Abstract
Resistant starch (RS) exacerbates health benefits on the host via modulation of the gut bacterial community. By far, these effects have been less well explored for RS of type 4. This study aimed at gaining a community-wide insight into the impact of enzymatically modified starch (EMS) on the cecal microbiota and hindgut fermentation in growing pigs. Castrated male pigs (n = 12/diet; 29-kg body weight) were fed diets with either 70% EMS or control starch for 10 days. The bacterial profile of each cecal sample was determined by sequencing of the V345 region of the 16S rRNA gene using the Illumina MiSeq platform. EMS diet reduced short-chain fatty acid concentrations in cecum and proximal colon compared to the control diet. Linear discriminant analyses and K means clustering indicated diet-specific cecal community profiles, whereby diversity and species richness were not different among diets. Pigs showed host-specific variation in their most abundant phyla, Firmicutes (55%), Proteobacteria (35%), and Bacteroidetes (10%). The EMS diet decreased abundance of Ruminococcus, Parasutterella, Bilophila, Enterococcus, and Lactobacillus operational taxonomic units (OTU), whereas Meniscus and Actinobacillus OTU were increased compared to those with the control diet (P < 0.05). Quantitative PCR confirmed results for host effect on Enterobacteriaceae and diet effect on members of the Lactobacillus group. The presence of less cecal short-chain fatty acids and the imputed metabolic functions of the cecal microbiome suggested that EMS was less degradable for cecal bacteria than the control starch. The present EMS effects on the bacterial community profiles were different than the previously reported RS effects and can be linked to the chemical structure of EMS.
INTRODUCTION
The commensal gut microbiota and their metabolic products interact with the host in many different ways, thereby influencing gut homeostasis, host metabolism, and health (1). Well-known nutrition-based strategies that offer a route to modulate the gut microbiota are the use of dietary fiber and analogous carbohydrates—among them resistant starches (RS) (1–3). Research interest in RS candidates is continuously high due to the many physiological benefits of prospering gut and systemic health that are associated with the variety of microbial metabolites originating from the fermentation of starch that escaped digestion in the small intestine (4–6). From the five types of RS described so far, the three types that can be found in nature (RS1, physically inaccessible starch; RS2, native granular starch consisting of ungelatinized granules; RS3, retrograded amylose) have been rigorously studied (4, 7). Comparatively little is known about types 4 (RS4) and 5 (RS5), representing starches that have been chemically modified by esterification, cross-linking, or transglycosylation and which comprise amylose-lipid complexes, respectively (4, 7). Due to the possibility to control the reduction in starch digestibility by targeted modification of the starch molecule, chemically modified starches (CMS) currently receive more attention in nutrition research as potential RS4 candidates (4). CMS can be commonly found in processed foods, where they are added to improve the rheological properties and texture of food items (4, 7, 8). Yet the specific RS4 and potential prebiotic properties still need to be established for many CMS. Due to the specific nature of CMS, their physiological and microbial effects may differ from those reported for RS2 and RS3 and between the various RS4 candidates, rendering it necessary to evaluate the impact of CMS on gut microbiota and gut homeostasis one by one (4, 7, 9). In using a deep pyrosequencing approach with the 16S rRNA gene, comparison of RS2 and RS4 intake was recently demonstrated to lead to diverging bacterial communities in human stool (9), which the authors associated with differential substrate-binding abilities of bacteria regarding RS2 and RS4. For instance, RS2 increased the fecal abundance of important butyrate producers Ruminococcus bromii and Eubacterium rectale, whereas RS4 enhanced Bifidobacterium adolescentis and Parabacteroides distasonis (9). Changes in the chemical structure of starch, such as cross-linking, esterification, or tranglycosylation, not only restrict the hydrolysis of the starch molecule by host enzymes but may also limit the starch hydrolysis by bacterial amylases (4). Gut bacterium-mediated starch breakdown includes α-amylase, type I pullulanases, and amylopullulanases (10, 11), and starch-degrading activity has been reported for three major phyla in the mammalian gut, Firmicutes, Bacteroidetes, and Actinobacteria, including Bifidobacterium, Butyrivibrio, Roseburia, Eubacterium, and Bacteroides strains (4, 12). By metabolic cross-feeding of fermentation metabolites, such as lactate and acetate, other gut bacteria and Archaea may profit from dietary starch as well (4, 12).
Generation of lactic acid and short-chain fatty acids (SCFA) from RS2 and RS3 breakdown is highest in the proximal hindgut and progressively decreases as digesta move toward the distal gut, leading to diverging fermentation metabolites and bacterial profiles in the different gut segments (13, 14, 15). Although fecal samples may be more easily obtained, it may be more relevant to evaluate effects of RS4 on the gut bacterial community at the gut sites with the highest fermentation intensity. Additionally, implementation of nontargeted novel deep-sequencing approaches may be useful to identify the bacterial taxa that interact with dietary RS4 candidates (4).
In the present study, we evaluated the impact of enzymatically modified starch (EMS) on the cecal digesta-associated bacterial microbiome and fermentation metabolites in growing pigs. We performed a comprehensive characterization of the porcine cecal microbiota by using Illumina MiSeq sequencing of 16S rRNA tags in combination with quantitative PCR (qPCR). Based on the expected changes in the chemical structure of the original standard waxy cornstarch, we hypothesized that the cecal bacterial community would show different profiles between pigs fed diets based on EMS and those fed on waxy cornstarch and that these differences at the community level would come along with differences in the profiles of primary fermentation metabolites along the gastrointestinal tract. As we observed the greatest effect of the EMS diet on SCFA in the cecum, we characterized the bacterial microbiomes in this segment. In addition, effects of EMS consumption on feed intake and body weight gain were assessed as evidence for potential systemic effects of EMS.
MATERIALS AND METHODS
Animals and diets.
All procedures involving animal handling and treatment were approved by the Institutional Ethics Committee of the University of Veterinary Medicine and the national authority according to §26 of “Law for Animal Experiments,” Tierversuchsgesetz—TVG (GZ 68.205/0063-WF/II3b/2014). Twenty-four castrated male pigs ([Landrace × Large White] × Piétrain; average body weight [BW], 29 ± 2.8 kg) were used in a block-randomized design with 3 blocks of 8 pigs. Pigs were housed individually in metabolism cages (1.20 m by 1.00 m) with Plexiglas walls. The cages were equipped with a heating lamp. Pigs had free access to demineralized water. During the 9-day adaptation prior to the experiment, pigs were fed a commercial cereal-based grower diet (metabolizable energy, 13.4 MJ/kg; crude protein, 16.8% on an as-fed basis). During the last 2 days of the adaptation, pigs were transitioned from the grower to the experimental diets by blending the grower diet with the experimental diets (50/50 vol/vol) to facilitate the diet change. Pigs were randomly allocated to the 2 feeding groups. Four pigs per block received one of the two experimental diets, resulting in a total of 12 observations per diet. The experimental diets were prepared as mash and were fed semi-ad libitum for 10 days. Pigs were offered feed three times daily at 8:00, 12:00, and 16:30 h. Feed allowances were calculated to surpass the pig's appetite. Feed weigh-backs (spillage and feed left in feeding bowls) were collected after feeding, and a subsample was dried (as described below) to determine the dry matter (DM) intake. One pig of the first block and receiving the EMS diet showed symptoms of illness during the experimental period and was removed from the experiment. All other pigs were clinically healthy throughout the experiment.
