Abstract
Migratory birds have the potential to transport exotic vectors and pathogens of human and animal health importance across vast distances. We systematically examined birds that recently migrated to the United States from the Neotropics for ticks. We screened both ticks and birds for tick-borne pathogens, including Rickettsia species and Borrelia burgdorferi. Over two spring seasons (2013 and 2014), 3.56% of birds (n = 3,844) representing 42.35% of the species examined (n = 85) were infested by ticks. Ground-foraging birds with reduced fuel stores were most commonly infested. Eight tick species were identified, including seven in the genus Amblyomma, of which only Amblyomma maculatum/Amblyomma triste is known to be established in the United States. Most ticks on birds (67%) were neotropical species with ranges in Central and South America. Additionally, a single Ixodes genus tick was detected. A total of 29% of the ticks (n = 137) and no avian blood samples (n = 100) were positive for infection with Rickettsia species, including Rickettsia parkeri, an emerging cause of spotted fever in humans in the southern United States, a species in the group of Rickettsia monacensis, and uncharacterized species and endosymbionts of unknown pathogenicity. No avian tick or blood samples tested positive for B. burgdorferi, the etiologic agent of Lyme disease. An extrapolation of our findings suggests that anywhere from 4 to 39 million exotic neotropical ticks are transported to the United States annually on migratory songbirds, with uncertain consequences for human and animal health if the current barriers to their establishment and spread are overcome.
INTRODUCTION
The large-scale seasonal movements of migrants provide opportunities for bird-associated parasites to rapidly disperse over large spatial scales, with implications for human and animal health (1, 2). Birds are increasingly recognized for their roles as reservoirs and hosts to vectors for a suite of emerging zoonotic diseases, including West Nile virus, Lyme disease, influenza A virus, and H5N1 avian influenza virus (3, 4), and they have been implicated in the range expansion and introduction of new pathogens. Additionally, migratory birds have the potential to disperse ectoparasites and their associated pathogens over long distances (5–7).
Migratory birds that overwinter in Central and South America are frequently infested with Amblyomma tick species (family Ixodidae), which are common carriers of Rickettsia species parasites (8–11). There are 45 Amblyomma species endemic to the Neotropics (12), where parasitism rates on birds have been found to vary from 6.5% in Panama (10) to 40% in the Brazilian Amazon (9). Rickettsia species of bacteria are transmitted to vertebrates by arthropod vectors, including Amblyomma ticks. The pathogenicity of many tick-borne Rickettsia species is unknown, but there are >25 recognized species in the zoonotic spotted fever group, including those that cause Rocky Mountain spotted fever, Mediterranean spotted fever, North Asian tick typhus, and Queensland tick typhus (13). Wild birds have been implicated as hosts of Amblyomma ticks and Rickettsia pathogens in South and Central America (8–11); however, the significance of wild birds in the epidemiology of these vectors and pathogens remains poorly understood (13).
The hard-tick species that attach to birds are characterized by a three-host life cycle, in which each active life stage (larva, nymph, and adult) will attach to a vertebrate host and feed for a few days to a week, after which they drop off from the host, molt to the next life stage, diapause, and repeat the cycle (14). In the adult stage, the females will mate and feed, drop off the host, and die, whereas males may not require a blood meal. While some tick species are generalists that can readily infest diverse avian or mammalian hosts at any life stage, other tick species have more rigid host preferences. During the off-host time period, in which the tick spends a majority of its life, the locomotion of ticks is typically limited to only meters, and accordingly, the movement and range expansion of ticks are largely attributed to the movement of the vertebrate hosts during the periods of tick attachment (15). Migratory birds can move hundreds of kilometers, including across the Gulf of Mexico and into the United States, within a short period of time; for example, the 12-g blackpoll warbler (Setophaga striata) can fly up to 2,770 km in 3 days (16). If such transcontinental movements coincide with attachment and feeding by ticks, avian migration can facilitate the rapid movement of ticks. In the only previous study to systematically examine northbound spring migrants for ticks immediately upon entry into the United States, Mukherjee et al. (7) found that 3% of songbirds hosted a tick, some of which were infected with spotted fever group Rickettsia species. Further, Central and South American Amblyomma species have been detected on northward-migrating birds as far north as Chicago, IL, and Canada (5, 6, 17, 18).
Despite the documented introductions of neotropical ticks on migratory birds, there is no evidence that these Central and South American tick species are established in the United States, presumably due to biotic or abiotic barriers that prevent their establishment. However, as global climate and the distributions of other vertebrate hosts change, the environment in the United States may become more suitable for tropical tick species, which might change the risk of tick-borne disease. A recent longitudinal study of European species found that climate change has influenced the distribution and abundance of parasites associated with many bird species, including ticks (19). In North America, the warming climate has also influenced tick species range and phenology (20). For example, Ixodes scapularis, a vector of Lyme disease, is thought to be expanding significantly northward in Canada, and the threshold number of immigrating ticks needed to establish new populations is expected to fall during the coming decades (20, 21).
Understanding the characteristics that lead to bird infestation helps predict future invasion scenarios. Ticks typically quest on low vegetation and contact potential hosts using either an ambush or hunting strategy (14). Accordingly, we expected higher infestation on ground-foraging than on canopy-foraging birds. Additionally, migrants with reduced fuel stores use more diverse foraging maneuvers, substrates, and heights than birds with greater fuel stores (22, 23). Therefore, we expected higher infestation in birds when fuel stores were reduced.
Our objectives were to (i) characterize tick-bird-pathogen associations during spring migration at a high-density stopover site on the northern coast of the Gulf of Mexico, (ii) test the hypothesis that tick infestation would be higher for intercontinental migrants that forage closer to the ground and have a reduced energy state, and (iii) estimate the number of neotropical ticks entering the United States annually on migratory birds.
MATERIALS AND METHODS
Bird capture and sampling.
We investigated the presence of ticks on northward migrants at a high-density stopover site on the northern coast of the Gulf of Mexico, The Nature Conservancy's Clive Runnells Family Mad Island Marsh Preserve (Mad Island) in Matagorda County, TX (Fig. 1). Mad Island is intermediate between the breeding and wintering grounds of many species and within the peak spring passage region for eastern songbird migrants in North America. The coastal woodlands at Mad Island provide some of the first resting and refueling habitats for northward migrants after hundreds of miles of nonstop flight across the Gulf of Mexico, and many species that occupy different ranges during breeding or wintering congregate there during spring. Therefore, Mad Island is well situated to capture an abundance of species from broad geographic regions. During spring migration in 2013 and 2014, we captured migrants throughout the period of peak passage (24). Birds were captured with mist nets (12 by 2.6 m or 6 by 2.6 m, 30-mm mesh) placed in a wooded habitat. The nets were opened daily between 8:00 and 17:00 CST, except in the case of rain, high winds, or extreme heat. We opened 31 individual nets, but daily netting effort varied with weather. Upon capture, each bird was banded with a unique U.S. Geological Survey (USGS) leg band, weighed to the nearest 0.1 g with an electronic scale, and assessed for subcutaneous fat (25).
FIG 1.
Birds were captured during northward spring migration on the northern coast of the Gulf of Mexico at The Nature Conservancy's Clive Runnells Family Mad Island Marsh Preserve in Matagorda County, TX (black circle). (Base map copyright ESRI.)
Birds were scanned for the presence of ticks by systematically searching the ear canals, back of the head, mandibular area, perimeter of the eyes, and cloacae (26). A straw was used to blow a stream of air to displace feathers, or feathers were parted with fine-tipped forceps. Ticks were removed with fine-tipped forceps and placed into a dry microcentrifuge tube or in 70% ethanol for later identification. Previously sampled birds were reexamined for ticks when they were recaptured on subsequent days or >3 h later on the same day. All captured birds were searched for ticks, except for rare occasions with extremely high capture rates of birds.
