippocampal astrocytes in neonatal whole-mount preparations, no reactive astrogliosis was observed in white matter nor grey matter GFP+ astrocytes during a 180-min reperfusion following modelled ischemia (Shannon et al., 2007). Furthermore, it was observed that the white matter astrocytes are much more sensitive to ischemia-reperfusion injury than the grey matter astrocytes. Even though the results from two groups were contradictory, the discrepancy could be attributed to the differences in the experimental models used and in the characteristics of the fibrous astrocytes of the optic nerve vs corpus colossal. In summary, astrocytes respond diversely to ischemia. Another study from Benesova et al., (Benesova et al., 2009) using the GFAP-EGFP mice revealed that there are two populations of cortical astrocytes in terms of their volume changes in response to OGD in brain slice preparations, the first exhibiting a large volume but the second a small volume increase. Furthermore, patch-clamp measurement of resting membrane potential (Vm) showed that one group significantly depolarized during OGD, while the second group only exhibited small changes in Vm toward more negative values. Their study provided evidence that there are two astrocytic populations in the cortex with different responses to ischemia. Given the complex of heterogeneity of astrocytes, further studies to identify the different responses such as morphological change and proliferation of astrocytes in response to ischemia should be performed using lineage analysis in the transgenic mice that express fluorescent markers in different types of astrocytes.
Reactive astrogliosis and behavioral recovery
After ischemia, the brain undergoes spontaneous recovery with improvement in behavioral deficits over time (Badan et al., 2003; Li et al., 2004; Clarkson et al., 2013). Focal ischemia-induced reactive astrocytes exhibit heterogeneity in morphology, GFAP expression and proliferation capability in a spatiotemporal dependent manner. This can be seen in Figure 2 where GFAP expression levels and proliferation capability eventually decrease after the first 4-day acute phase in a spatiotemporal dependent manner. One outstanding question is whether behavioral deficits are correlated to reactive astrogliosis and glial scar formation, and if so, are reactive astrocytes beneficial to functional recovery? To explore this, behavioral tests were conducted (Li et al., 2014). The time courses of forelimb shift asymmetricity, strength, and sensory motor impairments were evaluated. The functional deficits have a time window similar to that of infarct expansion, brain swelling, the fast cell proliferation and reactive astrocyte generation. Functional deficits improved from day 6 post PT when glial scar began to form, implying that glial scarring might have a beneficial effect by preventing the expansion of the ischemic core. Thus, the behavioral study results suggest that reactive astrocyte proliferation and astrogliosis correlate with neuronal remodeling and functional recovery. This can be supported by a study from Hayakwa et al. (2010). In their study, the effects of fluorocitrate, a metabolic inhibitor of astrocytes, on reactive gliosis and behavioral recovery were assessed. They found that fluorocitrate-treated mice have a significant decline in reactive astrocytes and neurovascular remodeling as well as a corresponding enhanced behavioral deficit compared with stroke-only controls, suggesting that reactive astrocytes in the penumbra may improve neurovascular remodeling, and thus promote stroke outcomes (Hayakawa et al., 2010). In another study using WT and glial fibrillary acidic protein and vimentin in double knockout (GFAP–/–Vimentin–/–) mice, Liu et al. (2014) compared the behavioral outcomes, axonal remodeling of the corticospinal tract (CST), and the spatio-temporal change of chondroitin sulfate proteoglycan (CSPG) expression after PT (Liu et al., 2014). The results showed that motor functional recovery and biotinylated dextran amine-positive CST axonal length in the denervated side of the cervical gray matter were significantly reduced in GFAP–/–Vimentin–/– mice as compared with WT mice. Immunostaining showed that the GFAP–/–Vimentin–/– mice exhibited attenuated astrocytic reactivity and a significant increase in CSPG in both hemispheres. However, these GFAP–/–Vimentin–/– mice showed a decrease in the ischemic lesion boundary zone when compared to the WT mice. This study suggests that attenuation of reactive astrogliosis reduces CST axonal remodeling in the denervated spinal cord and thus impairs or delays neurological recovery. These studies indicate that targeting reactive astrocytes might, therefore, lead to an important therapeutic strategy that can improve stroke outcomes.
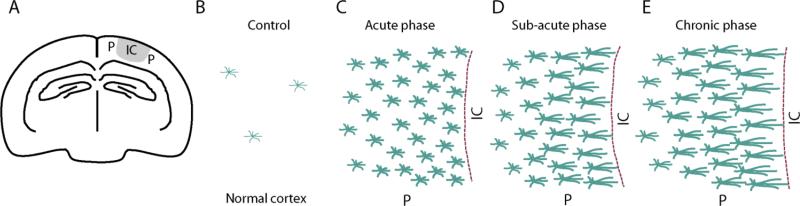
Figure 2. Schematic representations of dynamic reactive astrocytes and glial scar formation in the penumbra at different stages after a focal ischemic stroke.
A) Illustration of ischemic core and penumbra after PT in the mouse brain. The grey region indicates the ischemic core (IC) and the surrounding regions are penumbra (P). B) In control conditions, very low percentage of astrocytes expresses GFAP. C) In the acute phase after focal ischemia (days 1-4 post ischemia), astrocytes exhibit stellate morphology and hypertrophied GFAP positive processes and a high proliferating rate. D) During subacute phase after focal ischemia (days 4-8 post ischemia), astrocytes exhibit elongated processes pointing to the ischemic core, and a glial scar forms. The proliferating rate decrease significantly at this stage. E) Chronic phase after focal ischemia exceeds the 8-day post ischemia period. Astrocytes further extend processes toward the ischemic core and the glial scar matures during this period. The GFAP expression levels in reactive astrocytes surrounding the glial scar decrease and reactive astrocytes lose the capability of proliferation. Reactive astrocytes are heterogeneous in morphology, molecular expression, and proliferation depends on their location. Figure 2B-E were adapted from Ding (2015).
