Abstract
Background:
Chemokine receptors have been shown to play an important role in the development and metastatic spread of various malignancies. In this study, the gene expression profile of some key chemokine receptors involved in metastasis has been investigated in esophageal and breast cancer cell lines.
Methods:
In a descriptive study, gene expression profile of CCR1, CCR6, CCR7, CCR9, CXCR1, and CXCR4 in human esophageal cancer cell line (KYSE-30) and human breast cancer cell line (MCF7) were analyzed using real-time PCR and their results were compared accordingly.
Results:
We demonstrated for the first time the expression of CCR1, CCR6, CCR7, CCR9, CXCR1, and CXCR4 at transcriptional level in human esophageal cancer cell line. The expression of CCR1, CCR7 and CXCR4 were lower in esophageal compared with breast cancer cells, although without significant difference. CCR9 was highly expressed in esophageal cancer cells as compared to the breast cancer cells (P < 0.05). Similarly, the expression of CCR6 and CXCR1 were higher, although without significant difference.
Conclusion:
Esophageal cancer cells like breast cancer express some key chemokine receptors involved in metastasis. Targeting of proposed receptors in esophageal cancer may be a novel strategy for prevention of cancer metastasis.
Keywords: Esophageal cancer, Breast cancer, Metastasis, Chemokine receptors, Targeted cancer therapy
Introduction
Cancer is one the most common causes of morbidity and mortality in the world (1). Up regulation of oncogenes involved in bypassing cell cycle checkpoints leads to the uncontrolled cell division, transformation of cells towards cancer cells and finally tumor formation (2). Despite intensive research to apply novel therapeutic approaches, survival of rare cases with advanced cancers has been reported (1). One important factor that is involved in the progression of cancers is metastasis (3). Metastasis is the process in which cancer cells spread from the site of the original tumor to different parts of the body (4). More than 90% of cancers sufferings and deaths are associated with metastasis; making it most significant challenge in the management of disease. Application of proposed metastatic inhibitory agents against cancer metastasis is one of the significant therapeutic strategies. Studies have revealed that chemokines and chemokine receptors are key elements in the process of cancer metastasis (5, 6). Chemokines and their receptors are divided into four groups according to the position of cysteine residues: CC, CXC, CX3C and XC, where C represents the cysteine and X represents non-cysteine amino acids. Approximately 20 chemokine receptors and 50 chemokines have been characterized to date in humans. CC receptors (CCR1- 11) bind CC chemokines, CXC receptors (CXCR1- 7) bind CXC chemokines, CX3C (CX3CR1) receptor binds CX3C chemokine and the XC (XCR1) receptor binds the C chemokine (7, 8). Following activation of chemokine receptors, a wide range of intracellular signaling cascades initiate and typically lead to cell polarization, and directed cell motility (9).
Metastatic cancer cells bearing chemokine receptors migrate toward chemokine gradient. In addition to affecting metastasis, chemokines and chemokine receptors also seem to regulate tumor cell proliferation, angiogenesis, cell senescence and cell survival. Thus, chemokines and chemokine receptors have been considered valuable targets for cancer therapies (10). To achieve this goal a better understanding of chemokine receptors expressed by metastatic cancer cells, is urgently needed. In this study, we examined the expression levels of some important chemokine receptors in esophageal cancer cells compared with breast cancer. This pattern is well studied in breast cancer cells however; to date no inclusive analysis on expression of chemokine receptors in esophageal cancer has been reported. Esophageal cancer is one of the least studied and deadliest cancers worldwide.
Considering high incidence and low survival rate of esophageal cancer (11, 12) and lack of convinced information about chemokine receptors expression in these cells, we have evaluated the gene expression pattern of some chemokine receptors proved to have key role in cancer metastasis.
Materials and Methods
Cell lines
Esophageal cancer cell line (Kyse-30) was cultured in RPMI+Ham’s F12 media supplemented with 10% fetal bovine serum and 0.01% penicillin/streptomycine in 5% CO 2 environment at 37°C whereas DMEM-LG, supplemented with 10% FBS and 0.01% penicillin/streptomycine was used as culture medium for breast cancer cell line (MCF7).
