Abstract
The Salvador-Warts-Hippo pathway controls cell fate and tissue growth. The main function of the Hippo pathway is to prevent YAP and TAZ translocation to the nucleus where they induce the transcription of genes involved in cell proliferation, survival, and stem cell maintenance. Hippo signaling is thus a complex tumor suppressor, and its deregulation is a key feature in many cancers. Recent mounting evidence suggests that the overexpression of Hippo components can be useful prognostic biomarkers. Moreover, Hippo signaling appears to be intimately linked to some of the most important signaling pathways involved in cancer development and progression. A better understanding of the Hippo pathway is thus essential to untangle tumor biology and to develop novel anticancer therapies. Here, we comment on the progress made in understanding Hippo signaling and its connections, and also on how new drugs modulating this pathway, such as Verteporfin and C19, are highly promising cancer therapeutics.
Background
The Salvador-Warts-Hippo pathway (known as Hippo pathway) was first discovered to be involved in controlling tissue growth in Drosophila Melanogaster in 2002, and it has recently emerged as a critical tumor suppressor network (1). The Hippo pathway is a highly evolutionarily conserved pathway that controls multiple functions that are crucial to several carcinogenesis processes such as proliferation, apoptosis and stem cell maintenance.
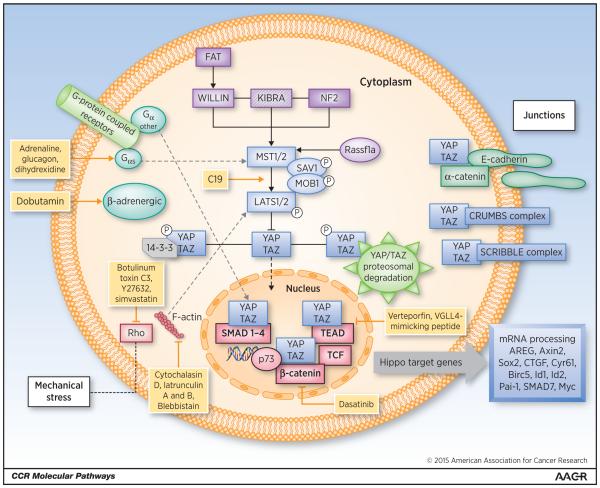
The core of the mammalian Hippo pathway consists of a protein complex that includes: kinases MST1 and MST2 (also known as STK4 and STK3), large tumor suppressors 1 and 2 (LATS1 and LATS2) and adaptor proteins SAV1 (Salvador homologue 1) and MOB kinase activator 1A and 1B (MOB1A and MOB1B). MST1 and MST2 facilitate LATS1 and LATS2 phosphorylation, which in turn facilitates LATS-dependent phosphorylation of the homologous oncoproteins YAP (Yes-associated protein; encoded by YAP1) and TAZ (PDZ-binding motif, also known as WWTR1). When the Hippo pathway is active, its main effectors, YAP and TAZ, are phosphorylated by the Hippo core complex and retained in the cytoplasm where they undergo proteasomal degradation. When the Hippo pathway is inactive or suppressed, YAP and TAZ translocate to the nucleus. In the nucleus, they regulate the activity of various transcription factors involved in cell proliferation, survival, micro-RNA processing, metastases development and stem cell maintenance such as SMADs, TEADs, TBX5 and RUNT1,2 and p73 (2-4). Based on this action mechanism, Hippo effectors YAP and TAZ can act either as tumor suppressors when located in the cytoplasm or as oncogenes by facilitating transcriptional activation in the nucleus (Fig. 1).
Figure 1.
