Abstract
Background
Fentanyl is commonly utilized in preterm infants. Relatively little is known regarding the neurodevelopmental outcomes of preterm infants exposed to fentanyl.
Objective
To investigate the association between cumulative fentanyl dose and brain injury and diameters in a cohort of preterm infants
Methods
Data on demographics, perinatal course, and neonatal course, including total fentanyl exposure prior to term equivalent age, were retrospectively evaluated for 103 infants born at ≤ 30 weeks gestational age who underwent magnetic resonance imaging at term equivalent age (mean gestational age 26.9 ± 1.8 weeks). Magnetic resonance images were evaluated for brain injury and regional brain diameters. Developmental testing was conducted at term equivalent and 2 years of age.
Results
Seventy-eight infants (76%) received fentanyl (median cumulative dose 3 μg/kg, interquartile range 1 – 441 μg/kg). Cumulative fentanyl dose in the first week of life correlated with the incidence of cerebellar hemorrhage after correction for covariates (OR 2.1, 95% confidence interval 1.1 – 4.1). Cumulative fentanyl dose before term equivalent age correlated with reductions in transverse cerebellar diameter after correction for covariates including the presence of cerebellar hemorrhage (r = 0.461, p = 0.002). No correlation was detected between cumulative fentanyl dose and development at 2 years of age.
Conclusions
Higher cumulative fentanyl dose in preterm infants correlated with a higher incidence of cerebellar injury and lower cerebellar diameter at term equivalent age. Our findings must be taken with caution, but emphasize the need for future prospective trials examining the risks and benefits of commonly utilized analgesic agents in preterm infants.
Keywords: premature infant, magnetic resonance imaging, neonatal intensive care, analgesics, opioid analgesics
INTRODUCTION
Control of pain and agitation is a fundamental component of neonatal intensive care. Preterm infants are uniquely susceptible to pain due to immature pain modulatory mechanisms and prolonged hyperalgesia after tissue injury.1 An adverse effect of pain in the preterm infant on cerebral development and neurological outcome has been demonstrated.2 However, limitations exist in the safety profile of the agents used to ameliorate pain in the preterm infant. Extensive preclinical data suggest that opioids may have a negative impact on brain growth and development, including both antiproliferative and apoptotic effects.3 The short- and long-term neurologic impact of morphine on preterm infants has been examined in large randomized controlled trials with additional doses of morphine being associated with an increased risk of brain injury (severe intraventricular hemorrhage [IVH]).4 A similar association has been observed with midazolam, possibly mediated by the hypotensive effects of both agents.5 Concerns regarding the use of morphine and midazolam have led to utilization of alternative agents for sedation and analgesia in preterm infants.
Fentanyl, a potent synthetic mu-opioid receptor agonist, is commonly utilized for sedation and analgesia in infants.6,7 There is a paucity of data regarding the neurodevelopmental outcomes of preterm infants exposed to fentanyl. Small, randomized trials have shown reduced stress responses in ventilated preterm infants receiving continuous infusion fentanyl versus placebo, with no difference in the incidence of IVH or periventricular leukomalacia (PVL) assessed by conventional cranial ultrasound.8–10 Notably, these studies were not designed or powered to assess brain injury.
Existing studies of sedatives and analgesics in preterm neonates employed conventional cranial ultrasound through the anterior fontanelle to assess brain injury. Conventional cranial ultrasound may underestimate the incidence of clinically important lesions, including cerebellar hemorrhage (CBH).11 Additionally, these trials do not report impacts on brain growth. Therefore, we utilized existing magnetic resonance images to retrospectively investigate the association between cumulative fentanyl dose and brain injury and diameters in a cohort of preterm infants. We hypothesized that early fentanyl exposure would be associated with white matter injury or cerebellar hemorrhage evident on magnetic resonance imaging (MRI). Additionally, we hypothesized that cumulative fentanyl exposure before term equivalent age would be associated with reduced regional brain size.