During 2014, we collected blood samples from all tick-infested birds and a subset of birds without ticks. We used brachial venipuncture with a 28-gauge needle and capillary tubes or jugular venipuncture with an insulin syringe to collect 50 μl of blood (for birds that weighed 12 to 19.9 g) and up to 100 μl of blood (for birds weighing ≥20 g). Blood was expelled into microcentrifuge tubes and stored at −20°C until processing. A variety of bird species that had no detectable tick infestation were sampled opportunistically.
Tick identification.
Ticks were identified by life stage and to the genus level, according to morphological keys, under a stereomicroscope. Ticks were assigned a relative engorgement score using a scale of 1 to 4, in which a score of 1 indicates a nearly flat tick removed from a host, and a score of 4 indicates a nearly replete tick. We assumed that tick engorgement and duration of feeding are positively associated based on experimental feeding trials (27), although the absolute duration of attachment was not able to be determined based on the engorgement score. Individual ticks were subjected to DNA extraction using the E.Z.N.A. tissue DNA kit (Omega Bio-Tek, Norcross, GA), with the exception of a group of >20 larvae that were removed from a single bird, which were divided into three pools of 7 to 9 larvae each before extraction. After the addition of lysis buffer, each tick was quartered with a sterile scalpel blade to open the exoskeleton to facilitate lysis. The ticks were incubated at 55°C overnight before the extraction was completed according to the manufacturer's instructions.
Ticks were identified to the species level using a PCR-DNA sequencing approach. For all tick samples, PCR to amplify the 12S mitochondrial ribosomal DNA (rDNA) was carried out using the T1B and T2A primers, resulting in a 360-bp product (28). For confirmatory purposes on a subset of samples, an additional PCR to amplify the internal transcribed spacer 2 (ITS2) region was carried out using the ITS2-7923-F and ITS2-7923-R primers, resulting in a 1.2-kb product (29). The reactions were performed in 15-μl volumes using 1.5 μl of extracted tick DNA as the template with 0.5 μM each primer and FailSafe PreMix E buffer and enzyme (Epicentre Technologies Corp., Chicago, IL). The PCR products were visualized on 1.5% agarose gels. The positive samples were purified with ExoSAP-IT (Affymetrix, Santa Clara, CA), and Sanger DNA sequencing was performed (Eton Biosciences, San Diego, CA). To facilitate identifications and examine species relationships, sequences were aligned and compared to a national database (NCBI Blast), and neighbor-joining phylogenetic trees were created using MEGA 6.0 (30).
Pathogen identification.
We screened ticks and blood samples taken from birds for Rickettsia species and Borrelia burgdorferi. The full volume of blood from each bird was subjected to DNA extraction, as described above, but with a 30-min lysis step. Rickettsia species were detected by amplification of a partial region of the gltA gene using the primers RrCS 372 and RrCS 989, resulting in a 617-bp product (31). Confirmatory testing on a subset of samples that tested positive on the initial assay was performed through the amplification of a 632-bp region in the ompA gene (32). Reactions were run, and DNA was sequenced using the same reagents, template volumes, and primer concentrations as indicated above. B. burgdorferi was detected using a quantitative real-time PCR to amplify the 16S rRNA gene using primers and a single TaqMan probe specific to B. burgdorferi, according to methods modified from those of Tsao et al. (33). Internal validations in our laboratory yielded a quantitation cycle (Cq) threshold of ≤33 as indicative of positive samples.
Characteristics of infested birds.
We tested the expectation that bird infestation with ticks was influenced by life history characteristics, including foraging guild, wintering range, and body condition. We categorized species based on where they forage (ground, understory, or subcanopy/canopy) (34, 35), where they overwinter (“intercontinental migrants” winter south of the Gulf of Mexico in Central and South America, and “local birds” winter at the study site on the northern coast of the Gulf of Mexico), and subcutaneous fat stores as an index of body condition (scale from 0 to 5, in which 0 is characterized by no visible fat, and 5 is characterized by furcular and abdominal fat deposits that are conspicuously mounded and convex [25]). We further categorized intercontinental migratory species by the extent of their stationary nonbreeding rages: Central America and the Caribbean (Central); Central America, South America, and the Caribbean (Central/South); and South America (South). We tested our expectations with zero-inflated negative binomial models for count data (hurdle function in library pscl for R [36]). We used a two-part model to account for the high number of zeroes (37). Two-part models first model the presence of ticks using a generalized linear model with a logit link and binomial error and then model the abundance of ticks, where they occurred, with a second generalized linear model with a negative binomial distribution and a log link (37). We included foraging guild, wintering range, and body condition as predictive variables.
Propagule pressure of neotropical ticks entering the United States.
Propagule pressure is a measure of the frequency and abundance of species introductions, an important determinant of the probability of a nonnative species becoming established (38). We estimated the propagule pressure of neotropical ticks entering the United States on migratory birds, using our results for species-specific tick infestation frequency combined with published estimates of bird species abundance for North America (39). We calculated the frequency of infestation for each bird species exclusively by exotic neotropical tick species (i.e., not including infestations with species known to be established in the United States) for migratory bird species that were frequently examined for ticks (>20 individuals sampled). Additionally, we estimated a range for annual neotropical tick propagule pressure using the minimum and maximum values from species-specific infestation frequency across infested bird species. Although our data come from only a single field site, we assume migrants captured at this site are representative of neotropical migrants entering the United States, because (i) migratory birds that stop over along the northern coast of the Gulf of Mexico during spring breed across North American latitudes (40, 41), and (ii) the only other study to systematically examine northbound spring migrants arriving in the United States found a remarkably similar exotic tick infestation prevalence (7).
Nucleotide sequence accession numbers.
The tick and Rickettsia sequences obtained from the DNA extracted from tick samples were assigned GenBank accession numbers KT386301 to KT386322.
RESULTS
Tick-bird associations.
During the spring of 2013 and 2014, we screened 3,844 captures of 85 bird species for ticks and found that 137 captures (3.56%) from 36 bird species were infested with ticks (Table 1). Tick infestation prevalence did not differ between years (3.82% of 1,729 captures from 26 species in 2013; 3.36% of 2,115 captures from 25 species in 2014; z = 0.766; P = 0.44). The infested birds included 26 intercontinental migrant species, 9 local wintering species, and 1 vagrant species that does not normally occur in Texas (yellow-green vireo [Vireo flavoviridis]). Infested intercontinental migrants overwintered in Central America and the Caribbean (51 individuals from 12 species), Central or South America (21 individuals from 8 species), and South America (16 individuals from 6 species). Most infested birds carried one detected tick (n = 114), 22 birds carried two to five individual ticks, and one bird, an Acadian flycatcher (Empidonax virescens), carried 27 ticks (Fig. 2).
TABLE 1.
