Summary
Background
Supplementary immunisation activities with oral poliovirus vaccines (OPVs) are usually separated by 4 week intervals; however, shorter intervals have been used in security-compromised areas and for rapid outbreak responses. We assessed the immunogenicity of monovalent type-1 oral poliovirus vaccine (mOPV1) given at shorter than usual intervals in Karachi, Pakistan.
Methods
This was a multicentre, randomised, controlled, four-arm, open-label, non-inferiority trial done at five primary health-care centres in low-income communities in and around Karachi, Pakistan. Eligible participants were healthy newborn babies with a birthweight of at least 2.5 kg, for whom informed consent was provided by their parent or guardian, and lived less than 30 km from the study clinic. After receiving a birth dose of trivalent OPV, we enrolled and randomly assigned newborn babies (1:1:1:1) to receive two doses of mOPV1 with an interval of 1 week (mOPV1–1 week), 2 weeks (mOPV1–2 weeks), or 4 weeks (mOPV1–4 weeks) between doses, or two doses of bivalent OPV (bOPV) with an interval of 4 weeks between doses (bOPV–4 weeks). We gave the first study dose of OPV at age 6 weeks. We did the randomisation with a centrally generated, computerised allocation sequence with blocks of 16; participants’ families and study physicians could not feasibly be masked to the allocations. Trial participants were excluded from local supplementary immunisation activities during the study period. The primary outcome was non-inferiority (within a 20% margin) between groups in seroconversion to type-1 poliovirus. The primary and safety analyses were done in the per-protocol population of infants who received all three doses of vaccine. This trial is registered with ClinicalTrials.gov, number NCT01586572, and is closed to new participants.
Findings
Between March 1, 2012, and May 31, 2013, we enrolled 1009 newborn babies, and randomly assigned 829 (82%) to treatment. 554 (67%) of the 829 babies were included in the per-protocol analysis. Proportions of seroconversion to type-1 poliovirus were 107/135 (79%, 95% CI 72.4–86.1) with mOPV1–1 week, 108/135 (80%, 73.2–86.8) with mOPV1–2 weeks, 129/148 (87%, 80.9–92.0) with mOPV1–4 weeks, and 107/136 (79%, 71.8–85.6) with bOPV–4 weeks. Non-inferiority was shown between groups and no significant differences were noted. Ten participants died during the trial. Seven of these deaths occurred during the lead-in period before randomisation (two from diarrhoea, five from unknown causes). Three infants died from sepsis after random assignment. No deaths were attributed to the procedures or vaccines. Additionally, we noted no events of vaccine-associated paralysis.
Interpretation
We identified no significant differences in responses to mOPV1 given with shorter intervals between doses than with the standard 4 week intervals. The short-interval strategy could be particularly beneficial when temporary windows of opportunity for safe access can be granted in areas of conflict—eg, during cease-fire periods. In such situations, we recommend shortening the interval between OPV doses to 7 days.
Funding
World Health Organization.
Introduction
Since 1988, when the World Health Assembly endorsed the eradication of poliomyelitis,1 substantial progress toward this goal has been made through implementation of the main strategies of the Global Polio Eradication Initiative. These strategies focus on high-quality supplemental immunisation activities that target children from birth to age 5 years with oral poliovirus vaccines (OPVs), and maintaining a sensitive system of acute flaccid paralysis surveillance. The number of people paralysed by poliovirus infection decreased to its lowest level in history in 2012— only 223 cases of paralytic disease caused by wild poliovirus were reported worldwide in this year.2 However, three countries (Afghanistan, Nigeria, and Pakistan) with uninterrupted circulation of polioviruses continue to be the source of spread to other countries, threatening the goal of global eradication. In 2013, outbreaks of poliomyelitis caused by wild polioviruses transmitted from endemic countries into previously polio-free countries were detected in the Horn of Africa and in the Middle East.2
Three serotypes of polioviruses have been described—types 1, 2, and 3. Indigenous type-2 wild poliovirus was eradicated in 1999,3 and the last known cases of type-3 wild poliovirus were identified in November, 2012. Type-3 wild polio virus might also therefore have been eliminated; however, more time without detection of the type-3 virus is needed for certification of eradication status. In 2013, all cases of poliomyelitis caused by wild polioviruses were due to type 1.4
In Pakistan, as elsewhere, the polio eradication programme strives to interrupt the circulation of polio-viruses by achieving high population coverage with OPVs through routine immunisation and supplemental immunisation activities. The supplemental activities in areas of high transmission risk are frequent, usually done every 1 or 2 months; additionally, national rounds of supplemental immunisation activities cover the entire country, usually done two to three times per year. In the tribal areas in northwest Pakistan, armed conflict poses an obstacle to interrupting poliovirus transmission.5 In 2012 and 2013, several attacks targeting polio-vaccination teams during supplemental immunisation activities resulted in deaths of health workers and security officials guarding health workers, and led to decreased coverage or cancellation of some planned rounds of immunisation in these areas. In 2013, nearly 90% of cases of paralytic polio were detected from security-challenged areas of Pakistan, such as the Federally Administered Tribal Areas, and parts of Khyber Pakhtunkhwa Province.2 Because of the continued threat to eradication posed by the international spread of wild polioviruses, on May 5, 2014, WHO declared the international spread of wild poliovirus a Public Health Emergency of International Concern.6
The OPVs used in poliovirus eradication are either trivalent (tOPV), which provide protection against all three poliovirus serotypes; bivalent OPV (bOPV), which protect against types 1 and 3; or monovalent OPV (mOPV1), which protect against type 1.7 The selection of OPV type for supplemental immunisation activities is driven by epidemiology and availability of the vaccine. All types of vaccine have been used in immunisation activities done in Pakistan. However, because type-3 wild poliovirus seems to be on the verge of eradication, mOPV1 will become even more important to achieve eradication of type-1 poliovirus.
