Abstract
Purpose
Recurrence score [RS] derived from a 21-gene RT-PCR assay stratifies patients with early-stage estrogen-receptor-positive, HER2-normal breast cancer into 3 groups based on 10-year distant metastasis risk: low, intermediate, and high. Published data are limited regarding the impact of RS on choice of adjuvant therapy for T1 breast cancer. We investigated the relationship between RS and choice of adjuvant therapy, prognosis, and benefit of chemotherapy in stage I breast cancer.
Methods
We reviewed the records of 1030 patients with estrogen-receptor-positive, HER2-normal stage I breast cancer and RS available. RSs were correlated with clinicopathologic characteristics, treatment, and outcome.
Results
Patients with pT1a, pT1b, and pT1c disease did not differ in distribution of low, intermediate, and high RS (P=.673). Overall, fewer than of 10% of patients had high RS. Histologic grade 1, nuclear grade 1 and low Ki67 expression had only 1%, 0% and 6% of high RS, respectively. Among patients with intermediate RS, 41% with pT1b and 46% with pT1c disease received CT. Among patients with intermediate RS, for pT1b disease, distant disease-free survival [DDFS] did not differ between HT alone and CT+HT (P=.752); for pT1c, DDFS was superior for CT+HT (P=.020). Histologic grade was the only independent prognostic factor of DDFS (P=.0007, 1 vs 3, P=.035, 2 vs 3); RS did not predict DDFS (P=.083, high vs low; P=.066, intermediate vs low).
Conclusion
The added value of RS to known prognostic factors appears limited to patients with pT1b breast cancer. However, this study lacked long-term follow-up.
Keywords: Breast Neoplasms, Chemotherapy, Adjuvant, Disease-Free Survival, Gene Expression Profiling, Receptors, Estrogen, Tumor Markers
Introduction
The 21-gene recurrence score (Oncotype DX) measures 21 genes by quantitative reverse transcriptase-polymerase chain reaction on formalin-fixed, paraffin-embedded tissues to determine a score that estimates the likelihood of distant metastasis at 10 years, and stratifies patients into 3 risk groups: low (RS <18), intermediate (RS 18–30), and high (RS >30) 1. It was first validated as an independent prognostic marker 1, and then validated as a predictor of response to tamoxifen in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 population, which consisted of patients with estrogen receptor (ER)-positive, node-negative early-stage breast cancer 2. In the NSABP B-20 cohort, which consisted of patients with ER-positive, node-negative disease treated with tamoxifen with or without chemotherapy, RS was assessed as a predictor of response to chemotherapy. High RS predicted benefit from addition of chemotherapy to hormonal therapy (HT); however, whether intermediate RS predicts benefit from addition of chemotherapy is unknown 3,4.
The 21-gene RS is commonly used to help clinicians predict the magnitude of benefit from chemotherapy for patients with early-stage breast cancer. The National Comprehensive Cancer Network recommend (Category 2A recommendation) considering the assay for patients with early-stage node-negative, ER-positive, and HER2 normal breast cancer. However, data are limited on the prognostic and predictive value of RS in patients with stage I breast cancer.
Studies have shown that in approximately 30% of cases, knowledge of RS changes the oncologist’s recommendation, in most cases from combined therapy to hormonal therapy alone 5–8. However, there are limited published data regarding the impact of RS on choice of adjuvant therapy for T1 breast cancers despite the fact that 62% of orders for the 21-gene assay are for patients with T1 disease 9.
The goals of this retrospective study, which we believe is the largest to date to evaluate the 21-gene RS in patients with stage I breast cancer, were to determine the impact of RS on choice of adjuvant therapy in patients with stage I breast cancer and to determine whether intermediate RS predicts benefit from chemotherapy and whether RS is an independent prognostic factor for patients with stage I breast cancer.
Methods
Patients
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this study (PA13-0937) and waived the requirement for informed consent. We used the electronic health record system of MD Anderson Cancer Center and the database of the Department of Breast Medical Oncology at MD Anderson to address research questions. We conducted a retrospective analysis of the medical records of all patients with stage I breast cancer who had a 21-gene assay performed during the period from September 2004, when the assay was first performed at MD Anderson, through September 2013. All data were double-checked to ensure high accuracy in our chart review.