Bird species captured on the northern coast of the Gulf of Mexico during spring migration (2013 and 2014), which were screened for ticksa
| Bird species | Family | Foragingb | Rangec | No. sampled for ticksd | No. of ticks of indicated species isolated |
Total no. (%) infested | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. auricularium | A. coelebs | A. geayi | A. longirostre | A. maculatum/A. triste | A. nodosum | A. ovale | Amblyomma spp. | Ixodes spp. | Unknown tick species | ||||||
| Eastern wood-pewee (Contopus virens) | Tyrannidae | C | M, S | 49 | 1 | 1 | 2 (4.1) | ||||||||
| Acadian flycatcher (Empidonax virescens) | Tyrannidae | C | M, C/S | 16 | 1 | 1 (6.3) | |||||||||
| Yellow-green vireo (Vireo flavoviridis) | Vireonidae | C | M, V | 1 | 1 | 1 (100) | |||||||||
| White-eyed vireo (Vireo griseus) | Vireonidae | C | L | 134 | 1 | 1 | 2 (1.5) | ||||||||
| Philadelphia vireo (Vireo philadelphicus) | Vireonidae | C | M, C | 32 | 1 | 1 (3.1) | |||||||||
| Red-eyed vireo (Vireo olivaceus) | Vireonidae | C | M, S | 148 | 1 | 6 | 1 | 8 (5.4) | |||||||
| House wren (Troglodytes aedon) | Troglodytidae | U | L | 14 | 1 | 1 (7.1) | |||||||||
| Veery (Catharus fuscescens) | Turdidae | G | M, S | 42 | 1 | 1 | 2 (4.8) | ||||||||
| Gray-cheeked thrush (Catharus minimus) | Turdidae | G | M, S | 39 | 1 | 1 | 1 | 3 (7.7) | |||||||
| Swainson's thrush (Catharus ustulatus) | Turdidae | G | M, C/S | 158 | 1 | 1 | 7 | 1 | 10 (6.3) | ||||||
| Wood thrush (Hylocichla mustelina) | Turdidae | G | M, C | 47 | 1 | 1 | 2 | 1 | 5 (10.6) | ||||||
| Gray catbird (Dumetella carolinensis) | Mimidae | U | M, C | 646 | 1 | 1 | 1 | 1 | 2 | 6 (0.9) | |||||
| Brown thrasher (Toxostoma rufum) | Mimidae | G | L | 7 | 1 | 1 (14.3) | |||||||||
| Ovenbird (Seiurus aurocapilla) | Parulidae | G | M, C | 42 | 1 | 1 (2.4) | |||||||||
| Worm-eating warbler (Helmitheros vermivorum) | Parulidae | U | M, C | 33 | 2 | 2 (6.1) | |||||||||
| Northern waterthrush (Parkesia noveboracensis) | Parulidae | G | M, C/S | 78 | 1 | 1 (1.3) | |||||||||
| Black-and-white warbler (Mniotilta varia) | Parulidae | C | M, C/S | 89 | 3 | 1 | 4 (4.5) | ||||||||
| Prothonotary warbler (Protonotaria citrea) | Parulidae | C | M, C/S | 14 | 1 | 1 (7.1) | |||||||||
| Tennessee warbler (Oreothlypis peregrina) | Parulidae | C | M, C/S | 208 | 1 | 1 | 2 (1.0) | ||||||||
| Kentucky warbler (Geothlypis formosa) | Parulidae | U | M, C/S | 59 | 4 | 1 | 1 | 6 (10.2) | |||||||
| Common yellowthroat (Geothlypis trichas) | Parulidae | U | L | 260 | 1 | 1 (0.4) | |||||||||
| Hooded warbler (Setophaga citrina) | Parulidae | U | M, C | 90 | 1 | 1 | 1 | 1 | 4 (4.4) | ||||||
| Bay-breasted warbler (Setophaga castanea) | Parulidae | C | M, S | 13 | 1 | 1 (7.7) | |||||||||
| Chestnut-sided warbler (Setophaga pensylvanica) | Parulidae | C | M, C/S | 19 | 2 | 2 (10.5) | |||||||||
| Yellow-breasted chat (Icteria virens) | Parulidae | U | M, C | 1 | 1 | 1 (100) | |||||||||
| White-throated sparrow (Zonotrichia albicollis) | Emberizidae | G | L | 1 | 1 | 1 (100) | |||||||||
| Savannah sparrow (Passerculus sandwichensis) | Emberizidae | G | L | 7 | 1 | 1 (14.3) | |||||||||
| Swamp sparrow (Melospiza georgiana) | Emberizidae | G | L | 12 | 3 | 3 (25.0) | |||||||||
| Lincoln's sparrow (Melospiza lincolnii) | Emberizidae | G | L | 58 | 2 | 1 | 3 (5.2) | ||||||||
| Rose-breasted grosbeak (Pheucticus ludovicianus) | Cardinalidae | C | M, C | 102 | 1 | 1 (1.0) | |||||||||
| Blue grosbeak (Passerina caerulea) | Cardinalidae | C | M, C | 19 | 1 | 1 (5.3) | |||||||||
| Indigo bunting (Passerina cyanea) | Cardinalidae | G | M, C | 397 | 1 | 7 | 7 | 1 | 16 (4.0) | ||||||
| Scarlet tanager (Piranga olivacea) | Cardinalidae | C | M, S | 46 | 1 | 1 | 2 (4.3) | ||||||||
| Painted bunting (Passerina ciris) | Cardinalidae | C | M, C | 157 | 1 | 2 | 3 (1.9) | ||||||||
| Summer tanager (Piranga rubra) | Cardinalidae | C | M, C/S | 54 | 2 | 2 | 4 (7.4) | ||||||||
| Northern cardinal (Cardinalis cardinalis) | Cardinalidae | C | L | 54 | 8 | 8 (14.8) | |||||||||
| Total | 3,097 | 3 | 2 | 3 | 35 | 32 | 22 | 7 | 4 | 1 | 3 | 112 (2.9) | |||
Species with at least one infested individual are shown (3,844 individuals of 85 species screened). Bird species were categorized according to foraging height during migration (ground, understory, and canopy/subcanopy) and whether their winter range was south of the Gulf of Mexico or included the study region (intercontinental migrant or local, respectively). We further categorized migrants on the extent of their wintering range (Central America and the Caribbean, Central and South America and the Caribbean, or South America). We captured one infested vagrant species from Central America that does not normally occur in Texas.
C, canopy/subcanopy; U, understory; G, ground.
Stationary nonbreeding range described as local (L) or migrant (M). C, Central America and the Caribbean; C/S, Central and South America and the Caribbean; S, South America; V, vagrant from Central America.
An additional 698 individuals of 49 species were sampled for ticks and were not infested.
FIG 2.

Acadian flycatcher (E. virescens) captured on 23 April 2014 carrying 27 larval ticks around its eyes. The identity of the pooled ticks was molecularly confirmed to be A. longirostre infected with R. amblyommii. (Courtesy of Tim Guida; reproduced with permission.)
We collected and identified 178 individual ticks, which were exclusively larvae and nymphs. Based on mitochondrial 12S rDNA and ITS2 sequences, we identified seven different Amblyomma species and a single Ixodes species (Table 1). Due to similar appearances and identical DNA sequences at the loci we examined, we could not differentiate between Amblyomma maculatum and Amblyomma triste, as was the case in a recent study of bird ticks (7). The single Ixodes tick shared 96% sequence homology with Ixodes minor recovered from a bird in Costa Rica (GenBank accession no. KF702338) based on analysis of the 12S rRNA, and it shared >90% sequence homology with Ixodes dentatus removed from a bird in Chicago (GenBank accession no. JQ868583) based on analysis of the ITS2 region. Ticks collected off four birds were determined to be in the genus Amblyomma but could not be identified to the species level. On the basis of 12S rRNA sequence analysis, these four sequences were identical to each other and shared 90% sequence homology with Amblyomma calcaratum removed from a southern tamandua in Peru (Tamandua tetradactyla) (GenBank accession no. AY225322) and 90% sequence homology with Amblyomma dubitatum removed from Didelphis albiventris in Brazil (GenBank accession no. AY342258). On the basis of ITS2 sequence analysis performed on two of the unknown Amblyomma ticks, the ticks were identical to each other and shared >93% sequence homology with Amblyomma aerolatum in Brazil (GenBank accession no. AF469611). No sequence was obtained from the genomes of ticks collected off three birds. When multiple ticks were collected from the same bird and processed separately, they were always identified to belong to the same tick species (n = 19 birds). Of the tick species for which we collected more than three individuals, the mean ± standard deviation (SD) engorgement scores were lowest for A. maculatum/A. triste (2.02 ± 0.70) yet generally similar across all species (mean ± SD for Amblyomma ovale, 2.13 ± 0.64; Amblyomma longirostre, 2.64 ± 0.96; Amblyomma nodosum, 2.65 ± 0.94).