MATERIALS AND METHODS
Patient population
This retrospective cohort study included assessment of MRI images obtained in preterm infants born between 23 and 30 weeks gestational age who were admitted to the neonatal intensive care unit (NICU) at St. Louis Children’s Hospital and enrolled in either an observational study (n = 59) or a randomized trial of early caffeine dosing (n = 74). The observational study incorporated serial MRI in a cohort of preterm infants enrolled within the first 72 hours of life between April 2007 and October 2008.12 The purpose of the observational study was examination of the association between microstructural changes in the preterm brain and neurodevelopmental disability in later life. The early caffeine dosing trial, also including serial MRI, randomized preterm infants to standard caffeine citrate (20 mg/kg loading dose) or high-dose caffeine citrate (80 mg/kg loading dose) within the first 24 hours of life between November 2008 and June 2010.13 All preterm infants admitted during the study periods were considered for enrollment in the observational or randomized study. Infants who had a known congenital anomaly, were moribund with severe sepsis, were in respiratory failure, or had severe brain injury identified by cranial ultrasound in the first 24 hours of life were excluded from both studies. Severe sepsis and/or respiratory failure were defined as physiologic instability requiring > 80% FiO2 for six hours and/or more than two inotropic drugs.
Data collection
Total exposure to all sedative/analgesic agents in the first week of life and from birth until term equivalent age or prior to discharge from the NICU was collected from the electronic medication administration records of infants enrolled in the observational study or randomized in the early, high-dose caffeine trial. Additionally, data regarding demographics (gender, gestational age, birth weight), perinatal course (maternal age and race, maternal alcohol and illicit drug use, antenatal steroids, mode of delivery, presence of chorioamnionitis, 5-minute APGAR score, Clinical Risk Index for Babies [CRIB] score at birth), and neonatal course (days of total parenteral nutrition [TPN] and ventilation; patent ductus arteriosus [PDA] requiring treatment; necrotizing enterocolitis [NEC] defined as Bell’s stage II or III; culture proven sepsis; caffeine regimen; and total exposure to inotropes, hydrocortisone, and dexamethasone) were also collected. Treatment for PDA included ibuprofen, indomethacin, or surgical ligation. Weight and occipitofrontal circumference (OFC) were recorded at birth and at the time of MRI to evaluate body and head growth, respectively.
Evaluation of brain injury and diameters
IVH was evaluated by cranial ultrasounds obtained at intervals determined by the clinicians caring for the infant. IVH was graded as I–IV by the clinical neuroradiologist. Research MRI was undertaken for enrolled infants at term equivalent age or at discharge. Brain injury was scored on T1- and T2-weighted MRI studies in four areas (white matter, cortical gray matter, deep nuclear gray matter, and cerebellum) using methods previously described.14 Bifrontal diameter (BFD), biparietal diameter (BPD), and transverse cerebellar diameter (TCD) were measured.15 The difference (d-) between each infant’s regional measure and the mean regional measure in healthy fetal MRI was calculated to correct for postmenstrual age at the time of MRI scan (d-BFD, d-BPD, and d-TCD).16 MRI analyses were undertaken by a single neuroradiologist blinded to the clinical characteristics of the infants.
Developmental assessment
Neurobehavioral outcome at term equivalent age was assessed using the NICU Network Neurobehavioral Scale (NNNS), Dubowitz Neurological Examination, and the Neonatal Oral Motor Assessment Scale (NOMAS). Term equivalent neurobehavioral testing was conducted by a single licensed occupational therapist certified to perform these evaluations. Outcomes assessed at term equivalent age included thirteen NNNS summary scores (attention, asymmetric reflexes, excitability, habituation, tolerance of handling, hypertonia, hypotonia, lethargy, quality of movement, regulation, non-optimal reflexes, stress, and arousal), the total optimality score and the 6 compound optimality scores on the Dubowitz (tone, tone patterns, reflexes, movements, deviant signs, and behavior), and result of the NOMAS (normal, disorganized, or dysfunctional).
Infants returned at two years of age for a developmental assessment using the Bayley Scales of Infant and Toddler Development, Third Edition. All testers were blinded to past medical history, including exposure to sedatives/analgesics, and imaging findings. Outcomes assessed included Bayley composite scores for language, motor, and cognitive function.
Statistical analysis
We performed statistical analyses using SPSS 19 (SPSS, Inc., Chicago, Illinois). To explore the relationship between cumulative fentanyl dose and outcome (brain injury and regional brain diameters), logistic or linear regression models were employed relating outcome measures to the log of total fentanyl dose in μg/kg with adjustment for potential covariates. Potential covariates with a significant association with the outcome variable tested (p < 0.1) on univariate analysis were included in the regression model.