Signaling pathways of reactive astrogliosis after ischemia
Over the last two decades, advancements in genetics and molecular biology have equipped researchers with unique tools, which have resulted in an extensive characterization of reactive astrocytes following CNS injury. Studies have identified a plethora of genes and molecular markers for reactive astrocytes and the list still continues to grow (Ridet et al., 1997; Zamanian et al., 2012; Colangelo et al., 2014). Moreover, many studies have provided clues that have shed some light on key signaling pathways that may regulate changes to genes and molecular markers during reactive astrogliosis in the brain and spinal cord following injury (Sofroniew and Vinters, 2010; Kang and Hebert, 2011; Karimi-Abdolrezaee and Billakanti, 2012). Despite such extensive characterization, an across-the-board understanding of the key signaling pathways regulating distinct aspects of the reactive astrogliosis still remains inadequate. Further complicating things is the highly context dependent nature of astrogliosis in the injured CNS (e.g., acute phase vs chronic phase, and white matter vs gray matter), which limits the one-size-fits-all approach for its manipulation in various CNS disorders (Zhang and Barres, 2010; Kang and Hebert, 2011; Anderson et al., 2014). To keep things in perspective, the following section is a brief summary of the major signaling mechanisms that have been identified to regulate reactive astrogliosis in stroke.
Intermediate filaments
The intermediate filament GFAP is predominantly expressed in astrocytes and is essential for astroglial cyto-architecture and cellular functions. Following CNS injury, including ischemic stroke, the peri-lesional astrocytes become reactivated and dramatically upregulate their GFAP in a temporally and spatially dependent manner. As a result, the role of intermediate filaments in the reactive astrogliosis has generated a widespread interest among neuroscientists trying to understand glial biology in the injured CNS. As a result, a number of genetic deletion studies of astrocyte intermediate filaments (especially GFAP and vimentin) have been undertaken to elucidate the importance of the intermediate filaments in ischemic injury, reactive astrogliosis and glial scar formation using mice with either a single deletion of GFAP or vimentin and a double deletion of GFAP and vimentin. Hanbury et al. (2003) found that deletion of GFAP is neuroprotective against metabolic insult and excitotoxicity which was attributed to increased production of the glial derived neurotrophic factor (GDNF) by GFAP knockout astrocytes (Hanbury et al., 2003). Pekny et al. showed that single deletion of either GFAP−/− or vimentin−/− from astrocytes produced little to no effect on reactive astrogliosis and resulted in scars similar to those found in WT animals after brain injury (Pekny et al., 1998; Pekny et al., 1999). In ischemia, studies demonstrated that GFAP−/− mice exhibited a more significant decrease in cortical cerebral blood flow, and relatively larger infarct volume than the WT mice after MCAo. Their results indicated that GFAP-null mice have a high susceptibility to cerebral ischemia and suggested that astrocytes and GFAP play an important role in the progression of brain damage after ischemia (Nawashiro et al., 2000). Their higher susceptibility to cerebral ischemia in GFAP-null mice could have resulted from the reduction in both astrocytic (EAAT1) and neuronal (EAAT3) glutamate transporter subtypes (Hughes et al., 2004). Thus, these results from single GFAP deletion were highly ambiguous. However, studies using GFAP−/−/Vimentin−/− double knockout mice have indicated that GFAP and vimentin are essential for retaining beneficial effects of reactive astrocytes after ischemia. These studies show that in acute ischemia, double deletion of GFAP and vimentin result in decreased glutamate uptake, increased susceptibility to oxidative stress and significantly increased infarct volumes (2-3 times compared to WT mice). Hence, the deletion of both intermediate filament proteins (GFAP and vimentin) in the acute stage disrupts glial scar formation and promotes neuronal regeneration after traumatic brain injury and spinal cord injury (Li et al., 2007; de Pablo et al., 2013). Whereas in chronic stages of stroke, attenuation of astroglial reactivity by GFAP and vimentin deletion impaired axonal remodeling and functional recovery (Liu et al., 2014). Together these studies suggest that GFAP and vimentin are essential to retain beneficial function of reactive astrocytes in ischemic as well as recovering nervous tissue.
p38 MAPK pathway
Mitogen activated protein kinases (MAPK) are an important family of enzymes transducing a wide range of extracellular signals like inflammation, growth factors, and toxic stimuli as well as integrating corresponding cellular responses (Kaminska, 2005). Among MAPKs, p38 is of particular importance since it is known to transduce cellular inflammation (Cuadrado and Nebreda, 2010). In an ischemic stroke, p38 MAPK can become activated in neurons, microglia and astrocytes (Nozaki et al., 2001; Ferrer et al., 2003; Krupinski et al., 2003). Furthermore, p38 inhibitors have proven effective in reducing infarct volume and improving functional recovery after experimental ischemia (Barone et al., 2001b; Barone et al., 2001a). Compared to neurons and microglia, the activation of p38 MAPK in astrocytes is delayed, reaching its peak during the chronic stages of injury following primary ischemic insult (Piao et al., 2002; Irving et al., 2000)., A similar pattern has been seen in GFAP upregulation during ischemia-reperfusion (Yamashita et al., 1996). Further, activation of p38 MAPK in astrocytes has been reported to induce matrix metalloprotease-9 (MMP-9) expression, reactive oxygen species (ROS) and cytokine production features that are manifested during astrocyte reactivation (Saha et al., 2007; Ralay Ranaivo et al., 2012; Nahirnyj et al., 2013). Recently, a study performed using an astrocyte-specific p38 MAPK knockout mouse model demonstrated a key role of p38 MAPK signaling in reactive astrogliosis induced by ischemic stroke (Roy Choudhury et al., 2014). This study demonstrated that delayed activation of p38 MAPK following ischemic injury corresponds with upregulation of GFAP in the penumbra region signifying an important role of p38 MAPK in signaling reactive astrogliosis following ischemic stroke. To further confirm this, the authors developed a novel conditional astrocyte specific p38 MAPK knockout mice line using a human GFAP promoter driven Cre-recombinase system and floxed p38 MAPK. Their results demonstrated that conditional deletion of p38 MAPK from astrocytes attenuated injury (both OGD and scratch injury) and induced GFAP over expression, MMP-9 secretion and astrocyte migration in vitro. In vivo, conditional GFAP-Cre/LoxP p38 mice had significantly fewer and smaller astrocytes in the penumbra following ischemia compared to WT animals. The conditional hGFAP Cre/LoxP p38 mice also had a significantly reduced expression of GFAP in the ischemic hemisphere compared to WT mice indicating attenuated reactive astrogliosis. However, attenuation of reactive astrogliosis by conditional deletion of p38 MAPK had no significant effect on motor functional recovery in the mice. Nito et al. (2012) found that p38 MAPK may be involved in the regulation of aquporin-4 expression in astrocytes and could potentially cause the development of lethal cerebral edema following ischemic injury. Taken together, these studies indicate that p38 MAPK signaling may be a critical transducing pathway in signaling reactive astrogliosis in ischemic stroke, and targeting p38 MAPK may provide an excellent opportunity to modulate reactive astrogliosis. However, extensive studies are still necessary to completely elucidate the role of p38 MAPK signaling in astrogliosis.
