Abstract
Ectomycorrhizal fungi play a key role in mobilizing nutrients embedded in recalcitrant organic matter complexes, thereby increasing nutrient accessibility to the host plant. Recent studies have shown that during the assimilation of nutrients, the ectomycorrhizal fungus Paxillus involutus decomposes organic matter using an oxidative mechanism involving Fenton chemistry (Fe2+ + H2O2 + H+ → Fe3+ + ˙OH + H2O), similar to that of brown rot wood-decaying fungi. In such fungi, secreted metabolites are one of the components that drive one-electron reductions of Fe3+ and O2, generating Fenton chemistry reagents. Here we investigated whether such a mechanism is also implemented by P. involutus during organic matter decomposition. Activity-guided purification was performed to isolate the Fe3+-reducing principle secreted by P. involutus during growth on a maize compost extract. The Fe3+-reducing activity correlated with the presence of one compound. Mass spectrometry and nuclear magnetic resonance (NMR) identified this compound as the diarylcyclopentenone involutin. A major part of the involutin produced by P. involutus during organic matter decomposition was secreted into the medium, and the metabolite was not detected when the fungus was grown on a mineral nutrient medium. We also demonstrated that in the presence of H2O2, involutin has the capacity to drive an in vitro Fenton reaction via Fe3+ reduction. Our results show that the mechanism for the reduction of Fe3+ and the generation of hydroxyl radicals via Fenton chemistry by ectomycorrhizal fungi during organic matter decomposition is similar to that employed by the evolutionarily related brown rot saprotrophs during wood decay.
INTRODUCTION
Boreal forests in the Northern Hemisphere are one of the major global sinks for carbon (C) (1). Carbon enters this system as plant litter and through below-ground allocation of photosynthates (2). Fungal communities play an important role in the turnover of this C. Saprotrophic fungi are thought to be the key decomposers of plant litter material (3–6). They can efficiently degrade recalcitrant lignocellulose polymers, which are the main components of plant litter material (7, 8). In contrast, the ectomycorrhizal (ECM) fungi are symbionts that depend on their plant hosts for their C sources. In return, the ECM fungi provide plants with nutrients such as nitrogen (N) (9). The soil organic matter that ECM fungi encounter is a heterogeneous environment consisting of humus-rich organic materials (10). Under such conditions, N is present mostly in organic forms that are associated with polyphenols and other degradation products of plant and microbial biopolymers (11, 12). Studies using stable isotopes have shown that ECM fungi have the capacity to mobilize at least some of this organic N (10). However, the molecular mechanism by which ECM fungi mobilize organic nutrients from the complex soil organic matter has not been well studied.
Recently, we showed that the ECM fungus Paxillus involutus (Basidiomycetes; Boletales) can decompose polysaccharides and lignin fragments while acquiring N from organic matter using a brown rot mechanism (13). Spectroscopy and transcriptome analyses revealed the action of a radical-based oxidation mechanism that is similar to that of brown rot wood-decaying fungi. Hydroxyl radicals (˙OH) are important oxidants in brown rot fungi and are produced via the Fenton reaction (Fe2+ + H2O2 + H+ → Fe3+ + ˙OH + H2O) (7, 14–16). The production of hydroxyl radicals requires the reduction of Fe3+ to Fe2+ (16). We demonstrated that P. involutus produced iron-reducing activity during the decomposition of organic matter extracts (13); however, the mechanism for reducing Fe3+ was not characterized.
Three different mechanisms have been proposed for the reduction of Fe3+ in wood-decaying brown rot basidiomycetes: (i) iron-reducing enzymes, such as cellobiose dehydrogenase (17), (ii) low-molecular-weight glycopeptides (18), and (iii) secondary metabolites, including hydroquinones, such as 2,5-dimethoxyhydroquinone (2,5-DMHQ), and catechols, such as 4,5-dimethoxycatechols (DMC) (16, 19). No genes encoding cellobiose dehydrogenase are found in the genome of P. involutus (20). Although genes encoding putative low-molecular-weight iron-reducing glycopeptides are present in P. involutus, they were not upregulated during organic matter degradation (13). Taken together, these findings suggest that secondary metabolites may act as the Fe3+ reductant that drives the Fenton-based decomposition mechanism in P. involutus.
Quinone-based redox cycles occur through the reduction of Fe3+ through a one-electron transfer to Fe3+ by secreted fungal hydroquinones and/or catechols (16, 19, 21, 22). Based on the Fe3+-reducing activity in vitro, Eastwood et al. (23) proposed that variegatic acid is the metabolite driving Fenton chemistry in the brown rot fungus Serpula lacrymans, a member of the order Boletales. Variegatic and xerocomic acids are hydroxylated derivatives of pulvinic acid (24). However, results from experiments performed more recently challenge the view that variegatic acid is the key reducing agent driving Fenton chemistry in S. lacrymans (25): variegatic acid was not detected in wood undergoing decay. It was demonstrated that the well-characterized Fe3+ reductant 2,5-DMHQ was produced by S. lacrymans under the growth conditions described.
The aim of this study was to isolate and characterize the iron-reducing compound(s) produced by the ECM fungus P. involutus during the decomposition of organic matter. Our results showed that the Fe3+-reducing activity was caused by one major compound that was secreted into the medium during organic matter decomposition. Mass spectrometry and nuclear magnetic resonance (NMR) analyses identified this compound as the secondary metabolite involutin, a diarylcyclopentenone. This pigment was described in 1967 (26), but its biological function has remained unknown. Our study demonstrates that a low-molecular-weight metabolite can function as the Fe3+ reductant in an ECM fungus during Fenton-based decomposition of organic matter.
MATERIALS AND METHODS
Reagents.
2,6-Dimethoxyhydroquinone (2,6-DMHQ), ferrozine [3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine], and 2-hydroxyterephthalic acid (h-TPA) were acquired from Sigma-Aldrich, Sweden. Disodium terephthalate (TPA) was purchased from Alfa Aesar, Sweden.