RNA extraction and real-time polymerase chain reaction (real-time PCR)
Total RNAs was isolated from cells using TriPure isolation reagent (Roche, Germany) according to the manufacturer’s instructions. Extracted RNA was treated with DNase I enzyme. One microgram of each RNA was used for cDNA synthesis by reverse transcriptase. The RNA sample was incubated at 65°C for 5 min with 1 μl Oligo dT (0.5 μg/μl) in a final volume of 12 μl, and then chilled on the ice, mixed with 4 μl 5X buffer, 2 μl dNTPs (10 mM), 0.5 μl Ribolock, 1 μl M-MLVRTase. After incubation at 42°C for 60 min, and at 70°C for 10 min, the mixture was reversely transcribed into cDNA. 2 ul cDNA of each sample were used for real-time PCR in a 20ul reaction mixture with 10ul of 2× SYBR Green PCR Mastermix (Parstous, Iran) and 1 μl of specific primer pair. The housekeeping gene β-actin was used to normalize target gene expression. The primer sequences used in this study are presented in Table 1. Each plate was run at 95°C for 10 min, then 40 cycles of 95°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec. All real-time RT-PCRs were performed in duplicate, and Relative mRNA of each target gene was determined by using the formula 2−ΔCT (CT, cycle threshold) where ΔCT=CT(target gene)−CT(β-actin). The comparative expression level of each target gene between different samples was 2−ΔΔCT. In addition, melting curves were used to evaluate non-specific amplification.
Table 1.
Description of the designed primers
| Gene | Primer sequence | Product size | |
|---|---|---|---|
| B-actin | Sense | 5′-GCTCAGGAGGAGCAAT-3′ | 187 |
| Antisense | 5′-GGCATCCACGAAACTAC-3′ | ||
| CCR1 | Sense | 5′-CACGGACAAAGTCCCTTGG-3′ | 134 |
| Antisense | 5′-CAAAGGCCCTCTCGTTCAC-3′ | ||
| CCR6 | Sense | 5′-GCTAGTGTTTCCTCTCATTTCC-3′ | 132 |
| Antisense | 5′-ACCTGTTCTGCCATTGTCC-3′ | ||
| CCR7 | Sense | 5′-AACCAATGAAAAGCGTGCTG-3′ | 120 |
| Antisense | 5′-CGAACAAAGTGTAGTCCACTG-3′ | ||
| CCR9 | Sense | 5′-TGATTGGCTCTTGACTGTGATG-3′ | 108 |
| Antisense | 5′-GAGGCAGCGGCATTGATG-3′ | ||
| CXCR1 | Sense | 5′-CATCAGTGTGGACCGTTACC-3′ | 120 |
| Antisense | 5′-GGCAGGGACAGATTCATAGAC-3′ | ||
| CXCR4 | Sense | 5′-ATCCCTGCCCTCCTGCTGACTATTC-3′ | 232 |
| Antisense | 5′-GAGGGCCTTGCGCTTCTGGTG-3′ |
Statistical analysis
In this descriptive study, the statistical differences were determined by student two-tailed test, and P < 0.05 was considered statistically significant. Results are presented as means ± SEM. Graph pad prism statistical software version 5.0 was used for all analysis.