On the other hand the role of another important member of MAPK in terms of cerebral ischemia, the Jun N-terminal kinase pathway (JNK), still remains unclear. Early activation of JNK in neurons and microglia during ischemia has been reported (Lennmyr et al., 2002), and its inhibition in the acute phase has proven protective (Borsello et al., 2003; Gao et al., 2005). Gadea et al. (2008) found that the JNK pathway plays a part in endothelin-1 induced astrocyte proliferation and reactive astrogliosis (Gadea et al., 2008). However, Murata et al. (2012) found that delayed inhibition of the JNK pathway following ischemic injury resulted in increased infarct volume and worsened the outcome after stroke. This seemingly contradictory information suggests the different roles of JNK in acute brain injury vs chronic stroke outcomes. Due to lack of effective pharmacological inhibitors and genetic models, many questions remain unanswered as to how and when JNK functions. Research is needed to determine if JNK is sequestrated or integrated with p38 MAPK pathway during activation. We know that activation of JNK induces changes in astrocytes akin to p38 MAPK activation that leads to the production of MMPs and cytokines as well as astrocyte migration. Future research should focus on how JNK impacts long-term stroke outcomes.
Notch signaling pathway
Notch signaling is a highly conserved pathway critical for the maintenance and self-renewal of progenitor cells and to inhibit precocious neurogenesis (Yoon and Gaiano, 2005). In developing CNS, Notch 1 is responsible for astrogliogenesis by forming a transcriptional activation complex with a GFAP gene thereby directing neural stem cells to differentiate into astrocytes (Ge et al., 2002) . Mature astrocytes are quiescent and do not divide in a healthy brain; however, they acquire a proliferative phenotype following cerebral injury (Barreto et al., 2011; Li et al., 2014). In an ischemic stroke, It was reported that Notch 1 signaling was activated in astrocytes in the peri-infarct region by 24 h (Marumo et al., 2013) and tamoxifen induced deletion of Notch 1 from GFAP positive cells resulted in a decreased number of proliferating astrocytes following injury. The study also suggested that Notch 1 signaling in reactive astrocytes may aid in suppression of immune response into the peri-infarct area as Notch 1 knockout animals had significantly higher invading immune cells (Shimada et al., 2011a). Some speculation existed among researchers concerning the source of Notch 1 in the peri-infarct area, as some scientists believed that NG2+ cells also expressed a Notch 1 positive stain along with reactive astrocytes, but genetic fate mapping analysis revealed that a subpopulation of reactive astrocytes—not NG2+ cells—activated Notch1 signaling in response to injury and became proliferative (Komitova et al., 2011; Marumo et al., 2013). In addition, it was suggested that activation of Notch 1 signaling in reactive astrocyte after stroke may redirect the cells to dedifferentiate into astrocytes derived neural stem cells (Shimada et al., 2012a). However, an important gap in knowledge still exists regarding Notch 1 signaling in regulating reactive astrocytes. Many questions remain to be explored such as: Why does only a subset of reactive astrocytes express Notch 1 signaling and become proliferative? What is the temporal and spatial pattern of Notch 1 signaling in the brain following ischemia? What is the fate of astrocyte-derived neural stem cells? Does inhibiting Notch 1 induced astrocyte proliferation have any effect on functional recovery? Until these gaps are filled, the definitive role of Notch 1 in reactive astrogliosis cannot be completely understood.
STAT3 signaling
Signal transducer and activator of transcription 3 (STAT3), a member of the Jak-STAT family, is a major downstream signaling pathway activated by peptides like hormones, growth factors or cytokines (Nicolas et al., 2013). Astrocyte marker GFAP is a STAT3 regulatory target and the role of STAT3 in reactive astrogliosis has been extensively studied in SCI models (Herrmann et al., 2008; Sofroniew, 2009; Karimi-Abdolrezaee and Billakanti, 2012; Wanner et al., 2013). Studies have showed that GFAP-cre-promoted deletion of STAT3 attenuates astrocyte hypertrophy, and astrocyte proliferation eventually causes failure of formation of a compact glial scar following spinal cord injury. In addition, studies indicate that STAT3 might be critical in migration of the astrocytes to the damaged site and may prevent its further spread (Okada et al., 2006; Herrmann et al., 2008; Wanner et al., 2013). In ischemic stroke, reperfusion induced increase in ROS and inflammatory cytokines are known to be powerful stimulants of STAT3 signaling (Lei et al., 2011; Zhu et al., 2013). But cell type specific activation of STAT3 in the post ischemic brain has been a matter of extensive debate. While some reports suggest neurons are the predominant source of activated STAT3 (Suzuki et al., 2001; Wen et al., 2001), others have shown that STAT3 is also activated in astrocytes along with neurons (Justicia et al., 2000; Choi et al., 2003). These conflicts in reports have been attributed to differences in insult models chosen and brain regions observed. More recent information, however, suggests that STAT3 can regulate reactive astrogliosis induced by diverse forms of neurotoxic insults where activation of STAT3 in astrocytes and subsequent increase in GFAP expression can be induced by direct neuronal death even in the absence of astrocyte damage (Sriram et al., 2004; O'Callaghan et al., 2014). Despite reports establishing STAT3 signaling as an important regulator of reactive astrogliosis in spinal cord injury, its role in driving astrogliosis in ischemic stroke remains open to exploration. This further reiterates the idea that reactive astrogliosis is highly dependent on its relationship to the context and its anatomical location in the CNS.