Hippo signaling pathway and main regulators. The core of Hippo signaling consists of a group of kinases (MST1/2, LATS1/2) and adaptor proteins SAV1 and MOB1, which by phosphorylating the Hippo pathway effectors YAP, and TAZ retain them in the cytoplasm. In the cytoplasm, YAP/TAZ undergo proteosomal degradation. YAP and TAZ, if not retained in the cytoplasm, can translocate to the nucleus where they regulate the activity of several transcription factors that induce the expression of Hippo target genes involved in cancer development and progression. Upstream regulation of Hippo includes: i) FERM domain-containing proteins (NF2 KIBRA and WILLIN) that activate the core kinase complex, ii) Polarization and Adhesion proteins like α-catenin, E-cadherin, CRUMBS and SCRIBBLE complexes that can sequester YAP/TAZ in the cytoplasm iii) GPCRs that can be divided in those signaling through GαS leading to activation of Hippo cascade or those signaling through other Gα subunits that favor YAP/TAZ nuclear translocation iv) Mechanical stress regulated by F-actin can also increase YAP/TAZ cytoplasmic location. Additionally, independent proteins like Rassf1a and several pathways regulate Hippo signaling. New molecules and drugs under development to modulate Hippo signaling are represented in yellow.
Hippo controls cell proliferation and survival mainly by sensing the physical state of cells within a tissue through several mechanisms. Unlike other pathways, Hippo does not have dedicated extracellular ligand/receptor complexes. Instead, the upstream signaling of Hippo involves many mechanisms and other signaling pathways. These upstream inputs can be divided into those controlling the pathway by retaining YAP/TAZ in the cytoplasm by physical interaction, and those that activate the core kinase complex MST/LATS leading to YAP/TAZ phosphorylation. Some of the most relevant upstream regulators of Hippo include:
i) FERM domain-containing proteins NF2 and KIBRA and WILLIN (5), ii) Polarization and Adhesion proteins including those controlling basolateral polarity like the SCRIBBLE complex (6), apical polarity like CRUMBS complex (7), and planar cell polarity like FAT and Dachsous family of cadherins (8). Cell adhesion proteins such as α-catenin and E-cadherin (9), iii) Extracellular factors, G protein-coupled receptors (GPCRs) and TKI receptors, and iv) Changes in mechanical forces changes regulated by Rho and F-actin polymerization that can also regulate YAP/TAZ nuclear localization (10).
Other proteins can also directly affect TAZ and YAP. These include WBP2, MASK proteins, HIPK2 and 14-3-3 proteins. Moreover, other proteins can interact with the upstream kinase complex. Of particular interest is Rassf1, a tumor suppressor frequently methylated in several tumors, which can act as a Hippo regulator. Rassf1a can bind MST regulating LATS1/2 phosphorylation and promoting proapoptotic signals through p73. For more detailed information on Hippo upstream regulators and downstream effectors, we refer the reader to some of the excellent reviews previously published (8, 11).
Clinical-Translational Advances
Disruptions in the correct function of this pathway have been found to take part in several tumorigenesis mechanisms. However, in human cancer, mutations in the Hippo components are infrequent (12). The most established mutation is located in the tumor suppressor NF2, an upstream Hippo regulator, causing the autosomal dominant syndrome Type 2 neurofibromatosis that entails, among others the development of meningiomas and schwanomas (13). In addition, mutations and deletions in LATS2 can be frequently found in malignant mesothelioma (14). In mice, mutations in Hippo components lead to developmental deficiencies within the intestinal epithelium, liver or skin keratinocytes (15-17). Although YAP/TAZ activating mutations have not been described, high YAP/TAZ nuclear levels are present in several cancer types (18). High YAP/TAZ nuclear levels favor cell proliferation and survival as well as the acquisition of a cancer-promoting phenotype. Stem cell maintenance is intimately linked to Hippo signaling in tumor cells. In breast tumors, in vitro studies show how TAZ acts as a stem cell promoter, providing stem cells with self-renewal and tumor initiating capacity. This property is acquired in part by forming a complex with the polarity complex SCRIBBLE that leads to induction of the epithelium-mesenchymal-transition (EMT) (6). In neuroblastoma cell lines, studies have shown how TAZ upregulates connective tissue growth factor (CTGF) known to induce EMT (19). Moreover, in oral cancer cells, TAZ has also been found to be involved in TGF-β1 induced EMT (20).
