Abstract
The colon tumor microenvironment (TME) is becoming increasingly recognized as a complex but central player in the development of many cancers. Previously, we identified an oncogenic role for the mRNA-binding protein IMP1 (IGF2BP1) in the epithelium during colon tumorigenesis. In the current study, we reveal the contribution of stromal IMP1 in the context of colitis-associated colon tumorigenesis. Interestingly, stromal deletion of Imp1 (Dermo1Cre;Imp1LoxP/LoxP, or Imp1ΔMes) in the azoxymethane/dextran sodium sulfate (AOM/DSS) model of colitis-associated cancer resulted in increased tumor numbers of larger size and more advanced histological grade than controls. In addition, Imp1ΔMes mice exhibited a global increase in pro-tumorigenic microenvironment factors, including enhanced inflammation and stromal components. Evaluation of purified mesenchyme from AOM/DSS-treated Imp1ΔMes mice demonstrated an increase in hepatocyte growth factor (HGF), which has not been associated with regulation via IMP1. Genetic knockdown of Imp1 in human primary fibroblasts confirmed an increase in HGF with Imp1 loss, demonstrating a specific, cell-autonomous role for Imp1 loss to increase HGF expression. Taken together, these data demonstrate a novel tumor-suppressive role for IMP1 in colon stromal cells and underscore an exquisite, context-specific function for mRNA-binding proteins, such as IMP1, in disease states.
Keywords: Imp1, colon tumor microenvironment, colitis-associated colon cancer, HGF
Introduction
The colon tumor microenvironment is becoming recognized increasingly as a central player in the development of colorectal cancer (CRC). Chronic inflammation, particularly in cases arising from colitis (colitis-associated CRC, CAC), is a major contributor to the pro-tumorigenic microenvironment (1–3). In addition to inflammatory pathways, growth factors such as epidermal growth factor (EGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF) produced by cancer-associated fibroblasts (CAFs) may potentiate tumor growth (reviewed in (4, 5)). For example, HGF from stromal myofibroblasts has been shown to facilitate Wnt signaling and “stemness” in colon cancer cells (6). Understanding mechanisms regulating these pathways is of utmost importance, as mounting evidence in patients suggests that high expression of certain growth factors in the stroma may confer chemoresistance to traditional therapeutics (7–9).
Post-transcriptional regulation of tissue homeostasis via mRNA-binding proteins, such as Igf2 mRNA binding protein 1 (IMP1), has been implicated in critical processes including development, stem cell dynamics, and cancer. While it has been appreciated that mRNA-binding proteins including IMP1, Lin28b, and Musashi are increased in epithelial tumor cells, it is not yet known if these factors play a role in the tumor microenvironment (10–13). Imp1-hypomorphic mice exhibit dwarfism, decreased organ size, morphologically defective intestines, and death near birth for a large portion of mice (14, 15). Recent studies demonstrated a specific role for IMP1 to promote expansion of fetal neural stem cells via post-transcriptional enhancement of self-renewal gene products including Hmga2 (16). Because of the severe phenotype of Imp1-hypomorphic mice, studying post-natal roles of Imp1 in these animals is not feasible.
IMP1 expression is low or absent in most adult tissues, yet is increased during tumorigenesis, likely due to its induction by and subsequent stabilization of oncogenic gene products including those within Wnt/βcatenin and cMyc pathways (12, 17–20). We and others have observed low IMP1 expression in the adult intestine and colon, suggesting a potential, functional homeostatic role in the adult gut (11, 12). Despite the severe intestinal phenotype observed in Imp1-hypomorphic mice (14), intestine-epithelium specific conditional knockout mice (VillinCre;Imp1LoxP/LoxP or Imp1ΔIEC) revealed a minimal gross phenotype during homeostasis, whereas crossing Imp1ΔIEC onto the ApcMin/+ background of intestinal tumors exhibited a dramatic reduction in tumor lesions (unpublished and (11)). We therefore reasoned that non-epithelial Imp1 might be important for normal homeostasis of the adult intestine and colon. In support of this hypothesis, a recent study revealed that Imp1 expression is upregulated in colonic stromal cells within days following focal wounding (21).
In the present study, we generated and characterized mice with mesenchyme-specific loss of Imp1 (Dermo1Cre;Imp1LoxP/LoxP, or Imp1ΔMes) during homeostasis and a mouse model of colitis-associated colorectal tumorigenesis. We report that stromal Imp1 is dispensable for adult tissue homeostasis and mice exhibit no changes in epithelial cell proliferation or differentiation. Induction of colitis-associated cancer, however, revealed a significant increase in tumor burden in Imp1ΔMes mice accompanied by up-regulation of known pro-tumorigenic factors previously unassociated with Imp1, including HGF. Taken together, our data suggest a novel role for mesenchymal Imp1 to modulate stromal factors in the context of colitis-associated cancer that directly influence the initiation and growth of epithelial tumors in the colon.
Materials and Methods
Animals
Previously generated Imp1-floxed mice (11) were maintained on a C57Bl/6 background and crossed with Dermo1Cre animals (Jackson Laboratories, Bar Harbor, ME), which were maintained as heterozygous animals by crossing with CD1 mice. The resulting Dermo1Cre;Imp1-floxed mice, referred to as Imp1ΔMes, were thus mixed strain. Control mice consisted of age-matched Dermo1Cre;Imp1+/+, Imp1fl/+, and Imp1fl/fl mice, all of which are functionally wild type and were thus all used to reduce overall animal numbers. Rosa-CAG-LSL-TdTomato mice (Jackson Laboratories) were used to confirm recombination in Dermo1Cre mice. All mice were cared for in accordance with University Laboratory Animal Resources requirements and under an IACUC approved protocol. Mice were housed in specific pathogen-free conditions and fed standard, irradiated chow and water ad libitum.
Azoxymethane/dextran sodium sulfate (AOM/DSS) tumor model
Adult (8–12 weeks), male and female control and Imp1ΔMes mice were given a single intraperitoneal injection of azoxymethane (AOM, Sigma, St Louis, MO) at a dose of 10mg/kg, followed by three cycles of 2.5% dextran sodium sulfate (DSS, Affymetrix, Santa Clara, CA) for 5 days in the drinking water ad libitum with 1 week of normal water in between each cycle. Mice were sacrificed 10 weeks after the AOM injection. A total of ≥9 mice per genotype were tested across 2 independent experiments. Upon sacrifice, gross colon lesions were evaluated in a blinded fashion (KEH) using a Nikon SMZ645 dissecting stereomicroscope. Colons were then processed further for histology and gene expression analysis. Pathological scoring was performed blinded by expert veterinary pathologist Dr. Elizabeth Buza of the University of Pennsylvania School of Veterinary Medicine’s Comparative Pathology Core using previously published scoring methods (22). For tumor stroma scoring, the presence of lymphocytes, plasma, and neutrophils was noted, as well as necrosis and crypt abscesses on a scale of 0 (not present) to 5 (marked).