Experimental diets were formulated to meet or exceed current recommendations for nutrient requirements (16) and were comprised mainly of cornstarch and casein (Table 1). The control diet contained standard rapidly digestible waxy cornstarch (Agrana Stärke GmbH, Gmünd, Austria). The test starch diet contained Agenanova (Agrana Stärke GmbH, Gmünd, Austria), which is an enzymatically modified cornstarch product (EMS) prepared from waxy cornstarch which consists of a uniform molecular weight distribution and increased branching of the starch molecules (17). The amylopectin fraction of the EMS contains twice as many α-1,6-glycosidic bonds (approximately 8%) as the nonmodified native waxy cornstarch (4% α-1,6-glycosidic bonds). The enzymatic treatment of waxy cornstarch also reduced the molecular weight. Measurement of the dextrose equivalent (DE), as a measure of the amount of reducing sugars relative to glucose (on a dry matter basis), of the EMS showed a value below 1, indicating that almost no low-molecular-weight sugars were present. Mono- and disaccharides were absent to the greatest possible extent.
TABLE 1.
Dietary ingredients and analyzed chemical composition of the control and EMS diets
| Parameter | Result for diet: |
|
|---|---|---|
| Control | EMS | |
| Ingredient (%) | ||
| Waxy cornstarch | 72.1 | |
| EMSa | 72.1 | |
| Casein | 18.0 | 18.0 |
| Lignocelluloseb | 4.0 | 4.0 |
| Rapeseed oil | 1.0 | 1.0 |
| Vitamin-mineral premixc | 4.3 | 4.3 |
| Monocalcium phosphate | 0.6 | 0.6 |
| Analyzed chemical composition on DM basis (g/kg) | ||
| DM | 938 | 949 |
| Crude protein | 167 | 166 |
| Total starchd | 668 | 687 |
| Resistant starche | 6.6 | 1.5 |
| Calcium | 8.1 | 8.0 |
| Phosphorus | 5.0 | 4.8 |
| Gross energy (MJ/kg) | 16.8 | 16.9 |
Agenanova (Agrana Stärke, Gmünd, Austria).
FiberCell (Agromed Austria GmbH, Kremsmünster, Austria).
Includes the following provided per kilogram of complete diet (Garant GmbH, Pöchlarn, Austria): 16,000 IU vitamin A, 2,000 IU vitamin D3, 125 mg vitamin E, 2.0 mg vitamin B1, 6.0 mg vitamin B2, 3.0 mg vitamin B6, 0.03 mg vitamin B12, 3.0 mg vitamin K3, 30 mg niacin, 15.0 mg pantothenic acid, 900 mg choline chloride, 0.15 mg biotin, 1.5 mg folic acid, 200 mg vitamin C, 4.6 g Ca, 2.3 g as digestible P, 2.4 g as Na, 2.0 g Cl, 3.2 g K, 1.0 g Mg, 50 mg Mn (as MnO), 100 mg Zn (as ZnSO4), 120 mg Fe (as FeSO4), 15.6 mg Cu (as CuSO4), 0.5 mg Se (as Na2SeO3), 1.9 mg of I [as Ca(IO3)2], and 3 g TiO2.
Total starch was determined using the K-TSTA total starch assay kit (Megazyme International Ireland, Ltd., Bray, Ireland).
Resistant starch was determined using the K-STAR resistant starch assay kit (Megazyme International Ireland, Ltd., Bray, Ireland).
Slaughtering and sampling.
On day 11, pigs were euthanized with 10 ml/kg body weight embutramide (MSD Animal Health, Vienna, Austria) after general anesthesia with 10 ml/kg body weight ketamine HCl (Narketan; Vétoquinol AG, Ittigen, Austria) or 3 ml/kg body weight azaperone (Stresnil; Biokema SA, Crissier, Switzerland) 2 to 3 h after their last meal to ensure that the upper gut would contain digesta. Thereafter, the abdominal cavity was opened, and the entire gastrointestinal tract was removed. The small and large intestines were carefully dissected from the mesentery. To prevent mixing of digesta among gut sites, gut sites (stomach, duodenum, jejunum, ileum, cecum, proximal, and mid- and distal colon) were immediately separated using clamps. Digesta from the stomach were collected by cutting the stomach wall 5 cm cranial to the pylorus and completely emptying its content into a sterile container. Digesta from the ileum (last 30 cm of the small intestine), cecum, and proximal colon (30 cm distal to the cecum) and midcolon (30 cm) were aseptically collected. Because the length of the gut varies between individuals, the colon was divided into 3 equal parts to identify the midcolon region. Collected digesta of each gut site were thoroughly homogenized before subsamples of digesta were taken. Digesta subsamples for bacterial community analysis were snap-frozen in liquid nitrogen, and stored at −80°C, whereas subsamples for measurements of fermentation metabolites (SCFA and lactate), pH, and dry matter were placed on ice until transfer to −20°C.
Chemical analysis.
Diets were analyzed in duplicate for dry matter, crude protein (N × 6.25), ash, Ca, and P using standard methods (18), and gross energy of diets was measured by combustion using a bomb calorimeter (C200 system; IKA-Werke GmbH & Co KG, Staufen, Germany). Dietary total starch and resistant starch contents were determined using enzymatic kits (K-TSTA and K-STAR; Megazyme International Ireland, Ltd., Bray, Ireland). The pH in cecal, proximal, and midcolonic digesta subsamples was measured using a Beckman Φ63 pH meter (Beckman Coulter, Fullerton, CA), and the DM content of digesta was determined (18). Double-distilled water was added to colonic digesta (vol/vol) to allow their pH to be read. For SCFA analysis, cecal and proximal and midcolonic digesta subsamples were thawed on ice, an aliquot of 1 g of digesta was mixed with 0.2 ml of 25% metaphosphoric acid, 1 ml of double-distilled water, and 200 μl of internal standard (4-methyl-valeric acid; Sigma-Aldrich, Vienna, Austria), and the mixture was centrifuged at 3,148 × g for 10 min (centrifuge 5810 R; Eppendorf, Hamburg, Germany). The supernatant was centrifuged at 15,000 × g for 25 min (centrifuge 5424; Eppendorf), and the clear supernatant was analyzed for SCFA (acetate, propionate, butyrate, iso-butyrate, valerate, iso-valerate, and caproate) using gas chromatography (19). d-Lactate and l-lactate in gastric, cecal, and colonic digesta were analyzed using a photometric method and a commercially available total lactate kit (Megazyme International Ireland, Ltd., Bray, Ireland) after deproteinization with 1 N perchloric acid.
Genomic DNA isolation and purification.
Total DNAs were extracted from 250-mg digesta samples from cecum using a PowerSoil DNA extraction kit (MoBio Laboratories, Inc., Carlsbad, CA) as described by Metzler-Zebeli et al. (20). To ensure proper lysis of bacteria, a heating step at 70°C for 10 min was introduced between mixing of the digesta sample with buffer C1 and the bead-beating step. DNA was dissolved in 60 μl of elution buffer provided by the extraction kit. Eluted DNA was purified using the NucleoSpin tissue kit (Macherey-Nagel, Düren, Germany). The genomic DNA concentration was determined by a Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA) using the Qubit double-stranded DNA (dsDNA) HS assay kit (Life Technologies) and ranged from 2 to 55 ng/μl. For qPCR assays, sample volumes were adjusted to achieve similar DNA concentrations across samples (21).
16S rRNA gene PCR, library preparation, and DNA sequencing.
Amplicon sequencing was performed using Illumina MiSeq sequencing technology. The V345 hypervariable region of bacterial 16S rRNA genes was amplified using the primer set 341F (CCTACGGGRSGCAGCAG) and 909R (TTTCAGYCTTGCGRCCGTAC) to generate an approximate amplicon size of 568 bp. 16S rRNA gene PCRs, library preparation, and sequencing were performed by Microsynth (Balgach, Switzerland). Libraries were constructed by ligating sequencing adapters and indices onto purified PCR products using the Nextera XT sample preparation kit (Illumina) according to the recommendations of the manufacturer. Equimolar amounts of each of the libraries were pooled and submitted for sequencing on an Illumina MiSeq personal sequencer using a 300-bp-read-length paired-end protocol. After sequencing, the corresponding overlapping paired-end reads were stitched by Microsynth.