Transforming growth factor Beta (TGF-β) signaling
An injury related peptide, TGF-β has been shown to increase in immunoreactivity in both the infarct and penumbra in stroke patients (Krupinski et al., 1996) as well as animal models of focal ischemic stroke (Wang et al., 1995; Ruocco et al., 1999; Ali et al., 2001). Despite opposing reports, TGF-β has been mostly regarded as neuroprotective particularly against the N-methyl-d-aspartate receptor (NMDA), which can induce neuronal cell death (Prehn et al., 1993; Ruocco et al., 1999). It is suggested that the protective action of TGF-β is mostly mediated by astrocytes (Buisson et al., 1998; Docagne et al., 1999). This was further confirmed when deletion of TGF-β in the CNS resulted in loss of astrocyte glutamate transporters, like GLT-1 (EAAT2) and GLAST (EAAT1) causing decreased glutamate uptake and enhanced neuronal death. Treatment of astrocytes with TGF-β reversed the phenotype suggesting a beneficial role of TGF-β signaling in astrocytes (Koeglsperger et al., 2013). Further, most recent data suggests that the early activation of TGF-β in astrocytes after injury promotes anti-inflammatory response and aids in neuroprotection (Cekanaviciute et al., 2014). Other studies have shown that TGF-β signaling in astrocytes induces the production of GFAP, CSPGs and promotes scar formation (Moon and Fawcett, 2001; Wang et al., 2008; Schachtrup et al., 2010) with aging being an important determinant of the outcome (Doyle et al., 2010; Yu et al., 2014).
In addition to the above mentioned signaling pathways, CD36, a class B scavenger receptor, has recently been reported to be involved in astrocyte activation and glial scar formation in ischemic stroke patients. Bao et al. (2012) demonstrated that functional absence of CD36 receptor reduced astrocyte proliferation, delayed wound healing and attenuated injury induced GFAP overexpression and glial scar formation in the brain.
Therapeutic potential of the reactive astrocytes in stroke
Despite tremendous efforts and advances in translational research, the treatment strategies for stroke are still limited. Tissue plasminogen activator (tPA) is the only FDA approved drug currently available for acute stroke treatment, but it is only effective within a narrow therapeutic window, that is, within a few hours after a stroke. Beyond this window, treatment of a stroke is primarily dependent on supportive care, secondary prevention and rehabilitation. Manipulation of the functional recovery process has emerged as an alternative strategy in stroke research. Since reactive astrocytes have intimate contact with neurons and vasculature, and undergo dynamic changes in Ca2+ signaling, morphology, proliferation and gene expression, modulation of reactive astrocytes might be a promising strategy for stroke therapy. Knowledge regarding these dynamic changes and signaling pathways that trigger reactive gliosis and modulate reactive astrocyte function may provide information for effective therapeutic strategies for ischemic brain disorders. However, most studies on the signaling pathway of reactive gliosis have been based on data obtained only from certain time point specific studies after ischemia. A recent study (Zamanian et al., 2012) on the genomic analysis of reactive astrocytes at different times after MCAo should help to define the properties of reactive astrocyte at different stages of response. On the other hand, the heterogeneity of astrocytes and reactive astrocytes in different regions adds another layer of complexity (Anderson et al., 2014). A recently developed approach, termed translating ribosome affinity purification (TRAP) provides a novel tool to study the translational status of transcripts present in reactive astrocytes after a stroke (Heiman et al., 2008; Doyle et al., 2008; Zhou et al., 2013; Hupe et al., 2014). This technology bypasses the lengthy and possibly disruptive cell purification procedures and purifies mRNAs that are associated with ribosomes in the entire cell body, thus enabling study of a translatome profile using high-throughput RNA-seq. With the development of astrocyte-specific TRAP mice (Heiman et al., 2008; Doyle et al., 2008), it would be feasible to study translatome and identify the critical signaling pathways involved in reactive gliosis at different times and in different brain regions after an ischemic stroke.
Data from signaling pathway and gene profile studies provide the base for modulation of reactive astrocytes through gain-and-loss of function approach. However, stroke outcomes can only be improved when large number of reactive astrocytes can be manipulated. Two in vivo genetic molecular approaches can be used to achieve this goal, i.e., the use of inducible knockout mice and viral transduction. Inducible knockout mice can be used to control temporal expression of genes by knocking down astrocyte-specific genes at a specific time point after ischemia using commercially available astrocyte-specific Cre reporter mice (Mori et al., 2006). Recent advances in self-complementary adeno-associated viruses (scAAV) have provided another promising approach to express transgenes in reactive astrocytes. scAAV can be transduced into the whole brain through intravenous administration in neonatal mice (Foust et al., 2009; Zhang et al., 2011). Since blood-brain barrier becomes permeable after ischemic stroke, this approach can be adapted in adult mice to knock down or overexpress certain genes in an entire penumbra. Combined with an astrocyte-specific promoter (Xie et al., 2010), scAAV would be an ideal approach for molecular manipulation in a large amount of reactive astrocytes in the penumbra, overcoming the disadvantages of invasive and low efficiency delivery of single strand AAV. Data of dynamics on reactive astrocyte and proliferation will provide the necessary information for the time frame of genetic manipulation after ischemia.
Many studies have shown that reactive astrocytes exhibit stem cell-like properties (Buffo et al., 2008; Sirko et al., 2013; Robel et al., 2011; Dimou and Gotz, 2014; Shimada et al., 2012b). They can express neural stem cell related proteins such as nestin and Sox2 (Shimada et al., 2012b) and doublecortin (DCX), an immature neural stem cell marker (Ohab et al., 2006; Magnusson et al., 2014). These studies suggest that reactive astrocytes can potentially transform into neurons under specific conditions such as overexpression of neurogenic factors. It has been recently reported that reactive astrocytes indeed can be converted to neuroblasts and neurons by forced expression of a single transcriptional factor such as Sox2 (Su et al., 2014), neurogenin-2 (Berninger et al., 2007; Heinrich et al., 2010), NeuroD1 (Guo et al., 2013), or a combination of multiple transcriptional factors such as ASCL1, LMX1B and NURR1 (Addis et al., 2011). The results of dynamics of reactive astrocytes provide an important implication for optimal timing to convert reactive astrocytes into neurons using genetic manipulation techniques such as astrocyte-specific inducible transgenic mice and viral transduction, which can possibly aid improvement of stroke outcomes in experimental as well as clinical studies on stroke therapy. However, astrocyte-to-neuron conversion has not been done in reactive astrocytes in the context of strokes.