Fungal strain and culture conditions.
Cultures of P. involutus (Batsch) Fr. (strain ATCC 200175) were maintained aseptically on minimum Melin-Norkrans (MMN) medium containing 1.5% agar. MMN medium consists of 2.5 g liter−1 d-glucose, 500 mg liter−1 KH2PO4, 200 mg liter−1 NH4Cl, 150 mg liter−1 MgSO4·7H2O, 25 mg liter−1 NaCl, 50 mg liter−1 CaCl2, 12 mg liter−1 FeCl3·6H2O, and 1 mg liter−1 thiamine-HCl at pH 4.0. The fungus was grown in petri dishes on a layer of glass beads immersed in liquid medium (13, 27). After 9 days of incubation at 18°C in the dark, the MMN medium was removed with a sterile pipette. The glass beads and the mycelium were washed with 10 ml of sterile Milli-Q (MQ) water, and 10 ml of MMN medium without N was added to induce an N-deprived mycelium (28). After 24 h, the mycelium was again washed in MQ water, and 10 ml of an organic matter extract was added (13).
The organic matter consisted of a maize compost material (designated MH) and was extracted with hot water, as described previously (13). The organic C content of this extract was 225 mg liter−1, and the iron concentration was 0.023 mg liter−1 (13). Previous experiments showed that the addition of glucose was required for Fenton-based decomposition of organic matter by P. involutus (29). Hence, the extracts were supplemented with glucose (final concentration, 2.5 g liter−1). The C/N ratio after the addition of glucose was ca 7.5 (13). The extracts were then sterilized by filtration with a 0.2-μm-pore-size filter. The cultures were incubated for 7 days at 18°C in the dark. Samples incubated for 7 days are designated MH7. Organic matter extracts that were not inoculated with fungi are designated MH0. The culture filtrate and mycelium were used for further analyses.
Ferrozine assay.
Fe3+-reducing activities were analyzed using the ferrozine assay (30). A 100-μl aliquot of the sample was mixed with 1.0 ml of 0.1 M acetate buffer (pH 4.4), 100 μl of freshly prepared 1.0 mM FeCl3, and 100 μl of 1% (wt/vol) aqueous ferrozine. The reaction mixture was incubated for 5 min. Fe3+ reduction was assayed spectrophotometrically at 562 nm. A standard curve was constructed using FeSO4 (0.0 to 3.0 mM).
Extraction of Fe3+-reducing compounds.
Fe3+-reducing compounds were extracted from the culture filtrates by adopting a procedure used for isolating such compounds from S. lacrymans (23). Briefly, equal volumes of culture filtrate and ethyl acetate (EtOAc) were mixed and vortexed. The EtOAc phase was recovered and was dried under a stream of N2. The dried EtOAc phase was redissolved in EtOAc. The extract was then adsorbed onto a 10-ml Bond Elut SI solid-phase (SP) fractionation cartridge (Agilent Technologies, Sweden), followed by sequential elution with cyclohexane, EtOAc, and methanol (MeOH). Fractions were designated SPCyclohex (the fraction resulting from SP extraction with cyclohexane), SPEtOAc, and SPMeOH. Fe3+-reducing activity was measured by using the ferrozine assay at every step of purification.
An intracellular mycelial extract was prepared by sonication followed by centrifugation, as described by Shah et al. (28). The supernatant was analyzed for Fe3+-reducing activity.
Metabolites were also extracted directly from the mycelium according to the procedure of Feling et al. (31). Briefly, mycelia from 20 petri dishes (∼0.5 g dry weight) were shaken with a mixture of acetone (0.5 liter), 2 M HCl (5 ml), and ascorbic acid (0.3 g) for 20 h. After filtration, the filtrate was extracted three times using 200 ml of EtOAc each time. The combined EtOAc phase was dried under N2 and was directly analyzed by mass spectrometry.
Mass spectrometry.
To identify secreted fungal metabolites, the MH0 SPEtOAc, MH7 SPEtOAc, and MH7 SPMeOH samples and the EtOAc phase of the MH7 mycelial extract were analyzed by liquid chromatography-mass spectrometry (LC-MS). The instrument used was an Accela high-performance LC (HPLC) system with an Exactive Orbitrap mass spectrometer equipped with a BetaSil C18 column (length, 150 mm; inside diameter, 2.1 mm; particle size, 3 μm) (Thermo Fisher Scientific, Germany). Samples were dissolved in 1 ml MeOH and were filtered (0.45-μm polytetrafluoroethylene [PTFE] filter; VWR, Germany). A gradient consisting of 0.1% (vol/vol) formic acid in water (solvent A) and 0.1% (vol/vol) formic acid in acetonitrile (solvent B) was applied at a flow rate of 0.2 ml min−1, with an initial hold for 1 min at 5% solvent B, followed by a linear increase to 98% solvent B within 16 min. These conditions were held for an additional 3 min. High-resolution electrospray ionization MS (HRESIMS) data were acquired in both positive and negative ionization modes. Data were analyzed using Xcalibur software (Thermo Fisher Scientific, USA) and the Reaxys database.
HPLC purification.
To purify secreted fungal metabolites, reverse-phase semipreparative HPLC was performed on the MH7 SPEtOAc fraction by using an Agilent 1200 HPLC system (Agilent Technologies, Germany) with a diode array detector and a Zorbax XDB C18 column (length, 250 mm; inside diameter, 9.4 mm; particle size, 5 μm). The sample was dissolved in MeOH. An isocratic run was applied using a solvent system with 92% solvent A (0.1% trifluoroacetic acid in water) plus 8% solvent B (acetonitrile) at a flow rate of 1 ml min−1. The chromatograms were recorded at 254, 260, 285, and 295 nm with peak-actuated scanning from 200 to 400 nm. Fractions collected according to peaks were analyzed for Fe3+-reducing activity. Fractions containing Fe3+-reducing activity were pooled, dried in a rotary evaporator, and freeze-dried to remove traces of trifluoroacetic acid. The fractions were analyzed by mass spectrometry and NMR.