The study was approved by the Human Research Protection Office at Washington University in St. Louis and all parents provided signed informed consent.
RESULTS
MRI was obtained at a mean postmenstrual age of 37.6 ± 1.8 weeks in 103 of the 133 infants enrolled in the observational or randomized study (77%). Thirty infants did not undergo an MRI scan due to death prior to scan (n = 20), transfer to another NICU (n = 5), and parental refusal (n = 5). Demographic and clinical characteristics were similar in those infants who underwent MRI and those in the overall cohort.
Demographic and clinical characteristics of the infants who underwent MRI are presented in Table 1. Of 103 infants, 25 infants (24%) received no sedative/analgesic medication before term equivalent age. For infants who received sedative/analgesic medication, the most commonly used agent was fentanyl, with 78 infants in the cohort (76%) receiving fentanyl before term equalivent age (median cumulative dose from birth to term equivalent age 3 μg/kg, interquartile range 1 – 441 μg/kg, range 1 – 3900 μg/kg). The median duration of therapy was 2 days (interquartile range 1 – 7 days, range 1 – 84 days). Bolus doses were used for both invasive procedures and at the clinicians’ discretion based on the subjective assessment of pain and agitation. Twenty-seven patients (26%) received a continuous infusion of fentanyl during the study period. The most common indication for continuous infusion of fentanyl was sedation during prolonged mechanical ventilation. Continuous infusions were generally initiated at 1 μg/kg/hour and titrated by 1 μg/kg/hour to approximately 5 μg/kg/hour as needed based on nursing assessment utilizing Premature Infant Pain Profile and COMFORT scores. Other sedative/analgesic agents were less commonly used. Eighteen infants (17%) received midazolam, with 5 (5%) of those receiving > 1 mg/kg total dose. Thirteen infants (13%) received morphine, with 5 (5%) of those receiving > 1 mg/kg total dose. Administration of other agents was limited to intermittent bolus doses.
Table 1.
Characteristics of infants who underwent magnetic resonance imaging at term equivalent age
| Characteristic | n = 103 |
|---|---|
| Gestational age (weeks)a | 26.9 ± 1.8 |
| Birth weight (g)a | 952 ± 251 |
| Growth restriction for weight at birth | 7 (7%) |
| Growth restriction for occipitofrontal circumference at birth | 13 (13%) |
| Male gender | 50 (49%) |
| Antenatal steroids | 86 (83%) |
| 5-minute APGAR scorea | 6.4 ± 2.0 |
| Clinical Risk Index for Babies scorea | 3.8 ± 3.6 |
| Ventilator daysb,c | 2 (1 – 13) |
| Total parenteral nutrition daysb | 16 (11 – 31) |
| Patent ductus arteriosus requiring treatment | 47 (46%) |
| Necrotizing enterocolitis | 7 (7%) |
| High loading dose of caffeined | 28 (27%) |
| Inotrope exposure | 39 (38%) |
| Fentanyl exposure | 78 (76%) |
| Morphine exposure | 13 (13%) |
| Midazolam exposure | 18 (17%) |
| Dexamethasone exposure | 9 (9%) |
| Hydrocortisone exposure | 16 (16%) |
Mean ± SD
Median (interquartile range)
All patients were intubated and received surfactant after birth
All study patients received maintenance caffeine citrate therapy at standard doses
Qualitative Brain Injury
Sixty-five of 133 study infants (49%) survived without brain injury. Higher cumulative fentanyl dose was associated with a lower incidence of survival without brain injury (OR 0.5, 95% confidence interval 0.4 – 0.7, p < 0.001). There was no correlation between cumulative fentanyl dose and the incidence of severe IVH or cystic white matter injury. However, cumulative fentanyl dose correlated with the incidence of CBH (Table 2).
Table 2.