Migrants from Central America and the Caribbean were infested with all seven Amblyomma species, while migrants from South America were infested with only three of those species (A. longirostre, A. nodosum, and A. geayi). The single Ixodes sp. tick was detected on a migrant from South America (gray-cheeked thrush [Catharus minimus]). Amblyomma auricularium and Amblyomma coelebs were detected on only migrants from Central America. Some tick species infested >10 bird species (A. longirostre, A. maculatum/A. triste, and A. nodosum), while others infested two to three bird species (A. auricularium, A. coelebs, and A. geayi; Table 1).
Of the 1,241 times birds were recaptured and rechecked for ticks, a single tick was removed from each of 10 different recaptured birds 1 to 10 days after their initial capture. Of these, seven A. maculatum/A. triste nymphs with engorgement scores of 1 to 3 were detected on avian species with winter ranges that include our study site. Two were A. longirostre larvae with engorgement scores of 2 and 3 and were detected on two intercontinental migrants (red-eyed vireo [Vireo olivaceus] and Tennessee warbler [Oreothlypis peregrina]) 1 and 2 days after the initial capture. Finally, one A. nodosum nymph with an engorgement score of 2 was detected on an intercontinental migrant (painted bunting [Passerina ciris]) recaptured 1 day after the initial capture. Eight birds were initially banded and checked for ticks in 2013 and recaptured and checked for ticks in 2014; all were local wintering birds checked for ticks on a combined total of 24 occasions, and no ticks were found at any time.
Two ticks were collected off bird banders on 10 May 2013. They were both identified as adult Dermacentor variabilis, with one female and one male.
Bird and tick infection.
We screened 137 ticks for Rickettsia species and B. burgdorferi. Thirty-eight individual ticks of six Amblyomma spp. were infected with at least five different Rickettsia species (Table 2 and Fig. 3). Rickettsia parkeri/R. rickettsii infected at least two neotropical tick species (A. nodosum and A. ovale) collected off four migratory bird species; sequencing of gltA and ompA did not distinguish between these two pathogenic species. Rickettsia amblyommii infected three neotropical tick species (A. auricularium, A. geayi, and A. longirostre) on five intercontinental migrant bird species. The Rickettsia endosymbiont of A. maculatum exclusively infected the A. maculatum/A. triste ticks collected off two local and four intercontinental migratory bird species. Rickettsia spp. in Brazil infected two neotropical tick species, A. geayi and A. longirostre, collected off nine migratory bird species. The single Ixodes sp. larva we collected off the migratory gray-cheeked thrush (C. minimus) was infected with a Rickettsia sp. in the group of Rickettsia monacensis, a spotted fever group human pathogen in Europe and Asia, on the basis of analysis of the gltA and ompA genes. The detected species shared >99% sequence homology to R. monacensis from a human blood sample in South Korea (GenBank accession no. KC993860) and a Rickettsia sp. from a questing Ixodes ricinus from Slovakia (GenBank accession no. AF140706) based on gltA analysis and >97% sequence homology to various R. monacensis strains, endosymbionts, and undescribed strains from Ixodes species in the southern United States and Central and South America (GenBank accession no. KJ507217, EF689735, KF702334, GQ902957, KF831361, EU544297, AF031535, and HM161773) on the basis of ompA analysis. No ticks tested positive for infection with B. burgdorferi.
TABLE 2.
Ticks collected off birds on the northern coast of the Gulf of Mexico during the spring of 2013 and 2014 and sampled for infection with Rickettsia species and B. burgdorferi
| Tick species | No. of ticks tested | No. (%) infecteda |
||||
|---|---|---|---|---|---|---|
| R. amblyommii | Rickettsia endosymbiont of A. maculatum | R. monacensis | R. parkeri/R. rickettsii | Rickettsia spp. in Brazil | ||
| A. auricularium | 7 | 2 (28.6) | ||||
| A. coelebs | 2 | |||||
| A. geayi | 3 | 1 (33.3) | 2 (66.7) | |||
| A. longirostre | 42 | 5 (11.9) | 16 (38.1) | |||
| A. maculatum/A. triste | 43 | 8 (18.6) | ||||
| A. nodosum | 26 | 1 (3.8) | ||||
| A. ovale | 8 | 2 (25) | ||||
| Other Amblyomma sp. | 5 | 1 (20) | ||||
| Ixodes sp. | 1 | 1 (100) | ||||
| Total | 137 | 8 (5.8) | 8 (5.8) | 1 (0.7) | 4 (2.9) | 18 (13.1) |
No ticks tested positive for B. burgdorferi.
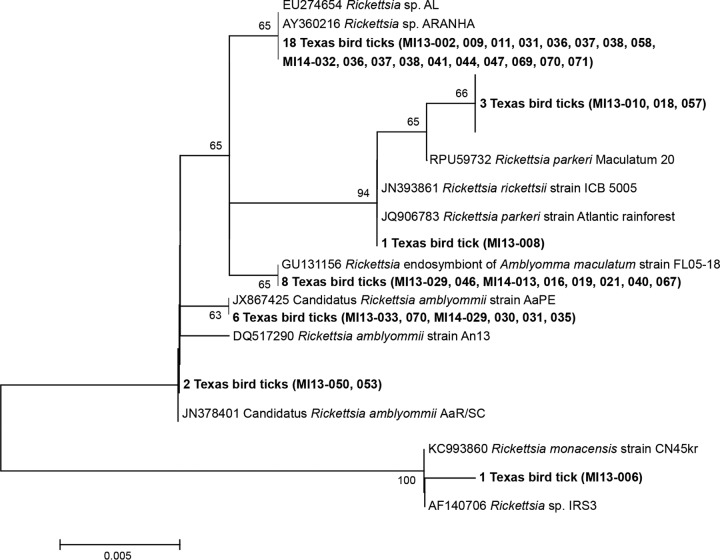
FIG 3.
Phylogenetic relationships of the Rickettsia species detected in ticks removed from birds in Texas, 2013 to 2014, based on partial gltA gene sequences (492 positions) and inferred by the neighbor-joining method. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches when they were ≥60%. The evolutionary distances were computed using the maximum composite likelihood method and are in the units of the number of base substitutions per site. The sequences generated in the current study are in bold. Sequences downloaded from GenBank for comparison are labeled with their accession numbers.
A total of 238 blood samples from 38 species were collected from birds in 2014, representing 11% of all birds that were checked for ticks in that year (n = 2,115; Table 1). Fifty of the samples (21%) were from infested birds (70% of infested birds in 2014), and the remaining 188 were from uninfested birds. None of the 238 samples were positive for Rickettsia spp. using the gltA assay. We subjected a random subset of 100 DNA extracts from these blood samples to the secondary ompA assay, and none were positive. No blood samples tested positive for infection with B. burgdorferi.
Characteristics of infested birds.