NMR analysis.
1H and 13C NMR spectroscopy of the isolated Fe3+-reducing activity was carried out on a Bruker Avance III instrument (Bruker, USA). The samples were dissolved in CD3OD (99.8%; Deutero, Germany). Signals were referenced to residual MeOH at 3.31 ppm. Spectra were analyzed using TopSpin software, version 3.1 (Bruker, USA). Involutin 1H NMR (600 MHz, CD3OD): δH 3.96 (d, 3J = 6.8 Hz, 1H, 5-H), 4.68 (d, 3J = 6.8 Hz, 1H, 4-H), 6.5 (dd, 3J = 8.1 Hz, 4J = 2.0 Hz, 1H), 6.61 (d, 4J = 2.0 Hz, 1H), 6.77 (d, 3J = 9.0 Hz, 2H), 6.72 (d, 3J = 8.1 Hz, 1H), 7.71 (d, 3J = 9.0 Hz, 2H). Involutin 13C NMR (125 MHz, CD3OD): δ 58.3 (C-5), 72.7 (C-4), 115.6 (CH), 116.1 (CH), 117.9 (CH), 122.8 (CH), 123.8 (C), 128.7 (C), 130.5 (CH), 145.6, 146.1, 157.2 (each C); signals for C-1, C-2, and C-3 not visible. HRESIMS (negative mode): m/z found 313.0719; m/z calculated 313.0718.
Detection of hydroxyl radicals.
TPA was used as a probe for measuring the generation of hydroxyl radicals during the Fenton reaction with involutin. TPA is selective for hydroxyl radicals and produces a fluorescent product, h-TPA, upon oxidation (32, 33). Briefly, a 100-μl aliquot of HPLC-purified involutin (∼0.6 mM) was allowed to react with TPA in a mixture containing 2.0 ml of 100 μM TPA, 1.0 ml of 3 μM H2O2, and 2.0 ml of 30 μM FeCl3 (in 0.1 M acetate buffer, pH 4.0). The increase in fluorescence intensity due to h-TPA production was measured at an excitation wavelength of 315 nm and an emission wavelength of 425 nm. The reaction mixture was incubated in the dark, and the increase in fluorescence intensity was measured after 0, 1, 2, and 3 h. 2,6-DMHQ (0.6 mM) was used as a positive control for hydroxyl radical production. The 2,6-DMHQ isomer is more stable than 2,5-DMHQ and has similar Fe3+-reducing properties; thus, it is the preferred isomer for this assay (25, 34). An assay mixture without involutin or 2,6-DMHQ was used as a negative control. An h-TPA standard curve (0.0 to 100.0 nM) was used for comparison (see Fig. S1 in the supplemental material).
RESULTS
Purification of secreted Fe3+-reducing compounds.
P. involutus was grown on a maize compost (MH) substrate. Previous studies using spectroscopic analyses showed that this substrate is decomposed by the fungus using a brown rot mechanism involving Fenton chemistry (13). The production of Fe3+-reducing activity was analyzed both in the mycelium and in the culture filtrate (Fig. 1). At the start of the experiment, trace amounts of Fe3+-reducing activity were detected in the culture filtrate (MH0). After 7 days of incubation, high levels of Fe3+-reducing activity were detected in the culture filtrate (97% of the total activity) but only minute amounts in the mycelium (3% of the total activity). Fe3+-reducing activity was not detected in the mineral nutrient medium (MMN) incubated with the fungus for 7 days.
FIG 1.
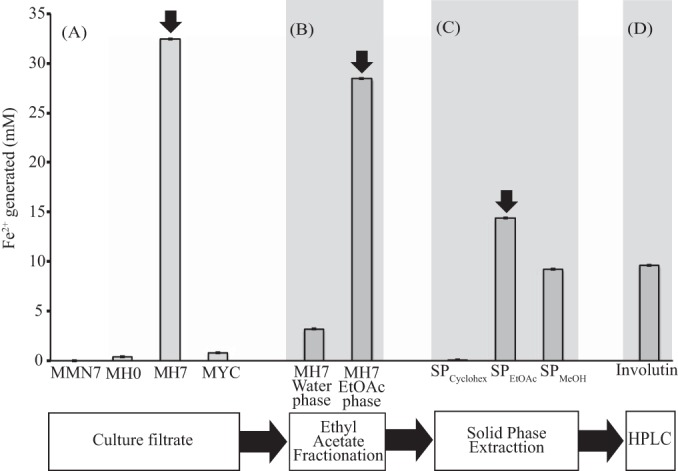
Purification of Fe3+-reducing activity secreted by P. involutus during the decomposition of an organic matter (maize compost) extract. Shown are the concentrations of Fe2+ generated by the reduction of Fe3+ in samples collected at different steps of the purification procedure. The amounts of Fe2+ are normalized per milliliter of culture filtrate. Fe2+ generated by the mycelium extract is normalized per gram (dry weight) of biomass (1 g biomass is produced through ∼40 ml culture extract). Sample details: MMN7, culture filtrate collected after 7 days of growth on a synthetic mineral nutrient medium (MMN) (n = 3); MH0, culture filtrate collected before the fungus was added to the maize compost substrate (MH) (n = 3); MH7, culture filtrate collected after 7 days of growth on the MH substrate (n = 3); MYC, extract of the mycelium grown for 7 days on the MH substrate (n = 3). Error bars indicate standard errors. Thick arrows indicate the samples that were transferred to the next purification step. (A) Activities in the crude culture filtrates and the mycelium extract. (B) Ethyl acetate (EtOAc) phase extraction of the MH7 culture filtrate. (C) Solid-phase fractionation of the EtOAc phase eluted with cyclohexane (SPCyclohex), EtOAc (SPEtOAc), or methanol (SPMeOH). (D) HPLC-purified involutin from the MH7 SPEtOAc eluate.