Cancer stem cells are believed to be resistant to chemotherapeutic agents. Recently, several works have linked Hippo effectors YAP and TAZ to chemoresistance in various cancer types. In breast cancer cell lines, the overexpression of TAZ correlated with taxol resistance. In this work, oncogenes Cyr61 and CTGF were identified as TAZ transcriptional targets. By knocking down both Cyr61 and CTGF through short hairpin-RNAs, TAZ-induced resistance to taxol was reversed (21). Moreover, in metastagenic breast cancer cells compared to non-metastagenic cells, TAZ was identified as a mediator of this metastagenic ability, conferring chemoresistance and migratory activity (22).
In colon cancer, chemoresistance to 5-fluorouracil (5-Fu) has been linked to a quiescent status favored by the selection of colon cancer stem cells resistant to cytotoxic drugs. In colorectal cancer cell lines resistant to 5-Fu it has been shown that in 5-Fu–free conditions, YAP silencing and subsequent reduction of YAP nuclear activity leads to induced cellular quiescence (23).
YAP/TAZ activity has also been implicated in chemoresistance in other tumor types. In hepatocarcinoma, the expression of SIRT1, a protein involved in tumorigenesis, metastasis and cell survival is upregulated, which correlates with CTGF mRNA levels. SIRT1 deacetylates YAP2, which in turn increases the YAP2/TEAD nuclear association leading to increased cell growth in hepatocellular carcinoma cells (24). In non-small lung cancer, it has been suggested that TAZ expression is an intrinsic mechanism of T790M-induced resistance to EGFR-TKIs (25).
As TAZ/YAP mutations are uncommon other mechanisms are likely responsible for Hippo’s relevance in cancer. Increasing evidence is suggesting that YAP/TAZ regulation derives from a complex network of interactions with other critical pathways that modulate YAP/TAZ activity such as TGFB, Notch, Wnt, EGFR and angiogenesis:
TGFβ signaling in cancer is complex, suppressing oncogenic events at an early stage and promoting an aggressive metastatic phenotype at later cancer stages. YAP and TAZ possess domains that facilitate the interaction with TGFβ-controlled SMADs (26). In response to TGFβ stimulation, TAZ binds SMADs 2, 3 and 4, thus regulating and promoting SMAD-mediated transcription. Nonetheless, YAP is also able to bind SMAD7, increasing the repressing role of SAMD7 over TGFβ signaling (27).
Notch signaling, essential in stem-cell renewal and differentiation, is also partially controlled by Hippo. The expression of the Notch ligand Jagged-1 is upregulated by nuclear YAP in colorectal and hepatocarcinoma tissue samples and correlates with the patient survival (28).
Wnt signaling is essential in embryonic development, and aberrant Wnt/β-catenin signaling contributes to cancer development and progression. Wnt activity is in part regulated by the Hippo pathway. Phosphorylated YAP/TAZ interacts with DVL in the cytoplasm, thus inhibiting nuclear β-catenin translocation and Wnt target gene expression (29). Additionally, TAZ and YAP can directly bind to β-catenin, leading to the cytoplasmic sequestration of the Hippo effectors suppressing their nuclear activity. In colon cancer cell lines with intact Wnt/β-catenin signaling, downregulation of Hippo signaling results in increased β-catenin activity (30). Interestingly, in colon cancer cell lines harboring mutations within Wnt signaling components, the Wnt pathway has been found to promote YAP expression through a β-catenin/TCF4 complex that directly binds a DNA enhancer element located in the first intron of the YAP gene (31).