Brain Injury in Relation to Log of Fentanyl Dose
| Outcome | Incidence n = 103 | Odds ratio | P value |
|---|---|---|---|
| Any brain injury | 49 (48%) | 1.6 (1.2 – 2.3) | 0.006 |
| Any intraventricular hemorrhage | 36 (35%) | 1.5 (1.0 – 2.1) | 0.03 |
| Grade III/IV intraventricular hemorrhage | 10 (10%) | 1.3 (0.8 – 2.1) | 0.4 |
| Cystic white matter injury | 8 (8%) | 0.8 (0.4 – 1.5) | 0.4 |
| Any cerebellar hemorrhage | 22 (21%) | 2.4 (1.6 – 3.7) | < 0.001 |
| Grade III/IV cerebellar hemorrhage | 7 (7%) | 2.4 (1.2 – 4.6) | 0.009 |
As hemorrhagic lesions most commonly occur in the first week of neonatal life, regression analysis was performed considering only fentanyl exposure during this time period. The association between cumulative fentanyl dose in the first week of postnatal life and the incidence of CBH remained significant after adjustment for gestational age at birth, 5-minute APGAR score, severity of early illness (CRIB score), PDA requiring treatment, inotrope exposure, and hydrocortisone exposure (OR 2.1, 95% confidence interval 1.1 – 4.1, p = 0.002) (Table 3).
Table 3.
Relationship of fentanyl dose (log mg/kg) in the first week of life to neonatal outcomes
| Univariate odds ratio (95% CI) | Multivariate odds ratio (95% CI)a | |
|---|---|---|
| Grade III/IV intraventricular hemorrhage | 1.1 (0.4 – 2.8) | |
| Cystic white matter injury | 0.8 (0.3 – 2.7) | |
| Any cerebellar hemorrhage | 2.7 (1.4 – 5.1) b | 2.1 (1.1 – 4.1) b |
| Death or any cerebellar hemorrhage | 2.8 (1.6 – 4.9) b | 2.1 (1.2 – 3.9) b |
Controlled for gestational age, 5-minute APGAR score, Clinical Risk Index for Babies score, patent ductus arteriosus requiring treatment, inotrope exposure, and hydrocortisone exposure. Death or cerebellar hemorrhage additionally controlled for length of stay.
p < 0.01
Brain Diameters
Cumulative fentanyl dose did not correlate with body weight at MRI scan or body growth between birth and term equivalent age. Cumulative fentanyl dose correlated with reductions in BFD (d-BFD: r = 0.376, p < 0.001) and BPD (d-BPD: r = 0.350, p = 0.001) as well as OFC at MRI scan (z-score: r = −0.363, p < 0.001). The association between higher cumulative fentanyl dose and lower cerebral diameters and OFC did not persist after adjustment for confounding factors.
For the cerebellum, a correlation was noted between cumulative fentanyl dose and reductions in cerebellar diameter (d-TCD: r = 0.531, p < 0.001)(Figure 1). The association between higher cumulative fentanyl dose and lower cerebellar diameter remained significant after adjustment for the potential confounders of gestational age at birth, z-score for weight at MRI scan, the presence of CBH, 5-minute APGAR score, severity of early illness (CRIB score), duration of mechanical ventilation, duration of TPN, PDA requiring treatment, NEC, inotrope exposure, hydrocortisone exposure, morphine exposure, and midazolam exposure (d-TCD: R2 = 0.461, p = 0.002).
Figure 1.
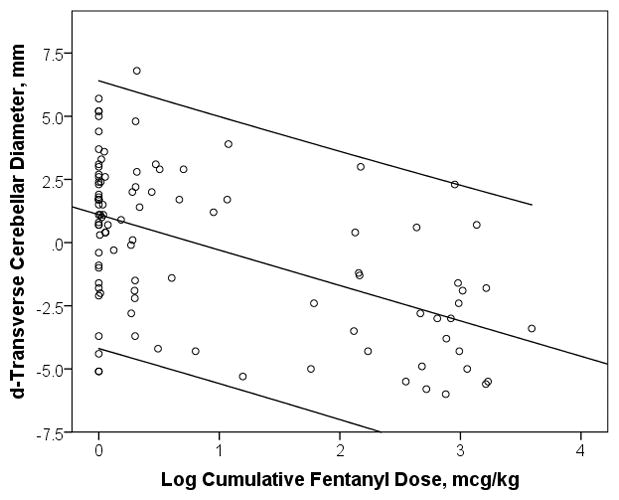
Log of Fentanyl Dose in Relation to Cerebellar Diameter (d-TCD). Center line represents fitted least-squares regression line and outer lines represent 95% confidence interval.