In another aspect, growing evidence suggests that an ischemic stroke dramatically increases neurogenesis in the subventricular zone (SVZ) and subgranular layers in dentate gyrus (Tobin et al., 2014). It is suggested that there is an association between endogenous neurogenesis and improved stroke outcomes (Lagace, 2012). Recent studies indicate that reactive astrocytes play an important role in neurogenesis after a stroke. Young et al. (2013) first showed that an ischemic stroke causes substantial reactive astrogliosis in SVZ. The hypertrophic reactive astrocytes and their tortuous processes disrupt the neuroblast migratory scaffold and cause SVZ reorganization after a stroke; thus, reactive astrocytes in SVZ might play a role in modulating neurogenesis and stroke recovery. Strokes also elicit a latent neurogenic program in striatal astrocytes in a mouse model. It is reported that striatal astrocytes produce neuroblasts and Notch 1 signaling is reduced in astrocytes after a stroke (Magnusson et al., 2014); furthermore, blocking Notch signaling triggers astrocytes in the striatum and the medial cortex to enter a neurogenic program even in the absence of a stroke, indicating that attenuated Notch 1 signaling is necessary for neurogenesis by striatal astrocytes. Future study is needed to explore whether astrocyte-derived neuroblasts can be differentiated into mature neurons and contribute to improvement of stroke outcomes.
Conclusions
Current neuron-centric strategies have not resulted in major breakthroughs in stroke therapy. Reactive astrogliosis is one of the most prominent pathological features in strokes and is an adaptive defense response that is both beneficial and detrimental to the injured CNS. Especially during the early time after ischemic injury, the main function of reactive astrocytes is to preserve integrity of the nervous tissue. However, with time, the process becomes increasingly unregulated and maladaptive, and progresses to form into a compact glial scar. The established glial scar further accentuates inflammation, generating a highly toxic microenvironment that inhibits migrating axons and interferes with long term motor functional recovery. Given the dualistic nature of reactive astrogliosis, a comprehensive understanding of reactive astrogliosis is extremely vital to the designing strategies that can aid in modulating reactive astrogliosis for healing tissue. The primary goal of research will be to enhance and retain the therapeutically beneficial functions of reactive astrocytes while completely eliminating undesired outcomes like glial scar formation. Since reactive astrocytes exhibit high plasticity in morphology, proliferation and gene expression, modulation of function, signaling pathways, neurogenic potential and neurogenesis through local reactive astrocytes is an attractive strategy for stroke therapy. Astrocyte-specific molecular genetic techniques including TRAP, inducible knockout animals, viral transduction and lineage analysis can elucidate the mechanisms of astrocyte-based stroke therapy, and targeting reactive astrocytes is a promising strategy to improve stroke outcomes.
Acknowledgements
This work was supported by the National Institutes of Health [R01NS069726] and the American Heart Association Grant in Aid Grant [13GRNT17020004] to SD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. Efficient Conversion of Astrocytes to Functional Midbrain Dopaminergic Neurons Using a Single Polycistronic Vector. PLoS ONE. 2011;6:e28719. doi: 10.1371/journal.pone.0028719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali C, Docagne F, Nicole O, Lesne S, Toutain J, Young A, Chazalviel L, Divoux D, Caly M, Cabal P, Derlon JM, MacKenzie ET, Buisson A, Vivien D. Increased expression of transforming growth factor-beta after cerebral ischemia in the baboon: an endogenous marker of neuronal stress? J Cereb Blood Flow Metab. 2001;21:820–827. doi: 10.1097/00004647-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neuroscience Letters. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated Glial Reactivity to Stroke in Aged Rats Correlates With Reduced Functional Recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Bao Y, Qin L, Kim E, Bhosle S, Guo H, Febbraio M, Haskew-Layton RE, Ratan R, Cho S. CD36 is involved in astrocyte activation and astroglial scar formation. J Cereb Blood Flow Metab. 2012;32:1567–1577. doi: 10.1038/jcbfm.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber PA, Demchuk AMH. Biochemistry Ischemic Stroke. Advances in Neurology. 2003;92:151–164. [PubMed] [Google Scholar]
- Barone FC, Irving EA, May AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, Erhardt JA, Nelson AH, Ohlstein EH, Hunter AJ, Ward K, Smith BR, Adams JL, Parsons AA. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther. 2001a;296:312–321. [PubMed] [Google Scholar]
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, Legos JJ, Erhardt JA, Ohlstein EH, Hunter AJ, Harrison DC, Philpott K, Smith BR, Adams JL, Parsons AA. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. J Pharmacol Exp Ther. 2001b;296:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Sun X, Xu L, Giffard RG. Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. Plos One. 2011;6:e27881. doi: 10.1371/journal.pone.0027881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus Coeruleus α-Adrenergic–Mediated Activation of Cortical Astrocytes In Vivo. Cerebral Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesova J, Hock M, Butenko O, Prajerova I, Anderova M, Chvatal A. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J Neurosci Res. 2009;87:96–111. doi: 10.1002/jnr.21828. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE, Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Current Opinion in Neurobiology. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. Functional Properties of Neurons Derived from In Vitro Reprogrammed Postnatal Astroglia. The Journal of Neuroscience. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-Linked IP3R-Dependent Ca2+ Signaling Does Not Mediate Neurovascular Coupling in Mouse Visual Cortex In Vivo. The Journal of Neuroscience. 2014;34:13139–13150. doi: 10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. PNAS. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson A, Nicole O, Docagne F, Sartelet H, MacKenzie ET, Vivien D. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. FASEB J. 1998;12:1683–1691. [PubMed] [Google Scholar]
- Burda J, Sofroniew M. Reactive Gliosis and the Multicellular Response to CNS Damage and Disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. The Journal of Neuroscience. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E, Fathali N, Doyle KP, Williams AM, Han J, Buckwalter MS. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia. 2014;62:1227–1240. doi: 10.1002/glia.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim SY, Cha JH, Choi YS, Sung KW, Oh ST, Kim ON, Chung JW, Chun MH, Lee SB, Lee MY. Upregulation of gp130 and STAT3 activation in the rat hippocampus following transient forebrain ischemia. Glia. 2003;41:237–246. doi: 10.1002/glia.10186. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab. 2013;33:716–723. doi: 10.1038/jcbfm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neuroscience Letters. 2014;565:59–64. doi: 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- de Pablo Y, Nilsson M, Pekna M, Pekny M. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen–glucose deprivation and reperfusion. Histochem Cell Biol. 2013;140:81–91. doi: 10.1007/s00418-013-1110-0. [DOI] [PubMed] [Google Scholar]