Results
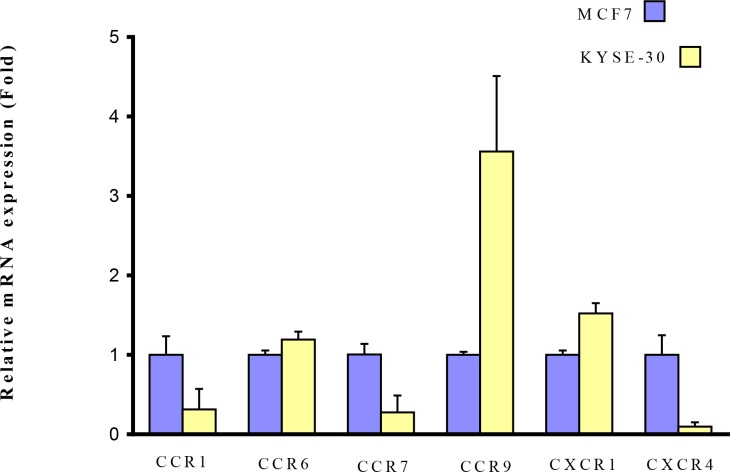
We performed real-time PCR to compare the expression profile of some regulator genes in metastasis between esophageal and breast cancer cells as control. These genes included CCR1, CCR6, CCR7, CCR9, CXCR1, and CXCR4. The housekeeping gene of β-actin was used as an internal control in the experiment. The expression pattern revealed that CCR1, CCR6, CCR7, CXCR1, and CXCR4 were expressed in both esophageal and breast cancer cells with no significant difference (P>0.05) (Fig. 1). Comparing to breast cancer cells, the expression level of CCR1, CCR7, and CXCR4 were lower. However, the CCR6 and CXCR1 in esophageal cells showed slightly higher expression level. As shown in Figure 1, CCR9 mRNA showed a significant ∼3.5-fold upregulation as compared to the breast cancer cells (P< 0.05) (Fig. 1). It is an interesting observation that the expression level of chemokine receptors in esophageal cancer cells is similar to a highly metastatic cancer type, breast cancer.
Fig.1:
Comparative relative mRNA expression profile of chemokine receptors in MCF7 (breast cancer cell line) and KYSE-30 (esophageal cancer cell line). All results are shown as mean ± standard deviation (SD), with significance indicated by *P < 0.05 vs. respective control group
Discussion
Metastatic cancer can be characterized by the metabolic disordering at genetic or protein level involving multiple and complex pathways i.e. ERK, Rac signaling, etc. (13). A significant association exists between the chemokine-chemokine receptor system and the metastasis of cancer cells (14). Considering the important potential role of chemokine receptors in this process, their expression in two common forms of metastatic cancers has been evaluated in this work. The present study for the first time has been demonstrated that esophageal cancer cells like breast cancer express some key chemokine receptors involved in metastasis.
It was demonstrated that increased expression of CCR1 significantly promotes invasion of prostate cancer cells by increasing secretion of MMPs 2 and 9 and by activating ERK and Rac signaling (13). Expression of CCR1 has been observed higher in liver metastases than the primary colorectal cancer as its expression, CCR1 has also been reported in breast cancer tissues (15, 16). In this study, expression of CCR1 was confirmed in breast cancer stem cells and additionally its expression was reported for the first time in esophageal cancer stem cells. Additionally our results indicated that expression of CCR1 was higher in breast cancer cells compared to esophageal cell line, although without significant difference.
The CCR6 plays a significant role in colorectal cancer metastasis. Its upregulated expression predicts poor survival in colorectal cancer patients and selective targeting of CCR6 has been applied to inhibit the growth of colorectal cancer in mice (17). Chemokines CCL19, CCL20 and CCL21 and their receptors CCR6 and CCR7, have been investigated as potential biomarkers of metastatic propagation in primary breast cancer (18). In our study, expression of CCR6 and CCR7 were examined in esophageal cancer cells compared with breast cancer. Quantitative RT-PCR revealed that CCR7 was expressed in both breast and esophageal cancer cells with significant difference although it was lower in esophageal cancer cells. Chemokine receptors have also been investigated as key receptors in the development of organ-specific metastases pattern. Involvement of CCR6 in the development of pleura metastases and CCR7 in the development of skin metastases has been reported recently in patients with positive primary breast cancer (19). In this study the expression of CCR6 and CCR7 were reported for the first time, however, their potential role in esophageal cancer invasion and also their potential metastatic site need to be investigated.