Immunohistochemistry
Mouse colons were Swiss-rolled and fixed in 10% formalin followed by paraffin embedding. H&E, alcian blue, Sirius red and Masson’s trichrome staining were performed using standard procedures. Immunofluorescence (IF) and/or immunohistochemistry (IHC) were performed using heat antigen-retrieval and staining with the following antibodies: Ki67 (1:250 dilution; Abcam, Cambridge, MA, 16667), α-SMA (1:2,000 dilution; Sigma A5228), IMP1 (1:100 dilution; Cell Signaling 2852S), βcatenin (1:100 dilution, Sigma, C2206). For IHC, biotin-conjugated secondary antibodies were as follows: anti-mouse IgG (1:250 dilution, Vector Laboratories, Burlingame, CA, BA-9200) and anti-rabbit IgG (1:250 dilution, Vector Laboratories BA-1000). For IF, anti-mouse Cy3-conjugated (Jackson ImmunoResearch, West Grove, PA) was used at 1:1,000.
Epithelial and mesenchymal isolations and antibody array
A one-centimeter piece of non-tumor tissue from mid-distal colon was excised from AOM/DSS-treated animals and subjected to epithelial dissociation using EDTA and DTT (23). The epithelium was then mechanically dissociated from the tissue via shaking, and remaining mesenchyme washed twice with PBS. Epithelial and mesenchymal fractions were then lysed in Triton lysis buffer (1% Triton X-100, 50mM Tris-HCl pH 7.5, 100mM NaCl, 5mM EDTA, 40mM β-glycerophosphate, 5% glycerol, 50mM NaF) plus protease and phosphatase inhibitors (Pierce, Rockford, IL, and Sigma, respectively). Total protein was quantified using Pierce BCA assay and tested for purity via Western blot for E-cadherin (epithelial cells, 1:1,000 dilution; BD Biosciences, San Diego, CA, 610182) and α-SMA (mesenchyme/fibroblasts, 1:2,000 dilution; Sigma A5228). Protein samples were assayed using the RayBiotech, Inc. (Norcross, GA) Mouse Quantibody Array 4000, which includes 200 targets.
Immunoblotting
Cells were lysed in Triton lysis buffer and total protein quantified using Pierce BCA assay (Thermo Scientific, Waltham, MA, 23225). Protein lysates were resolved in reducing conditions on 4–12% gradient gels for electrophoresis and detected with ECL Prime Western Blotting Detection Reagent (Amersham, Kingsport, TN, RPN2232) according to manufacturer instructions. Primary antibodies were as follows: Imp1 (1:400 dilution; Cell Signaling, Danvers, MA, 2852S), mouse HGF (1:1,000 dilution, Abcam 83760), human HGFα (1:1,000 dilution; Santa Cruz H-145), GAPDH (1:20,000 dilution; Chemicon, Temecula, CA, MAb374). Secondary antibodies from GE Healthcare (Michelton, NJ) ECL anti-rabbit IgG, HRP-linked and ECL anti-mouse IgG, HRP-linked were used at 1:1,000–1:10,000 dilutions.
Gene expression analysis
Total RNA was isolated from cell lines using the GeneJet RNA purification kit (Thermo Scientific) and reverse transcribed using the Applied Biosystems/Life Technologies Taqman Reverse Transcriptase Reagents. qRT-PCR was performed using Taqman Fast Universal Master Mix (Applied Biosystems) using the following primer/probe sets: human Hgf (Hs00300159) and housekeeping gene Hmbs (Hs00609297).
Cell lines
Human fetal esophageal fibroblast lines (FEF 2008 and FEF 3303) were generated by the Rustgi laboratory as described previously (24). All cells were cultured in DMEM with 10% fetal bovine serum plus penicillin/streptomycin and maintained at 37° with 5% CO2.
Cell proliferation assays
Fetal esophageal fibroblast cell proliferation was evaluated by staining with Trypan blue and enumeration via the Countess Automated Cell Counter (Life Technologies, Omaha, NE). Manual cell counting via hemocytometer was also used to confirm results obtained via automated cell counting. Cell counts were performed in duplicate across 4 independent experiments.
Statistical Analyses
For AOM/DSS treated animals, statistical significance of comparisons between control and experimental mice was determined by applying student’s t-test, with P < 0.05 as statistically significant. For all other analyses, unless noted otherwise, data from at least three experiments were presented as mean ± standard error and analyzed by applying student’s t-test, with P < 0.05 as statistically significant.
Results
Stromal Imp1 is dispensable for normal intestinal development and homeostasis
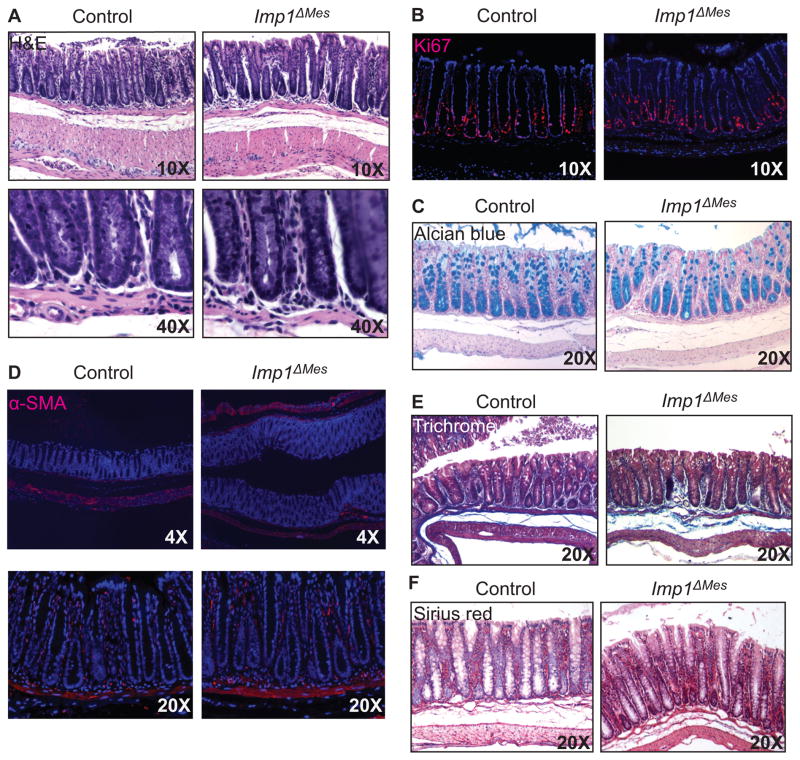
Imp1-hypomorphic mice exhibit severe growth defects in the intestine. Intriguingly, intestine-epithelium specific conditional knockout mice (Imp1ΔIEC) are phenotypically normal during homeostasis (unpublished). We therefore generated mice with mesenchyme-specific loss of Imp1 (Imp1ΔMes) by crossing Imp1-floxed mice (11) with Dermo1Cre mice (25, 26), causing Imp1 disruption in mesoderm-derived tissues, including fibroblasts, macrophages, and endothelial cells, starting at E9.5. We confirmed Cre-recombination in the mesenchymal compartment of the intestine and colon by crossing Dermo1Cre mice with Rosa-LSL-tdTomato mice and evaluating tdTomato expression grossly and histologically (Supplementary Fig. 1A&B) as well as Imp1 expression (Supplementary Fig. 1C). Imp1ΔMes mice were born at comparable ratios to wild type littermates and exhibited no differences in gross phenotype into adulthood. Initial histological examination of adult Imp1ΔMes mice revealed normal crypt architecture of the colon (Fig. 1A), with no gross abnormalities. Furthermore, we found no difference in proliferation (Ki67) in Imp1ΔMes mice (Fig. 1B). Evaluation of goblet cells (alcian blue) also revealed comparable numbers of goblet cells between control and Imp1ΔMes mice (Fig. 1C), suggesting that lineage specification of the epithelium was unaffected. Analysis of proliferation and cell lineages in the small intestine were also unaffected (data not shown).