To purify the Fe3+-reducing activity secreted by P. involutus after 7 days of growth in the MH substrate, the activity was extracted with EtOAc, fractionated on solid-phase silica gel cartridges, and purified by HPLC. Approximately 87% of the Fe3+-reducing activity present in the culture filtrate was extracted with EtOAc (Fig. 1). This activity was further separated into two fractions by solid-phase fractionation, 51% in the SPEtOAc fraction and 32% in the SPMeOH fraction. No activity was detected in the SPCyclohex fraction (Fig. 1).
Identification of involutin.
LC-MS analysis of the MH0 SPEtOAc, MH7 SPEtOAc, and MH7 SPMeOH fractions revealed that one major compound was formed during the decomposition of the organic material (Fig. 2). Based on the HPLC and HRESIMS total-ion chromatogram, the signal corresponded to a single compound with m/z 313.0719 and thus was tentatively identified as involutin (Fig. 2B, inset). The total ion current profiles for the MH7 SPEtOAc and MH7 SPMeOH fractions were similar except that the peak associated with involutin was considerably lower in the MH7 SPMeOH fraction than in the MH7 SPEtOAc fraction (Fig. 2B and C).
FIG 2.
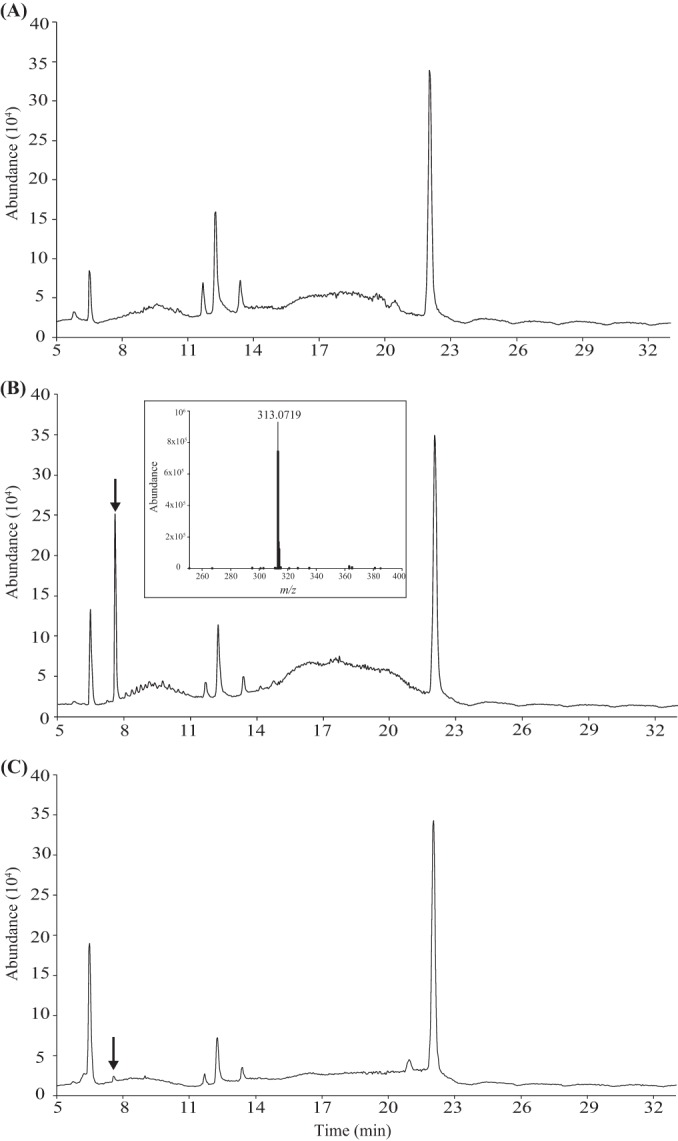
Analysis of secondary metabolites secreted by P. involutus during growth on organic matter. Shown are total ion current profiles from the HRESIMS (negative mode) spectra of the MH0 SPEtOAc eluate, recovered from the initial organic material (A), and of the MH7 SPEtOAc (B) and MH7 SPMeOH (C) fractions, sampled after 7 days of growth. Note that the MH7 SPEtOAc sample contains one major peak, indicated by the arrow (involutin), that is not present in the MH0 sample. Involutin is also present in MH7 SPMeOH. (Inset) HRESIMS profile of involutin.
To examine whether involutin was responsible for the Fe3+-reducing activity produced during the decomposition of organic matter, the MH7 SPEtOAc fraction was further analyzed by semipreparative HPLC using an isocratic gradient. Scanning of the chromatograms recorded at different wavelengths revealed that only one peak detected at 254 nm had Fe3+-reducing activity (Fig. 1 and 3A). HRESIMS showed that the peak contained a single compound with an m/z of 313.0718 calculated for C17H13O6 (found 313.0719) and UV absorption maxima at 228 nm and 248 nm with the solvent mixture of acetonitrile and water; thus, the compound was tentatively identified as involutin (26, 31). The structure of involutin was finally confirmed by NMR analysis (Fig. 3B) (see Materials and Methods). Purification yielded 0.4 mg of involutin with >90% purity, which corresponds to a titer of ∼7.5 μg ml−1 of organic matter extract.
FIG 3.