Sequence processing and sequence analysis of gene amplicons.
Sequencing data were processed using the software package mothur (22), following the MiSeq standard operating procedure (SOP) (23, 24). Briefly, sequences shorter than 500 bp or with an average quality score of less than 35, using a window size of 50 or containing ambiguities, were removed. After trimming, the reads from each sample were randomly subsampled to yield approximately 50,000 reads per sample, and subsequently the reads from all samples were merged. Reads were aligned using the SILVA SSU 119 reference database, PCR errors were reduced using the “pre.cluster” option in mothur, and chimeric sequences were removed using “chimera.uchime.” Sequences were clustered into operational taxonomic units (OTU) using a 16S rRNA distance of 0.03. Taxonomy was assigned to the OTU using the Ribosomal Database Project (RDP) naive Bayesian rRNA classifier (25) and the RDP reference training set version 9 available at the mothur website. All OTU containing less than 10 sequences were removed. A heat map was created using JColorGrid (26) and Adobe Illustrator CS2 (version 12.0.1; Adobe Systems, Inc., San Jose, CA). The Venn diagram was calculated in mothur. Cluster analysis was done with Cluster 3.0 (Clustering Library version 1.52 [27]). OTU detected in both feeding groups (347 OTU) were centralized and K means clustering (8 clusters) was performed based on the similarity metric “Euclidean distance.” The file was visualized in TREEVIEW (28).
qPCR.
Based on the sequencing data, qPCR assays were designed and performed with the Stratagene Mx3000P qPCR system (Agilent Technologies, Santa Clara, CA) to quantify the abundance of 16S rRNA genes of total bacteria and five target bacterial groups (i.e., Lactobacillus group, Enterobacteriaceae, Clostridium cluster IV, Clostridium cluster I, and Campylobacter spp.) using previously reported primer sets and amplification conditions (20). Quantification of DNA in digesta samples was performed using Brilliant II SYBR green qPCR low ROX master mix (Agilent Technologies), forward and reverse primers (62.5 pmol/μl), and 1 μl of genomic DNA in a final volume of 25 μl. All qPCR amplifications consisted of initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, annealing for 30 s, and elongation at 72°C for 30 s (20). Fluorescence was measured at the last step of each cycle. Standards and samples were run on the same plate in duplicate. Negative controls without template DNA were included in triplicate. Melting curve analysis was performed to determine the specificity of the amplification. The dissociation of PCR products was monitored by slow heating with an increment of 0.1°C/s from 55 to 95°C, with fluorescence measurement at 0.1°C intervals. The PCR product length was additionally verified by horizontal gel electrophoresis of a 10-μl aliquot in a 2% agarose gel in Tris-acetate-EDTA (40 mM Tris acetate, 1 mM EDTA, pH 8.5) containing Midori green advanced DNA stain (Nippon Genetics Europe GmbH, Dueren, Germany) for visualization of DNA bands under UV light.
For quantification of bacterial 16S rRNA gene copies, standards were prepared by making serial dilutions (107 to 103 molecules/μl) of the purified and quantified PCR products generated by standard PCR and genomic DNA from pig intestinal digesta using the universal primer set 27F-1492R and group-specific primers (20). Amplification efficiencies (E) were calculated according to the following equation: E = 10−1/slope (93 to 97%). Linear relationships between quantification cycle (Cq) and log of DNA concentration were observed for each primer pair (R2 = 0.996 to 0.999). Gene copy numbers of total bacteria and target bacterial groups were determined by relating the Cq values to standard curves (20). The final copy numbers of total bacteria and target bacterial groups per gram of digesta were calculated using the following equation: (QM × C × DV)/(S × V), where QM is the quantitative mean of the copy number, C was the DNA concentration of each sample, DV is the dilution volume of extracted DNA, S is the DNA amount (nanograms) subjected to analysis, and V is the weight of the sample (grams) subjected to DNA extraction (20). To minimize the errors of absolute quantification of DNA from gut digesta samples, cecal abundance of target bacterial groups was expressed relative to the amplification of reference primers utilizing the experimentally derived amplification efficiency for each primer set (29). The percentage of each target bacterial group was obtained by dividing the gene copies of the 16S rRNA gene of the target group by the 16S rRNA genes amplified with the universal bacterial primer set (30).
Predictive functional profiling of microbial communities.
The tool PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) was used to predict the metagenome functional content from the 16S rRNA data set. PICRUSt is a computational approach based on evolutionary modeling that relies on the assumption that phylogeny can predict the gene content (31). For PICRUSt, controlled quality sequences were aligned to Greengenes reference OTU (downloaded from http://greengenes.secondgenome.com/downloads/database/13_5), clustered based on a 0.03-distance limit, and processed in the online Galaxy PICRUSt interface (http://galaxyproject.org/) with a workflow described by the developers (http://picrust.github.com/picrust/tutorials/quickstart.html#quickstartguide). Sequences were categorized by function based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in PICRUSt.
Statistical analyses.
To compare differences among the two diets, data were subjected to analysis of variance (ANOVA) using the MIXED procedure of SAS (version 9.3; SAS Institute, Inc., Cary, NC) using the pig as the experimental unit. Means were reported as least-squares means ± standard error of the mean (SEM), and P ≤ 0.05 and 0.05 < P < 0.10 were defined as indicating significance and trend, respectively. For cecal metagenome and qPCR data, fixed effects of diet and block as well as the random effect of pig within period were included in the main model. SCFA and lactate data were analyzed as repeated measures over gut sites, including the fixed effects of block, diet, gut site, and diet × gut site. Degrees of freedom were approximated using the Kenward-Roger method (ddfm = kr), where ddfm represents the denominator degrees of freedom for the model and kr represents the Kenward-Roger type. Linear discriminate analysis (LDA) was performed using JMP10 software (SAS Stat, Inc.) with the 30 most abundant genera as covariates and diet as the categorical variable. The LDA results were visualized using the first 2 principal components of the scores plot to identify characteristic trends or grouping among pigs fed the two diets.
Sequencing data accession number.
Raw sequencing data are available in NCBI's BioProject SRA database under accession no. PRJEB8696.
RESULTS
Dietary nutrient composition, performance, and digesta characteristics.
The chemical compositions of the diets are presented in Table 1. Diets were iso-caloric and iso-nitrogenous and contained about 68% total starch and 17% crude protein on a dry matter basis. The RS content of the EMS diet when determined with a commercial enzymatic kit was smaller than that of the control diet. Pigs fed the EMS diet had a 270-g higher (P < 0.05) daily dry matter intake than pigs fed the control diet (Table 2). However, average daily gain was not different between pigs fed the two diets (P > 0.1), resulting in the higher feed conversion ratio for pigs fed the EMS diet.
TABLE 2.
Feed intake and growth performance of pigs fed the EMS or control dieta
| Parameter | Result for diet group: |
SEM | P value | |
|---|---|---|---|---|
| Control | EMS | |||
| Dry matter intake (g) | 978 | 1,248 | 35.5 | <0.001 |
| Avg daily gain (g) | 779 | 813 | 32.8 | 0.471 |
| Feed conversion ratio (g/g) | 1.25 | 1.56 | 0.07 | 0.003 |
Data are presented as least-square means ± SEM. n =8 pigs in the control diet group, and n = 7 pigs in the EMS diet group.