Figure 1. Stromal Imp1 is dispensable for colon homeostasis.
A. Representative H&E staining from control and Imp1ΔMes mice demonstrate no morphological differences in colon crypts or mesenchymal layers. B. Ki67 staining is comparable between control and Imp1ΔMes mice. C. Staining for goblet cell lineage via Alcian blue showed no significant differences between control and Imp1ΔMes mice. To evaluate stromal components, staining for αSMA (D), Masson’s trichrome (E), and Sirius red (F) was performed; however, no differences were observed between control and Imp1ΔMes mice.
As Imp1 deletion was targeted to the sub-epithelial compartment, we specifically evaluated stromal cells and extracellular matrix components in these mice. There were no changes in αSMA-positive fibroblasts, which appeared in the same location and frequency in Imp1ΔMes and control mice (Fig. 1D). Trichrome staining for collagen and muscle fibers similarly revealed no significant difference between groups (Fig. 1E), nor did Sirius red staining for collagen (Fig. 1F). This is intriguing, since Imp1-hypomorphic mice exhibited a decrease in genes encoding extracellular matrix components, including multiple procollagens (types Iα1, Iα2, Vα1, VIα2, VIα3, XIVα1), galectin-1, lumican, and tenascin-C (14). Taken together, these data indicate that Imp1 expression is dispensable in the mesenchymal compartment for homeostasis in the adult colon.
Mice lacking stromal Imp1 exhibit enhanced tumor burden in the colon
We have established previously a direct, oncogenic role of epithelial Imp1 in CRC xenografts and ApcMin/+ tumorigenesis (11). In addition, studies of IMP1 have demonstrated specific roles for IMP1 in cytoskeletal organization and cell movement (27–30). Most recently, studies have reported that IMP1 may regulate cell migration and polarization via regulation of MAPK4 and PTEN in osteosarcoma and ovarian tumor cell lines, and may promote wound repair in colonic mesenchymal stem cells via stabilization of Ptgs2 mRNA (21, 31). We sought to determine the consequences of Imp1 loss in the mesenchyme in the setting of inflammation-associated colon tumorigenesis. Imp1ΔMes mice were treated with a single dose of azoxymethane (AOM) followed by three cycles of dextran sodium sulfate (DSS) to include colitis-associated tumors. The AOM/DSS model is an ideal approach to study the tumor microenvironment, as it combines a chemical-carcinogen in the setting of inflammation to recapitulate tumor initiation and progression in a defined chronology (32). Furthermore, this model is characterized by activating mutations in epithelial pathways observed in humans, including Wnt/Apc/βcatenin and K-Ras (32, 33). We sacrificed mice at ten weeks post-AOM injection in order to evaluate the early establishment of adenomas and/or adenocarcinomas.
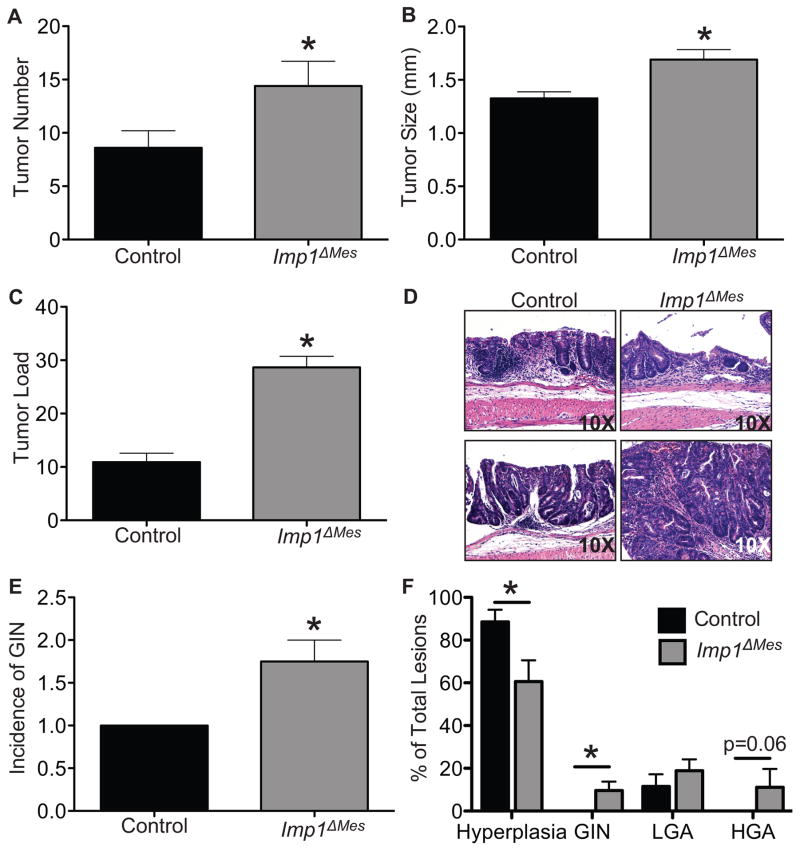
Intriguingly, loss of mesenchymal Imp1 promoted, rather than attenuated, tumor growth. Male Imp1ΔMes mice exhibited a significant increase in tumor number (14.4 ± 2.3 versus 8.6 ± 1.6; Fig. 2A). Tumor size was also significantly increased in Imp1ΔMes mice (1.7 ± 0.09 versus 1.3 ± 0.06), as well as tumor load (Fig. 2B–D). A non-significant trend was observed in female mice (data not shown), which is not surprising as female mice are less susceptible to DSS (34). Blinded, histological scoring by an expert veterinary pathologist supported increases in gross tumor number in Imp1ΔMes mice. Furthermore, Imp1ΔMes mice had a higher incidence of both low-grade and high-grade adenomas and gastrointestinal neoplasia (GIN) compared to controls (Fig. 2E&F), whereas the majority of lesions found in control mice were characterized as hyperplasia (Fig. 2F). Histological evidence of adenocarcinoma was observed in Imp1ΔMes mice but not controls; however, those lesions did not meet the full criteria for adenocarcinoma based on published scoring systems (22). Together, these data suggest that Imp1ΔMes mice exhibit enhanced tumor initiation and progression compared to controls.