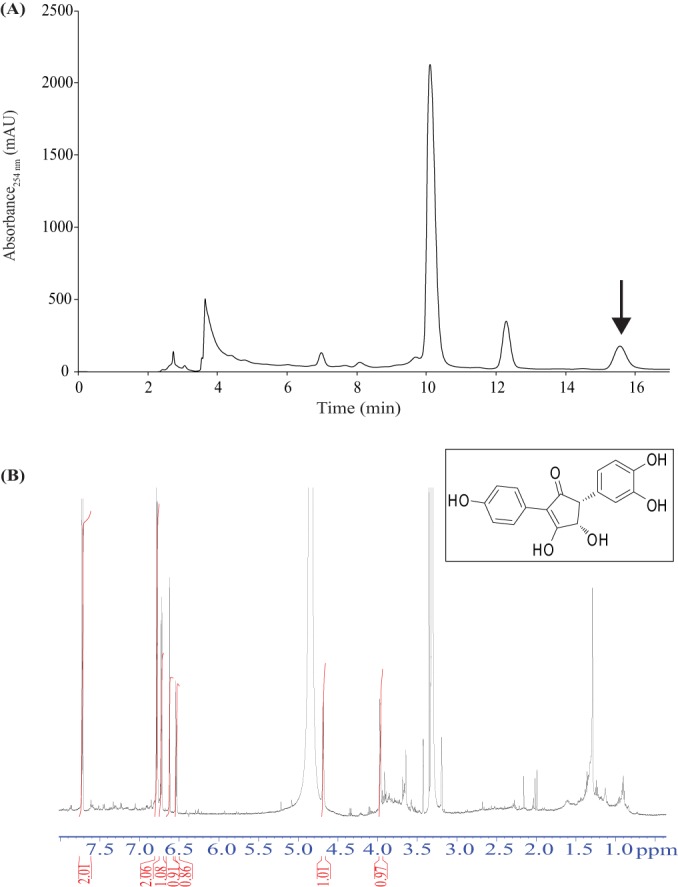
HPLC purification of the Fe3+-reducing compound secreted by the ECM fungus P. involutus during the decomposition of organic matter. (A) Chromatogram of the MH7 SPEtOAc eluate (see Fig. 1) separated by isocratic reverse-phase chromatography. Fractions were collected according to peaks (λ = 254 nm). Fe3+-reducing activity was detected in one peak, indicated by the arrow, and the compound was identified as involutin by HRESIMS (see Fig. 2 inset). (B) 1H NMR spectrum of HPLC-purified involutin (>90% pure). The red lines/numbers represent the integrator trace. (Inset) Chemical structure of involutin.
Other metabolites.
LC-MS analysis also detected traces of involutin in the mycelium grown on the organic matter extract (see Fig. S2A in the supplemental material). Other compounds detected in the mycelial extract were (i) C7H11O5N, m/z 188.0553, and (ii) C10H16O3, m/z 183.1024 (see Fig. S2B and C in the supplemental material). These compounds have not been reported previously in P. involutus.
The culture filtrate and mycelial extract were searched for the presence of hydroquinones, such as 2,5-DMHQ (C8H10O2, m/z 138.1638), its oxidized form, 2,5-dimethoxybenzoquinone (DMBQ) (C8H8O4, m/z 168.0423), and catechols, such as DMC (C8H10O4, m/z 170.0579), by using HPLC-HRESIMS data from authentic compounds. These metabolites were not detected in the culture filtrate or in the mycelial extract of P. involutus grown in the organic matter. Similar techniques, as described above, were used to search for pulvinic acid-derived metabolites, such as variegatic and xerocomic acids; however, these compounds were not detected.
Ability of involutin to generate hydroxyl radicals.
To examine whether involutin may play a role in the production of hydroxyl radicals during organic matter decomposition by P. involutus, we analyzed the ability of purified involutin to drive Fenton chemistry in vitro via Fe3+ reduction. In the presence of involutin, FeCl3, and H2O2, significant amounts of h-TPA were produced, suggesting the production of hydroxyl radicals (Fig. 4). Under these conditions, the generation of h-TPA by involutin was similar to that of the hydroquinone 2,6-DMHQ. In the absence of H2O2, no hydroxyl radicals were formed in the presence of either involutin or 2,6-DMHQ. It has been shown previously that hydroxyl radical production requires the reduction of Fe3+ in the presence of H2O2 (33). Thus, in our experimental system, the metabolite involutin drives a Fenton reaction via Fe3+ reduction.
FIG 4.
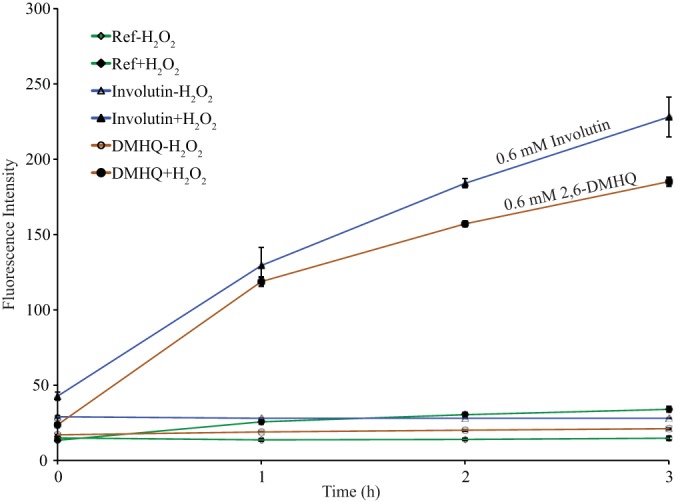
In vitro Fenton reaction with involutin. Shown is the generation of h-TPA, an indicator of hydroxyl radical production, by involutin and 2,6-DMHQ, as measured by fluorescence spectroscopy at an excitation wavelength of 315 nm and an emission wavelength of 425 nm. Error bars indicate standard errors (n = 3).
DISCUSSION
Ectomycorrhizal fungi have evolved from saprotrophic ancestors multiple times (20). During evolution, ECM fungi have lost many of the genes encoding plant cell wall-degrading enzymes, suggesting a reduced decay capacity. Nevertheless, we recently showed that the ECM fungus P. involutus decomposes lignocellulosic material during the assimilation of organic N from organic matter extracted from forest soil or maize compost (13). Data from spectroscopic analyses and transcriptome profiling suggested the involvement of a Fenton-based oxidation mechanism similar to that used by brown rot fungi for decaying lignin in wood tissues (13). In the present study, the oxidation reaction was further examined by characterizing the mechanism involved in the production of the Fe2+ required for the Fenton reaction.