Digesta DM content tended (P < 0.1) to be lower by 2 to 3% in pigs fed the EMS diet compared to pigs fed the control diet and increased (P < 0.010) by 13% from cecum to midcolon (Table 3). Likewise, digesta pH was affected (P < 0.001) by gut site and increased from cecum to midcolon, whereas diet type had little influence on digesta pH. Only cecal pH tended (P < 0.1) to be 0.2 log units higher in pigs fed the EMS diet than that in pigs fed the control diet. Lactate concentrations in digesta were affected neither by gut site nor by diet type (Table 3). The concentration of total SCFA and, except for iso-valerate and caproate, the concentrations of all individual SCFA in digesta of the cecum and proximal colon were higher than those in midcolonic digesta (P < 0.05) (Table 3). The EMS diet reduced (P < 0.05) propionate, iso-valerate, and valerate and tended (P < 0.1) to reduce iso-butyrate in digesta of the cecum and proximal colon, thereby reducing the total SCFA concentration in cecum by 20 μmol/g compared to that with the control diet (P < 0.05). There were diet × gut site interactions (P < 0.05) for propionate and iso-valerate, indicating that propionate and iso-valerate concentrations were affected by diet in the cecum and proximal colon but not in the midcolon.
TABLE 3.
DM content, pH, and concentrations of lactate and SCFA of gut digesta in pigs fed the EMS or control dieta
| Parameter | Result for diet groupb |
SEM | Diet P valuec | |||||
|---|---|---|---|---|---|---|---|---|
| Cecum |
Proximal colon |
Midcolon |
||||||
| Control | EMS | Control | EMS | Control | EMS | |||
| DM (%) | 21.2 A | 18.8 B | 25.9 A | 23.4 B | 34.2 A | 31.8 B | 0.96 | 0.030 |
| pH | 7.5 B | 7.7 A | 8.0 | 8.0 | 8.7 | 8.7 | 0.10 | 0.328 |
| Concn of fermentation acids (μmol/g digesta) | ||||||||
| Total lactate | 0.99 | 1.71 | 1.61 | 1.81 | 1.25 | 1.04 | 0.37 | 0.439 |
| l-Lactate | 0.82 | 1.23 | 1.13 | 1.2 | 1.08 | 0.82 | 0.22 | 0.702 |
| d-Lactate | 0.17 | 0.47 | 0.48 | 0.62 | 0.17 | 0.22 | 0.16 | 0.230 |
| Total SCFA | 127.0 a | 104.5 b | 117.6 | 106.4 | 56.4 | 60.2 | 6.52 | 0.057 |
| Acetate | 79.7 A | 68.9 B | 73.3 | 69.8 | 33.1 | 36.0 | 4.37 | 0.295 |
| Propionate | 30.9 a | 22.5 b | 28.2 a | 22.0 b | 13.3 | 13.5 | 1.59 | 0.001 |
| Iso-butyrate | 4.0 A | 3.5 B | 4.2 A | 3.7 B | 2.9 | 3.2 | 0.22 | 0.039 |
| Butyrate | 6.9 | 5.6 | 6.4 | 6.5 | 3.5 | 3.6 | 0.68 | 0.564 |
| Iso-valerate | 2.8 a | 1.9 b | 2.9 a | 2.2 b | 2.2 | 2.5 | 0.20 | 0.003 |
| Valerate | 2.5 a | 1.9 b | 2.4 a | 1.9 b | 1.3 | 1.3 | 0.16 | 0.017 |
| Caproate | 0.22 | 0.19 | 0.21 | 0.21 | 0.17 | 0.17 | 0.03 | 0.700 |
Data are presented as least-square means ± SEM. n = 8 pigs in the control diet group, and n = 7 pigs in the EMS diet group.
Different capital letters at one gut site indicate a tendency (P < 0.1) for a difference between the EMS and control diets at this gut site. Different lowercase letters at one gut site indicate significant difference (P < 0.05) between the EMS and control diets at this gut site.
Gut site affected (P < 0.05) all variables except lactate, iso-valerate, and caproate. Diet × gut site interactions are shown for propionate (P = 0.032) and iso-valerate (P = 0.028).
Sequencing metrics.
Shifts in the cecal bacterial community structure were examined using MiSeq Illumina 16S rRNA gene amplicon sequencing to identify bacterial OTU that responded to the change in the dietary starch source. A total of 14,165,641 stitched reads were generated from the 23 cecal digesta samples. After subsampling and quality control, 1,133,253 reads remained, accounting for approximately 50,000 paired reads per digesta sample (range, 47,016 to 51,149). Sequences were classified into 2,762 OTU containing at least 10 sequences per OTU. These OTU were used for downstream analysis. The Venn diagram (see Fig. S1 in the supplemental material) revealed a high overlap of OTU among both diets. Overall, 1,374 OTU were shared among cecal digesta of all pigs, whereas 841 and 547 OTU were specifically associated with the intake of the control and EMS diets, respectively. Although only 49.7% of all OTU were shared among the two diets, these shared OTU represented the abundant OTU and corresponded to 95% of all reads. Analysis of community diversity and community richness of the cecal communities (see Table S1 in the supplemental material) revealed that both diversity and richness were not related (P < 0.1) to the dietary starch source.
General cecal community composition.
The most abundant phyla for all samples were the Firmicutes (55.0%), Proteobacteria (34.6%), and Bacteroidetes (9.7%) (Table 4), whereas other phyla were far less abundant (<0.25%). At the genus level, seven genera had >3% relative abundance: Escherichia (31.6%), Clostridium cluster IV (9.8%), Ornithobacterium (8.5%), Clostridium sensu stricto (6.2%), Turicibacter (4.7%), Acetivibrio (3.9%), and Gracilibacter (3.1%) (see Table S2 in the supplemental material). Of the classifiable species, Escherichia (26.6%) was the predominant OTU, and only 8 other species had >1% relative abundance belonging to the genera Ornithobacterium, Turicibacter, Eubacterium, Clostridium cluster IV (2 OTU), Clostridium sensu stricto, Desulfovibrio, Acetivibrio, and Gracilibacter (see Table S3 and Fig. S2 in the supplemental material).
TABLE 4.
Microbiome composition at the phylum level for 16S rRNA sequences in cecal digesta of pigs fed the EMS or control dieta
| Phylumb | Relative abundance (%) by diet |
SEM | P value | |
|---|---|---|---|---|
| Control | EMS | |||
| Firmicutes | 59.9 | 50.0 | 4.038 | 0.098 |
| Proteobacteria | 29.6 | 39.5 | 5.070 | 0.182 |
| Bacteroidetes | 9.7 | 9.8 | 2.153 | 0.965 |
| Verrucomicrobia | 0.21 | 0.02 | 0.124 | 0.306 |
| Actinobacteria | 0.11 | 0.11 | 0.017 | 0.994 |
| Synergistetes | 0.02 | 0.02 | 0.007 | 0.852 |
| Elusimicrobia | 0.02 | 0.01 | 0.010 | 0.583 |
| Tenericutes | 0.03 | 0.02 | 0.008 | 0.215 |
| Planctomycetes | 0.016 | 0.004 | 0.007 | 0.231 |
Data are presented as least-square means ± SEM. n = 8 pigs in control diet group, and n = 7 pigs in the EMS diet group.
The most abundant phyla are shown, accounting for 99.6 and 99.4% of the sequences for the control diet and EMS diet, respectively.
Diet-related changes in the cecal bacterial community.
The phyla Firmicutes and Proteobacteria showed numerical differences in the relative abundance of 10% between the two pig groups (Table 4). However, only for the Firmicutes phylum could a trend (P < 0.1) for a lower abundance in pigs fed the EMS diet compared to the control diet be established. Of the 30 most abundant genera, Ruminococcus and Parasutterella were less abundant (P < 0.01) in pigs fed the EMS diet than in those fed the control diet (Table 5). The EMS diet further tended (P < 0.1) to decrease Gracilibacter and Anaerovibrio and to increase the relative abundance of Meniscus, Campylobacter, and Acetanaerobacterium compared to the control diet. At the species level, diet effects were investigated at an average relative abundance of >0.03%. Alterations in the relative species abundance were in accordance with those at the genus level: OTU tentatively identified as Meniscus glaucopis (P < 0.05), Campylobacter lanienae (P < 0.1), Oscillibacter valericigenes (P < 0.1), and Actinobacillus capsulatus (P < 0.05) were more abundant, whereas Ruminococcus bromii (P < 0.05), Parasutterella (P < 0.05), Desulfovibrio desulfuricans (P < 0.05), Lactobacillus gasseri (P < 0.1), and Enterococcus durans (P < 0.05) decreased in pigs fed the EMS diet compared to pigs fed the control diet (Table 6). Due to animal-to-animal variation, the observed 10% higher abundance of the Escherichia OTU in pigs fed the EMS diet compared to pigs fed the control diet was numerical only.