A comparative analysis of CCR9 expression in aggressive breast cancer cell line (MDA-MD-231) and less aggressive MCF-7 cell line has been performed. Functional interactions between CCR9 and its only known ligand, CCL25 in breast cancer cell lines (MDA-MB-231 and MCF-7) have been assessed by migration assay. Neutralizing CCR9-CCL25 interactions resulted in impaired cellular migration and invasion of breast cancer cells (20). It is for the first time that expression of CCR9 has been reported in esophageal cancer cells, which is significantly higher than breast cancer cells.
Among the genes overexpressed in the breast cancer stem cell (CSCs) population, CXCR1, a receptor that binds the proinflammatory chemokines of IL-8/CXCL8 and CXCL6, appeared to have an important role in growth and metastasis of breast CSCs (21–23). CXCR1 blockade induced apoptosis, retardation and reduction in systemic metastasis of breast cancer and tumor growth in NOD/SCID mice (24). Inhibition of metastases in human colon cancer was observed by small molecule antagonists for CXCR1 (25). In our study, expression of CXCR1 was detected in esophageal cancer cell line like breast cancer without significant difference.
The chemokine receptor of CXCR4 and its sole ligand CXCL12 plays important role in the malignancy of cancer (26). Actin polymerization in breast cancer triggered when CXCR4-CXCL12 receptor-ligand start interactions allowing tumor cells to invade surrounded tissues (27). Anti-CXCR4 or CXCL12 antibodies inhibit the CXCL12-CXCR4 interactions and impairment of these migratory responses has been observed as 63–76% and 60–62%, respectively (28).
Taking all together it seems that the expression of chemokine receptors is similar in breast cancer cells compared to esophageal ones. It may explain high invasive character of esophageal cancer cells. To our best of knowledge, this study is reporting key chemokine receptors in esophageal cancer cells compared with breast cancer for the first time. Understanding the distinct molecular and cellular characters of cancer cells may therefore lead to new therapeutic opportunities, and improve the quality of life and overall survival of patients.
Conclusion
To our knowledge, this is the first report on gene expression profile of chemokine receptors in esophageal cancer cells. Neutralization of chemokine receptors in vivo by antibodies, inhibitory peptide, or siRNA may attenuate the progress of cancer.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
Thanks to Piraste Norouzi and Mojgan Fazli for providing technical support and Muhammad Irfan-Maqsood for editing the manuscript. This work was supported in part by Grant 9247 from Shahroud University and by the ACECR grant, 710. The authors declare that there is no conflict of interest.
References
- 1. Bray F, Jemal A, Grey N, Ferlay J, Forman D. (2012). Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol, 13( 8): 790–801. [DOI] [PubMed] [Google Scholar]
- 2. Maqsood MI, Matin MM, Bahrami AR, Ghasroldasht MM. (2013). Immortality of cell lines: challenges and advantages of establishment. Cell Biol Int, 37( 10): 1038–45. [DOI] [PubMed] [Google Scholar]
- 3. Chambers AF, Groom AC, MacDonald IC. (2002). Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer, 2( 8): 563–72. [DOI] [PubMed] [Google Scholar]
- 4. Yilmaz M, Christofori G. (2010). Mechanisms of motility in metastasizing cells. Mol Cancer Res, 8( 5): 629–42. [DOI] [PubMed] [Google Scholar]
- 5. Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. (2014). CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World J Gastroenterol, 20( 7): 1681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soffietti R, Trevisan E, Ruda R. (2012). Targeted therapy in brain metastasis. Curr Opin Oncol, 24( 6): 679–86. [DOI] [PubMed] [Google Scholar]
- 7. Vinader V, Afarinkia K. (2012). The emerging role of CXC chemokines and their receptors in cancer. Future Med Chem, 4( 7): 853–67. [DOI] [PubMed] [Google Scholar]
- 8. Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. (2013). Chemokines in tumor progression and metastasis. Oncotarget, 4( 12): 2171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thelen M. (2001). Dancing to the tune of chemokines. Nat Immunol, 2( 2): 129–34. [DOI] [PubMed] [Google Scholar]