We tested our expectations regarding life history characteristics of infested birds, with samples from 56 intercontinental migrant species (n = 3,177 captures) and 28 local species (n = 665 captures). We sampled 21 ground-foraging (n = 912 captures), eight understory (n = 1,148 captures), and 21 canopy/subcanopy (n = 1,784 captures) species. Local and intercontinental migrant groups included species in each of the three foraging groups. We sampled birds with completely depleted fuel stores (fat score = 0; n = 1,634 captures), low fuel stores (fat score = 1; n = 1,259 captures), and moderate to considerable fuel (fat score = 2 to 4; n = 949 captures). Fat score was not recorded for two individuals, and the Acadian flycatcher with 27 ticks was removed from analysis as an outlier. Migratory status, foraging guild, and energetic condition did not influence the abundance of ticks for birds that were infested (Table 3). However, birds with reduced fuel stores that foraged closer to the ground were most likely to be infested (Table 3). Intercontinental migrants were not more likely to be infested than local wintering birds (Table 3).
TABLE 3.
Factors influencing the occurrence and abundance of tick infestation on birds sampled during spring migrationa
| Effect | Binomial model |
Count model |
||||
|---|---|---|---|---|---|---|
| Estimate | χ2 | P | Estimate | χ2 | P | |
| Intercept | −2.62 ± 0.29 | −9.24 ± 99.60 | ||||
| Intercontinental migrantsb | −0.16 ± 0.26 | 0.37 | 0.546 | −1.01 ± 0.65 | 0.52 | 0.104 |
| Understoryc | −0.88 ± 0.27 | 14.20 | 0.001 | 0.40 ± 0.62 | 1.44 | 0.486 |
| Canopy and subcanopyc | −0.71 ± 0.22 | 14.20 | 0.001 | −0.41 ± 0.60 | 1.44 | 0.486 |
| Fatd | −0.37 ± 0.13 | 7.89 | 0.005 | −0.20 ± 0.34 | 0.37 | 0.543 |
The binomial model tests for relationships in the presence or absence of ticks, and the count model tests for relationships within positive samples (n = 3,839 samples; df = 11).
Compared to birds that overwintered locally.
Compared to ground-foraging birds.
Fat score increased from completely depleted fuel (score 0) to low fuel (score 1) to moderate and considerable fuel (scores 2 to 4).
Propagule pressure of neotropical ticks entering the United States.
Twenty-five migrant species screened were infested with one or more exotic neotropical tick species (A. auricularium, A. coelebs, A. geayi, A. longirostre, A. nodosum, and A. ovale). Nineteen of those species were screened sufficiently to derive the frequencies of infestation with neotropical ticks (mean ± SD, 128.32 ± 153.40 birds sampled), and the species-specific frequency of infestation varied (mean ± SD, 0.036 ± 0.02 ticks). Propagule pressure of neotropical ticks entering the United States annually on migratory songbirds was >19 million (19,418,653), derived from species-specific infestation frequencies and North American abundance estimates. Minimum (0.008; gray catbird [Dumetella carolinensis]) and maximum (0.074; summer tanager [Piranga rubra]) neotropical tick infestation frequencies applied across infested species resulted in low and high estimates of >4 million (4,224,240) and >39 million (39,074,220) propagules of neotropical ticks entering the United States annually on migratory birds.
DISCUSSION
Every spring, birds migrate northward into the United States from Central and South America, where they have spent the winter. After crossing the Gulf of Mexico, migrants congregate in coastal habitats before moving on to breeding areas throughout North America (40, 41). Migrating songbirds can move thousands of kilometers in just a few days (16, 42), and we found that 3% of the migrants in coastal Texas harbored ticks across the Gulf of Mexico, over two-thirds (67%) of which were neotropical tick species not known to occur in the United States. Extrapolation of our data yields an estimate of a bird-associated propagule pressure of >19 million exotic neotropical ticks imported to the United States each spring. Although this extrapolation is limited by uncertainties in the estimates of North American breeding bird abundance (39), our estimate of exotic tick propagule pressure is likely conservative given that our calculation includes data from only 19 infested bird species that were commonly captured, combined with a previous study's finding that 33% of ticks on birds may fail to be detected by bird banders (4).
There have been a growing number of observations of neotropical ticks on birds throughout the eastern half of the United States and Canada (4–6, 17, 18, 43, 44). The infestation prevalence we found is remarkably similar to that found in the only other standardized survey of spring migrants arriving in the United States, which found that 2.4% of migrants were infested (7). Nonetheless, no data sets are available to provide evidence that any of these neotropical tick species have become established locally within the United States. We found that <1% of locally recaptured birds were infested, and when they were, it was primarily with A. maculatum/A. triste; both A. maculatum and A. triste are known to be established in Texas (45). Although we detected two neotropical ticks (A. longirostre and A. nodosum) on recaptured birds, our methods do not allow a determination of whether these ticks failed to be detected on first capture or whether they might have been acquired locally. Despite the activity of juvenile Amblyomma americanum across the southern states in the spring, we did not detect this species on birds in our study. At least three reasons may contribute to its lack of detection in our study: (i) our work was done in a coastal marsh habitat and not the preferred woodland habitat of A. americanum (46), (ii) passerine birds seem to be less utilized by A. americanum than other vertebrate species (47), and (iii) the majority of birds we examined had recently arrived from the southern tropics, where A. americanum is not distributed (46).
The pathway of species invasion includes five stages, in which (i) an exotic species is in the invasion pathway, (ii) it is transported and released alive, (iii) a new population establishes itself, (iv) the population spreads, and (v) ecological, human health, or economic impacts result (48). Our data support that the first two stages of the invasion pathway are under way. The barriers to establishment may be both biotic and abiotic, including host species or climatic limitations. For example, although these neotropical ticks feed on diverse wild bird species in their larval and nymphal stages, the adult life stages typically feed upon wild mammalian hosts that do not exist in the southern United States. For example, A. coelebs feeds on tapirs (49), A. longirostre feeds on porcupines (50), and A. nodosum feeds on neotropical anteaters (51). In contrast, the host range of A. ovale in the Neotropics includes felids, rodents, and carnivores (12, 52, 53), and A. auricularium feeds on armadillos (Dasypodidae) (52, 54), all of which are abundant in south Texas. Nonetheless, an adult A. longirostre tick was recently found crawling on a propane tank outside a home in Oklahoma in the fall, which might represent a bird-imported nymph that arrived in the spring and successfully molted (55).
Ongoing changes to the climate may alter the species ranges and phenology of tropical tick species, resulting in unknown disease consequences. A longitudinal study found that climate change has already influenced the abundance and distribution of tick species associated with European birds (19), and models found that Ixodes scapularis will expand significantly northward to Canada (20, 21). Further, climate warming trends correlated with earlier (as much as 3 weeks) activity of I. scapularis in the spring (20). At the same time, migratory songbirds have not advanced their arrival timing across the Gulf of Mexico over the past 2 decades, but they are arriving earlier to breeding grounds throughout North America (24). Therefore, migrants may be compensating for advancing spring phenology by migrating faster within North America (56), potentially moving farther during the period of time when they are infested with neotropical ticks. Such distributional and seasonal expansions place ticks in contact with additional humans and afford more opportunities for pathogen transfer.
An increasing number of spotted fever group Rickettsia species are recognized to cause disease in humans (13, 57), including R. parkeri, which we detected in ticks on northward migrants. R. parkeri was first described in 1939 in A. maculatum ticks from Texas, and it has only recently been implicated in human disease in the southern United States (58), where cross-reaction with R. rickettsii (an agent of Rocky Mountain spotted fever) occurs and the human burden of disease is therefore difficult to discern. The role of birds in the ecology of R. parkeri is unknown, but R. parkeri-like organisms have been detected from at least three species of bird-derived neotropical Amblyomma ticks in Mexico and Brazil (59, 60). Further, the possible pathogenic effects of neotropical Rickettsia species on humans are largely unknown (61). Biting of humans by many of the neotropical ticks we detected has been documented (e.g., 62, 63), suggesting that the establishment of these ticks might be associated with human biting and the opportunity for bridging of pathogens with human health consequences.