Supplemental immunisations in geographical areas harbouring infection are typically separated by a 1 month interval. This interval between OPV doses is based on the interval of 4 weeks between tOPV doses used in routine immunisation schedules in most developing countries.8–12 The 4 week interval in routine immunisation schedules provides the optimum immune response to tOPV and also coincides with the schedule for the diphtheria, tetanus, and pertussis vaccine in childhood. However, shorter-interval rounds, referred to as short-interval additional doses (SIAD), with OPV have been done in Somalia and Pakistan, and elsewhere, with the aim of rapidly increasing immunity in populations in outbreak situations, or as a means to rapidly immunise populations living in areas with little access to vaccines because of social or political insecurity. Unpublished data from WHO’s polio teams show that SIADs have led to fast interruption of poliovirus trans mission in areas where they have been used, and might thus be an attractive strategy for accelerating interruption efforts against the circulation of type-1 poliovirus in 2015. However, very few data exist for immuno genicity of OPV given in shorter intervals. We therefore aimed to assess the non-inferiority of two doses of mOPV1 given at shorter intervals (7 or 14 days), compared with two doses of mOPV1 or bivalent OPV1 and 3 given at the standard 30 day (4 week) interval, in achievement of seroconversion to type-1 poliovirus.
Methods
Study design and participants
We did a multicentre, randomised, controlled, four-arm, open-label, non-inferiority trial at five primary health-care centres in Karachi, Pakistan. The study area consisted of five low-income communities in and around Karachi (including contiguous coastal villages at the outskirts of Karachi, and one urban squatter-settlement with Pashtun predominance). The participating centres are operated by the Department of Paediatrics and Child Health Research Programme of the Aga Khan University and provide free primary health-care services to children from these communities (appendix). The study was approved by the Ethical Review Committee of Aga Khan University (Karachi, Pakistan), the National Bioethics Committee of Pakistan, and the Ethical Review Committee of WHO, Geneva (Switzerland).
To recruit participants, health-centre staff approached women at home either during their pregnancy or immediately after delivery, informed them about the trial, and invited them to enrol their child into the trial. Eligible participants were healthy newborn babies with a birthweight of at least 2.5 kg, for whom informed, written consent was provided by their parent or guardian, and lived less than 30 km from the study clinic, and whose family did not intend to move during the study period. Newborn babies who had any clinical sign of illness established with the WHO Integrated Management of Neonatal and Childhood Illness and needed to be admitted to hospital, or were at risk of immunodeficiency (through a family history screen), were excluded from the trial. Additionally, trial participants were excluded from local supplemental immunisation activities during the study period through close coordination with officials from local polio-immunisation campaigns.
Randomisation and masking
Study physicians at each site enrolled infants at birth to the trial. Infants meeting inclusion criteria at age 6 weeks were randomly assigned to one of four treatment groups: two doses of mOPV1 given 1 week apart (mOPV1–1 week), two doses of mOPV1 given 2 weeks apart (mOPV1–2 weeks), two doses of mOPV1 given 4 weeks apart (mOPV1–4 weeks), or bOPV given 4 weeks apart (bOPV–4 weeks). We did the random assignment using a randomisation sequence list generated by the Clinical Trials Unit at Aga Khan University. We did the computerised block randomisation with blocks of 16 and no restrictions. The study physicians opened the envelope containing information about treatment assignment when the infants were aged 6 weeks, and accordingly assigned participants to the study group. The families of the study participants, and the study physicians, could not feasibly be masked to allocation groups.
Procedures
At birth, we obtained umbilical cord or peripheral blood and gave tOPV to all trial participants, as per Pakistan’s national immunisation programme.13 At age 42 days (6 weeks), we collected a second blood sample, and gave the first study dose of OPV according to the random assignment. We obtained a third blood sample on day 30 after the last dose of OPV. Thus, the last blood sample was collected at age 79 days in group mOPV1–1 week, 86 days in group mOPV1–2 weeks, and 102 days in groups mOPV1–4 weeks and bOPV–4 weeks.
We used OPV from WHO-prequalified producers: mOPV1 from GlaxoSmithKline, tOPV from Sanofi Pasteur, and bOPV from Novartis. The tOPV contained at least 1 million of the 50% tissue culture infective dose (TCID50) of Sabin-strain type-1 poliovirus, at least 100 000 TCID50 of Sabin-strain type-2 poliovirus, and at least 1 × 105.8 TCID50 of Sabin-strain type-3 poliovirus. The mOPV1 contained at least 1 million TCID50 of Sabin-strain type-1 poliovirus. The bOPV contained at least 1 million 50% cell culture infective dose (CCID50) of Sabin strain type-1 poliovirus and 1 × 105.8 CCID50 of type-3 Sabin poliovirus. The blood specimens obtained at the sites were allowed to clot and centrifuged to separate the serum. We transported the serum samples to the Infectious Disease Research Laboratory at the Aga Khan University, where they were stored at −20°C until being shipped to the Centers for Disease Control and Prevention (Atlanta, GA, USA), and tested for the presence of poliovirus-neutralising antibodies with standard neutralisation assays.14
Outcomes
The primary outcome was non-inferiority (within a 20% margin) between groups in seroconversion to type-1 poliovirus after participants received all three vaccine doses in each treatment group.
Secondary endpoints were seroprevalence with median reciprocal titres in each group, seroconversion after two study doses of OPV in per-protocol infants who did not seroconvert after birth OPV, vaccine safety, and factors associated with low or no seroconversion to type-1 poliovirus after birth OPV and two doses of study OPV in per-protocol infants.
Per-protocol status was defined as infants who completed all the scheduled visits within 2 days of the expected dates, provided three blood samples, and did not receive OPV other than the study doses. We also did an intention-to-treat analysis.
Seropositivity was defined as reciprocal titres of poliovirus-neutralising antibodies of more than 8. Seroconversion was defined as a change from seronegative to seropositive in infants without detectable maternal anti bodies at birth, or as an increase in titres by at least four times more than the expected value, and an assumption of negative exponential decay of maternal antibody titres with a half-life of 28 days. A high concentration of maternal antibodies was defined as a titre of at least 64 at birth.15,16 Interval–seroconversion was defined as seroconversion achieved by the end of the two study doses of OPV given at short or standard intervals to per-protocol infants who did not seroconvert after the birth dose. Adverse events were identified by site investigators and reviewed by the principal investigator. Serious adverse events (any deaths or occurrences of vaccine-associated paralysis) were reported and reviewed by a data and safety monitoring board and by ethical review committees based in Geneva, Switzerland, and Karachi, Pakistan.