Developmental outcome
Developmental testing was completed in 84 infants at term equivalent age and 86 infants at 2 years of age. Cumulative fentanyl dose correlated with sub-optimal reflexes (r = 0.310, p = 0.004) and hypertonicity (r = 0.277, p = 0.011) on the NNNS, which did not persist after correction for gestational age at birth (p > 0.05). There was no correlation between cumulative fentanyl dose and other measures of developmental outcome at term equivalent or 2 years of age.
DISCUSSION
Our study raises concerns regarding fentanyl exposure in the preterm infant. Preterm infants in our institution were exposed to high cumulative doses of fentanyl during their NICU course. Fentanyl exposure was associated with an increased risk of cerebellar injury and impaired cerebellar size in a dose-dependent manner. Cumulative fentanyl dose was not independently associated with developmental outcomes at term equivalent or 2 years of age.
While there are no large studies of the effects of fentanyl on acute brain injury in preterm infants, studies of the effects of morphine may be instructive, as the repercussions of morphine exposure have been evaluated in several randomized, controlled trials. In the NEOPAIN trial, continuous infusion of morphine was compared to placebo in ventilated preterm infants.4 Morphine infusion did not alter the frequency of severe IVH, PVL, or death. However, open-label intermittent boluses of morphine were associated with a higher incidence of severe IVH in the morphine group and a higher incidence of severe IVH, PVL, and death in the placebo group. The authors hypothesized that pre-existing hypotension, potentially exacerbated by morphine, may play a role in the development of these adverse outcomes. The NEOPAIN trial did not examine the incidence of CBH. The identification of CBH has been enhanced by improved neuroimaging techniques, particularly posterolateral fontanel ultrasonographic imaging and MRI.17 We must emphasize that the retrospective nature of our analysis precludes conclusion regarding the role of fentanyl in the pathology of CBH in these infants. As fentanyl use may be a sensitive marker of concomitant morbidity in our cohort, we view this association as highly tentative, but worthy of attention in future analyses.
Preterm infants in our observational cohort with higher cumulative fentanyl dose before term equivalent age displayed marked reduction in cerebellar diameter, even after controlling for CBH and other clinical variables. Preterm infants have lower brain volumes at term equivalent postmenstrual age compared to term controls,18 and these regional volumetric reductions correlate strongly with simple measures of regional brain diameter.15 As with brain injury, both prematurity and corresponding morbidities likely contribute to the multifactorial pathophysiology of impaired brain growth. Vulnerability of the developing cerebellum to opioid exposure has been suggested in animal models.19 Opioids are implicated in the modulation of proliferation, survival, and differentiation of neuroblasts and astroglia of the cerebellum.20 Further, the nuclei of fetal brain cells have a greater capacity to bind and retain opioids, as compared to maternal hosts.21 Animal models have shown significant impact on cerebellar growth from both acute doses of morphine and more chronic morphine exposure, though it has been difficult to separate direct opioid toxicity from the behavioral influence of opioids exposure on feeding and nutrition during a critical phase of development.22 However, a more direct opioid inhibition of Purkinje cell survival in the cerebellum has been demonstrated in mouse models.23
Additionally, opioid exposure alters astrocyte morphology, increasing both calcium and carbonyl oxidation resulting in promotion of neuronal apoptosis.24 Finally, opioids inhibit DNA synthesis in the external granular layer of the cerebellum, leading to the inhibition of neuroblast proliferation.25 These preclinical findings lend plausibility to the associations observed in our retrospective cohort. Of great concern, a similar association between increased opioid exposure and impaired cerebellar growth has been reported recently in an independent retrospective cohort.26
Finally, cumulative fentanyl dose was not independently associated with developmental outcomes at term equivalent or 2 years of age in our cohort. This correlates with findings from a previous observational study.27 However, the previous study suffered from several limitations, including lack of prospective enrollment, exclusion of patients with severe brain injury on cranial ultrasound, and utilization of Bayley scores at one year of age as the sole marker of neurodevelopmental outcome.28 In the case of our study, we are not reassured by this null finding at 2 years of age. This finding may suggest that the potentially negative impact of opioid exposure on the cerebellum are balanced by positive impacts such as reduction of pain and agitation. However, it must also be considered that the developmental assessments utilized in this cohort may not have been sensitive to subtle changes in neurobehavior or early developmental alterations. Cellebellar hemorrhage is associated with subsequent neurologic abnormalities, including impaired receptive and expressive language, socialization-behavorial deficits, and autism. Cerebellar underdevelopment is associated with deficits in executive functions and visual-spatial and linguistic abilities.29 These deficits may be difficult to detect until later childhood. We will evaluate this cohort to school-age with particular interest in impairments commonly associated with cerebellar abnormalities.