- Dimou L, Gotz M. Glial Cells as Progenitors and Stem Cells: New Roles in the Healthy and Diseased Brain. Physiol Rev. 2014;94:709–737. doi: 10.1152/physrev.00036.2013. [DOI] [PubMed] [Google Scholar]
- Ding F, O'Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–394. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. GLIA. 2009;57:767–776. doi: 10.1002/glia.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. In vivo astrocytic Ca2+ signaling in health and brain disorders. Future Neurology. 2013;8:529–554. doi: 10.2217/fnl.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. Ca2+ Signaling in Astrocytes and its Role in Ischemic Stroke. Adv Neurobiol. 2014;11:189–211. doi: 10.1007/978-3-319-08894-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. Dynamic reactive astrocytes after focal ischemia. Neural Regenerative Research. 2015 doi: 10.4103/1673-5374.147929. in poress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced Astrocytic Ca2+ Signals Contribute to Neuronal Excitotoxicity after Status Epilepticus. J Neurosci. 2007;27:10674–10684. doi: 10.1523/JNEUROSCI.2001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional Knock-Out of Kir4.1 Leads to Glial Membrane Depolarization, Inhibition of Potassium and Glutamate Uptake, and Enhanced Short-Term Synaptic Potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docagne F, Nicole O, Marti HH, MacKenzie ET, Buisson A, Vivien D. Transforming growth factor-beta1 as a regulator of the serpins/t-PA axis in cerebral ischemia. FASEB J. 1999;13:1315–1324. doi: 10.1096/fasebj.13.11.1315. [DOI] [PubMed] [Google Scholar]
- Dong Qp, He Jq, Chai Z. Astrocytic Ca2+ waves mediate activation of extrasynaptic NMDA receptors in hippocampal neurons to aggravate brain damage during ischemia. Neurobiology of Disease. 2013;58:68–75. doi: 10.1016/j.nbd.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a Translational Profiling Approach for the Comparative Analysis of CNS Cell Types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGF-β signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, MacVicar BA. In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes. The Journal of Neuroscience. 1996;16:71–81. doi: 10.1523/JNEUROSCI.16-01-00071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Bonvento G. Targeted Activation of Astrocytes: A Potential Neuroprotective Strategy. Mol Neurobiol. 2008;38:231–241. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Friguls B, Dalfo E, Planas AM. Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta Neuropathol. 2003;105:425–437. doi: 10.1007/s00401-002-0661-2. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotech. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, Luo Y, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G, Weinmaster G, Sun YE. Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res. 2002;69:848–860. doi: 10.1002/jnr.10364. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GCR, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In-Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer Disease Model. Cell Stem Cell. 2013;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbury R, Ling ZD, Wuu J, Kordower JH. GFAP knockout mice have increased levels of GDNF that protect striatal neurons from metabolic and excitotoxic insults. J Comp Neurol. 2003;461:307–316. doi: 10.1002/cne.10667. [DOI] [PubMed] [Google Scholar]
- Haupt C, Witte OW, Frahm C. Up-regulation of Connexin 43 in the glial scar following photothrombotic ischemic injury. Molecular and Cellular Neuroscience. 2007;35:89–99. doi: 10.1016/j.mcn.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, Iwasaki K, Jin G, Lo EH, Mishima K, Fujiwara M. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30:871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nature Reviews Neuroscience. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Su+írez-Fari+¦as M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A Translational Profiling Approach for the Molecular Characterization of CNS Cell Types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. Directing Astroglia from the Cerebral Cortex into Subtype Specific Functional Neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Bergles DE, Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Current Opinion in Neurobiology. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Molecular Brain Research. 2004;124:114–123. doi: 10.1016/j.molbrainres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Hupe M, Li MX, Gertow Gillner K, Adams RH, Stenman JM. Evaluation of TRAP-sequencing technology with a versatile conditional mouse model. Nucleic Acids Research. 2014;42:e14. doi: 10.1093/nar/gkt995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77:6, 65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab. 2014;34:1599–1603. doi: 10.1038/jcbfm.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justicia C, Gabriel C, Planas AM. Activation of the JAK/STAT pathway following transient focal cerebral ischemia: signaling through Jak1 and Stat3 in astrocytes. Glia. 2000;30:253–270. doi: 10.1002/(sici)1098-1136(200005)30:3<253::aid-glia5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. GLIA. 1998;23:1–10. [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. 2005. pp. 253–262. 2005/10/04. [DOI] [PubMed]
- Kang W, Hebert J. Signaling Pathways in Reactive Astrocytes, a Genetic Perspective. Mol Neurobiol. 2011;43:147–154. doi: 10.1007/s12035-011-8163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012a;46:251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Billakanti R. Reactive Astrogliosis after Spinal Cord InjuryΓÇöBeneficial and Detrimental Effects. Mol Neurobiol. 2012b;46:251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- Koeglsperger T, Li S, Brenneis C, Saulnier JL, Mayo L, Carrier Y, Selkoe DJ, Weiner HL. Impaired glutamate recycling and GluN2B-mediated neuronal calcium overload in mice lacking TGF-beta1 in the CNS. 2013. pp. 985–1002. 2013/03/29. [DOI] [PMC free article] [PubMed]
- Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. 2011. pp. 800–809. 2011/02/26. [DOI] [PMC free article] [PubMed]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Slevin M, Marti E, Catena E, Rubio F, Gaffney J. Time-course phosphorylation of the mitogen activated protein (MAP) kinase group of signalling proteins and related molecules following middle cerebral artery occlusion (MCAO) in rats. Neuropathol Appl Neurobiol. 2003;29:144–158. doi: 10.1046/j.1365-2990.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- Lagace DC. Does the endogenous neurogenic response alter behavioral recovery following stroke? Behavioural Brain Research. 2012;227:426–432. doi: 10.1016/j.bbr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- Lei C, Deng J, Wang B, Cheng D, Yang Q, Dong H, Xiong L. Reactive oxygen species scavenger inhibits STAT3 activation after transient focal cerebral ischemia-reperfusion injury in rats. Anesth Analg. 2011;113:153–159. doi: 10.1213/ANE.0b013e31821a9fbe. [DOI] [PubMed] [Google Scholar]
- Lennmyr F, Karlsson S, Gerwins P, Ata KA, Terent A. Activation of mitogen-activated protein kinases in experimental cerebral ischemia. Acta Neurol Scand. 2002;106:333–340. doi: 10.1034/j.1600-0404.2002.01313.x. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang N, Lin H, Yu Y, Cai QM, Li H, Zhang N, Lin H, Yu Y, Cai QM. Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neuroscience. 2014;15:58. doi: 10.1186/1471-2202-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang N, Sun G, Ding S. Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN NEURO. 2013;5:e00117. doi: 10.1042/AN20130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2007;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Experimental Neurology. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. GLIA. 2014;62:2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszevicz AC, Sampaio N, Guegan C, Benchoua A, Couriaud C, Chevalier E, Sola B, Lacombe P, Onteniente B. High Sensitivity of Protoplasmic Cortical Astroglia to Focal Ischemia. J Cereb Blood Flow Metab. 2002;22:289–298. doi: 10.1097/00004647-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EMK, Lindvall O, Kokaia Z, Frisen J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- Marumo T, Takagi Y, Muraki K, Hashimoto N, Miyamoto S, Tanigaki K. Notch signaling regulates nucleocytoplasmic Olig2 translocation in reactive astrocytes differentiation after ischemic stroke. Neurosci Res. 2013;75:204–209. doi: 10.1016/j.neures.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. GLIA. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Research Reviews. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, Rose CR, Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. GLIA. 2008;56:1127–1137. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- Mestriner RgG, Saur L, Bagatini PB, Baptista PPA, Vaz SP, Ferreira K, Machado SA, Xavier LdL, Netto CA. Astrocyte morphology after ischemic and hemorrhagic experimental stroke has no influence on the different recovery patterns. Behavioural Brain Research. 2015;278:257–261. doi: 10.1016/j.bbr.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Moon LD, Fawcett JW. Reduction in CNS scar formation without concomitant increase in axon regeneration following treatment of adult rat brain with a combination of antibodies to TGF-β1 and β2. Eur J Neurosci. 2001;14:1667–1677. doi: 10.1046/j.0953-816x.2001.01795.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial gliaΓÇöA valuable tool for functional and lineage analysis. GLIA. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murata Y, Fujiwara N, Seo JH, Yan F, Liu X, Terasaki Y, Luo Y, Arai K, Ji X, Lo EH. Delayed inhibition of c-Jun N-terminal kinase worsens outcomes after focal cerebral ischemia. J Neurosci. 2012;32:8112–8115. doi: 10.1523/JNEUROSCI.0219-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahirnyj A, Livne-Bar I, Guo X, Sivak JM. ROS detoxification and proinflammatory cytokines are linked by p38 MAPK signaling in a model of mature astrocyte activation. Plos One. 2013;8:e83049. doi: 10.1371/journal.pone.0083049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM. High Susceptibility to Cerebral Ischemia in GFAP-Null Mice. J Cereb Blood Flow Metab. 2000;20:1040–1044. doi: 10.1097/00004647-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. GLIA. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S. The role of JAK-STAT signaling within the CNS. Jak-Stat. 2013;2:e22925. doi: 10.4161/jkst.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nito C, Kamada H, Endo H, Narasimhan P, Lee YS, Chan PH. Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes. J Neurotrauma. 2012;29:2404–2412. doi: 10.1089/neu.2012.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizar K, et al. In vivo Stimulus-Induced Vasodilation Occurs without IP3 Receptor Activation and May Precede Astrocytic Calcium Increase. The Journal of Neuroscience. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka D, Rogozinska K, Aleksy M, Witte OW, Skangiel-Kramska J. Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp. 2008;68:155–68. doi: 10.55782/ane-2008-1685. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB. Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. Plos One. 2014;9:e102003. doi: 10.1371/journal.pone.0102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of Astrocytic Domain Organization in the Epileptic Brain. The Journal of Neuroscience. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A Neurovascular Niche for Neurogenesis after Stroke. The Journal of Neuroscience. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general considerations. GLIA. 2005;50:287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze V, Kang J, Bergles D. Norepinephrine Controls Astroglial Responsiveness to Local Circuit Activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Eliasson C, Chien CL, Kindblom LG, Liem R, Hamberger A, Betsholtz C. GFAP-Deficient Astrocytes Are Capable of Stellationin VitroWhen Cocultured with Neurons and Exhibit a Reduced Amount of Intermediate Filaments and an Increased Cell Saturation Density. Experimental Cell Research. 1998;239:332–343. doi: 10.1006/excr.1997.3922. [DOI] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J. Abnormal Reaction to Central Nervous System Injury in Mice Lacking Glial Fibrillary Acidic Protein and Vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN, Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Che Y, Han PL, Lee JK. Delayed and differential induction of p38 MAPK isoforms in microglia and astrocytes in the brain after transient global ischemia. Brain Res Mol Brain Res. 2002;107:137–144. doi: 10.1016/s0169-328x(02)00456-4. [DOI] [PubMed] [Google Scholar]
- Prehn JH, Backhauss C, Krieglstein J. Transforming growth factor-beta 1 prevents glutamate neurotoxicity in rat neocortical cultures and protects mouse neocortex from ischemic injury in vivo. 1993:521–525. doi: 10.1038/jcbfm.1993.67. 1993/05/01. [DOI] [PubMed] [Google Scholar]