In agreement with the findings for several brown rot wood decayers (16, 19, 21–23, 25), we showed that in P. involutus, the Fe2+ involved in Fenton chemistry is formed by the activity of a low-molecular-weight Fe3+ reductant. Four observations support the conclusion that this reductant is the previously known pigment involutin (26, 31). First, the Fe3+-reducing activity that was secreted into the medium during organic matter decomposition was due largely to a single compound. The compound was identified by mass spectrometry and NMR as involutin. Second, the production of the Fe3+-reducing activity was significantly enhanced during the decomposition of the organic matter extract. In contrast, only trace amounts of extracellular Fe3+-reducing activity were detected when the fungus was grown on a mineral nutrient medium. Third, a large part of the involutin that was synthesized during organic matter decomposition was secreted into the medium. Fourth, a purified fraction of involutin had the ability to drive a Fenton reaction in the presence of H2O2 in vitro.
The order Boletales is one of the major groups of the Agaricomycetes, containing ca. 1,300 described species (35). The ancestor of the Boletales was likely a brown rot saprotroph; however, lineages with ECM species have evolved several times within the order (36). Fungi within the Boletales have been extensively examined for secondary metabolites (24, 37). So far, involutin has been detected from the fruiting bodies in only three species: P. involutus, Gyrodon lividus, and Melanogaster broomeianus (24, 38). These species belong to the family Paxillaceae, which was recently characterized as a well-resolved lineage of the suborder Boletineae (39). At present, only ECM species are known in Paxillaceae. Involutin is probably synthesized from the terphenylquinone atromentin, which is a key intermediate in the biosynthetic pathways of many basidiomycete secondary metabolites (24, 40). Among them are the pulvinic acid-derived pigments variegatic acid and xerocomic acid, which are commonly found in species of the Boletales but not in species of the Paxillaceae (24, 37). The lack of these pigments in P. involutus was confirmed in this study. These observations suggest that the gain of a biosynthetic pathway for involutin in the family Paxillaceae has been accompanied by a loss of the pathways leading to variegatic acid and xerocomic acid.
The ecological function of the terphenylquinones and related derivatives is not well known. It has been proposed that variegatic acid can function as a Fe3+ reductant during Fenton-based wood decay in the bolete S. lacrymans (23). A more recent experiment has provided evidence that the hydroquinone 2,5-DMHQ is a primary Fe3+-reducing agent expressed by S. lacrymans during brown rot decay of aspen wood (25). There are also studies showing that 2,5-DMHQ is the reductant involved in Fenton-based wood decay in species from two other divergent agaricomycete lineages, the Gloeophyllales and the Polyporales (41, 42). Although the biosynthetic pathway for 2,5-DMHQ is not known, the data imply that this pathway was present in the ancestor of the Agaricomycetes (25). Neither 2,5-DMHQ nor the more stable isomer 2,5-DMBQ was detected in the mycelium or the culture filtrates during organic matter decomposition by P. involutus. Since studies of brown rot fungi have shown that the production of iron-reducing compounds and iron chelators can be affected by the composition of the medium, including the concentrations of C, N, and iron (43, 44), the possibility that P. involutus has the capacity to synthesize other Fe3+-reducing metabolites and iron chelators in addition to involutin cannot be ruled out. However, considering the fact that P. involutus was grown under conditions promoting oxidative decomposition, our results suggest that involutin is a key Fe3+ reductant secreted during Fenton-based decomposition of organic matter by P. involutus.
It has been shown that in the brown rot wood-decaying system, 2,5-DMHQ has a dual role and drives Fenton chemistry by reducing both Fe3+ and O2 (to produce H2O2), generating hydroxyl radicals (16). In our experiments, involutin produces hydroxyl radicals only in the presence of H2O2, suggesting that involutin functions as a Fe3+ reductant. H2O2 can also be generated by the activity of a number of oxidases, including laccases, glucose-methanol-choline (GMC) oxidoreductases, and copper radical oxidases, and genes encoding such enzymes are expressed by P. involutus during the decomposition of organic matter (13).
The formation of the Fe2+ that is involved in Fenton-based decomposition of lignocellulose has been extensively studied in brown rot wood-decaying fungi (45). Apart from a mechanism to reduce Fe3+ to Fe2+, an additional mechanism is needed for solubilizing the iron from the insoluble Fe (oxyhydr)oxide complexes found in plant tissues. Brown rot fungi are thought to retrieve such iron by secreting oxalic acid, which binds to and solubilizes Fe3+ from Fe (oxyhydr)oxide complexes. The oxalate tightly chelates Fe3+, making it unreactive with hydroquinones (46). Studies with Postia placenta have shown that the hydroquinone 2,5-DMHQ can be oxidized to a semiquinone by the activity of an extracellular laccase and that this reaction could generate a complete Fenton reaction system (42). The mycelia of ECM fungi are present mainly in the humus-rich region of soil horizons, where Fe3+ is found in iron oxide crystals with very low solubility, such as hematite, goethite, and ferrihydrite (47). Fe3+ is solubilized from such minerals only when it is reduced to Fe2+ or when it forms complexes with ligands such as citrate or humic acids (47). It remains to be demonstrated whether involutin is capable of releasing and reducing Fe3+ from these solid phases or whether cooperative processes, including the action of other metabolites and enzymes, such as laccases, are involved.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Research Council (VR) (to A.T. and P.P.) and the Knut and Alice Wallenberg Foundation (KAW) (to A.T.). D.S. acknowledges a doctoral fellowship from the International Leibniz Research School for Microbial Interactions (ILRS).
We thank A. Perner and H. Heinecke (Hans-Knöll-Institute Jena) for recording mass and NMR spectra, respectively.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02312-15.