- 10. Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. (2010). The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev, 21( 1): 27–39. [DOI] [PubMed] [Google Scholar]
- 11. Kamangar F, Dores GM, Anderson WF. (2006). Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol, 24( 14): 2137–50. [DOI] [PubMed] [Google Scholar]
- 12. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. (2011). Global cancer statistics. CA Cancer J Clin, 61( 2): 69–90. [DOI] [PubMed] [Google Scholar]
- 13. Kato T, Fujita Y, Nakane K, Mizutani K, Terazawa R, Ehara H, Kanimoto Y, Kojima T, Nozawa Y, Deguchi T, Ito M. (2013). CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine, 64( 1): 251–7. [DOI] [PubMed] [Google Scholar]
- 14. Swamydas M, Ricci K, Rego SL, Dreau D. (2013). Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adh Migr, 7( 3): 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho YB, Lee WY, Choi SJ, Kim J, Hong HK, Kim SH, Choi YL, Kim HC, Yun SH, Chun HK, Lee KU. (2012). CC chemokine ligand 7 expression in liver metastasis of colorectal cancer. Oncol Rep, 28( 2): 689–94. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Yao F, Yao X, Yi C, Tan C, Wei L, Sun S. (2009). Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24-phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncol Rep, 21( 4): 1113–21. [PubMed] [Google Scholar]
- 17. Liu J, Ke F, Xu Z, Liu Z, Zhang L, Yan S, Wang Z, Wang H, Wang H. (2014). CCR6 Is a Prognostic Marker for Overall Survival in Patients with Colorectal Cancer, and Its Overexpression Enhances Metastasis In Vivo. PLoS One, 9( 6): e101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassier PA, Treilleux I, Bachelot T, Ray-Coquard I, Bendriss-Vermare N, Ménétrier-Caux C, Trédan O, Goddard-Léon S, Pin JJ, Mignotte H, Bathélémy-Dubois C, Caux C, Lebecque S, Blay JY. (2011). Prognostic value of the expression of C-Chemokine Receptor 6 and 7 and their ligands in non-metastatic breast cancer. BMC Cancer, 11: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, Soria JC, Cristofanilli M. (2006). Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol, 17( 6): 945–51. [DOI] [PubMed] [Google Scholar]
- 20. Johnson-Holiday C, Singh R, Johnson E, Singh S, Stockard CR, Grizzle WE, Lillard JW., Jr (2011). CCL25 mediates migration, invasion and matrix metalloproteinase expression by breast cancer cells in a CCR9-dependent fashion. Int J Oncol, 38( 5): 1279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. (2009). Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res, 15; 69( 4): 1302– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu W, Wang J, Luo G, Luo B, Wu C, Wang W, Xiao Y, Li J. (2013). Proteomics-based analysis of differentially expressed proteins in the CXCR1-knockdown gastric carcinoma MKN45 cell line and its parental cell. Acta Biochim Biophys Sin (Shanghai). 45( 10): 857–66. [DOI] [PubMed] [Google Scholar]
- 23. Jiang QF, Wu TT, Yang JY, Dong CR, Wang N, Liu XH, Liu ZM. (2013). 17beta-estradiol promotes the invasion and migration of nuclear estrogen receptor-negative breast cancer cells through cross-talk between GPER1 and CXCR1. J Steroid Biochem Mol Biol, 138: 314–24. [DOI] [PubMed] [Google Scholar]
- 24. Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. (2010). CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest, 120( 2): 485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. (2011). Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett, 300( 2): 180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. (2004). Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J, 18( 11): 1240–2. [DOI] [PubMed] [Google Scholar]
- 27. Verschueren H, Van der Taelen I, Dewit J, De Braekeleer J, De Baetselier P. (1994). Metastatic competence of BW5147 T-lymphoma cell lines is correlated with in vitro invasiveness, motility and F-actin content. J Leukoc Biol, 55( 4): 552–6. [DOI] [PubMed] [Google Scholar]
- 28. Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature, 410( 6824): 50–6. [DOI] [PubMed] [Google Scholar]