Figure 2. Imp loss in stromal cells promotes enhanced tumorigenesis.
Tumors were induced in control and Imp1ΔMes mice using the azoxymethane/dextran sodium sulfate (AOM/DSS) model. Tumors were evaluated 10 weeks after the initial AOM injection. A–D. Imp1ΔMes mice exhibited a significant increased in tumor number, size, and load compared to control mice. N ≥ 5 animals per genotype. E. Histological analysis of AOM/DSS tumors revealed an increase in the incidence of gastrointestinal neoplasia (GIN) in Imp1ΔMes mice compared to controls. Furthermore, Imp1ΔMes mice exhibited a significantly higher percentage of more histologically advanced lesions compared to controls, whereas control lesions were most often characterized as hyperplasia (F; LGA= low grade adenoma, HGA= high grade adenoma). Evidence of adenocarcinoma was only observed in Imp1ΔMes mice; however, lesions did not meet the full scoring criteria and were thus considered HGA. P-values are indicated for individual histograms; p<0.05 is considered statistically significant.
Imp1 loss enhances the colon tumor microenvironment
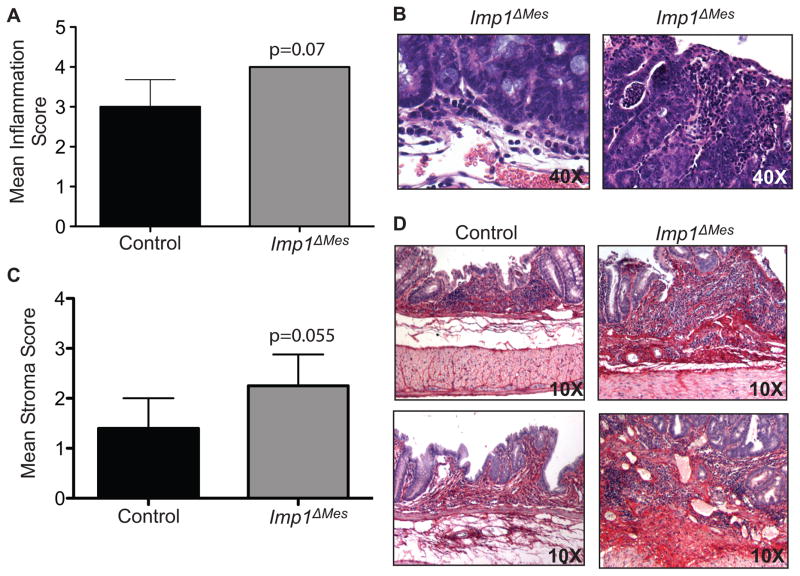
Histological examination of tumor stroma revealed higher inflammation and activated stroma scores in Imp1ΔMes mice compared to controls (Fig. 3A–C). Inflammatory infiltrates consisted of mostly lymphocytes and plasma cells. Inflammation and necrosis within tumors tended to be more severe in the high-grade adenomas versus low-grade adenomas versus hyperplasia, and inflammation within the neoplasms had more of a neutrophilic component. Overall stromal scores included the extent of inflammatory infiltrate, as well as evidence of necrosis, crypt abscesses and crypt loss with replacement by fibrosis (Fig. 3D). Evidence of herniation was observed in Imp1ΔMes mice only. Sirius red scoring revealed a trend for enhanced fibrosis in Imp1ΔMes mice (scoring not shown), and tumor stromal fibrosis tended to be more prominent within the higher-grade adenomas. Collectively, we demonstrate that the increase in tumor burden in Imp1ΔMes mice is accompanied by enhanced inflammation and stromal components, suggesting that Imp1 in the colon mesenchyme may normally function to attenuate the effects of chronic inflammatory damage.
Figure 3. Imp1ΔMes mice exhibit increased tumor inflammation and stroma compared to controls.
A. Blinded histological scoring of inflammation in AOM/DSS colons revealed a trend for increased inflammation in Imp1ΔMes mice compared to control. Examples from the more severe inflammation and necrosis within tumors of higher histological grade from Imp1ΔMes mice are shown in (B). C. The mean stroma score in Imp1ΔMes mice approached significance compared to control. D. Representative images of Sirius red staining, where dark-red staining, collagen-positive staining is enhanced in Imp1ΔMes mice. P-values are indicated for individual histograms.