TABLE 5.
Genus-level differences in cecal digesta of pigs fed the EMS or control dieta
| Genus | Relative abundance (%) by diet |
SEM | P valueb | |
|---|---|---|---|---|
| Control | EMS | |||
| Gracilibacter | 4.62 | 1.61 | 1.290 | 0.084 |
| Anaerovibrio | 3.01 | 1.75 | 0.464 | 0.069 |
| Meniscus | 1.26 | 2.46 | 0.407 | 0.051 |
| Campylobacter | 0.31 | 2.05 | 0.671 | 0.084 |
| Ruminococcus | 1.21 | 0.075 | 0.265 | 0.007 |
| Acetanaerobacterium | 0.63 | 0.29 | 0.132 | 0.085 |
| Parasutterella | 0.61 | 0.17 | 0.100 | 0.006 |
Data are presented as least-square means ± SEM. n = 8 pigs in control diet group, and n = 7 pigs in the EMS diet group.
Statistical comparisons were made for the 30 most abundant genera. Only values for genera that were different (significance, P < 0.05, or trend, P < 0.1) are presented.
TABLE 6.
Species-level differences in cecal digesta of pigs fed the EMS or control dieta
| OTU no. | Sequence taxonomy (accession no.) | Similarity (%) | Relative abundance (%) by diet |
SEM | P valueb | |
|---|---|---|---|---|---|---|
| Control | EMS | |||||
| OTU 10 | Meniscus glaucopis ATCC 29398 (GU269545) | 83.0 | 0.87 | 1.89 | 0.310 | 0.032 |
| OTU 14 | Campylobacter lanienae NCTC 13004 (AF043425) | 96.8 | 0.24 | 1.72 | 0.567 | 0.080 |
| OTU 22 | Oscillibacter valericigenes NBRC 101213 (AB238598) | 91.2 | 0.11 | 0.76 | 0.223 | 0.054 |
| OTU 38 | Ruminococcus bromii ATCC 27255 (NR_025930) | 99.0 | 0.46 | 0.001 | 0.116 | 0.011 |
| OTU 46 | Parasutterella sp. strain YIT 12071 (AB491209) | 97.1 | 0.29 | 0.05 | 0.067 | 0.024 |
| OTU 60 | Desulfovibrio desulfuricans ATCC 29577, Essex 6 (AF192153) | 89.4 | 0.15 | 0.09 | 0.020 | 0.046 |
| OTU 82 | Lactobacillus gasseri ATCC 33323 (AF519171) | 99.3 | 0.15 | 0.02 | 0.046 | 0.064 |
| OTU 119 | Enterococcus durans DSM20633 (AJ276354) | 99.0 | 0.04 | 0.01 | 0.009 | 0.041 |
| OTU 153 | Actinobacillus capsulatus CCUG 12396 (AY362886) | 96.2 | 0.01 | 0.05 | 0.013 | 0.037 |
Data are presented as least-square means ± SEM. n = 8 pigs in the control diet group, and n = 7 pigs in the EMS diet group. Taxonomy for each OTU is provided at the highest classifiable level. OTU are based on an OTU definition of a 16S rRNA distance of 0.03. Similarity values to best hit to type strains in the ribosomal database project (RDP) are given.
Statistical comparisons were made only on those OTU whose average among pigs exceeded 0.03% reads. Only values for OTU that were different (significance, P < 0.05, or trend, P < 0.1) are presented.
qPCR data.
Total bacterial 16S rRNA gene abundance and diet-affected bacterial taxa belonging to Proteobacteria and Firmicutes with known importance for porcine gut health were quantified using qPCR. Overall, total bacterial gene copy numbers were not affected by diet (Table 7). The relative abundance of the Lactobacillus group ranged from 0.22 to 60.5% of total bacterial 16S rRNA gene copy numbers and was reduced (P = 0.013) by 19% in cecal digesta of pigs fed the EMS diet compared to pigs fed the control diet. The qPCR data confirmed the wide abundance range for Enterobacteriaceae, ranging from 3 to 95% of total bacterial 16S rRNA gene copies in the 23 pigs. However, the cecal abundances of Clostridium cluster IV, Clostridium cluster I, Enterobacteriaceae, and Campylobacter spp. were not different in pigs fed the two diets.
TABLE 7.
Effect of diet and abundance range of the gene copy numbers of total bacterial and group-specific 16S rRNA genes in cecal digesta of pigs fed the EMS or control dieta
| Parameter | Range | Result by diet |
SEM | P value | |
|---|---|---|---|---|---|
| Control | EMS | ||||
| Total bacteria (log10 gene copies/g digesta) | 7.8–10.3 | 9.7 | 9.5 | 0.18 | 0.348 |
| % of total eubacterial 16S rRNA | |||||
| Total coverageb | 11–118 | 73.0 | 56.6 | 10.54 | 0.288 |
| Lactobacillus group | 0.22–60.5 | 26.8 | 8.1 | 4.72 | 0.013 |
| Clostridium cluster IV | 0.01–17.2 | 7.7 | 5.1 | 1.18 | 0.583 |
| Clostridium cluster I | <0.01–3.0 | 0.25 | 0.32 | 0.24 | 0.837 |
| Enterobacteriaceae | 3.2–94.6 | 35.5 | 45.0 | 8.85 | 0.461 |
| Campylobacter spp. | 0–3.3 | 0.25 | 0.83 | 0.272 | 0.288 |
Data are presented as least-square means ± SEM. n = 8 pigs in the control diet group, and n = 7 pigs in the EMS diet group.
Coverage of total bacterial 16S rRNA gene copies by the five bacterial group primers.
Linear discriminant and cluster analyses.
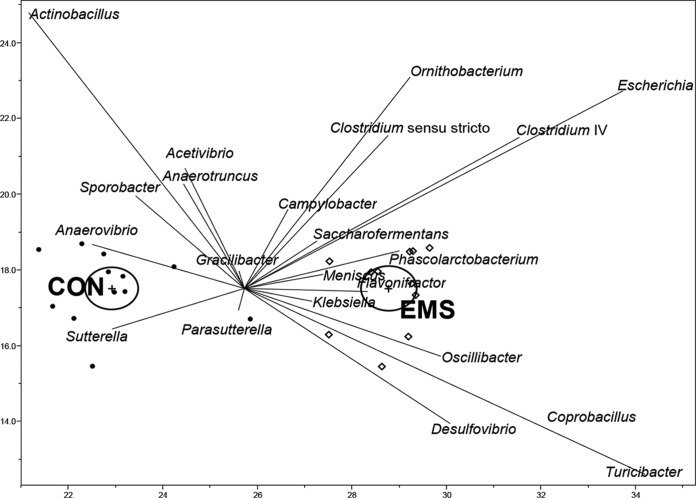
To examine the potential relationships between diets and OTU and to approximate OTU in clusters with similar responses toward the diets, multivariate and cluster analyses were performed. The LDA divided the effect of diet of the 30 most abundant genera in cecal digesta into 2 clearly distinguishable clusters (Fig. 1). The greatest influence to distinguish the cecal bacterial communities from pigs fed the EMS diet from those of pigs fed the control diet was exerted by Flavonifractor, Meniscus, and Klebsiella, which discriminated the best for the EMS diet, whereas Anaerovibrio and Sutterella correlated best with the control diet. Cluster analyses (see Fig. S3 and Table S4 in the supplemental material) revealed 86.7% of OTU belonging to clusters that depicted OTU being constitutively distributed or only slightly differently distributed between the two feeding groups (clusters I, II, IV, and VI). Clusters III and VII (consisting of 26 OTU) depicted OTU that were moderately and highly abundant in the control diet group compared to the EMS diet group. Clusters included representatives (more than one OTU) from the genera Acetivibrio, Clostridium, and Saccharofermentans. Clusters V and VIII (20 OTU in total) depicted OTU moderately and highly abundant in the EMS diet group compared to the control group. These clusters included also representatives from the genera Acetivibrio and Clostridium and from the genus Sporobacter. OTU within Eubacterium and Campylobacter were shown to be highly abundant in the EMS diet. In total, 3.3% of all OTU belonged to clusters that were highly differently distributed between feeding groups (clusters V and VII).