Statistical analysis
We estimated a sample size of 139 eligible infants per group at 80% power and α was set at 0.05 to detect differences of no more than 20% in seroconversion between standard and short-interval groups. We assumed that around 20% of participants would drop out between enrolment at birth and randomisation at 6 weeks (with a long lead-time in between), and an additional 30% attrition or non-per-protocol status after randomisation; therefore we set a target to enrol a minimum of 1000 infants at birth to have a sufficient number of infants in the primary, per-protocol analysis. We did the statistical analyses with Stata version 12. We used one way ANOVA-Tukey’s multiple comparison test to compare the proportions of seroconversion and median titres with 95% CIs across short versus long intervals between groups in the per-protocol population. We tested univariate analyses of factors associated with no seroconversion with binary logistic regression (SPSS version 19). We considered variables with a p value less than 0.25 in the univariate for inclusion in the multivariable logistic regression. We used a parsimonious model-building strategy to select variables with significance on multivariable analysis. The trial protocol was registered at ClinicalTrials.gov, number NCT01586572.
Role of the funding source
The funder of the study assisted in study design, trial monitoring, and contributed to writing of the report, but had no role in data collection, data analysis, or data interpretation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 1, 2012, and May 31, 2013, we enrolled 1009 newborn babies, and randomly assigned 829 (82%) to treatment. 554 (67%) of the 829 babies were included in the per-protocol analysis (figure 1). Most infants (597/829; 72%) received the birth dose of tOPV within first 24 h of life; the remaining infants received tOPV within 72 h of birth (table 1). Baseline characteristics did not differ between groups (table 1).
Figure 1. Trial profile.
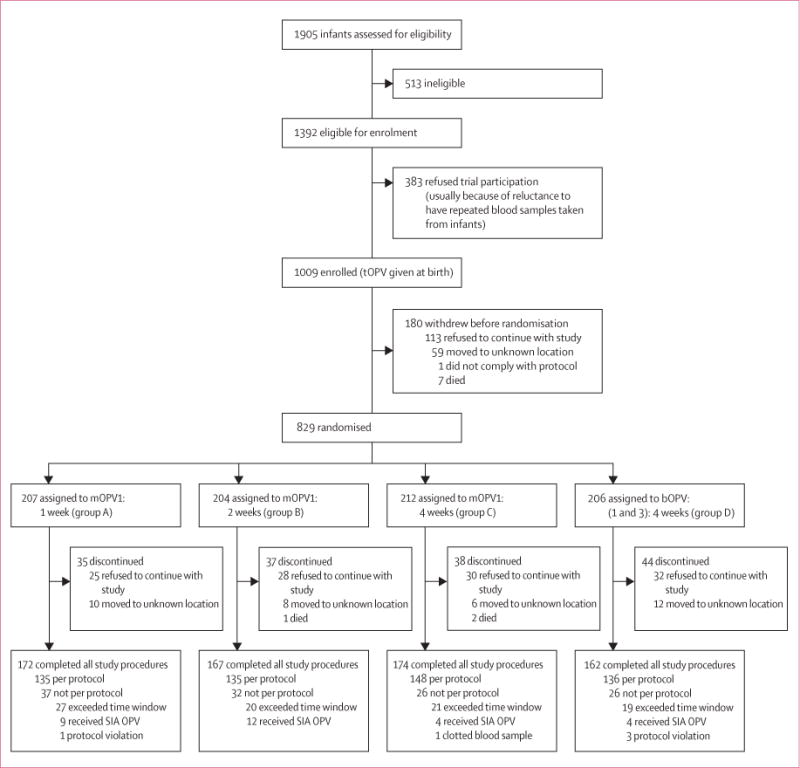
tOPV=trivalent oral polio vaccine. mOPV1=monovalent oral polio vaccine type 1. bOPV=bivalent OPV type 1 and 3. SIA=supplementary immunisation activity.
Table 1.
Baseline characteristics
| mOPV1: 1 week interval between doses (N=207) | mOPV1: 2 week interval between doses (N=204) | mOPV1: 4 week interval between doses (N=212) | bOPV: 4 week between doses interval (N=206) | |
|---|---|---|---|---|
| Sex of baby | ||||
| Female | 103/207 (50%) | 100/204 (49%) | 105/212 (50%) | 99/206 (48%) |
| Male | 104/207 (50%) | 104/204 (51%) | 107/212 (50%) | 107/206 (52%) |
|
| ||||
| Completion of primary education by parent (usually by age 9–11 years) | ||||
| Father | 74/207 (36%) | 64/204 (31%) | 59/212 (28%) | 76/206 (37%) |
| Mother | 42/207 (20%) | 43/204 (21%) | 52/212 (25%) | 56/206 (27%) |
|
| ||||
| Home birth | 173/207 (84%) | 179/204 (88%) | 183/212 (86%) | 178/206 (86%) |
|
| ||||
| Birthweight, kg | 2.96 (0.38) | 2.97 (0.41) | 2.99 (0.39) | 2.90 (0.38) |
|
| ||||
| Interval between birth and giving tOPV, h | 17 (8–31) | 14 (7.3–24) | 16.5 (7.3–24) | 16 (7.8–25) |
|
| ||||
| Family size | 8 (6–12) | 8 (6–12) | 8 (5–10) | 8 (6–11) |
|
| ||||
| Households with monthly income <10 000 Pakistani rupees | 136/207 (66%) | 144/204 (71%) | 147/212 (69%) | 135/206 (66%) |
Data are n/N (%), mean (SD), or median (IQR). mOPV1=monovalent oral polio vaccine type 1. bOPV=bivalent OPV type 1 and 3. tOPV=trivalent oral polio vaccine.
For the primary outcome of seroconversion to type-1 poliovirus at the end of the study in per-protocol infants (figure 2) receiving the birth dose of OPV and two additional doses of OPV given at short or standard intervals, no significant differences were noted between groups: 107/135 (79%, 95% CI 72.4–86.1) with mOPV1–1 week, 108/135 (80%, 73.2–86.8) with mOPV1–2 weeks, 129/148 (87%, 80.9–92.0) with mOPV1–4 weeks, and 107/136 (79%, 71.8–85.6) with bOPV–4 weeks. Non-inferiority of short-interval dosing was thus shown. High proportions of seroconversion to type-2 poliovirus were also noted, although only the birth dose contained vaccine against type-2 poliovirus.
Figure 2. Seroconversion after three doses of oral polio vaccine.