The single Ixodes sp. larva in our study was infected with a Rickettsia sp. in the group of R. monacensis, a spotted fever group pathogen that is associated with I. ricinus ticks in Europe and North Africa (64, 65) and which is the causative agent of a Mediterranean spotted fever-like illness in humans (66). Our finding may reflect a rickettsemic blood meal within the tick, indicative of an infected and potentially reservoir-competent avian host, or it may reflect a systemically infected tick that had been infected transovarially from an infected female tick. The infected tick was removed from a gray-cheeked thrush, a species that winters in South America, east of the Andes, and breeds in spruce forests in Alaska and across northern Canada. To our knowledge, this agent was not previously reported in the Americas (57).
No avian tick or blood sample in our study was infected with B. burgdorferi. Although none of the neotropical Amblyomma species in our study have been evaluated as a candidate vector of B. burgdorferi, the overwhelming evidence from decades of work with A. americanum indicates that A. americanum is not a vector of B. burgdorferi due to a borreliacidal agent in the saliva (67), and it is extrapolated that the genus Amblyomma likely does not contribute to Lyme disease epidemiology or ecology. Testing for B. burgdorferi in our study was not performed to identify vectors but rather to potentially learn about enzootic maintenance of the pathogen in local or migratory birds and their ticks, given that spirochetes in the avian blood could have been detected by our approach of testing the blood directly or testing the engorged ticks. Birds play a key role in the ecology of Lyme disease through contributing to the range expansion of ticks and maintaining B. burgdorferi in the environment in areas where Lyme disease is recognized as endemic or emerging (4, 26, 68–70). Regional studies of pathogen prevalence are increasingly important from ecological and human health perspectives, especially considering that the annual movement patterns of many of the birds connect geographic zones in which Lyme disease is endemic with those in which it is nonendemic.
We found that ground-foraging birds were more likely to be infested with ticks than those that forage elsewhere, a finding that is congruent with many other studies over different geographic regions (e.g., 7, 70, 71) and that reflects the ground-level host-seeking behavior of ticks (14). We also found that birds with reduced fat stores were more likely to harbor ticks. When fuel stores are depleted during migration, birds expand their foraging heights and substrates (22, 23) and may therefore be increasingly exposed to questing ticks on low vegetation. Although it is possible for a migrant to carry a tick from South America in a few days, migrants from South America may also acquire ticks during migratory stopovers in Central America. Three of the tick species we detected, A. longirostre, A. nodosum, and A. maculatum/A. triste, occur broadly across Central and South America (51), and these species infested many bird species from both Central and South America, as well as local nonmigratory birds. Six of the seven Amblyomma species were detected on bird species that winter in Central America, and three Amblyomma species that occur in both Central and South America, A. auricularium, A. coelebs, and A. ovale, were detected only on migrants from Central America. Only A. geayi was detected exclusively on canopy-foraging migrants from South America (red-eyed vireo and scarlet tanager). It is possible that these long-distance migrants transported A. geayi from South America, where it is known to infest birds in the Amazon (9), to the United States, but A. geayi is also known to occur in Central America (72). The broad geographic distribution of the tick and bird species in this study limits our ability to identify the geographic location of infestation.
Exotic neotropical ticks and associated pathogens are being transported to and presumably released alive in the United States. Recommendations specific to this stage in the invasion pathway include monitoring for early invasions to allow a rapid response and providing authority and funding for eradication and control programs (48). However, these introduction events via the natural migrations of native bird species have likely been ongoing for millennia with no perceived economic or health impact to date, given the apparent lack of establishment of the invading species. Nonetheless, as climates and ranges of potential vertebrate host species change, future studies to elucidate the origin and destination of ticks and pathogens carried by birds, as well as detailed studies of local vertebrate hosts that could be used to support establishing populations of exotic ticks and pathogens, will be important for more fully understanding avian migration in the field of disease ecology.
ACKNOWLEDGMENTS
We thank the bird banders, Timothy Guida, Trischa Thorne, Sean McElaney, and Gunner Kramer, and the preserve managers, Julie Sullivan and Steven Goertz. Lorenza Beati provided support in tick sequence analysis.
E.B.C. and S.A.H. wrote the first draft, and all authors provided comments. E.B.C. and P.P.M. designed and coordinated the field work, and S.A.H. and L.D.A. designed and performed lab work.
This work was supported by Helen Runnells DuBois, The Nature Conservancy, The Trull Foundation, and the Texas A&M University College of Veterinary Medicine and Biomedical Sciences. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Peterson AT, Andersen MJ, Bodbyl-Roels S, Hosner P, Nyári Á, Oliveros C, Papeş M. 2009. A prototype forecasting system for bird-borne disease spread in North America based on migratory bird movements. Epidemics 1:240–249. doi: 10.1016/j.epidem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Benskin CMH, Wilson K, Jones K, Hartley IR. 2009. Bacterial pathogens in wild birds: a review of the frequency and effects of infection. Biol Rev Camb Philos Soc 84:349–373. doi: 10.1111/j.1469-185X.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 3.Reed KD, Meece JK, Henkel JS, Shukla SK. 2003. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, influenza A and enteropathogens. Clin Med Res 1:5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden NH, Lindsay LR, Hanincová K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O'Callaghan CJ, Schwartz I, Thompson RA. 2008. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol 74:1780–1790. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamer SA, Goldberg TL, Kitron UD, Brawn JD, Anderson TK, Loss SR, Walker ED, Hamer GL. 2012. Wild birds and urban ecology of ticks and tick-borne pathogens, Chicago, Illinois, USA, 2005–2010. Emerg Infect Dis 18:1589. doi: 10.3201/eid1810.120511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JD, Lee M-K, Fernando K, Durden LA, Jorgensen DR, Mak S, Morshed MG. 2010. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J Vector Ecol 35:124–139. doi: 10.1111/j.1948-7134.2010.00068.x. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee N, Beati L, Sellers M, Burton L, Adamson S, Robbins RG, Moore F, Karim S. 2014. Importation of exotic ticks and tick-borne spotted fever group rickettsiae into the United States by migrating songbirds. Ticks Tick Borne Dis 5:127–134. doi: 10.1016/j.ttbdis.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogrzewalska M, Uezu A, Labruna MB. 2011. Ticks (Acari: Ixodidae) infesting wild birds in the Atlantic Forest in northeastern Brazil, with notes on rickettsial infection in ticks. Parasitol Res 108:665–670. doi: 10.1007/s00436-010-2111-8. [DOI] [PubMed] [Google Scholar]