REFERENCES
- 1.Myneni RB, Dong J, Tucker CJ, Kaufmann RK, Kauppi PE, Liski J, Zhou L, Alexeyev V, Hughes MK. 2001. A large carbon sink in the woody biomass of Northern forests. Proc Natl Acad Sci U S A 98:14784–14789. doi: 10.1073/pnas.261555198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ. 2001. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–780. doi: 10.1038/35081058. [DOI] [PubMed] [Google Scholar]
- 3.Rayner ADM, Boddy L. 1988. Fungal communities in the decay of wood. Adv Microb Ecol 10:115–166. doi: 10.1007/978-1-4684-5409-3_4. [DOI] [Google Scholar]
- 4.Schneider T, Keiblinger KM, Schmid E, Sterflinger-Gleixner K, Ellersdorfer G, Roschitzki B, Richter A, Eber L, Zechmeister-Boltenstern S, Riedel K. 2012. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J 6:1749–1762. doi: 10.1038/ismej.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voříšková J, Brabcová V, Cajthaml T, Baldrian P. 2014. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol 201:269–278. doi: 10.1111/nph.12481. [DOI] [PubMed] [Google Scholar]
- 6.Baldrian P, Kolařík M, Štursová M, Kopecký J, Valášková V, Větrovský T, Zifčáková L, Snajdr J, Rídl J, Vlček C, Voříšková J. 2012. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J 6:248–258. doi: 10.1038/ismej.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson K-E, Blanchette RA, Ander P. 1990. Microbial and enzymatic degradation of wood and wood components. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 8.Horwarth W. 2007. Carbon cycling and formation of soil organic matter, p 303–309. In Paul EA. (ed), Soil microbiology, ecology, and biochemistry, 3rd ed Academic Press, Oxford, United Kingdom. [Google Scholar]
- 9.Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd ed Academic Press, London, United Kingdom. [Google Scholar]
- 10.Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD. 2007. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620. doi: 10.1111/j.1469-8137.2006.01936.x. [DOI] [PubMed] [Google Scholar]
- 11.Piccolo A. 2001. The supramolecular structure of humic substances. Soil Sci 166:810–832. doi: 10.1097/00010694-200111000-00007. [DOI] [Google Scholar]
- 12.Nannipieri P, Eldor P. 2009. The chemical and functional characterization of soil N and its biotic components. Soil Biol Biochem 41:2357–2369. doi: 10.1016/j.soilbio.2009.07.013. [DOI] [Google Scholar]
- 13.Rineau F, Roth D, Shah F, Smits M, Johansson T, Canbäck B, Olsen PB, Persson P, Grell MN, Lindquist E, Grigoriev IV, Lange L, Tunlid A. 2012. The ectomycorrhizal fungus Paxillus involutus converts organic matter in plant litter using a trimmed brown-rot mechanism involving Fenton chemistry. Environ Microbiol 14:1477–1478. doi: 10.1111/j.1462-2920.2012.02736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatakka A, Hammel KE. 2010. Fungal biodegradation of lignocelluloses, p 319–340. In Hofrichter M. (ed), Mycota, vol X, 2nd ed Industrial applications. Springer, Berlin, Germany. [Google Scholar]
- 15.Goodell B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. 1997. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol 53:133–162. doi: 10.1016/S0168-1656(97)01681-7. [DOI] [Google Scholar]
- 16.Jensen KA Jr, Houtman CJ, Ryan ZC, Hammel KE. 2001. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol 67:2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldrian P, Valášková V. 2008. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 18.Vanden Wymelenberg A, Gaskell J, Mozuch M, Sabat G, Ralph J, Skyba O, Mansfield SD, Blanchette RA, Martinez D, Grigoriev I, Kersten PJ, Cullen D. 2010. Comparative transcriptome and secretome analysis of wood decay fungi Postia placenta and Phanerochaete chrysosporium. Appl Environ Microbiol 76:3599–3610. doi: 10.1128/AEM.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcombe D, Paszczynski A, Gajewska W, Kröger M, Feis G, Crawford R. 2002. Production of small molecular weight catalysts and the mechanism of trinitrotoluene degradation by several Gloeophyllum species. Enzyme Microb Technol 30:506–517. doi: 10.1016/S0141-0229(02)00014-5. [DOI] [Google Scholar]
- 20.Kohler AK, Kuo A, Nagy L, Morin E, Barry K, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa M, Doré J, Floudas D, Gay G, Girlanda M, Henrissat B, Herrmann S, Hess J, Högberg N, Johansson T, Khouja H-R, LaButti K, Lahrmann U, Levasseur A, Lindquist E, Lipzen A, Marmeisse R, Martino E, Murat C, Ngan CY, Nehls U, Plett J, Pringle A, Ohm R, Perotto S, Riley R, Rineau F, Ruytinx J, Salamov A, Shah F, Sun H, Tarkka M, Tritt A, Veneault-Fourrey C, Zuccaro A, Tunlid A, Grigoriev I, Hibbett D, Martin F. 2015. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet doi: 10.1038/ng.3223. [DOI] [PubMed] [Google Scholar]
- 21.Kerem Z, Jensen KA Jr, Hammel KE. 1999. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven Fenton reaction. FEBS Lett 446:49–54. doi: 10.1016/S0014-5793(99)00180-5. [DOI] [PubMed] [Google Scholar]
- 22.Paszczynski A, Crawford R, Funk D, Goodell B. 1999. De novo synthesis of 4,5-dimethoxycatechol and 2,5-dimethoxyhydroquinone by the brown rot fungus Gloeophyllum trabeum. Appl Environ Microbiol 65:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastwood DC, Floudas D, Binder M, Majcherczyk A, Schneider P, Aerts A, Asiegbu FO, Baker SE, Barry K, Bendiksby M, Blumentritt M, Coutinho PD, Cullen D, de Vries RP, Gathman A, Goodell B, Henrissat B, Ihrmark K, Kauserud H, Kohler A, LaButti K, Lapidus A, Lavin JL, Lee YH, Lindquist E, Lilly W, Lucas S, Morin E, Murat C, Oguiza JA, Park J, Pisabarro AG, Riley R, Rosling A, Salamov A, Schmidt O, Schmutz J, Skrede I, Stenlid J, Wiebenga A, Xie X, Kües U, Hibbett DS, Hoffmeister D, Högberg N, Martin F, Grigoriev IV, Watkinson SC. 2011. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333:762–765. doi: 10.1126/science.1205411. [DOI] [PubMed] [Google Scholar]