FIG 1.
Scores plot of linear discriminant analysis between diets and 30 most abundant genera in cecal digesta of pigs fed the enzymatically modified starch (EMS) diet or control (CON) diet. Genera that did not discriminate for the two diets were Acetanaerobacterium, Pseudoflavonifractor, Eubacterium, Lactobacillus, Subdoligranulum, Ruminococcus, Akkermansia, and Anaerophaga.
Imputed metabolic potential.
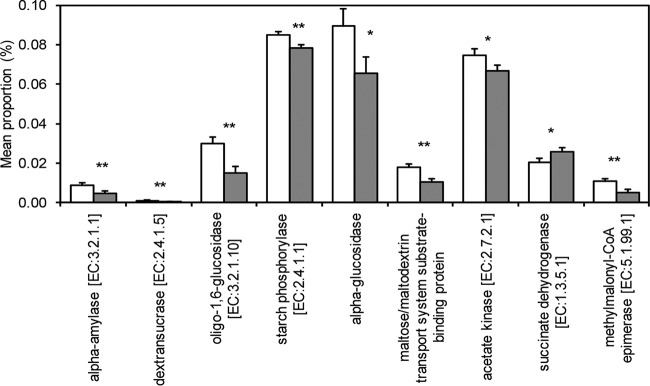
Given the few differences in single OTU abundances among diets, we then examined whether the two diets rather led to functional changes in the cecal microbiomes. To estimate this effect, PICRUSt was applied on the 16S rRNA sequencing data (31) and the imputed relative abundances of KEGG pathways in each respective sample were used to predict alterations in metabolic function of the present cecal microbiomes of pigs fed the EMS or control diet (Table 8). The KEGG pathways that were significantly less abundant with the EMS compared to the control diet included membrane transporters and lipid metabolism, amino acid metabolism, biosynthesis of secondary metabolites, and particularly pathways related to carbohydrate metabolism, such as fructose and mannose metabolism, galactose metabolism, glycolysis/gluconeogenesis, pentose and glucuronate interconversions, and starch and sucrose metabolism. Additionally, the KEGG pathways of protein folding and associated processing were negatively affected (P < 0.05) by the EMS diet compared to the control diet. Otherwise, pigs fed the EMS diet had a greater abundance of KEGG pathways related to phosphatidylinositol signaling system and vitamin metabolism, such as folate biosynthesis, nicotinate and nicotinamide metabolism, and biotin metabolism, compared to pigs fed the control diet. Due to the reduction in the KEGG pathways related to carbohydrate metabolism, we surveyed important KEGG modules within the respective pathways and found that many key enzymes in starch degradation were predicted to be less abundant with the EMS versus control diet, such as α-amylase, dextran sucrase, oligo-1,6-glucosidase, and starch phosphorylase (P < 0.05) (Fig. 2). As the SCFA synthesis appeared to be decreased in cecal digesta of pigs fed the EMS diet, we also compared the abundances of important KEGG modules in the respective pathways (Fig. 2), which predicted differences in the abundance of acetate kinase (P = 0.073), methylmalonyl coenzyme A (methylmalonyl-CoA) epimerase (P = 0.026), and succinate dehydrogenase (P = 0.052) between the two diets.
TABLE 8.
Imputed metagenomic profiles using KEGG pathway maps in cecal digesta of pigs fed the EMS or control dieta
| KEGG pathway | Predicted function | Relative abundance (%) by dietb |
SEM | P value | |
|---|---|---|---|---|---|
| Control | EMS | ||||
| Predicted functions more abundant with EMS diet | |||||
| Environmental information processing | |||||
| Membrane transport | Secretion system | 1.732 | 1.905 | 0.0663 | 0.081 |
| Signal transduction | Phosphatidylinositol signaling system | 0.087 | 0.091 | 0.0014 | 0.029 |
| Metabolism | |||||
| Energy metabolism | Oxidative phosphorylation | 0.945 | 0.990 | 0.0185 | 0.096 |
| Sulfur metabolism | 0.257 | 0.281 | 0.0084 | 0.054 | |
| Metabolism of cofactors and vitamins | Folate biosynthesis | 0.336 | 0.357 | 0.0061 | 0.027 |
| Nicotinate and nicotinamide metabolism | 0.402 | 0.424 | 0.0069 | 0.037 | |
| Riboflavin metabolism | 0.231 | 0.266 | 0.0117 | 0.051 | |
| Biotin metabolism | 0.127 | 0.139 | 0.0034 | 0.026 | |
| Metabolism of other amino acids | β-Alanine metabolism | 0.218 | 0.248 | 0.0119 | 0.090 |
| Unclassified | |||||
| Genetic information processing | Replication, recombination, and repair proteins | 1.327 | 1.636 | 0.1233 | 0.093 |
| Metabolism | Glycan biosynthesis and metabolism | 0.076 | 0.095 | 0.0078 | 0.095 |
| Predicted functions less abundant with EMS diet | |||||
| Environmental information processing | |||||
| Membrane transport | Transporters | 7.807 | 7.120 | 0.1672 | 0.009 |
| Signaling molecules and interaction | Bacterial toxins | 0.085 | 0.070 | 0.0059 | 0.084 |
| Metabolism | |||||
| Biosynthesis of other secondary metabolites | Butirosin and neomycin biosynthesis | 0.058 | 0.048 | 0.0038 | 0.093 |
| Flavone and flavonol biosynthesis | 0.013 | 0.006 | 0.0019 | 0.029 | |
| Flavonoid biosynthesis | 0.008 | 0.003 | 0.0011 | 0.009 | |
| Phenylpropanoid biosynthesis | 0.151 | 0.132 | 0.0064 | 0.005 | |
| Carbohydrate metabolism | Amino sugar and nucleotide sugar metabolism | 1.248 | 1.179 | 0.0249 | 0.067 |
| Fructose and mannose metabolism | 0.741 | 0.683 | 0.0138 | 0.008 | |
| Galactose metabolism | 0.592 | 0.501 | 0.1729 | 0.050 | |
| Glycolysis/gluconeogenesis | 1.049 | 1.030 | 0.0062 | 0.039 | |
| Pentose and glucuronate interconversions | 0.556 | 0.518 | 0.0127 | 0.048 | |
| Starch and sucrose metabolism | 0.904 | 0.837 | 0.0185 | 0.019 | |
| Glycan biosynthesis and metabolism | Other glycan degradation | 0.269 | 0.197 | 0.0276 | 0.083 |
| Lipid metabolism | Glycerolipid metabolism | 0.411 | 0.372 | 0.0072 | 0.001 |
| Sphingolipid metabolism | 0.186 | 0.140 | 0.0172 | 0.075 | |
| Metabolism of other amino acids | Cyanoamino acid metabolism | 0.266 | 0.240 | 0.0091 | 0.057 |
| d-Arginine and d-ornithine metabolism | 0.007 | 0.004 | 0.0009 | 0.062 | |
| Phosphonate and phosphinate metabolism | 0.068 | 0.057 | 0.0027 | 0.011 | |
| Unclassified | |||||
| Genetic information processing | Protein folding and associated processing | 0.666 | 0.642 | 0.0070 | 0.026 |
| Metabolism | Carbohydrate metabolism | 0.174 | 0.152 | 0.0043 | 0.002 |
Data are presented as least-square means ± SEM. n = 8 pigs in control diet group, and n = 7 pigs in the EMS diet group.