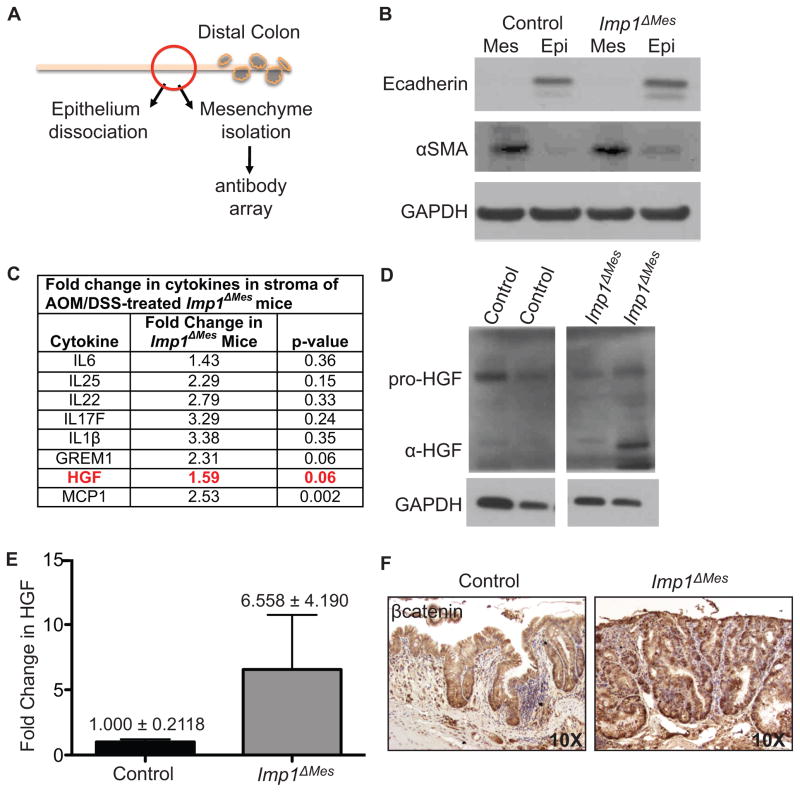
To elucidate mechanisms underlying enhanced tumorigenesis in Imp1ΔMes mice, we isolated mesenchyme from Imp1ΔMes and control mice treated with AOM/DSS and performed a cytokine/growth factor array of 200 targets. Tissues from the mid-distal colon, at least 1 centimeter from the closest gross lesion were evaluated in order to ensure minimal contamination of sample with tumor epithelium (Fig. 4A). Stromal enrichment and epithelial depletion of isolated mesenchyme were confirmed via western blotting for epithelial marker E-cadherin and activated fibroblast marker α-SMA (Fig. 4B). There was a global increase in several pro-inflammatory cytokines and chemokines, including IL6, IL1β, and MCP1 in stroma from Imp1ΔMes mice (Fig. 4C), although not all were statistically significant. In addition, the BMP pathway inhibitor Gremlin 1, which has been implicated in colorectal cancer progression (35), was also upregulated in Imp1ΔMes mice. Interestingly, there was also a 60% increase in stromal hepatocyte growth factor (HGF), a growth factor produced by fibroblasts with known roles in stimulation epithelial cell growth in the gut (36). We confirmed upregulation of HGF in stromal lysates of AOM/DSS-treated Imp1ΔMes mice (Fig. 4D&E). HGF has been implicated in the pathogenesis of CRC not only via direct stimulation of pathways downstream of its receptor, cMet, but also via synergy with the Wnt/βcatenin pathway in epithelial tumor cells (37). We therefore evaluated the presence of nuclear βcatenin in hyperplastic areas of control and Imp1ΔMes mice (Fig. 4F). Indeed, Imp1ΔMes mice exhibited enhanced nuclear βcatenin compared to controls, suggesting that the tumor microenvironment in Imp1ΔMes mice indeed contributes to the increase tumorigenesis in these mice.
Figure 4. Stromal analysis revealed increased pro-inflammatory and growth factors in Imp1ΔMes mice.
A. Schematic of epithelial/mesenchymal preparation from AOM/DSS-treated animals for downstream analyses. A small, non-tumor area of mid-distal colon was used for dissociation. B. Western blot for epithelial (E-cadherin) and mesenchymal (α-smooth muscle actin, α-SMA) markers confirmed enrichment of respective fractions. C. Antibody array results showed a global increase in cytokines and growth factors in Imp1ΔMes mice, with significant or near significant increases in both MCP1 and HGF. D. Western blotting confirmed the increase in HGF in mesenchyme from AOM/DSS-treated Imp1ΔMes mice, particularly the α-HGF isoform. E. Densitometric analysis for changes in α-HGF normalized to GAPDH confirmed this increase. F. Staining of lesions for βcatenin demonstrated an increase in nuclear localization in Imp1ΔMes mice compared to controls.
Fibroblasts lacking Imp1 exhibit increased HGF expression and fibroblast proliferation in vitro
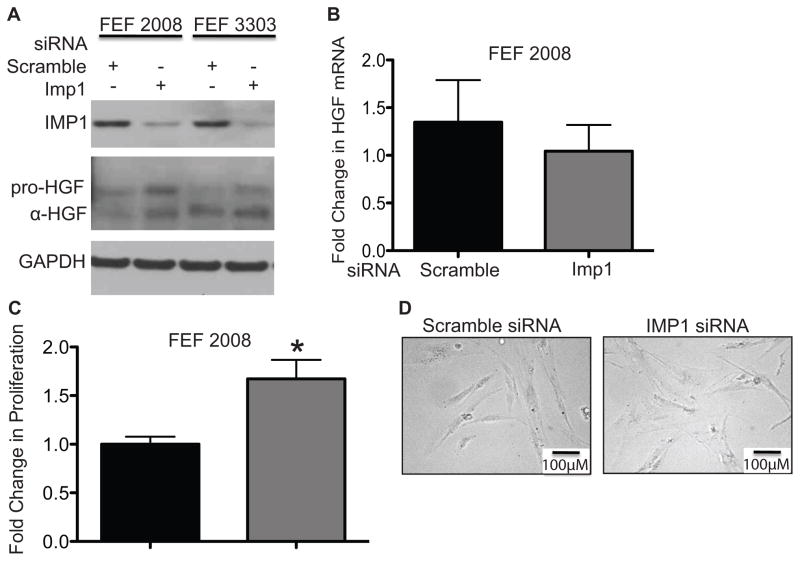
HGF has been implicated in the pathogenesis of CRC (38–40) and is a key paracrine signaling component relevant to several human cancers, including CRC. In order to determine if the increase in AOM/DSS tumorigenesis is due to direct signaling from Imp1-deficient fibroblasts, we evaluated the expression of HGF following Imp1 knockdown in human fibroblast cell lines and human colon primary fibroblasts. We first evaluated Imp1 knockdown in human fetal esophageal fibroblasts (FEF2008 and FEF 3303), as these cells produce low to intermediate levels of HGF and are highly amenable to transfection (24, 41). In addition, FEF cell lines recapitulate phenotypically the proliferative and invasive behavior characteristic of cancer associated fibroblasts “CAFs”, and were thus appropriate model for the current study (41). In both fibroblast cell lines evaluated, there was an increase in both pro-HGF as well as the active, αHGF isoform with Imp1 knockdown (Fig. 5A). Interestingly, we do not observe a significant difference in Hgf mRNA with IMP1 knockdown (Fig. 5B). This is consistent with IMP1 regulating HGF at the level of post-transcription/translation. We next evaluated the proliferative capacity of Imp1 knockdown fibroblasts, and found a 67% increase in proliferation via automated cell counting 72 hours after knockdown (Fig. 5C). The increase in HGF and proliferation with Imp1 knockdown was found in the absence of any differences in morphology of Imp1 knockdown fibroblasts and controls (Fig. 5D). Together, these data indicate that Imp1 knockdown may promote a “CAF-like” phenotype in fibroblasts.