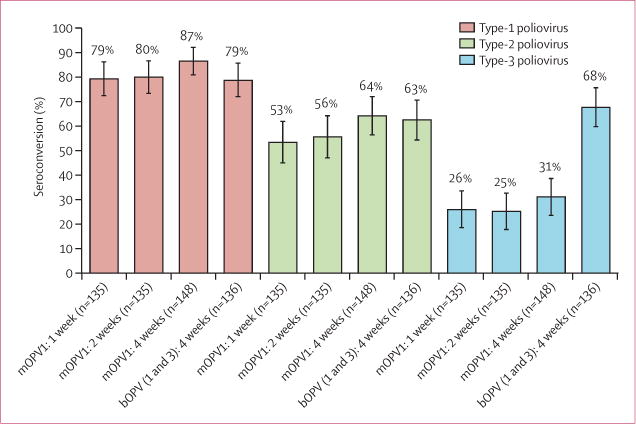
Data are proportions (95% CIs) from per-protocol population (n=554). Type-1 poliovirus: mOPV1-1 week 95% CI 72.4–86.1. mOPV1-2 week 73.2–86.8. mOPV1-4 week 80.9–92.0. bOPV (1&3)-4 week 71.8–85.6. Type 2 poliovirus: mOPV1-1 week 95% CI 44.9–61.8. mOPV1-2 week 47.1–64.0. mOPV1-4 week 56.4–72.0. bOPV(1&3)-4 week 54.3–70.7. Type 3 poliovirus: mOPV1-1 week 95% CI 18.5–33.4. mOPV1-2 week 17.8–32.6. mOPV1-4 week 23.6–38.6. bOPV (1&3)-4 week 59.7–75.6. mOPV1=monovalent oral polio vaccine type 1. bOPV=bivalent oral polio vaccine type 1 and 3.
Baseline seroprevalence (ie, concentrations of maternal antibodies) did not differ between groups and was more than 90% for types 1 and 2 and more than 70% for type 3 (table 2). Final seroprevalence achieved after three doses of OPV doses for types 1 and 2 did not differ between groups, and was more than 95% for serotype 1, and more than 75% for serotype 2. Final seroprevalence for serotype 3 was significantly higher in the bOPV–4 weeks group than in the other groups, as expected, because infants in the mOPV1 groups did not receive the type-3 vaccine after the birth dose.
Table 2.
Seroprevalence of poliovirus antibodies in per-protocol infants (n=554)
| Group A: mOPV1–1 week (N=135) |
Group B: mOPV1–2 weeks (N=135) |
Group C: mOPV1–4 weeks (N=148) |
Group D: bOPV 1 and 3–4 weeks (N=136) |
p value
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| A vs C | A vs D | B vs C | B vs D | C vs D | |||||
| At birth | |||||||||
|
| |||||||||
| Type-1 poliovirus | 125/135 (93%) | 132/135 (98%) | 135/148 (91%) | 129/136 (95%) | 0.961 | 0.860 | 0.092 | 0.738 | 0.566 |
| Reciprocal titres | 113.8 (22.6–227.5), 72.0–126.1 | 90.5 (28.4–227.5), 72.0–113.8 | 68.0 (18.0–181.0), 41.2–100.8 | 90.5 (28.4–343.5), 58.1–178.1 | 0.163 | 0.671 | 0.374 | 0.667 | 0.318 |
| Type-2 poliovirus | 125/135 (93%) | 126/135 (93%) | 135/148 (91%) | 125/136 (92%) | 0.973 | 0.997 | 0.911 | 0.972 | 0.996 |
| Reciprocal titres | 90.5 (22.6–288.0), 56.9–113.8 | 90.5 (22.6–362.0), 83.0–181.0 | 56.9 (14.2–227.5), 28.4–90.5 | 90.5 (14.2–288.0), 56.9–144.0 | 0.185 | 0.851 | 0.099 | 0.586 | 0.131 |
| Type-3 poliovirus | 102/135 (76%) | 99/135 (73%) | 113/148 (76%) | 106/136 (78%) | 0.999 | 0.968 | 0.935 | 0.814 | 0.990 |
| Reciprocal titres | 18.0 (9.0–144.0), 14.2–28.4 | 18.0 (7.1–72.0), 14.2–28.4 | 22.6 (9.0–90.5), 18.0–36.0 | 22.6 (9.0–113.8), 14.2–31.7 | 0.479 | 0.503 | 0.278 | 0.670 | 0.374 |
|
| |||||||||
| Age 42 days | |||||||||
|
| |||||||||
| Type-1 poliovirus | 120/135 (89%) | 126/135 (93%) | 129/148 (87%) | 124/136 (91%), 45.3–113.8 | 0.962 | 0.923 | 0.307 | 0.934 | 0.671 |
| Reciprocal titres | 56.9 (18.0–288.0), 41.5–90.5 | 56.9 (18.0–288.0), 36.0–72.0 | 45.3 (11.3–288.0), 28.4–90.5 | 90.5 (18.0–288.0) | 0.789 | 0.248 | 0.679 | 0.129 | 0.131 |
| Type-2 poliovirus | 127/135 (94%) | 129/135 (96%), | 136/148 (92%) | 120/136 (88%), 113.8–362.0 | 0.899 | 0.266 | 0.649 | 0.104 | 0.649 |
| Reciprocal titres | 181.0 (36.0–910.2), 113.8–362.0 | 113.8 (36.0–1152.1), 90.5–288.0 | 288.0 (45.3–1152.1), 181.0–455.1 | 227.5 (24.1–1152.1) | 0.425 | 0.948 | 0.194 | 0.247 | 0.464 |
| Type-3 poliovirus | 74/135 (55%) | 78/135 (58%) | 86/148 (58%) | 81/136 (60%), | 0.944 | 0.860 | 0.99 | 0.991 | 0.995 |
| Reciprocal titres | 11.3 (5.7–56.9), 7.1–18.0 | 11.3 (5.7–90.5), 7.1–14.2 | 11.3 (5.7–90.5), 8.2–14.2 | 11.3 (57–85.9) | 0.907 | 0.668 | 0.622 | 0.426 | 0.748 |
|
| |||||||||
| At completion | |||||||||
|
| |||||||||
| Type-1 poliovirus | 132/135 (98%) | 131/135 (97%) | 143/148 (97%) | 126/136 (93%) | 0.959 | 0.134 | 0.998 | 0.250 | 0.316 |
| Reciprocal titres | 910.2 (181.0–1448.2), 663.9–1152.1 | 910.2 (144.0–1448.2), 663.9–1152.1 | 910.2 (288.0–1448.2), 724.1–1152.1 | 817.1 (121.3–1448.2), 455.1–1152.1 | 0.739 | 0.948 | 0.968 | 0.763 | 0.789 |
| Type-2 poliovirus | 111/135 (82%) | 105/135 (78%) | 117/148 (79%) | 107/136 (79%) | 0.913 | 0.889 | 0.994 | 0.998 | 0.999 |
| Reciprocal titres | 181.0 (11.3–1152.1), 56.9–399.9 | 227.5 (9.0–1152.1), 32.9–504.2 | 362.0 (12.0–1448.2), 130.6–724.1 | 371.6 (11.3–1448.2), 92.3–576.0 | 0.245 | 0.584 | 0.590 | 0.761 | 0.649 |
| Type-3 poliovirus | 60/135 (44%) | 50/135 (37%) | 65/148 (44%) | 107/136 (79%), | 0.999 | <0.0001 | 0.616 | <0.0001 | <0.0001 |
| Reciprocal titres | 5.7 (5.7–72.0), 5.7–9.9 | 5.7 (5.7–45.3), 5.7–5.8 | 5.7 (5.7–136.4), 5.7–9.0 | 181.0 (9.0–910.2), 90.5–455.1 | 0.695 | <0.0001 | 0.173 | <0.0001 | <0.0001 |
Data are n/N (%) for proportion of infants with seroprevalence, and median (IQRs) and 95% CIs for reciprocal titres in these infants. mOPV1=monovalent oral polio vaccine type 1. bOPV1 and 3=bivalent OPV type 1 and 3. mOPV1–1 week=1 week interval between two doses of mOPV1. mOPV1–2 weeks=2 week interval between two doses of mOPV1. mOPV1–4 weeks=4 week interval between two doses of mOPV1. bOPV1 and 3–4 weeks=4 week interval between two doses of bOPV1 and 3.