The relative abundances of different KEGG pathway maps encoded in each imputed sample metagenome are presented (P < 0.1).
FIG 2.
Imputed metagenomic profiles in pigs fed the EMS diet or control diet. The relative abundances (mean proportion percentage) of different KEGG modules between the enzymatically modified starch (EMS; gray bars) and control (CON; white bars) diets related to the KEGG pathways starch and sucrose and metabolism are shown. **, P < 0.05; *, P < 0.1.
DISCUSSION
We used in-depth sequencing of the 16S rRNA gene and qPCR to evaluate the modulatory potential of EMS on the cecal microbiome of growing pigs during a 10-day short-term feeding experiment. Sequencing results demonstrated that pigs fed the EMS diet had a diverging cecal bacterial community compared to pigs fed the control diet. However, the impact of the EMS on the cecal bacterial community structure and fermentation metabolites differed from previous observations in pigs fed diets with RS2 and RS3 (32, 33), thereby clearly demonstrating that gut bacterial effects may largely vary among the different RS types.
Feeding semipurified diets has the advantage that confounding effects of and interactions with other carbohydrate fractions in plant feedstuffs can be minimized (32). However, this simple dietary composition was likely the reason for the predominance of Proteobacteria in cecal digesta, whereas the phylum typically associated with the hindgut, Bacteroidetes (34), represented only 10% of the present cecal microbiome. Hence, EMS effects on the gut microbiota may diverge from the present results when EMS is included in diets containing other carbohydrates and proteins of plant origin. Aside from diet effects, our results also displayed a certain host specificity of the cecal bacterial community on a single OTU level, which was best demonstrated by the relative abundances of Firmicutes and Proteobacteria. They actually differed by 10% between diets without reaching statistical significance. Quantitative PCR analysis supported the plasticity of the cecal microbiota among pigs that were fed the same diet. Aside from differences in bacterial species, host factors may have played a role in influencing the extent of digestion and absorption due to differences in feed intake, digestive enzyme activity, and intestinal retention time.
The specific structural modification leading to 2-fold more α-1,6-glycosidic bonds in the EMS was supposed to reduce the starch digestibility of the host, thereby stimulating large intestinal fermentation. Increased intestinal SCFA generation with RS2 and RS3 has been reported to reduce feed intake and allay the sensation of hunger—the effect being mediated via intestinal secretion of incretins and insulin response (35, 36). The present EMS did obviously not show comparable properties as pigs fed the EMS diet consumed more feed and had lower SCFA concentrations in cecal digesta than pigs fed the control diet. Aside from slight differences in texture when mixing the EMS and control diets with water, pigs fed the EMS diet might have increased their feed intake to achieve similar digestible energy and nutrient intakes to cover the pig's needs for growth and thus to compensate for the lower digestibility of the EMS compared to the control diet. The decreased SCFA concentrations in cecal digesta indicated that EMS was not only less digestible for the host but also less accessible for microbial enzymes compared to the control starch (4). This assumption was supported by the predicted reduced abundances in starch and sucrose metabolism KEGG pathways and of key enzymes needed for starch degradation in cecal microbiomes of pigs fed the EMS diet. The predicted reduced abundance of acetate kinase and methylmalonyl-CoA epimerase with the EMS diet versus the control diet corresponded to the decreased cecal concentrations of acetate and propionate. A slower microbial degradation of EMS was further indicated by the facts that the negative effect of EMS on SCFA diminished until the midcolon and the SCFA profile was only little influenced by the type of starch. As a potential EMS effect on satiety could be only examined by feeding pigs to their appetite, effects may vary when pigs eat the same amount of feed. Also, changes in lactate or SCFA absorption or digesta retention time in the cecum in response to the EMS diet versus the control diet may have occurred. Classically, RS consumption has been associated with changes in the SCFA profile, most characteristically with enhanced intestinal propionate and butyrate proportions (32, 33). As luminal SCFA have many important physiological effects at the gut and systemic levels (35), lower SCFA concentrations in pigs fed the EMS diet may have consequences for gut function and systemic effects. Using a catheterization model, we could recently show that the EMS diet modified serum phospholipid profiles in both the pre- and postprandial phases, which might be linked to intestinally generated SCFA (17). Reduced intestinal dry matter content and enlarged cecum in growing pigs fed RS2 and RS3 due to enhanced fermentation were previously reported (14, 33), but the presently lower cecal and colonic dry matter contents in pigs fed the EMS diet compared to those fed the control diet were rather related to greater substrate availability in the large intestine.
Diet-related differences in the gut community structure were clearly visible among pigs as indicated by LDA and K-means clustering. The alpha diversity of the cecal microbiome and total bacterial 16S rRNA gene abundance in each gram of cecal digesta were little affected by the type of starch, whereas the Venn diagram illustrated that the EMS diet supported other bacteria in cecal digesta than the control diet as only half of the OTU were shared by pigs fed the two diets. In general, EMS-related bacterial alterations were different from previously reported effects of RS2 and RS3 (9, 33, 36). As pointed out by Haenen et al. (36), most studies investigated RS effects on the colonic and fecal bacterial microbiota in pigs; thus, less information is generally available for the cecal bacterial response to RS, and even less is known about RS4 effects. Haenen et al. (36) observed major differences between the cecal and colonic bacterial community profiles, demonstrating that the gut sites need to be viewed individually regarding potential health effects. Alterations in the bacterial profiles were likely caused by changes in the chemical structure of RS from cecum to colon, thereby modifying the bacterial accessibility of RS (36). In line with this, our data indicate that bacterial amylases could not attack the EMS in a comparable manner to the initial starch in the present study. For instance, the genera Acetanaerobacterium and Ruminococcus, belonging to Ruminococcaceae, as well as Enterocococcus and Lactobacillus species were largely depressed in pigs fed the EMS diet compared to the control diet and were probably replaced by other starch-fermenting genera, such as Meniscus. Dietary casein is almost completely digestible until the distal ileum in pigs (16). Nevertheless, cecal digesta of pigs fed the EMS diet contained proportionally more proteolytic bacteria, particularly Escherichia, although not reaching statistical significance, which may be linked to protein flow into the cecum or increased cecal mucus secretion with the EMS diet compared to the control diet. Likewise, Haenen et al. (36) found a greater abundance of Rhodobacter and Brachyspira and Leptospira in cecal digesta of pigs fed RS3, whereas in the colon, Proteobacteria abundance decreased. Quantitative PCR supported the depressing effect of EMS on Lactobacillus abundance, but results diverged between sequencing and qPCR as qPCR failed to confirm the proportional differences for cluster I and IV bacteria and Campylobacter. Previously, dietary RS inclusion has been shown to promote the amylolytic genera Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcus, Eubacterium, and Roseburia in the gut of humans and pigs, with the bacterial effects depending on the actual type of RS, gut site, and host (9, 32, 33, 36).
Although only few significant changes in the cecal microbiome were observed, imputed functional prediction indicated that the EMS diet caused changes in a number of metabolic pathways which may stand in relation to the reduced capability to degrade EMS and thus to gain energy for the bacterial metabolism. Almost all predicted pathways that were linked to carbohydrate metabolism were less abundant in pigs fed the EMS diet, but also lipid and amino acid metabolism, membrane transporters, protein folding, and biosynthesis of secondary metabolites were negatively affected by the EMS diet. Interestingly, succinate dehydrogenase as a key enzyme in many metabolic pathways, such as the citrate cycle, oxidative phosphorylation, and fatty acid metabolism, and several pathways in relation to vitamin metabolism were predicted to be more abundant with the EMS diet. In contrast, Umu et al. (33) found altered imputed bacterial metabolic potential in feces related to a downregulation of fatty acid metabolism when feeding RS3 to pigs, whereas their imputed polysaccharide degradation was consistent for RS3 and control diets.