The EGFR pathway has a wide map of molecular interactions that includes the Hippo pathway. The EGFR downstream branch Ras-MAPK regulates YAP and TAZ promoting their nuclear translocation acting upstream or at LATS level (32). Other Hippo upstream factors like Rassf1a, intimately linked to EGFR signaling, can inhibit LATS activity by disrupting the apoptosis inhibitory complex formed by Raf and MST2 allowing YAP/TAZ nuclear translocation and association with the proapoptotic protein p73. Rassf1a also regulates the expression of the EGFR ligand amphiregulin (AREG) through Hippo activation (33). In colon cancer, AREG expression is associated with the prognosis of KRAS wild-type patients treated with anti-EGFR therapies. In KRAS mutant patients, Rassf1a activity through Hippo signaling might also be of critical importance as mutant KRAS binds directly to Rassf1 to activate Hippo signaling and promotes apoptosis through p53 (34). In esophageal cancer, YAP1 has been shown to induce EGFR overexpression leading to chemotherapy resistance (35). Moreover, in pancreatic KRAS mutant mice models, YAP was found to be an essential oncogenic KRAS effector (36). Hippo also regulates the other EGFR downstream branch PI3K-AKT-mTOR (37).
Hippo signaling is also involved in one of the key processes that favor tumor progression, angiogenesis. It is believed that intratumor hypoxia increases the percentage of breast cancer stem cells. This molecular mechanism is not well understood but could be explained, in part, by the fact that hypoxic conditions regulate TAZ and YAP in cancer cells (38). The hypoxia hallmark HIF1-α binds directly to WWTR1 gene (that codifies for TAZ) activating the TAZ mRNA transcription which helps maintain a stem cell phenotype (39). In bone metastasis samples from breast cancer, HIF1-α has been described to co-localize with TAZ in the nucleus. Under hypoxic conditions, nuclear HIF1-α and TAZ favor tumorigenesis by leading to increased transactivation by HIF1-α DNA binding (40). This increased rate of gene expression likely affects several target genes. Angiopoetin-2, an important angiogenesis regulator, has recently been found to be one of the YAP transcriptional targets in vitro and in vivo (41).
Many other pathways interact and regulate Hippo signaling. Of interest is the G-protein couple receptor (GPCR) pathway that can either stimulate or inhibit Hippo tumor suppression activity depending on the ligand (42).
The Hippo pathway is the common link of multiple critical pathways involved in the development and progression of cancer. This fact makes it an exciting and important pathway for the discovery of new biomarkers like the expression of YAP and/or TAZ. Most studies correlate nuclear or nuclear and cytoplasmic YAP/TAZ overexpression with a worse outcome (43-46). Additionally, a gene expression signature reflecting the activation of YAP1 has been associated with poor prognosis and cetuximab resistance in KRAS wild-type patients (47). On the other hand, YAP/TAZ cytoplasmic expression is correlated with a more favorable prognosis (48). The expression of other Hippo components such as LATS has also been associated with a better outcome. In non-small cell cancer patients, LATS overexpression and the consequent negative regulation of YAP correlate with longer overall survival (49).
Besides YAP/TAZ expression, pharmacogenomic studies have evaluated several genetic variants within Hippo components as potential biomarkers. In localized prostate cancer, a polymorphism that affects STK3 (MST2) expression has been reported to correlate with biochemical recurrence (50). In patients with cutaneous melanoma, single nucleotide polymorphisms (SNPs) in YAP and TEAD4 have been associated with the survival of these patients (51). In colorectal cancer polymorphisms in the Hippo pathway also have been found to affect the recurrence rate in high risk stage II and stage III colon cancer treated with 5-Fu based chemotherapy (52). Moreover, some of these polymorphisms have also been found to influence progression-free and overall survival in metastatic colorectal cancer patients treated with cetuximab (53).
The clinical significance of Hippo signaling makes it a highly attractive targetable pathway. Currently, there are no approved cancer therapies targeting the Hippo pathway, but several molecules and pathway modulators are in early stages of clinical development (Table 1, Fig. 1). The Hippo pathway plays an essential role in tissue growth, regeneration and repair, raising the concern of potential side effects with inhibitors of Hippo signaling.
Table 1.