The immunological response to tOPV given at birth, measured at 42 days, was 171/554 (31%, 95% CI 27.1–34.9) for type 1, 263/554 (48%, 43.3–51.7) for type 2, and 112/554 (20%, 17.0–23.9) for type 3 in per-protocol infants. For all three types, the proportion of immune responses to the birth dose of OPV was significantly lower in infants with a high titre of maternal antibodies (proportion of immune response in those with or without high maternal antibodies: type 1.93/554 [17%] with, 279/554 [50%] without; type 2.172/554 [31%] with, 379/554 [68%] without; type 3.63/554 [11%] with, 134/554 [24%] without).
Seroconversion achieved by two doses of mOPV1 or bOPV given at short or standard intervals was also assessed in per-protocol infants who did not seroconvert after the birth dose (figure 3). No significant differences were noted for interval–seroconversion for type-1 poliovirus between groups. For type 2, the interval–sero-conversion was significantly higher for bOPV–4 weeks than mOPV1–1 week (p=0.001) and mOPV1–2 weeks (p=0.013). For type 3, interval–seroconversion was significantly higher for bOPV–4 weeks than all other groups (p<0.0001 for all; figure 3).
Figure 3. Seroconversion after two doses of oral polio vaccine.
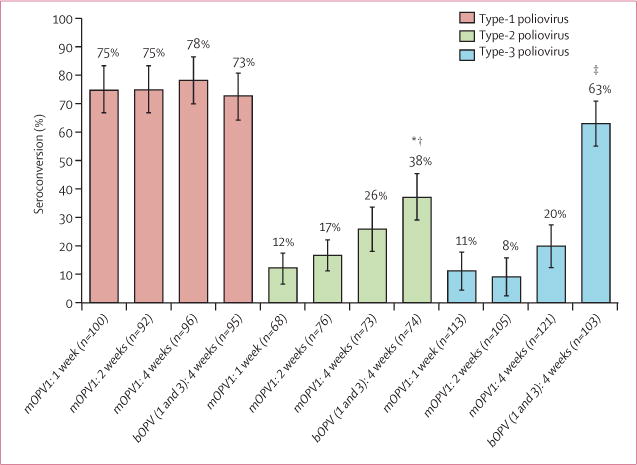
Data from per-protocol population who did not show seroconversion after being vaccinated at birth.mOPV1=monovalent oral polio vaccine type 1. bOPV(1and 3) =bivalent oral polio vaccine type 1 and 3. *p=0.001 for mOPV1–1 week vs bOPV1 and 3–4 weeks. †p=0.013 for mOPV1–2 weeks vs bOPV1 and 3–4 weeks. ‡p<0.0001 for mOPV1–1 week, mOPV1–2 weeks, and mOPV1–4 weeks vs bOPV1 and 3–4 weeks.
No significant differences were noted between median titres of neutralising antibodies in all groups and all visits except for serotype 3 for which, as expected, it was significantly higher with bOPV–4 weeks at completion of the trial (table 2). In the intention-to-treat analysis, no significant differences were noted between groups for seroconversion of type-1 poliovirus (appendix), as in the per-protocol analysis. However, the significant difference noted in the per-protocol analysis between mOPV1–1 week and bOPV–4 weeks for seroprevalence of type-1 poliovirus at completion of the study was no longer seen in the intention-to-treat analysis (appendix).
No differences for type-1 immunogenicity were noted between groups; therefore we did the multivariable analysis for all study groups combined. High con centrations of maternal antibodies were a risk factor for low seroconversion against type-1 poliovirus at study completion (table 3). Other predictors significantly associated with risk of low seroconversion for type-1 poliovirus in the multivariable analysis were receiving OPV in warmer months (between March and October) and having diarrhoeal disease soon before receiving at least one dose of OPV.
Table 3.