Figure 5. IMP1 knockdown is sufficient to increase HGF expression in fibroblasts.
A. Representative western blot demonstrating siRNA-mediated knockdown of IMP1 compared to scramble controls. IMP1 knockdown increased HGF expression in 2 different fetal esophageal fibroblast (FEF) cell lines, FEF 2008 and FEF 3303. B. Evaluation of Hgf mRNA levels in FEF 2008 cells revealed no significant difference between control or IMP1 knockdown cells. C. Proliferation of FEF 2008 cells was evaluated in IMP1 knockdown 72 hours after siRNA transfection. IMP1 knockdown increased FEF proliferation by 60% compared to controls. D. Morphological analysis of FEF 2008 cells with IMP1 knockdown did not reveal any difference between control and knockdown cells via bright field microscopy. Scale bar indicates 100μM. Data represent n≥3 independent experiments.
Discussion
In the present study, we demonstrate dispensability of stromal Imp1 for homeostasis in the adult colon and that Imp1 loss renders mice more susceptible to a pro-tumorigenic microenvironment and thus increases tumor burden in a mouse model of colitis-associated cancer. The lack of phenotype in Imp1ΔMes mice during homeostasis is perhaps not surprising, as expression of IMP1 in the mesenchyme of untreated adult mice is nearly undetectable; however, dramatic intestine defects in mice with global Imp1 deletion remains unexplained. It is possible that Cre-mediated excision of Imp1-floxed alleles using Dermo1Cre (or VillinCre, unpublished) does not occur early enough during development to recapitulate any of the global hypomorphic phenotype. It is also possible that Imp1 must be lost in both epithelial and mesenchymal compartments simultaneously in order to elicit a phenotype at homeostasis, which will be a focus of future studies.
Our finding that Imp1ΔMes mice exhibit increased tumor burden was unexpected, as our previous studies in xenograft and genetic models identified an oncogenic role for epithelial Imp1 in the intestine (11). We now demonstrate a dichotomous role for Imp1 in the adult colon, whereby loss of Imp1 in the mesenchymal compartment promotes, rather than attenuates, inflammation-associated colon tumorigenesis. These data support a growing literature in which the discrete roles of Imp1 within specific contexts reveal either oncogenic or tumor-suppressive roles. For example, transgenic expression of Imp1 in mouse breast tissue promotes tumor growth; whereas other studies have shown that ectopic Imp1 expression can attenuate metastatic potential in breast cancer cell lines via modulation of cell motility (28, 42, 43). In CRC, IMP1 expression correlates with poor clinical outcome (12, 20); however, no studies have evaluated IMP1 expression in tumor stroma of CRC patients.
Our data demonstrating that Imp1 loss promotes an enhanced tumor microenvironment is the first to evaluate the non-epithelial roles of Imp1 in this context. While analyses of specific inflammatory targets revealed few statistically significant upregulated targets, several pro-inflammatory cytokines exhibited a trend for increased expression with Imp1 loss. This, together with pathological scoring of inflammation support the hypothesis that stromal Imp1 loss promotes an inflammatory environment in this context. Recent studies in a colon wound-healing model revealed that IMP1 expression is upregulated in the mesenchyme, where it binds to and promotes induction of Ptgs2 mRNA during the initial phase of wound healing (21). This implicates IMP1 as a facilitator of the temporal orchestration of wound healing in the colon; however, it likely has a divergent role in chronic inflammation such as that found in the current study in AOM/DSS-treated mice. Interestingly, the tumor progression locus-2 (TPL2) kinase has also been shown to regulate Ptgs2 during colon homeostasis, suggesting alternative or potentially overlapping pathways (44). In AOM/DSS-treated Imp1ΔMes mice, the enhanced tumor microenvironment promotes tumorigenesis characterized by increased tumor load together with more advanced pathological grading and increased nuclear βcatenin in epithelial cells. In addition to increased inflammation, Imp1ΔMes mice express also increased HGF levels, which are produced by stromal fibroblasts and promotes proliferation of epithelial cells (36). HGF is also thought to promote invasive growth of CRC cells via cooperation with βcatenin signaling, and has been shown to confer resistance to EGFR inhibitors by colon tumor-initiating cells (37, 45, 46). As such, we confirmed that Imp1 loss does indeed upregulate HGF in fibroblasts in vitro, implicating a novel, cell autonomous role for Imp1 in the modulation of HGF in fibroblasts. In addition, our data demonstrating that Imp1 loss increased fibroblast cell growth, while likely due to increased HGF, may also be attributed to other factors. While the direct mechanism(s) for IMP1 modulation of Hgf are still under investigation, possible roles include 1) direct binding of IMP1 to Hgf mRNA, 2) binding and modulation of negative regulators of HGF such as TPL2, which has been shown to have other phenotypic similarities to Imp1 (40), and 3) modulation of mature HGF cleavage events, amongst others possibilities.
There are likely additional factors, including those identified in our antibody array and other cytokines (Fig. 4C), that contribute to the tumorigenic microenvironment in mice lacking stromal Imp1. For example, we demonstrate that GREM 1, an inhibitor of the BMP pathway, is upregulated with Imp1 loss. This is intriguing as GREM 1 has recently been implicated in the promotion and progression of colorectal cancer when aberrantly expressed in epithelial cells or via loss of cancer cell differentiation at invasive fronts, respectively (35, 47). The role of IMP1 in modulating the BMP pathway through suppression of GREM 1 will be a focus of future studies.
In addition to factors revealed in our antibody screen, previous studies revealed that IMP1 contributes to activation of the NFκB pathway by stabilizing mRNA of βTrCP1 that results in increased levels of βTrCP1 protein and accelerated degradation of IκBs (17). Interestingly, βTrCP1 is minimally expressed in fibroblasts, whereas its homologue βTrCP2 (HOS) is a predominantly expressed gene in fibroblasts. Since IMP1 does not affect the expression of βTrCP2, the present data may suggest that knockout of Imp1 fails to inhibit NFκB in fibroblasts, potentially due to the absence of βTrCP1. Additional cell-type specific Cre-mice will be employed in future studies to target Imp1 specifically in fibroblasts or immune cells to evaluate the specific contribution of Imp1 loss on NFκB and other pro-inflammatory pathways in the context of inflammation-associated cancer CRC.