- Ralay Ranaivo H, Hodge JN, Choi N, Wainwright MS. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. 2012:68. doi: 10.1186/1742-2094-9-68. 2012/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Privat A, Malhotra SK, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Roy Choudhury G, Ryou MG, Poteet E, Wen Y, He R, Sun F, Yuan F, Jin K, Yang SH. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke. Braon Research. 2014;1551:45–58. doi: 10.1016/j.brainres.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco A, Nicole O, Docagne F, Ali C, Chazalviel L, Komesli S, Yablonsky F, Roussel S, MacKenzie ET, Vivien D, Buisson A. A transforming growth factor-beta antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischemic brain injury. J Cereb Blood Flow Metab. 1999;19:1345–1353. doi: 10.1097/00004647-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. Journal of Neuroscience Methods. 2002;117:43–49. doi: 10.1016/s0165-0270(02)00072-9. [DOI] [PubMed] [Google Scholar]
- Shannon C, Salter M, Fern R. GFP imaging of live astrocytes: regional differences in the effects of ischaemia upon astrocytes. Journal of Anatomy. 2007;210:684–692. doi: 10.1111/j.1469-7580.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Ishii Y, Xu G, Dang TC, Hamashima T, Matsushima T, Yamamoto S, Hattori Y, Takatsuru Y, Nabekura J, Sasahara M. PDGFR-β as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:353–367. doi: 10.1038/jcbfm.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, Borders A, Aronshtam A, Spees JL. Proliferating reactive astrocytes are regulated by Notch-1 in the peri-infarct area after stroke. Stroke. 2011a;42:3231–3237. doi: 10.1161/STROKEAHA.111.623280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J Neurosci. 2012a;32:7926–7940. doi: 10.1523/JNEUROSCI.4303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, Borders A, Aronshtam A, Spees JL. Proliferating Reactive Astrocytes Are Regulated by Notch-1 in the Peri-Infarct Area After Stroke. Stroke. 2011b;42:3231–3237. doi: 10.1161/STROKEAHA.111.623280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL. Self-Renewal and Differentiation of Reactive Astrocyte-Derived Neural Stem/Progenitor Cells Isolated from the Cortical Peri-Infarct Area after Stroke. The Journal of Neuroscience. 2012b;32:7926–7940. doi: 10.1523/JNEUROSCI.4303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirko S, et al. Reactive Glia in the Injured Brain Acquire Stem Cell Properties in Response to Sonic Hedgehog. Cell Stem Cell. 2013;12:426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in neurosciences. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M, Vinters H. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O'Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- Stapf C, Mohr JP. Ischemic stroke therapy. Annual Review of Medicine. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Progress in Neurobiology. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014:5. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-Dependent Neuroglial Calcium Signaling Differs Between Young and Adult Brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol. 2001;170:63–71. doi: 10.1006/exnr.2001.7701. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Current Molecular Medicine. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Takata N, Hirase H. Cortical Layer 1 and Layer 2/3 Astrocytes Exhibit Distinct Calcium Dynamics In Vivo. PLoS ONE. 2008;3:e2525. doi: 10.1371/journal.pone.0002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. Cerebral Blood Flow Modulation by Basal Forebrain or Whisker Stimulation Can Occur Independently of Large Cytosolic Ca2+ Signaling in Astrocytes. PLoS ONE. 2013;8:e66525. doi: 10.1371/journal.pone.0066525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. PNAS. 2012;109:18974–18979. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DC, Scheibe J, Glocke I, Weise G, Deten A, Boltze J, Kranz A. Object-based analysis of astroglial reaction and astrocyte subtype morphology after ischemic brain injury. Acta Neurobiol Exp (Wars) 2012;73:79–87. doi: 10.55782/ane-2013-1923. [DOI] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neuroscience. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, White RF, Barone FC, Feuerstein GZ. Transforming growth factor-beta 1 exhibits delayed gene expression following focal cerebral ischemia. Brain Res Bull. 1995;36:607–609. doi: 10.1016/0361-9230(94)00243-t. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. The Journal of Neuroscience. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TC, Peng H, Hata R, Desaki J, Sakanaka M. Induction of phosphorylated-Stat3 following focal cerebral ischemia in mice. Neurosci Lett. 2001;303:153–156. doi: 10.1016/s0304-3940(01)01711-6. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. In Vivo Calcium Imaging Reveals Functional Rewiring of Single Somatosensory Neurons after Stroke. J Neurosci. 2008;28:6592–6606. doi: 10.1523/JNEUROSCI.0622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S. Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience. 2010;170:992–1003. doi: 10.1016/j.neuroscience.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Vogel P, Fritze K, Back T, Hossmann KA, Wiessner C. Monitoring the temporal and spatial activation pattern of astrocytes in focal cerebral ischemia using in situ hybridization to GFAP mRNA: comparison with sgp-2 and hsp70 mRNA and the effect of glutamate receptor antagonists. Brain Res. 1996;735:285–297. doi: 10.1016/0006-8993(96)00578-1. [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Young CC, van der Harg JM, Lewis NJ, Brooks KJ, Buchan AM, Szele FG. Ependymal Ciliary Dysfunction and Reactive Astrocytosis in a Reorganized Subventricular Zone after Stroke. Cerebral Cortex. 2013;23:647–659. doi: 10.1093/cercor/bhs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Yu P, Chen H, Geller HM. Targeted inhibition of KCa3.1 attenuates TGF-β-induced reactive astrogliosis through the Smad2/3 signaling pathway. J Neurochem. 2014;130:41–49. doi: 10.1111/jnc.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic Analysis of Reactive Astrogliosis. The Journal of Neuroscience. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G. Several rAAV Vectors Efficiently Cross the Blood-brain Barrier and Transduce Neurons and Astrocytes in the Neonatal Mouse Central Nervous System. Mol Ther. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1962–1971. doi: 10.1038/jcbfm.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Current Opinion in Neurobiology. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zheng W, Talley Watts L, Holstein DM, Wewer J, Lechleiter JD. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab. 2013;33:600–611. doi: 10.1038/jcbfm.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly Isolated Astrocytes From Rat Hippocampus Show Two Distinct Current Patterns and Different [K+]oUptake Capabilities. J Neurophysiol. 2000;84:2746–2757. doi: 10.1152/jn.2000.84.6.2746. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zhang Y, Ma Q, Gu F, Day DS, He A, Zhou B, Li J, Stevens SM, Romo D, Pu WT. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purification. PNAS. 2013;110:15395–15400. doi: 10.1073/pnas.1304124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zou L, Tian J, Du G, Gao Y. SMND-309, a novel derivative of salvianolic acid B, protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway. Eur J Pharmacol. 2013;714:23–31. doi: 10.1016/j.ejphar.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]