Factors associated with failure of seroconversion
| Seroconversion failure (N=104) | Seroconversion (N=450) | OR (95% CI); univariate analysis | Adjusted OR (95% CI); final model with multivariable analysis | |
|---|---|---|---|---|
|
Titre of maternal antibodies to poliovirus 1
| ||||
| High, ≥1/64 | 91/104 (87.5%) | 231/450 (51%) | 6.636 (3.607–12.212) | 6.243 (3.316–11.754) |
| Low, <1/64 | 13/104 (12.5%) | 219/450 (49%) | .. | .. |
|
| ||||
|
All doses of OPV*
given in warm months†
| ||||
| Yes | 58/104 (56%) | 199/450 (44%) | 1.590 (1.035–2.443) | .. |
| No | 46/104 (44%) | 251/450 (56%) | .. | .. |
|
| ||||
|
All OPV doses*
in cold months
| ||||
| Yes | 14/104 (13%) | 100/450 (22%) | 0.544 (0.297–0.997) | .. |
| No‡ | 90/104 (87%) | 350/450 (78%) | .. | .. |
|
| ||||
|
First study dose of OPV given in warm months after birth dose
| ||||
| Yes | 69/104 (66%) | 164/450 (36%) | 3.438 (2.193–5.391) | 2.856 (1.779–4.586) |
| No | 35/104 (34%) | 286/450 (64%) | .. | .. |
|
| ||||
|
Diarrhoeal illness ≤14 days before ≥1 dose of mOPV or bOPV
| ||||
| Yes | 12/104 (12%) | 27/450 (6%) | 2.043 (0.998–4.183) | 3.132 (1.395–7.028) |
| No | 92/104 (88%) | 423/450 (94%) | .. | .. |
|
| ||||
|
Sex
| ||||
| Male | 60/104 (58%) | 216/450 (48%) | 0.677 (0.440–1.041) | .. |
| Female | 44/104 (42%) | 234/450 (52%) | .. | .. |
|
| ||||
|
Household size
| ||||
| ≥8 people | 68/104 (65%) | 247/450 (55%) | 0.644 (0.413–1.005) | 1.602 (0.993–2.585) |
| <8 people | 36/104 (35%) | 203/450 (45%) | .. | .. |
Data assessed for type-1 poliovirus after three doses of oral polio vaccine (given at birth and two further study doses) in the per-protocol population (N=554). OR=odds ratio. OPV=oral polio vaccine. mOPV=monovalent oral polio vaccine. bOPV=bivalent oral polio vaccine.
Study vaccine doses excluding birth trivalent dose.
March to October.
Excluding birth trivalent OPV.
Ten infants died during the study. Seven of these deaths occurred during the trial lead-in period before randomisation (two from diarrhoea, five from unknown causes). Three infants died from sepsis after random assignment. No deaths were attributed to the study procedures by the principal investigator or the safety and monitoring board. Additionally, no vaccine-associated paralysis was noted.
Discussion
Our data suggest that the proportion of infants with seroconversion to type-1 poliovirus and immune response after two doses of mOPV1 given at an interval of 7 or 14 days is non-inferior to two doses of mOPV1 given 30 days apart (panel). These findings lend support to the rationale for the expansion of short-interval immunisation campaigns as an eradication strategy in high-risk areas with short periods of access to vaccines or where rapid outbreak responses are needed. The data further suggest that, for type-1 poliovirus, the seroconversion and seroprevalence achieved with bOPV with a 30 day interval (79% and 93%) might be slightly lower than with mOPV1 with a 30 day interval (87% and 97%) in the population of Pakistan, although neither differences were significant. This finding might have implications for immunisation programmes to preferentially use mOPV1 rather than bOPV in supplemental immunisation activities in Pakistan, especially because no cases resulting from type 3 wild poliovirus have been identified in Pakistan or Afghanistan since April, 2012.
Interval–seroconversion for type-2 poliovirus in infants who did not seroconvert after the birth dose of tOPV was high in those who received bOPV study doses (38%), although they received no further doses of type 2 vaccine. This high level of conversion might be attributable to a combination of three factors: priming with the birth dose of tOPV, environmental exposure to type 2 OPV from community recipients of tOPV with longer exposure time in the standard-interval groups, and potential cross-reactivity with the type-1 and type-3 polioviruses contained in bOPV.19,20 Further studies will be needed to quantify the effect of bOPV on potentiating a serotype-2 response. This response will be an important factor to consider in the worldwide switch from tOPV to bOPV in routine immunisation planned for April, 2016.21 Similarly, the non-significant increase in seroconversion with mOPV1–4 weeks compared with the shorter-interval groups is probably due to environ mental exposure to type-1 vaccine-derived virus rather than a higher immunogenicity with the 30 day schedule.
A high proportion of infants were protected by maternal antibodies against all three poliovirus serotypes at birth. This finding suggests high levels of protection against polioviruses in women in the study areas of Karachi. High concentrations of maternal antibodies (defined as titres higher than 1/64)16,22,23 were a risk factor for seroconversion after giving OPV; this finding is in agreement with previous studies describing the neutralising capability of maternal antibodies on OPVs.24–26
Significantly fewer children vaccinated in hotter months (March to October) had seroconversion than those vaccinated between November and February. Other reports27–29 have also described lower immunogenicity of OPVs in warmer months, which coincides with the season of high enterovirus transmission. The presence of other enteroviruses might therefore interfere with the poliovirus immune-response, and this possibility needs to be studied further. Crowding (ie, more than eight people in one household) was also associated with lower seroconversion.30 This finding is probably a surrogate for poor sanitation and hygiene, and exposure to enteroviral infections in household contacts.22 Diarrhoeal disease before vaccination was also associated with an increased risk of non-seroconversion; this finding has also been reported previously.31–34
Our investigation had several limitations. Of the randomly assigned infants, 33% did not finish the study or finished the study not per protocol; these include 29 enrolled infants who received a dose of OPV in a round of supplemental immunisation activities during the study period and were therefore excluded from the analysis. We had very tight definitions for time windows that were judged as per protocol (within 2 days of the target day); 121 infants (22%) were excluded because they were vaccinated outside this window. The most common reason for missing a visit was the child’s illness. Because of the long lead-in period, 19% of newborn babies enrolled at birth were lost to follow-up before randomisation could occur at age 42 days; in other cases, parents changed their minds about their child’s participation (115/829; 14%). Analysis of baseline data showed no significant differences between children who finished all study procedures per protocol and children who did not. Two factors that might have positively affected interval–seroconversion estimates in participants were priming35–38 in those who did not respond to the birth dose of tOPV, and exposure to vaccine-derived viruses from close contacts who received OPV during the study period from supplemental immunisation activities or routine immunisation.39–41 Generalisability of findings on short-interval dosing might be restricted to geographical areas with similar exposure to background use of OPV.
Our data show that the proportion of infants with serconversion and immunogenicity from mOPV1 given in shorter intervals (1–2 weeks) is non-inferior to vaccine doses given 30 days apart. Application of the SIAD strategy with mOPV1 can be useful to rapidly strengthen population immunity to control outbreaks, quickly reduce the circulation of virus from importations, or accelerate eradication of polioviruses. The short-interval strategy could be especially beneficial when temporary windows of opportunity for safe access can be granted in areas of conflict—eg, during cease-fire periods. In such situations, we recommend shortening the interval between doses of OPV to 7 days. Additionally, SIAD campaigns might have the highest chance of interrupting circulation of the virus during the winter months because of the higher immunogenicity noted with OPV in colder weather.