Recent genomic approaches have identified in patients a significant correlation between tumor stromal components and the most aggressive, therapy-resistant CRCs (48, 49). Specifically, stromal markers and genes associated with CAFs correlate with higher disease recurrence, resistance to therapeutics, and enhancement of tumor-initiating cells. Our data that stromal Imp1 loss promotes colon tumorigenesis indicate that stromal Imp1 may normally play a tumor-suppressive role in the colon. As such, it would be interesting to evaluate IMP1 expression in the stroma of CRC patient samples in the future to determine its potential as a diagnostic or prognostic marker.
Supplementary Material
Implications.
The tumor-suppressive role of stromal IMP1 and its ability to modulate pro-tumorigenic factors suggest that IMP1 status is important for the initiation and growth of epithelial tumors.
Acknowledgments
Financial support: National Institutes of Health K01 DK100485 (KEH); National Institutes of Health (NIH) R01 DK056645, U01 DK085551, P30 DK050306 to KEH, PC, ETL, SFA, VG, PDH, AKR); Fonds de Recherche en Santé du Québec (P-Giroux-27692 to VG); NIH R21 CA191550 to VSS.
We acknowledge Drs. M.P. Tetreault, K. Whelan, A. Rhim, D. Worthley, and T. Stappenbeck, and B. Madison for helpful discussions. We thank A. Bedenbaugh, D. Budo, and R. Hasan (Penn Molecular Pathology and Imaging Core Facility); D. Heitjan (Penn Biostatistics); S. Keilbaugh and L. Chua (Penn Molecular Biology Core); E. Roberston (Penn Cell Culture Core).
Footnotes
Conflicts of interest: none
References
- 1.Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer cell. 2013;23(5):634–46. doi: 10.1016/j.ccr.2013.03.022. Epub 2013/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature immunology. 2011;12(8):715–23. doi: 10.1038/ni.2060. Epub 2011/07/21. [DOI] [PubMed] [Google Scholar]
- 3.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–16. doi: 10.1053/j.gastro.2011.01.057. Epub 2011/05/03. [DOI] [PubMed] [Google Scholar]
- 4.Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2010;3(1):149–66. doi: 10.1007/s12307-010-0038-3. Epub 2011/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Seminars in cell & developmental biology. 2010;21(1):19–25. doi: 10.1016/j.semcdb.2009.10.002. Epub 2009/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12(5):468–76. doi: 10.1038/ncb2048. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 7.Liska D, Chen CT, Bachleitner-Hofmann T, Christensen JG, Weiser MR. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res. 2011;17(3):472–82. doi: 10.1158/1078-0432.CCR-10-0568. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–4. doi: 10.1038/nature11183. Epub 2012/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum D, LaBarge S. Registered report: Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. eLife. 2014;3 doi: 10.7554/eLife.04034. Epub 2014/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madison BB, Liu Q, Zhong X, Hahn CM, Lin N, Emmett MJ, et al. LIN28B promotes growth and tumorigenesis of the intestinal epithelium via Let-7. Genes Dev. 2013;27(20):2233–45. doi: 10.1101/gad.224659.113. Epub 2013/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton KE, Noubissi FK, Katti PS, Hahn CM, Davey SR, Lundsmith ET, et al. IMP1 promotes tumor growth, dissemination and a tumor-initiating cell phenotype in colorectal cancer cell xenografts. Carcinogenesis. 2013;34(11):2647–54. doi: 10.1093/carcin/bgt217. Epub 2013/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitriadis E, Trangas T, Milatos S, Foukas PG, Gioulbasanis I, Courtis N, et al. Expression of oncofetal RNA-binding protein CRD-BP/IMP1 predicts clinical outcome in colon cancer. International journal of cancer Journal international du cancer. 2007;121(3):486–94. doi: 10.1002/ijc.22716. Epub 2007/04/07. [DOI] [PubMed] [Google Scholar]
- 13.Rezza A, Skah S, Roche C, Nadjar J, Samarut J, Plateroti M. The overexpression of the putative gut stem cell marker Musashi-1 induces tumorigenesis through Wnt and Notch activation. J Cell Sci. 2010;123(Pt 19):3256–65. doi: 10.1242/jcs.065284. Epub 2010/09/10. [DOI] [PubMed] [Google Scholar]
- 14.Hansen TV, Hammer NA, Nielsen J, Madsen M, Dalbaeck C, Wewer UM, et al. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol Cell Biol. 2004;24(10):4448–64. doi: 10.1128/MCB.24.10.4448-4464.2004. Epub 2004/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhraldeen SA, Clark RJ, Roopra A, Chin EN, Huang W, Castorino J, et al. Two Isoforms of the RNA Binding Protein, Coding Region Determinant-binding Protein (CRD-BP/IGF2BP1), are Expressed in Breast Epithelium, and Support Clonogenic Growth of Breast Tumor Cells. J Biol Chem. 2015 doi: 10.1074/jbc.M115.655175. Epub 2015/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino J, Kim S, Zhu Y, Zhu H, Morrison SJ. A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. eLife. 2013;2:e00924. doi: 10.7554/eLife.00924. Epub 2013/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noubissi FK, Elcheva I, Bhatia N, Shakoori A, Ougolkov A, Liu J, et al. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature. 2006;441(7095):898–901. doi: 10.1038/nature04839. Epub 2006/06/17. [DOI] [PubMed] [Google Scholar]
- 18.Elcheva I, Goswami S, Noubissi FK, Spiegelman VS. CRD-BP protects the coding region of betaTrCP1 mRNA from miR-183-mediated degradation. Mol Cell. 2009;35(2):240–6. doi: 10.1016/j.molcel.2009.06.007. Epub 2009/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noubissi FK, Nikiforov MA, Colburn N, Spiegelman VS. Transcriptional Regulation of CRD-BP by c-myc: Implications for c-myc Functions. Genes Cancer. 2010;1(10):1074–82. doi: 10.1177/1947601910395581. Epub 2011/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongroo PS, Noubissi FK, Cuatrecasas M, Kalabis J, King CE, Johnstone CN, et al. IMP-1 displays cross-talk with K-Ras and modulates colon cancer cell survival through the novel proapoptotic protein CYFIP2. Cancer Research. 2011;71(6):2172–82. doi: 10.1158/0008-5472.CAN-10-3295. Epub 2011/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manieri NA, Drylewicz MR, Miyoshi H, Stappenbeck TS. Igf2bp1 Is Required for Full Induction of Ptgs2 mRNA in Colonic Mesenchymal Stem Cells in Mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.03.037. Epub 2012/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124(3):762–77. doi: 10.1053/gast.2003.50094. Epub 2003/03/04. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton KE, Crissey MA, Lynch JP, Rustgi AK. Culturing adult stem cells from mouse small intestinal crypts. Cold Spring Harbor protocols. 2015;2015(4) doi: 10.1101/pdb.prot078303. pdb prot078303. Epub 2015/04/04. [DOI] [PubMed] [Google Scholar]