Modulators of Hippo signaling pathway
| Action mechanism |
Compound/molecule | Target | Effect | Development stage |
Ref. |
|---|---|---|---|---|---|
| Interaction with Hippo’s core complex |
C19 | MST/LATS | Phosphorylation of MST/LATS |
In vivo: inhibition of tumor growth by 90% |
54 |
| Direct target YAP/TAZ or its complexes |
Verteporfin | YAP-TEAD | Inhibition of the YAP-TEAD interaction |
In vitro: inhibition of YAP1 expression and sensitizes cells to cytotoxic drugs In vivo: inhibition of tumor growth in Nf2-depleted mouse model of liver cancer Clinical trial: phase III for the treatment of multiple basal cell carcinoma (NCT00049959) |
35, 60 |
| VGLL4-mimicking peptide |
TEAD | Competition with YAP for TEAD binding |
In vitro and in
vivo: inhibition of tumor growth |
56 | |
| Targeting upstream regulators |
Adrenaline, glucagon, dihydrexidine |
GPCRs GαS |
Agonists of GαS. Increase YAP phoshorylation |
In vitro | 42 |
| Dobutamin | GPC-β- adrenergic receptor |
Inhibition of YAP nuclear location |
In vitro | 57 | |
| Cytochalasin D, latrunculin A and B, blebbistain |
F-actin | Destabilizers of F- actin. Inhibition of YAP nuclear localization |
In vitro | 58 | |
| Botulinum toxin C3, Y27632 |
Rho | Rho inhibition. Inhibition of YAP/TAZ nuclear location |
In vitro | 59 | |
| Simvastatin | Rho GTPases |
Inhibition of the SREBP/mevalonate cascade needed for RhoGTPase activity |
In vitro: inhibition of TAZ and dowregulation of TAZ, YAP, and CTGF transcripts Clinical trials: ongoing in several cancer types (Clinicaltrials.gov). |
20, 59 |
|
| Dasatinib | YES1 | Inhibition of YES1 needed for the YAP-β-catenin complex |
Clinical trials: ongoing in several cancer types (Clinicaltrials.gov) |
55 |
Abbreviations: VGLL4, vestigial-like family member 4; YES1, Yamaguchi sarcoma viral oncogene homolog 1.
The goal of targeting Hippo for cancer treatment is to increase its function as a tumor suppressor. For example, C19, a small-molecule, can induce phosphorylation of MST/LATS, leading to increased TAZ cytoplasmic levels and TAZ degradation by the GSK3-β–associated destruction complex involved in Wnt signaling (54).
The broad-spectrum tyrosine kinase inhibitor Dasatinib inhibits YES1, which is required for the YAP-β-catenin complex and might impact Hippo signaling in β-catenin dependent tumors. Dasatinib is currently being investigated in colorectal cancer with promising results (55).
Directly targeting TAZ/YAP is no easy challenge, as these proteins do not have a known catalytic activity and function mainly by protein-protein interactions. Therefore, one option is to target YAZ/TAZ complexes. A small molecule inhibiting YAP-TEAD, Verteporfin, is able to reduce YAP1 and EGFR expression in chemotherapy-resistant cells re-sensitizing those cells to cytotoxic drugs (35). A peptide mimicking VGLL4, a TEAD interacting partner, can also negatively regulate the YAP-TEAD4 complex competing with YAP for TEAD binding and suppressing tumor growth in vitro and in vivo (56).
Upstream regulators of Hippo can also be used to modulate the signaling cascade. GPCRs can either stimulate or repress YAP nuclear activity: GPCRs acting through GαS inhibit nuclear YAP, thus agonist of GαS like adrenaline, glucagon or dihydrexidine increase YAP phosphorylation and cytoplasmic location (42). Dobutamin, a GPC β-adrenergic receptor agonist can prevent nuclear accumulation of YAP/TAZ in osteoblastoma cell lines (57). F-actin, essential for cellular adhesion, regulates Hippo signaling though RHO. Several F-actin destabilizers including cytochalasin D, latrunculin A and B, the non-muscle myosin II inhibitor blebbistain as well as RHO inhibitors, botulinum toxin C3 and RHO kinase inhibitor Y27632 are able to modulate Hippo by exporting YAP/TAZ from the nucleus (58). Interestingly, the SREBP/mevalonate cascade is needed to activate RhoGTPase. As this metabolic pathway is inhibited by statins, simvastatin has also been reported to repress TAZ, leading to potent anti-cancer effects (20, 59).