This study has limitations. We excluded moribund infants with early sepsis or respiratory failure, which may limit the generalizability of our findings. However, we would expect the adverse effects of fentanyl may be greater in this vulnerable population and their exclusion decreases the likelihood of positive associations between fentanyl exposure and brain injury and compromised brain growth. The study was retrospective and observational, and fentanyl was administered at the clinicians’ discretion with practice varying between physicians. We can draw no conclusions regarding the potential causitive role of fentanyl in the observed associations and, as stated previously, these associations should be viewed as highly tentitive. The sample size was relatively small and confounded by co-morbidities, including prolonged mechanical ventilation and NEC, resulting in higher cumulative doses of fentanyl in more moribund infants. These co-morbidities are also significant risk factors for adverse neurodevelopmental outcome, although the pathway to such impairments is not fully understood. It must be considered that fentanyl use in our cohort may be a sensitive indicator of neonatal disease severity. We attempted to control for this effect in regression analyses, although it is not possible to consider all potential predictors of brain injury and compromised brain growth or completely address collinearity among the predictors included in our models. For example, we were unable to analyze the influence of other sedative/analgesic agents, as their use coincided with higher cumulative fentanyl dose. However, the cumulative doses of morphine and midazolam in our cohort were 10 times the standard single dose in fewer than ten infants, compared with 39 infants receiving greater than 100 times the standard single dose of fentanyl. Despite these limitations, and based on the strength of our findings after adjusting for numerous potential confounders, our data suggest potential risk to the immature brain from high cumulative fentanyl exposure.
Conclusions
Our study raises concerns over potential detrimental effects of high cumulative fentanyl exposure in the developing brain of the preterm infant. However, we wish to emphasize that our findings do not suggest suspension of appropriate analgesia to preterm infants. Our data emphasize the need for future prospective trials examining the risks and benefits of commonly utilized sedative and analgesic agents in preterm infants, particularly in relation to brain growth. Additionally, further preclinical work is necessary to determine the role of opioid receptors in the development and pathology of the neonatal brain.
Acknowledgments
Divyen Shah, Claudine Vavassseur, Han Tjoeng, Karen Lukas, and Amit Mathur for their assistance in the recruitment and execution of this cohort study. We also wish to acknowledge Amanda Felber Brown, Nathalie Bednarek, Hiroyuki Kidokoro, Jim Alexopoulos, Reginald Lee, and Joshua Shimony for their assistance in image and data analysis.
Funding: This work was supported by the National Institute of Child Health and Development (R01 HD057098); the Intellectual and Developmental Disabilities Research Center at Washington University (NIH/NICHD P30 HD062171); and the Doris Duke Charitable Foundation.
Footnotes
Declaration of conflicting interests: The authors declare no conflicts of interest.
Presentations: Preliminary results were presented as posters at the Pediatric Academic Societies’ Annual Meeting in Denver, CO (April 2011).
Contributor Information
Christopher McPherson, Clinical Pharmacist, Departments of Pediatric Newborn Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA, USA.
Matthew Haslam, Research Assistant, Department of Pediatrics, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO, USA.
Roberta Pineda, Research Assistant Professor, Departments of Pediatrics and Occupational Therapy, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO, USA.
Cynthia Rogers, Assistant Professor, Department of Psychiatry, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO, USA.
Jeffrey J. Neil, Associate Professor, Department of Neurology, Boston Children’s Hospital, 333 Longwood Avenue, Boston, MA, USA.
Terrie E. Inder, Chair, Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA, USA.