- 24.Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21(21):2788–803. doi: 10.1101/gad.1544507. Epub 2007/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130(13):3063–74. doi: 10.1242/dev.00491. Epub 2003/05/21. [DOI] [PubMed] [Google Scholar]
- 26.Geske MJ, Zhang X, Patel KK, Ornitz DM, Stappenbeck TS. Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development. 2008;135(17):2959–68. doi: 10.1242/dev.020453. Epub 2008/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a beta-actin mRNA zipcode-binding protein. Mol Cell Biol. 1997;17(4):2158–65. doi: 10.1128/mcb.17.4.2158. Epub 1997/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapidus K, Wyckoff J, Mouneimne G, Lorenz M, Soon L, Condeelis JS, et al. ZBP1 enhances cell polarity and reduces chemotaxis. J Cell Sci. 2007;120(Pt 18):3173–8. doi: 10.1242/jcs.000638. Epub 2007/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberman F, Rand K, Maizels Y, Rubinstein AM, Yisraeli JK. VICKZ proteins mediate cell migration via their RNA binding activity. RNA. 2007;13(9):1558–69. doi: 10.1261/rna.559507. Epub 2007/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vainer G, Vainer-Mosse E, Pikarsky A, Shenoy SM, Oberman F, Yeffet A, et al. A role for VICKZ proteins in the progression of colorectal carcinomas: regulating lamellipodia formation. J Pathol. 2008;215(4):445–56. doi: 10.1002/path.2376. Epub 2008/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stohr N, Kohn M, Lederer M, Glass M, Reinke C, Singer RH, et al. IGF2BP1 promotes cell migration by regulating MK5 and PTEN signaling. Genes Dev. 2012;26(2):176–89. doi: 10.1101/gad.177642.111. Epub 2012/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karim BO, Huso DL. Mouse models for colorectal cancer. American journal of cancer research. 2013;3(3):240–50. Epub 2013/07/11. [PMC free article] [PubMed] [Google Scholar]
- 34.Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. The American journal of physiology. 1998;274(3 Pt 1):G544–51. doi: 10.1152/ajpgi.1998.274.3.G544. Epub 1998/04/08. [DOI] [PubMed] [Google Scholar]
- 35.Karagiannis GS, Musrap N, Saraon P, Treacy A, Schaeffer DF, Kirsch R, et al. Bone morphogenetic protein antagonist gremlin-1 regulates colon cancer progression. Biological chemistry. 2015;396(2):163–83. doi: 10.1515/hsz-2014-0221. Epub 2014/08/26. [DOI] [PubMed] [Google Scholar]
- 36.Goke M, Kanai M, Podolsky DK. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. The American journal of physiology. 1998;274(5 Pt 1):G809–18. doi: 10.1152/ajpgi.1998.274.5.G809. Epub 1998/06/05. [DOI] [PubMed] [Google Scholar]
- 37.Rasola A, Fassetta M, De Bacco F, D’Alessandro L, Gramaglia D, Di Renzo MF, et al. A positive feedback loop between hepatocyte growth factor receptor and beta-catenin sustains colorectal cancer cell invasive growth. Oncogene. 2007;26(7):1078–87. doi: 10.1038/sj.onc.1209859. Epub 2006/09/06. [DOI] [PubMed] [Google Scholar]
- 38.Otte JM, Schmitz F, Kiehne K, Stechele HU, Banasiewicz T, Krokowicz P, et al. Functional expression of HGF and its receptor in human colorectal cancer. Digestion. 2000;61(4):237–46. doi: 10.1159/000007764. Epub 2000/07/06. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Li Q, Zhu L. Expression of the hepatocyte growth factor and c-Met in colon cancer: correlation with clinicopathological features and overall survival. Tumori. 2012;98(1):105–12. doi: 10.1177/030089161209800115. Epub 2012/04/13. [DOI] [PubMed] [Google Scholar]
- 40.Koliaraki V, Roulis M, Kollias G. Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J Clin Invest. 2012;122(11):4231–42. doi: 10.1172/JCI63917. Epub 2012/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grugan KD, Miller CG, Yao Y, Michaylira CZ, Ohashi S, Klein-Szanto AJ, et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A. 2010;107(24):11026–31. doi: 10.1073/pnas.0914295107. Epub 2010/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tessier CR, Doyle GA, Clark BA, Pitot HC, Ross J. Mammary Tumor Induction in Transgenic Mice Expressing an RNA-Binding Protein. Cancer Research. 2004;64(1):209–14. doi: 10.1158/0008-5472.can-03-2927. [DOI] [PubMed] [Google Scholar]
- 43.Gu W, Katz Z, Wu B, Park HY, Li D, Lin S, et al. Regulation of local expression of cell adhesion and motility-related mRNAs in breast cancer cells by IMP1/ZBP1. Journal of cell science. 2012;125(Pt 1):81–91. doi: 10.1242/jcs.086132. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V, et al. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci U S A. 2014;111(43):E4658–67. doi: 10.1073/pnas.1415762111. Epub 2014/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luraghi P, Reato G, Cipriano E, Sassi F, Orzan F, Bigatto V, et al. MET signaling in colon cancer stem-like cells blunts the therapeutic response to EGFR inhibitors. Cancer Res. 2014;74(6):1857–69. doi: 10.1158/0008-5472.CAN-13-2340-T. Epub 2014/01/23. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N, Yamada Y, Furuta K, Honma Y, Iwasa S, Takashima A, et al. Serum levels of hepatocyte growth factor and epiregulin are associated with the prognosis on anti-EGFR antibody treatment in KRAS wild-type metastatic colorectal cancer. Br J Cancer. 2014;110(11):2716–27. doi: 10.1038/bjc.2014.230. Epub 2014/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis H, Irshad S, Bansal M, Rafferty H, Boitsova T, Bardella C, et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat Med. 2015;21(1):62–70. doi: 10.1038/nm.3750. Epub 2014/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015 doi: 10.1038/ng.3225. Epub 2015/02/24. [DOI] [PubMed] [Google Scholar]
- 49.Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015 doi: 10.1038/ng.3224. Epub 2015/02/24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.