Conclusions
The importance of Hippo signaling deregulation in several mechanisms involved in cancer development, progression and resistance to cancer treatment has underlined the critical role of the Hippo pathway as a tumor suppressor. Increasing evidence demonstrates that Hippo activity affects the patients’ prognosis. As a result, new therapeutics targeting Hippo are currently under development and, although in initial stages, studies suggest that some new and old molecules and drugs can modulate Hippo signaling and reduce cancer progression. The Hippo pathway is, however, involved in multiple physiological processes maintaining tissue homoeostasis thus potential side effects should be closely monitored.
Overall, the Hippo pathway is the newest and probably the most complex tumor suppressor. Better understanding Hippo signaling and modulation will surely lead to new therapeutic approaches against cancer.
Acknowledgments
Grant Support
A. Sebio is a recipient of a Juan Rodés contract from Instituto de Salud Carlos III, Spain (JR00006). H.-J. Lenz is supported in part by in part by the NCI of the NIH under award number P30CA014089 and the Daniel Butler Research Fund.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH.
References
- 1.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–91. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 2.Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–93. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seton-Rogers S. Tumour suppressors: Hippo promotes microRNA processing. Nat Rev Cancer. 2014;14:216–7. doi: 10.1038/nrc3715. [DOI] [PubMed] [Google Scholar]
- 5.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–72. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 7.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J Cell Sci. 2009;122:2587–96. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 8.Irvine KD. Integration of intercellular signaling through the Hippo pathway. Semin Cell Dev Biol. 2012;23:812–7. doi: 10.1016/j.semcdb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–11. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–14. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 11.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 13.Evans DG. Neurofibromatosis 2 [Bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II] Genet Med. 2009;11:599–610. doi: 10.1097/GIM.0b013e3181ac9a27. [DOI] [PubMed] [Google Scholar]
- 14.Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–83. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 15.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–42. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–9. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Xu Z, An Q, Jiang D, Wang L, Liang B, et al. TAZ promotes epithelial to mesenchymal transition via the upregulation of connective tissue growth factor expression in neuroblastoma cells. Mol Med Rep. 2015;11:982–8. doi: 10.3892/mmr.2014.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang W, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol. 2015;9:1091–105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–38. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 22.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–90. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 23.Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monte D, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–46. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F, et al. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468–74. doi: 10.1038/onc.2013.88. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, Wei Y, Wu S, Wang Y, Wang Z, Sun Y, et al. Up-regulation of the Hippo pathway effector TAZ renders lung adenocarcinoma cells harboring EGFR-T790M mutation resistant to gefitinib. Cell Biosci. 2015;5:7. doi: 10.1186/2045-3701-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–48. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 27.Ferrigno O, Lallemand F, Verrecchia F, L'Hoste S, Camonis J, Atfi A, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–84. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- 28.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144 doi: 10.1053/j.gastro.2013.02.009. 1530-42 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–91. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–22. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konsavage WM, Jr., Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–9. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–71. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn EY, Kim JS, Kim GJ, Park YN. RASSF1A-mediated regulation of AREG via the Hippo pathway in hepatocellular carcinoma. Mol Cancer Res. 2013;11:748–58. doi: 10.1158/1541-7786.MCR-12-0665. [DOI] [PubMed] [Google Scholar]