References
- 1.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 2.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Durrmeyer X, Vutskits L, Anand KJ, Rimensberger PC. Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res. 2010;67:117–27. doi: 10.1203/PDR.0b013e3181c8eef3. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJ, Hall RW, Desai N, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 5.Anand KJ, Barton BA, McIntosh N, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med. 1999;153:331–8. doi: 10.1001/archpedi.153.4.331. [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–87. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Walker JK, Hurt KM, Bennett KM, Grosshans N, Fotis MA. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J Pediatr. 2008;152:412–5. doi: 10.1016/j.jpeds.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 8.Orsini AJ, Leef KH, Costarino A, Dettorre MD, Stefano JL. Routine use of fentanyl infusions for pain and stress reduction in infants with respiratory distress syndrome. J Pediatr. 1996;129:140–5. doi: 10.1016/s0022-3476(96)70201-9. [DOI] [PubMed] [Google Scholar]
- 9.Lago P, Benini F, Agosto C, Zacchello F. Randomised controlled trial of low dose fentanyl infusion in preterm infants with hyaline membrane disease. Arch Dis Child Fetal Neonatal Ed. 1998;79:F194–7. doi: 10.1136/fn.79.3.f194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ancora G, Lago P, Garetti E, et al. Efficacy and safety of continuous infusion of fentanyl for pain control in preterm newborns on mechanical ventilation. J Pediatr. 2013;163:645–51.e1. doi: 10.1016/j.jpeds.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Miall LS, Cornette LG, Tanner SF, Arthur RJ, Levene MI. Posterior fossa abnormalities seen on magnetic resonance brain imaging in a cohort of newborn infants. J Perinatol. 2003;23:396–403. doi: 10.1038/sj.jp.7210941. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt E, Inder TE, Alexopoulos D, et al. Regional impairments of cortical folding in premature infants. Ann Neurol. 2015;77:154–62. doi: 10.1002/ana.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson C, Neill JJ, Tjoeng TH, Pineda R, Inder TE. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res. 2015;78:198–204. doi: 10.1038/pr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34:2208–14. doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol. 2009;30:125–31. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garel C. MR imaging of the fetal brain: normal development and cerebral pathologies. Berlin; New York: Springer; 2004. [Google Scholar]
- 17.Limperopoulos C, Benson CB, Bassan H, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116:717–24. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 18.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–77. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 19.Golalipour MJ, Ghafari S. Purkinje cells loss in off spring due to maternal morphine sulfate exposure: a morphometric study. Anat Cell Biol. 2012;45:121–7. doi: 10.5115/acb.2012.45.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer RP, Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology. 1989;10:475–83. [PubMed] [Google Scholar]
- 21.Steele WJ, Johannesson T. Distribution of 14C-morphine and macromolecules in the brain and liver and their nuclei in pregnant rats and their foetuses after infusion of morphine into pregnant rats at near-term. Acta Pharmacol Toxicol (Copenh) 1975;37:265–73. doi: 10.1111/j.1600-0773.1975.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 22.Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacology. 1977;15:276–82. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- 23.Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp Neurol. 1994;130:95–105. doi: 10.1006/exnr.1994.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp Neurol. 1998;151:70–6. doi: 10.1006/exnr.1998.6788. [DOI] [PubMed] [Google Scholar]
- 25.Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W., 3rd Opioids intrinsically inhibit the genesis of mouse cerebellar granule neuron precursors in vitro: differential impact of mu and delta receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000;12:1281–93. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwicker JG, Miller SP, Grunau RE, et al. Morphine exposure is associated with altered cerebellar growth in premature newborns [abstract #1528.465]. Poster presented at: Pediatric Academic Societies Annual Meeting; 2012; Boston, MA. [Google Scholar]
- 27.Lammers EM, Johnson PN, Ernst KD, et al. Association of fentanyl with neurodevelopmental outcomes in very-low-birth-weight infants. Ann Pharmacother. 2014;48:335–42. doi: 10.1177/1060028013514026. [DOI] [PubMed] [Google Scholar]
- 28.Harris SR, Megens AM, Backman CL, Hayes VE. Stability of the Bayley II Scales of Infant Development in a sample of low-risk and high-risk infants. Dev Med Child Neurol. 2005;47:820–3. doi: 10.1017/S0012162205001738. [DOI] [PubMed] [Google Scholar]
- 29.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24:1085–104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]