Supplementary Material
Panel: Research in context.
Systematic review
We did a literature review of available English-language, peer-reviewed data on immunogenicity of oral poliovirus vaccines (OPV) in different settings and in different age groups with the search terms “immunogenicity”, “oral poliovirus vaccines”, “children”, “adults”, and “developing countries” using the PubMed database. We also reviewed unpublished reports from the Polio Eradication Initiative in Pakistan and elsewhere about data from the programme on use of OPV in short intervals. We identified around 90 publications from our search, of which two directly examined short-interval administration of oral poliovirus vaccines.17,18 The report from the Centers for Disease Control and Prevention included use of OPV in short intervals in Afghanistan and Pakistan from Jan, 2009 to Dec, 2009, to reach previously unreached children, or to give a dose within 1–2 weeks of a previous dose during negotiated periods of security. Success in terms of the number of missed children was not reported. We concluded that a shortage of scientific data exists for the immunogenicity of OPV given in a shorter than 30 day interval. A summary of the experience with use of OPV, including their use with different intervals between doses, was published in 2001.14
Interpretation
To our knowledge, ours is the first randomised, clinical trial to generate data about the use of OPV with short-interval dosing. The results have been shared with the Polio Eradication Initiative and provided the basis for making strategic decisions to do short-interval campaigns with OPV in several countries. We are aware of a similar study assessing giving bivalent OPV in shorter intervals; the results from both studies will provide new data about the novel use of OPV to accelerate the eradication of polioviruses.
Acknowledgments
FM has received research training support from the National Institute of Health’s Fogarty International Center (1 D43 TW007585-01). The Centers for Disease Control and Prevention supported the project in-kind by provision of laboratory testing and skills in interpretation. We thank Shahida Qureshi, Aneeta Hotwani, and Kumail Ahmed (the Infectious Disease Research Laboratory, Aga Khan University) for laboratory handling and shipment of blood samples, Salah Tumsah (WHO team leader at Polio Program Sindh) for technical advice, Mazhar Khamisani (Project Director, Department of Health, Government of Sindh), officials at EPI Sindh, and all deputy commissioners, district officers and deputy town health officers at the Department of Health, Government of Sindh in Bin Qasim and Landhi townships for their constant support during the project. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention or WHO.
Footnotes
Contributors
FM and FQ contributed equally to the manuscript. FM oversaw study implementation and data collection, wrote the first draft of the manuscript, analysed and interpreted the data, and finalised the manuscript. FQ implemented the investigation at the field sites, supervised data collection, wrote the first draft of the methods, and analysed and interpreted the data. OM was involved in trial design, trial monitoring, planned the data analysis, interpreted the data, and finalised the manuscript. IA supervised the data analysis. ZB and NuR analysed the data. AK was responsible for quality control in field sites during study procedures and data collection. ED gave technical advice throughout the project and contributed to the discussion section. MS was responsible for trial monitoring and compliance with Good Clinical Practice guidelines. SMO and WCW did the neutralisation antibody assays and finalised the manuscript. RWS was the senior adviser for trial design and implementation, and data interpretation. AKMZ was the principal investigator for the study and the overall guarantor, and oversaw the study design, study implementation, data analysis, and writing of the manuscript. All authors reviewed and approved the final manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Robertson SE, Chan C, Kim-Farley R, Ward N. Worldwide status of poliomyelitis in 1986, 1987 and 1988, and plans for its global eradication by the year 2000. World Health Stat Q. 1990;43:80–90. [PubMed] [Google Scholar]
- 2.WHO. Wild poliovirus 2009–14. http://www.polioeradication.org/Portals/0/Document/Data&Monitoring/Wild_poliovirus_list_2009_2014_29JUL.pdf (accessed Sept 16, 2014)
- 3.Centers for Disease Control and Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep. 2001;50:222–24. [PubMed] [Google Scholar]
- 4.Global polio eradication initiative. Wild poliovirus (WPV) case breakdown by country. 2013 http://www.polioeradication.org/dataandmonitoring/poliothisweek.aspx (accessed Sept 16, 2014)
- 5.Boone J. Taliban leader bans polio vaccinations in protest at drone strikes. The Guardian; London: Jun 26, 2012. http://www.theguardian.com/world/2012/jun/26/taliban-bans-polio-vaccinations (accessed Aug 26, 2014) [Google Scholar]
- 6.WHO. Statement on the meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus. 2014 who.int/mediacentre/news/statements/2014/polio-20140505/en/ (accessed May 5, 2014)
- 7.Baicus A. History of polio vaccination. World J Virol. 2012;1:108–14. doi: 10.5501/wjv.v1.i4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec. 2014;89:73–92. [PubMed] [Google Scholar]
- 9.Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ. 1985;63:1151–69. [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin AB. Oral, live poliovirus vaccine for elimination of poliomyelitis. Arch Intern Med. 1960;106:5–9. doi: 10.1001/archinte.1960.03820010007003. [DOI] [PubMed] [Google Scholar]
- 11.Salisbury D. Immunisation policies and programmes in Europe. Biologicals. 1994;22:313–15. doi: 10.1006/biol.1994.1046. [DOI] [PubMed] [Google Scholar]
- 12.Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine— live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th. London: WB Saunders; 2013. pp. 598–645. [Google Scholar]
- 13.WHO. WHO vaccine-preventable diseases: monitoring system. 2014 global summary. http://apps.who.int/immunization_monitoring/globalsummary(accessed May 21, 2015)