- 34.Matallanas D, Romano D, Al-Mulla F, O'Neill E, Al-Ali W, Crespo P, et al. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol Cell. 2011;44:893–906. doi: 10.1016/j.molcel.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clin Cancer Res. 2015;21:2580–90. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–74. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan L, Cai Q, Xu Y. Hypoxic conditions differentially regulate TAZ and YAP in cancer cells. Arch Biochem Biophys. 2014;562:31–6. doi: 10.1016/j.abb.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, et al. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–27. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendinelli P, Maroni P, Matteucci E, Luzzati A, Perrucchini G, Desiderio MA. Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WWdomain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer. Eur J Cancer. 2013;49:2608–18. doi: 10.1016/j.ejca.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 42.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, et al. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wang Y, Xie C, Li Q, Xu K, Wang E. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biol. 2013;34:2169–74. doi: 10.1007/s13277-013-0751-x. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9:e91770. doi: 10.1371/journal.pone.0091770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu ZH, et al. Prognostic significance of TAZ expression in resected non-small cell lung cancer. J Thorac Oncol. 2012;7:799–807. doi: 10.1097/JTO.0b013e318248240b. [DOI] [PubMed] [Google Scholar]
- 47.Lee KW, Lee SS, Kim SB, Sohn BH, Lee HS, Jang HJ, et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res. 2015;21:357–64. doi: 10.1158/1078-0432.CCR-14-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun PL, Kim JE, Yoo SB, Kim H, Jin Y, Jheon S, et al. Cytoplasmic YAP expression is associated with prolonged survival in patients with lung adenocarcinomas and epidermal growth factor receptor tyrosine kinase inhibitor treatment. Ann Surg Oncol. 2014;21(Suppl 4):S610–8. doi: 10.1245/s10434-014-3715-5. [DOI] [PubMed] [Google Scholar]
- 49.Lin XY, Zhang XP, Wu JH, Qiu XS, Wang EH. Expression of LATS1 contributes to good prognosis and can negatively regulate YAP oncoprotein in non-small-cell lung cancer. Tumour Biol. 2014;35:6435–43. doi: 10.1007/s13277-014-1826-z. [DOI] [PubMed] [Google Scholar]
- 50.Huang CY, Huang SP, Lin VC, Yu CC, Chang TY, Juang SH, et al. Genetic variants in the Hippo pathway predict biochemical recurrence after radical prostatectomy for localized prostate cancer. Sci Rep. 2015;5:8556. doi: 10.1038/srep08556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan H, Liu H, Liu Z, Zhu D, Amos CI, Fang S, et al. Genetic variants in Hippo pathway genes YAP1, TEAD1 and TEAD4 are associated with melanoma-specific survival. Int J Cancer. 2015;137:638–45. doi: 10.1002/ijc.29429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sebio Garcia A, Zhang W, Yang D, Stremitzer S, Stintzing S, Ning Y, et al. Germline polymorphisms in genes involved in the Hippo pathway to predict recurrence in locally advanced colon cancer. J Clin Oncol. 32;2014;(suppl) abstr e14505. [Google Scholar]
- 53.Sebio A, Stintzing S, Heinemann V, Zhang W, Sunakawa Y, Ichikawa W, et al. A genetic variant in RASSF1A, a key regulator of HIPPO pathway, predicts survival in two independent cohorts of mCRC patients treated with cetuximab-based chemotherapy [oral presentation]. Proceedings of the European Cancer Congress 2015; 2015 Sep 25–29; Vienna, Austria. 2015. Brussels (Belgium): European CanCer Organisation (ECCO) [Google Scholar]
- 54.Basu D, Lettan R, Damodaran K, Strellec S, Reyes-Mugica M, Rebbaa A. Identification, mechanism of action, and antitumor activity of a small molecule inhibitor of hippo, TGF-beta, and Wnt signaling pathways. Mol Cancer Ther. 2014;13:1457–67. doi: 10.1158/1535-7163.MCT-13-0918. [DOI] [PubMed] [Google Scholar]
- 55.Strickler JH, McCall S, Nixon AB, Brady JC, Pang H, Rushing C, et al. Phase I study of dasatinib in combination with capecitabine, oxaliplatin and bevacizumab followed by an expanded cohort in previously untreated metastatic colorectal cancer. Invest New Drugs. 2014;32:330–9. doi: 10.1007/s10637-013-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 58.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–66. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 60.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Amnders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]