- 24.Gill M, Steglich W. 1987. Pigments of fungi (Macromycetes). Prog Chem Org Nat Prod 51:1–317. [DOI] [PubMed] [Google Scholar]
- 25.Korripally P, Timokhin VI, Houtman CJ, Mozuch MD, Hammel KE. 2013. Evidence from Serpula lacrymans that 2,5-dimethoxyhydroquinone is a lignocellulolytic agent of divergent brown rot basidiomycetes. Appl Environ Microbiol 79:2377–2383. doi: 10.1128/AEM.03880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards RL, Elsworthy GC, Kale N. 1967. Constituents of the higher fungi. Part IV. Involutin, a diphenylcyclopenteneone from Paxillus involutus. J Chem Soc C 6:405–409. [Google Scholar]
- 27.van Schöll L, Hoffland E, van Breemen N. 2006. Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol 170:153–163. doi: 10.1111/j.1469-8137.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 28.Shah F, Rineau F, Canbäck B, Johansson T, Tunlid A. 2013. The molecular components of the extracellular protein-degradation pathways of the ectomycorrhizal fungus Paxillus involutus. New Phytol 200:875–887. doi: 10.1111/nph.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rineau F, Shah F, Smits MM, Persson P, Johansson T, Carleer T, Troein C, Tunlid A. 2013. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J 7:2010–2022. doi: 10.1038/ismej.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodell B, Daniel G, Jellison J, Qian Y. 2006. Iron-reducing capacity of low molecular-weight compounds produced in wood by fungi. Holzforschung 60:630–636. [Google Scholar]
- 31.Feling R, Polborn K, Steglich W, Muhlbacher J, Bringmann G. 2001. The absolute configuration of the mushroom metabolites involutin and chamonixin. Tetrahedron 57:7857–7863. doi: 10.1016/S0040-4020(01)00761-X. [DOI] [Google Scholar]
- 32.Page SE, Arnold WA, McNeill K. 2010. Terephthalate as a probe for photochemically generated hydroxyl radical. J Environ Monit 12:1658–1665. doi: 10.1039/c0em00160k. [DOI] [PubMed] [Google Scholar]
- 33.Freinbichler W, Colivicchi MA, Fattori M, Ballini C, Tipton KF, Linert W, Corte LD. 2008. Validation of a robust and sensitive method for detecting hydroxyl radical formation together with evoked neurotransmitter release in brain microdialysis. J Neurochem 105:738–749. doi: 10.1111/j.1471-4159.2007.05168.x. [DOI] [PubMed] [Google Scholar]
- 34.Sheh L, Li CJ, Dai HY, Cheng V, Chiang CD, Shen NK, Yu CY. 1992. Cytotoxicity studies on some novel 2,6-dimethoxyhydroquinone derivatives. Anticancer Drug Des 7:315–327. [PubMed] [Google Scholar]
- 35.Binder M, Larson K-H, Matheny PB, Hibbett DS. 2010. Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102:865–880. doi: 10.3852/09-288. [DOI] [PubMed] [Google Scholar]
- 36.Binder M, Hibbett DS. 2006. Molecular systematics and biological diversification of Boletales. Mycologia 98:971–981. doi: 10.3852/mycologia.98.6.971. [DOI] [PubMed] [Google Scholar]
- 37.Besl H, Bresinsky A. 1997. Chemosystematics of Suillaceae and Gomphidiaceae (suborder Suillineae). Plant Syst Evol 206:223–242. doi: 10.1007/BF00987949. [DOI] [Google Scholar]
- 38.Besl H, Dorsch R, Fischer M. 1996. On the systematic position of the genus Melanogaster (Melanogastraceae, Basidiomycetes). Z Mykol 62:195–199. [Google Scholar]
- 39.Nuhn ME, Binder M, Taylor AFS, Halling RE, Hibbett DS. 2013. Phylogenetic overview of the Boletineae. Fungal Biol 117:479–511. doi: 10.1016/j.funbio.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z-Y, Liu J-K. 2010. Pigments of fungi (macromycetes). Nat Prod Rep 27:1531–1570. doi: 10.1039/c004593d. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. 2006. Fungal hydroquinones contribute to brown rot of wood. Environ Microbiol 8:2214–2223. doi: 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- 42.Wei DS, Houtman CJ, Kapich AN, Hunt CG, Cullen D, Hammel KE. 2010. Laccase and its role in the production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl Environ Microbiol 76:2091–2097. doi: 10.1128/AEM.02929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varela E, Mester T, Tien M. 2003. Culture conditions affecting biodegradation components of the brown-rot fungus Gloeophyllum trabeum. Arch Microbiol 180:251–256. doi: 10.1007/s00203-003-0583-y. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Jellison J. 1994. Regulation of hyphal sheath formation and iron-chelator production by the brown rot fungi Gloeophyllum trabeum and Postia placenta. Document IRG/WP 94-10074. International Research Group on Wood Preservation, Stockholm, Sweden. [Google Scholar]
- 45.Arantes V, Jellison J, Goodell B. 2012. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl Microbiol Biotechnol 94:323–338. doi: 10.1007/s00253-012-3954-y. [DOI] [PubMed] [Google Scholar]
- 46.Varela E, Tien M. 2003. Effect of pH and oxalate on hydroquinone-derived hydroxyl radical formation during brown rot wood degradation. Appl Environ Microbiol 69:6025–6031. doi: 10.1128/AEM.69.10.6025-6031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwertmann U, Taylor RM. 1989. Chapter 8. Iron oxides, p 379–438. In Dixon JB, Weed SB (ed), Minerals in soil environments, 2nd ed Soil Science Society of America, Madison, WI. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