- 9.Ogrzewalska M, Uezu A, Labruna MB. 2010. Ticks (Acari: Ixodidae) infesting wild birds in the eastern Amazon, northern Brazil, with notes on rickettsial infection in ticks. Parasitol Res 106:809–816. doi: 10.1007/s00436-010-1733-1. [DOI] [PubMed] [Google Scholar]
- 10.Miller MJ, Esser HJ, Loaiza JR, Herre EA, Aguilar C, Quintero D, Alvarez E, Bermingham E. 2014. Molecular insights into Neotropical bird-tick ecological associations and the role of birds in tick-borne disease ecology. arXiv:1411.6686. [Google Scholar]
- 11.Pacheco RC, Arzua M, Nieri-Bastos FA, Moraes-Filho J, Marcili A, Richtzenhain LJ, Barros-Battesti DM, Labruna MB. 2012. Rickettsial infection in ticks (Acari: Ixodidae) collected on birds in southern Brazil. J Med Entomol 49:710–716. doi: 10.1603/ME11217. [DOI] [PubMed] [Google Scholar]
- 12.Guglielmone AA. 2003. Ticks (Acari: Ixodida) of the Neotropical zoogeographic region. Universiteit Utrecht, Utrecht, the Netherlands. [Google Scholar]
- 13.Eremeeva ME, Dasch GA. 2015. Challenges posed by tick-borne rickettsiae: eco-epidemiology and public health implications. Front Public Health 3:55. doi: 10.3389/fpubh.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph SE. 2014. Ecology of non-nidicolous ticks, p 3–60. In Sonenshine DE, Roe RM (ed), Biology of ticks. Oxford University Press, New York, NY. [Google Scholar]
- 15.Randolph SE. 1998. Ticks are not insects: consequences of contrasting vector biology for transmission potential. Parasitol Today 14:186–192. doi: 10.1016/S0169-4758(98)01224-1. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca WV, Woodworth BK, Rimmer CC, Marra PP, Taylor PD, McFarland KP, Mackenzie SA, Norris DR. 2015. Transoceanic migration by a 12 g songbird. Biol Lett 11:20141045. doi: 10.1098/rsbl.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, Morshed MG. 2001. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol 38:493–500. doi: 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- 18.Scott JD, Durden LA. 2015. First record of Amblyomma rotundatum tick (Acari: Ixodidae) parasitizing a bird collected in Canada. Syst Appl Acarol 20:155–161. doi: 10.11158/saa.20.2.1. [DOI] [Google Scholar]
- 19.Møller AP, Merino S, Soler JJ, Antonov A, Badás EP, Calero-Torralbo MA, de Lope F, Eeva T, Figuerola J, Flensted-Jensen E, Garamszegi LZ, Gonzalez-Braojos S, Gwinner H, Hanssen SA, Heylen D, Ilmonen P, Klarborg K, Korpimaki E, Martinez J, Martinez-de la Puente J, Marzal A, Matthysen E, Matyjasiak P, Molina-Morales M, Moreno J, Mousseau TA, Nielsen JT, Pap PL, Rivero-de Aguilar J, Shurulinkov P, Slagsvold T, Szep T, Szöliõsi E, Török J, Vaclav R, Valera F, Ziane N. 2013. Assessing the effects of climate on host-parasite interactions: a comparative study of European birds and their parasites. PLoS One 8:e82886. doi: 10.1371/journal.pone.0082886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostfeld RS, Brunner JL. 2015. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci 370:20140051. doi: 10.1098/rstb.2014.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogden NH, Maarouf A, Barker IK, Bigras-Poulin M, Lindsay LR, Morshed MG, O'Callaghan CJ, Ramay F, Waltner-Toews D, Charron DF. 2006. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int J Parasitol 36:63–70. doi: 10.1016/j.ijpara.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Loria DE, Moore FR. 1990. Energy demands of migration on red-eyed vireos, Vireo olivaceus. Behav Ecol 1:24–35. [Google Scholar]
- 23.Yong W, Moore FR. 2005. Long-distance bird migrants adjust their foraging behavior in relation to energy stores. Acta Zool Sin 51:12–23. [Google Scholar]
- 24.Cohen EB, Németh Z, Zenzal TJ Jr, Paxton KL, Diehl R, Paxton EH, Moore FR. 2015. Spring resource phenology and timing of songbird migration across the Gulf of Mexico, p 63–82. In Wood EM, Kellermann JL (ed), Phenological synchrony and bird migration: changing climate and seasonal resources in North America. CRC Press, Boca Raton, FL. [Google Scholar]
- 25.Helms CW, Drury WH. 1960. Winter and migratory weight and fat field studies on some North American buntings. Bird-Banding 31:1–40. doi: 10.2307/4510793. [DOI] [Google Scholar]
- 26.Hamer SA, Hickling GJ, Sidge JL, Rosen ME, Walker ED, Tsao JI. 2011. Diverse Borrelia burgdorferi strains in a bird-tick cryptic cycle. Appl Environ Microbiol 77:1999–2007. doi: 10.1128/AEM.02479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falco RC, Fish D, Piesman J. 1996. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol 143:187–192. doi: 10.1093/oxfordjournals.aje.a008728. [DOI] [PubMed] [Google Scholar]
- 28.Beati L, Keirans JE. 2001. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol 87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Zahler M, Gothe R, Rinder H. 1995. Genetic evidence against a morphologically suggestive conspecificity of Dermacentor reticulatus and D. marginatus (Acari: Ixodidae). Int J Parasitol 25:1413–1419. doi: 10.1016/0020-7519(95)00081-X. [DOI] [PubMed] [Google Scholar]
- 30.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson PC, Billingsley PM, Teltow GJ, Seals JP, Turnbough MA, Atkinson SF. 2010. Borrelia, Ehrlichia, and Rickettsia spp. in ticks removed from person, Texas, USA. Emerg Infect Dis 16:441–446. doi: 10.3201/eid1603.091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Jin J, Fu X, Raoult D, Fournier P-E. 2006. Genetic differentiation of Chinese isolates of Rickettsia sibirica by partial ompA gene sequencing and multispacer typing. J Clin Microbiol 44:2465–2467. doi: 10.1128/JCM.02272-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A 101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrow WC, Chen C-C, Hamilton RB, Ouchley K, Spengler TJ. 2000. Disruption and restoration of en route habitat, a case study: the Chenier Plain. Stud Avian Biol 20:71–87. [Google Scholar]
- 35.Poole A. 2005. The birds of North America online. Cornell Laboratory of Ornithology, Ithaca, NY: http://bna.birds.cornell.edu/bna/. [Google Scholar]
- 36.R Development Core Team. 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 37.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Springer, New York, NY. [Google Scholar]
- 38.Kolar CS, Lodge DM. 2001. Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. doi: 10.1016/S0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 39.Rich TD, Beardmore CJ, Berlanga H, Blancher PJ, Bradstreet MSW, Butcher GS, Demarest DW, Dunn EH, Hunter WC, Iñigo-Elias EE, Kennedy JA, Martell AM, Panjabi AO, Pashley DN, Rosenberg KV, Rustay CM, Wendt JS, Will TC. 2004. Partners in flight: North American landbird conservation plan. Cornell Laboratory of Ornithology, Ithaca, NY: http://www.partnersinflight.org/cont_plan/PIF4_AppendicesWEB.pdf. [Google Scholar]
- 40.Langin KM, Marra PP, Nemeth Z, Moore FR, Kurt Kyser T, Ratcliffe LM. 2009. Breeding latitude and timing of spring migration in songbirds crossing the Gulf of Mexico. J Avian Biol 40:309–316. doi: 10.1111/j.1600-048X.2008.04496.x. [DOI] [Google Scholar]
- 41.Paxton KL, Moore FR. 2015. Carry-over effects of winter habitat quality on en route timing and condition of a migratory passerine during spring migration. J Avian Biol 46:495–506. doi: 10.1111/jav.00614. [DOI] [Google Scholar]