- 14.Sutter RW, Pallansch MA, Sawyer LA, Cochi SL, Hadler SC. Defining surrogate serologic tests with respect to predicting protective vaccine efficacy: poliovirus vaccination. Ann N Y Acad Sci. 1995;754:289–99. doi: 10.1111/j.1749-6632.1995.tb44462.x. [DOI] [PubMed] [Google Scholar]
- 15.Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. J Infect Dis. 1997;175(suppl 1):S215–27. doi: 10.1093/infdis/175.supplement_1.s215. [DOI] [PubMed] [Google Scholar]
- 16.el-Sayed N, el-Gamal Y, Abbassy AA, et al. Monovalent type 1 oral poliovirus vaccine in newborns. N Engl J Med. 2008;359:1655–65. doi: 10.1056/NEJMoa0800390. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–Afghanistan and Pakistan, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:268–72. [PubMed] [Google Scholar]
- 18.Deming MS, Jaiteh KO, Otten MW, Jr, et al. Epidemic poliomyelitis in The Gambia following the control of poliomyelitis as an endemic disease. II. Clinical efficacy of trivalent oral polio vaccine. Am J Epidemiol. 1992;135:393–408. doi: 10.1093/oxfordjournals.aje.a116300. [DOI] [PubMed] [Google Scholar]
- 19.Hale JH, Doraisingham M, Kanagaratnam K, Leong KW, Monteiro ES. Large-scale use of Sabin type 2 attenuated poliovirus vaccine in Singapore during a type 1 poliomyelitis epidemic. Br Med J. 1959;1:1541–49. doi: 10.1136/bmj.1.5137.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashkenazi A, Melnick JL. Heterotypic antibody response after feeding of monovalent attenuated live poliovaccine. N Engl J Med. 1962;267:1228–30. doi: 10.1056/NEJM196212132672404. [DOI] [PubMed] [Google Scholar]
- 21.Global polio eradication initiative. Polio eradication & endgame strategic plan 2013–18. http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf (Oct 26, 2013)
- 22.Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and The Gambia. World Health Organization collaborative study group on oral poliovirus vaccine. J Infect Dis. 1995;171:1097–106. doi: 10.1093/infdis/171.5.1097. [DOI] [PubMed] [Google Scholar]
- 23.Parent du Chatelet I, Merchant AT, Fisher-Hoch S, et al. Serological response and poliovirus excretion following different combined oral and inactivated poliovirus vaccines immunization schedules. Vaccine. 2003;21:1710–18. doi: 10.1016/s0264-410x(02)00523-6. [DOI] [PubMed] [Google Scholar]
- 24.Isomura S, Mubina A, Dure-Samin A, et al. Virological and serological studies on poliomyelitis in Karachi, Pakistan. I. Outbreaks in 1990–91. Acta Paediatr Jpn. 1993;35:382–86. doi: 10.1111/j.1442-200x.1993.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 25.Linder N, Handsher R, Fruman O, et al. Effect of maternal immunization with oral poliovirus vaccine on neonatal immunity. Pediatr Infect Dis J. 1994;13:959–62. doi: 10.1097/00006454-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Yin-Murphy M, Tan KL, Lim GN, Quek JH, Ishak B, Phoon MC. Poliovirus neutralising antibody in infants and cord blood. Ann Acad Med Singapore. 1993;22:281–85. [PubMed] [Google Scholar]
- 27.Deming MS, Linkins RW, Jaiteh KO, Hull HF. The clinical efficacy of trivalent oral polio vaccine in The Gambia by season of vaccine administration. J Infect Dis. 1997;175(suppl 1):S254–57. doi: 10.1093/infdis/175.supplement_1.s254. [DOI] [PubMed] [Google Scholar]
- 28.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7:369–74. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis. 2012;205:1554–61. doi: 10.1093/infdis/jis241. [DOI] [PubMed] [Google Scholar]
- 30.Sabin AB, Ramos-Alvarez M, Alvarez-Amezquita J, et al. Live, orally given poliovirus vaccine. Effects of rapid mass immunization on population under conditions of massive enteric infection with other viruses. JAMA. 1960;173:1521–26. doi: 10.1001/jama.1960.03020320001001. [DOI] [PubMed] [Google Scholar]
- 31.Feldman RA, Holguin AH, Gelfand HM. Oral poliovirus vaccination in children: a study suggesting enterovirus interference. Pediatrics. 1964;33:526–33. [PubMed] [Google Scholar]
- 32.Maldonado YA, Pena-Cruz V, de la Luz Sanchez M, et al. Host and viral factors affecting the decreased immunogenicity of Sabin type 3 vaccine after administration of trivalent oral polio vaccine to rural Mayan children. J Infect Dis. 1997;175:545–53. doi: 10.1093/infdis/175.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myaux JA, Unicomb L, Besser RE, et al. Effect of diarrhea on the humoral response to oral polio vaccination. Pediatr Infect Dis J. 1996;15:204–09. doi: 10.1097/00006454-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Posey DL, Linkins RW, Oliveria MJ, Monteiro D, Patriarca PA. The effect of diarrhea on oral poliovirus vaccine failure in Brazil. J Infect Dis. 1997;175(suppl 1):S258–63. doi: 10.1093/infdis/175.supplement_1.s258. [DOI] [PubMed] [Google Scholar]
- 35.Resik S, Tejeda A, Sutter RW, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med. 2013;368:416–24. doi: 10.1056/NEJMoa1202541. [DOI] [PubMed] [Google Scholar]
- 36.Diez-Domingo J, Cantarino MV, Torrenti JM, et al. A randomized, multicenter, open-label clinical trial to assess the immunogenicity of a meningococcal C vaccine booster dose administered to children aged 14 to 18 months. Pediatr Infect Dis J. 2010;29:148–52. doi: 10.1097/INF.0b013e3181b9a831. [DOI] [PubMed] [Google Scholar]
- 37.Mallet E, Belohradsky BH, Lagos R, et al. A liquid hexavalent combined vaccine against diphtheria, tetanus, pertussis, poliomyelitis, Haemophilus influenzae type B and hepatitis B: review of immunogenicity and safety. Vaccine. 2004;22:1343–57. doi: 10.1016/j.vaccine.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol. 1999;162:5011–18. [PubMed] [Google Scholar]
- 39.Contreras G, Dimock K, Furesz J, et al. Genetic characterization of Sabin types 1 and 3 poliovaccine virus following serial passage in the human intestinal tract. Biologicals. 1992;20:15–26. doi: 10.1016/s1045-1056(05)80003-x. [DOI] [PubMed] [Google Scholar]
- 40.John TJ. Anomalous observations on IPV and OPV vaccination. Dev Biol (Basel) 2001;105:197–208. [PubMed] [Google Scholar]
- 41.Pavlov DN, Van Zyl WB, Van Heerden J, Grabow WO, Ehlers MM. Prevalence of vaccine-derived polioviruses in sewage and river water in South Africa. Water Res. 2005;39:3309–19. doi: 10.1016/j.watres.2005.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