In conclusion, despite the presence of host-specific bacterial communities, we could show that EMS promoted a different bacterial community structure in cecal digesta of pigs compared to the control diet. Reduced cecal and colonic SCFA concentrations and the imputed metabolic functions of the cecal microbiome supported that EMS was less degradable for cecal bacteria than the control starch. Moreover, the present data demonstrate the importance of evaluating potential RS4 candidates as present EMS effects on the gut microbiota differed from previously reported effects of RS2 and RS3.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Austrian Research Promotion Agency (FFG), BRIDGE project no. 836447—“Healthy Carbohydrates.”
We thank A. Dockner, M. Wild, M. Hollmann, S. Sharma, and M. Finsterböck of the Institute of Animal Nutrition and Functional Plant Compounds for assistance with sampling and technical assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02756-15.
REFERENCES
- 1.Flint HJ, Duncan SH, Scott KP, Louis P. 2015. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 74:13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- 2.Lattimer JM, Haub MD. 2010. Effects of dietary fiber and its components on metabolic health. Nutrients 2:1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzler-Zebeli BU, Hooda S, Pieper R, Zijlstra RT, van Kessel AG, Mosenthin R, Gänzle MG. 2010. Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl Environ Microbiol 76:3692–3701. doi: 10.1128/AEM.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M, Schalinske K, Scott MP, Whitley EM. 2013. Resistant starch: promise for improving human health. Adv Nutr 4:587–601. doi: 10.3945/an.113.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura T. 2013. The regulatory effects of resistant starch on glycaemic response in obese dogs. Arch Anim Nutr 67:503–509. doi: 10.1080/1745039X.2013.857081. [DOI] [PubMed] [Google Scholar]
- 6.Zaman SA, Sarbini SR. 2015. The potential of resistant starch as a prebiotic. Crit Rev Biotechnol 7:1–7. doi: 10.3109/07388551.2014.993590. [DOI] [PubMed] [Google Scholar]
- 7.Singh J, Dartois A, Kaur L. 2010. Starch digestibility in food matrix: a review. Trends Food Sci Technol 21:168–180. doi: 10.1016/j.tifs.2009.12.001. [DOI] [Google Scholar]
- 8.Topping DL, Clifton PM. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064. [DOI] [PubMed] [Google Scholar]
- 9.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. 2010. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erra-Pujada M, Debeire P, Duchiron F, O'Donohue MJ. 1999. The type II pullulanase of Thermococcus hydrothermalis: molecular characterization of the gene and expression of the catalytic domain. J Bacteriol 181:3284–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacGregor EA, Janecek S, Svensson B. 2001. Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim Biophys Acta 1546:1–20. doi: 10.1016/S0167-4838(00)00302-2. [DOI] [PubMed] [Google Scholar]
- 12.Louis P, Scott KP, Duncan SH, Flint HJ. 2007. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 13.Bird AR, Jackson M, King RA, Davies DA, Usher S, Topping DL. 2004. A novel high-amylose barley cultivar (Hordeum vulgare var. Himalaya 292) lowers plasma cholesterol and alters indices of large-bowel fermentation in pigs. Br J Nutr 92:607–615. [DOI] [PubMed] [Google Scholar]
- 14.Bird AR, Vuaran M, Brown I, Topping DL. 2007. Two high-amylose maize starches with different amounts of resistant starch vary in their effects on fermentation, tissue and digesta mass accretion, and bacterial populations in the large bowel of pigs. Br J Nutr 97:134–144. doi: 10.1017/S0007114507250433. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane S, Macfarlane GT. 2003. Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 16.Nutrient Research Council. 2012. Nutrient requirements of swine, 10th ed National Academies Press, Washington, DC. [Google Scholar]
- 17.Metzler-Zebeli BU, Eberspächer E, Grüll D, Kowalczyk L, Molnar T, Zebeli Q. 2015. Enzymatically modified starch ameliorates postprandial serum triglycerides and lipid metabolome in growing pigs. PLoS One 10:e0130553. doi: 10.1371/journal.pone.0130553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naumann C, Bassler R. 2012. Die Chemische Untersuchung von Futtermitteln. VDLUFA Verlag, Darmstadt, Germany. [Google Scholar]
- 19.Deckardt K, Metzler-Zebeli BU, Zebeli Q. 13 January 2015. Processing barley grain with lactic acid and tannic acid ameliorates rumen microbial fermentation and degradation of dietary fibre in vitro. J Sci Food Agric doi: 10.1002/jsfa.7085. [DOI] [PubMed] [Google Scholar]
- 20.Metzler-Zebeli BU, Mann E, Schmitz-Esser S, Wagner M, Ritzmann M, Zebeli Q. 2013. Changing dietary calcium-phosphorus level and cereal source selectively alters abundance of bacteria and metabolites in the upper gastrointestinal tracts of weaned pigs. Appl Environ Microbiol 79:7264–7272. doi: 10.1128/AEM.02691-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins CR, Mamedova LK, Carpenter AJ, Ying Y, Allen MS, Yoon I, Bradford BJ. 2013. Analysis of rumen microbial populations in lactating dairy cattle fed diets varying in carbohydrate profiles and Saccharomyces cerevisiae fermentation product. J Dairy Sci 96:5872–5881. doi: 10.3168/jds.2013-6775. [DOI] [PubMed] [Google Scholar]
- 22.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD. 2013. Secondary structure improves OTU assignments of 16S rRNA gene sequences. ISME J 7:457–460. doi: 10.1038/ismej.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joachimiak MP, Weisman JL, May BCH. 2006. JColorGrid: software for the visualization of biological measurements. BMC Bioinformatics 7:225. doi: 10.1186/1471-2105-7-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Hoon MJ, Imoto S, Nolan J, Miyano S. 2004. Open source clustering software. Bioinformatics 20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 28.Page RD. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson DM, Weimer PJ. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 75:165–174. doi: 10.1007/s00253-006-0802-y. [DOI] [PubMed] [Google Scholar]
- 30.Muyzer G, de Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regmi PR, Metzler-Zebeli BU, Gänzle MG, van Kempen TA, Zijlstra RT. 2011. Starch with high amylose content and low in vitro digestibility increases intestinal nutrient flow and microbial fermentation and selectively promotes bifidobacteria in pigs. J Nutr 141:1273–1280. doi: 10.3945/jn.111.140509. [DOI] [PubMed] [Google Scholar]
- 33.Umu ÖC, Frank JA, Fangel JU, Oostindjer M, da Silva CS, Bolhuis EJ, Bosch G, Willats WG, Pope PB, Diep DB. 2015. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 3:16. doi: 10.1186/s40168-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Looft T, Allen HK, Cantarel BL, Levine UY, Bayles DO, Alt DP, Henrissat B, Stanton TB. 2014. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J 8:1566–1576. doi: 10.1038/ismej.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Requena T, Cotter P, Shahar DR, Kleiveland CR, Marinez-Cuesta MC, Pelaez C, Lea T. 2013. Interactions between gut microbiota, food and the obese host. Trends Food Sci Technol 34:44–53. doi: 10.1016/j.tifs.2013.08.007. [DOI] [Google Scholar]
- 36.Haenen D, Zhang J, Souza da Silva C, Bosch G, van der Meer IM, van Arkel J, van den Borne JJ, Pérez Gutiérrez O, Smidt H, Kemp B, Müller M, Hooiveld GJ. 2013. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr 143:274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.