- 42.Bairlein F, Norris DR, Nagel R, Bulte M, Voigt CC, Fox JW, Hussell DJT, Schmaljohann H. 2012. Cross-hemisphere migration of a 25 g songbird. Biol Lett 8:505–507. doi: 10.1098/rsbl.2011.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lord CC, Day JF. 2000. First record of Amblyomma auricularium (Acari: Ixodidae) in the United States. J Med Entomol 37:977–978. doi: 10.1603/0022-2585-37.6.977. [DOI] [PubMed] [Google Scholar]
- 44.Morshed MG, Scott JD, Fernando K, Beati L, Mazerolle DF, Geddes G, Durden LA. 2005. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J Parasitol 91:780–790. doi: 10.1645/GE-3437.1. [DOI] [PubMed] [Google Scholar]
- 45.Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF. 2010. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J Med Entomol 47:707–722. doi: 10.1093/jmedent/47.5.707. [DOI] [PubMed] [Google Scholar]
- 46.Childs JE, Paddock CD. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol 48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 47.Harmon JR, Scott MC, Baker EM, Jones CJ, Hickling GJ. 2015. Molecular identification of Ehrlichia species and host bloodmeal source in Amblyomma americanum L. from two locations in Tennessee, United States. Ticks Tick Borne Dis 6:246–252. doi: 10.1016/j.ttbdis.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Lodge DM, Williams S, MacIsaac HJ, Hayes KR, Leung B, Reichard S, Mack RN, Moyle PB, Smith M, Andow DA, Carlton JT, McMichael A. 2006. Biological invasions: recommendations for U.S. policy and management. Ecol Appl 16:2035–2054. doi: 10.1890/1051-0761(2006)016[2035:BIRFUP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Labruna MB. 2009. Ecology of Rickettsia in South America. Ann N Y Acad Sci 1166:156–166. doi: 10.1111/j.1749-6632.2009.04516.x. [DOI] [PubMed] [Google Scholar]
- 50.Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LMA, Camargo EP, Popov V, Walker DH. 2004. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondonia, Western Amazon, Brazil. J Med Entomol 41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- 51.Voltzit OV. 2013. A review of Neotropical Amblyomma species (Acari: Ixodidae). Acarina 15:3–134. [Google Scholar]
- 52.Guglielmone AA, Estrada-Peña A, Luciani CA, Mangold AJ, Keirans JE. 2003. Hosts and distribution of Amblyomma auricularium (Conil 1878) and Amblyomma pseudoconcolor Aragão, 1908 (Acari: Ixodidae). Exp Appl Acarol 29:131–139. doi: 10.1023/A:1024251020035. [DOI] [PubMed] [Google Scholar]
- 53.Jones EK, Clifford CM, Keirans JE, Kohls GM. 1972. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigh Young Univ Sci Bull-Biol Ser 17:1–40. [Google Scholar]
- 54.Horta MC, do Nascimento GF, Martins TF, Labruna MB, Machado LC, Nicola PA. 2011. Ticks (Acari: Ixodida) parasitizing free-living wild animals in the Caatinga biome in the State of Pernambuco, northeastern Brazil. Syst Appl Acarol 16:207–211. [Google Scholar]
- 55.Noden BH, Arnold D, Grantham R. 2015. First report of adult Amblyomma longirostre (Acari: Ixodidae) in Oklahoma. Syst Appl Acarol 20:468–470. doi: 10.11158/saa.20.5.2. [DOI] [Google Scholar]
- 56.Marra PP, Francis CM, Mulvihill RS, Moore FR. 2005. The influence of climate on the timing and rate of spring bird migration. Oecologia 142:307–315. doi: 10.1007/s00442-004-1725-x. [DOI] [PubMed] [Google Scholar]
- 57.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier P-E, Raoult D. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SLF, Tamminga CL, Ohl CA. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis 38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 59.Ogrzewalska M, Pacheco RC, Uezu A, Richtzenhain LJ, Ferreira F, Labruna MB. 2009. Rickettsial infection in Amblyomma nodosum ticks (Acari: Ixodidae) from Brazil. Ann Trop Med Parasitol 103:413–425. doi: 10.1179/136485909X451744. [DOI] [PubMed] [Google Scholar]
- 60.Torga K, Tolesano-Pascoli G, Vasquez JB, Júnior ELDS, Labruna MB, Martins TF, Ogrzewalska M, Szabó MPJ. 2013. Ticks on birds from Cerrado forest patches along the Uberabinha River in the Triângulo Mineiro region of Minas Gerais, Brazil. Cienc Rural 43:1852–1857. doi: 10.1590/S0103-84782013005000121. [DOI] [Google Scholar]
- 61.Eremeeva ME. 2012. Molecular epidemiology of rickettsial diseases in North America. Ticks Tick Borne Dis 3:332–337. doi: 10.1016/j.ttbdis.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 62.Garcia MV, Matias J, Aguirre ADAR, Csordas BG, Szabo MPJ, Andreotti R. 2015. Successful feeding of Amblyomma coelebs (Acari: Ixodidae) nymphs on humans in Brazil: skin reactions to parasitism. J Med Entomol 52:117–119. doi: 10.1093/jme/tju060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guglielmone AA, Beati L, Barros-Battesti DM, Labruna MB, Nava S, Venzal JM, Mangold AJ, Szabó MPJ, Martins JR, González-Acuña D, Estrada-Peña A. 2006. Ticks (Ixodidae) on humans in South America. Exp Appl Acarol 40:83–100. doi: 10.1007/s10493-006-9027-0. [DOI] [PubMed] [Google Scholar]
- 64.Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TJ, Munderloh UG. 2002. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microbiol 68:4559–4566. doi: 10.1128/AEM.68.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dib L, Bitam I, Bensouilah M, Parola P, Raoult D. 2009. First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clin Microbiol Infect 15:261–262. doi: 10.1111/j.1469-0691.2008.02277.x. [DOI] [PubMed] [Google Scholar]
- 66.Jado I, Oteo JA, Aldámiz M, Gil H, Escudero R, Ibarra V, Portu J, Portillo A, Lezaun MJ, García-Amil C, Rodríguez-Moreno I, Anda P. 2007. Rickettsia monacensis and human disease, Spain. Emerg Infect Dis 13:1405–1407. doi: 10.3201/eid1309.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stromdahl EY, Nadolny RM, Gibbons JA, Auckland LD, Vince MA, Elkins CE, Murphy MP, Hickling GJ, Eshoo MW, Carolan HE, Crowder CD, Pilgard MA, Hamer SA. 2015. Borrelia burgdorferi not confirmed in human-biting Amblyomma americanum ticks from the southeastern United States. J Clin Microbiol 53:1697–1704. doi: 10.1128/JCM.03454-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brinkerhoff RJ, Folsom-O'Keefe CM, Tsao K, Diuk-Wasser MA. 2009. Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen Borrelia burgdorferi. Front Ecol Environ 9:103–110. doi: 10.1890/090062. [DOI] [Google Scholar]
- 69.Schneider SC, Parker CM, Miller JR, Page Fredericks L, Allan BF. 2015. Assessing the contribution of songbirds to the movement of ticks and Borrelia burgdorferi in the midwestern United States during fall migration. Ecohealth 12:164–173. doi: 10.1007/s10393-014-0982-3. [DOI] [PubMed] [Google Scholar]
- 70.Newman EA, Eisen L, Eisen RJ, Fedorova N, Hasty JM, Vaughn C, Lane RS. 2015. Borrelia burgdorferi sensu lato spirochetes in wild birds in northwestern California: associations with ecological factors, bird behavior and tick infestation. PLoS One 10:e0118146. doi: 10.1371/journal.pone.0118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinsey AA, Durden LA, Oliver JH Jr. 2000. Tick infestations of birds in coastal Georgia and Alabama. J Parasitol 86:251–254. doi: 10.2307/3284764. [DOI] [PubMed] [Google Scholar]
- 72.Martins TF, Scofield A, Oliveira WBL, Nunes PH, Ramirez DG, Barros-Battesti DM, Sá LRM, Ampuero F, Souza JC Jr, Labruna MB. 2013. Morphological description of the nymphal stage of Amblyomma geayi and new nymphal records of Amblyomma parkeri. Ticks Tick Borne Dis 4:181–184. doi: 10.1016/j.ttbdis.2012.11.015. [DOI] [PubMed] [Google Scholar]