The following information was extracted from patient records: age at diagnosis, menopausal status, histologic subtype, pathologic tumor size and nodal status (pN0 or pNmi), ER and progesterone receptor (PR) expression level by immunohistochemistry (IHC), HER2 expression level by IHC and fluorescence in situ hybridization, nuclear grade (including Black’s modified nuclear grade 1–3), histologic grade (Nottingham grade 1–3), proliferation index (Ki67 expression), and presence or absence of intramammary lymphovascular invasion. We also recorded the RS and the adjuvant treatment received.
We excluded patients with the following characteristics: male sex, bilateral disease, history of cancer in the past 5 years (except basal cell carcinoma or squamous cell carcinoma of the skin), neoadjuvant treatment (as we evaluated pathologic stage), ER-negative disease (defined as ER expression in < 10% of cells by IHC, by local institutional standards and some landscape studies), HER2-positive disease (amplification ratio of ≥ 2.2 by FISH or if FISH not available, IHC 3+), and RS missing.
Recurrence score
The 21-gene RS assay was performed by Genomic Health, Inc., on formalin-fixed, paraffin-embedded tissue samples, and RS was reported on a scale from 0 to 100. Patients with RS less than 18 were classified as low risk, patients with RS from 18 to 30 were classified as intermediate risk, and patients with RS more than 30 were classified as high risk.
Study design and end points
The relationships between RS and selection of adjuvant therapy, benefit from chemotherapy, and survival were investigated in the overall study population and in the subsets of patients with pT1a disease (pathologic tumor size ≤ 5 mm), pT1b disease (pathologic tumor size > 5 mm but ≤ 10 mm), or pT1c disease (pathologic tumor size > 10 mm but ≤ 20 mm). Similar to landscape studies of the 21-gene assay, the primary endpoint was distant disease-free survival (DDFS), in which distant metastasis or death, whichever happens first were counted as an event.
Statistical analysis
Descriptive statistics were used for continuous and categorical variables separately. Kruskal-Wallis test and either chi-square test or Fisher exact test, as appropriate, were used for the group comparison for continuous and categorical variables, respectively. Kaplan-Meier survival analysis and Cox regression analysis were used to assess the effect of categorical and continuous covariates, respectively, on DDFS. All computations were carried out in SAS 9.3 (SAS Institute Inc., Cary, NC) and Splus 8.2 (TIBCO Software Inc, Palo Alto, CA).
Results
Patient demographics and RSs
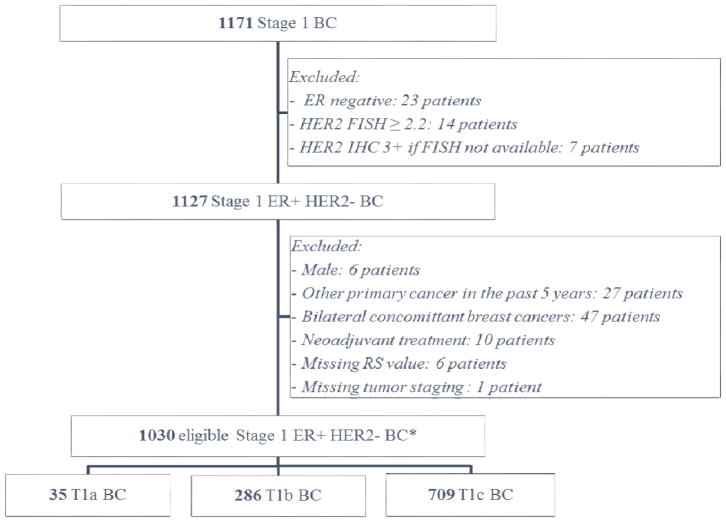
A total of 1030 patients met the eligibility criteria and were included in the study (Figure 1). Of these, 35 patients had pT1a, 286 had pT1b, and 709 had pT1c breast cancer. The only baseline demographic and clinical characteristics that differed significantly between patients with pT1a, pT1b, and pT1c disease were age at diagnosis, histologic grade, and intramammary lymphovascular invasion (Table 1).
Figure 1.
Consort diagram.
Table 1.
Baseline demographic and clinical characteristics of all eligible patients.
| Characteristics | All patients
|
Patients by pT stage
|
P-value | ||
|---|---|---|---|---|---|
| pT1a (n=35) No(%) |
pT1b (n=286) No(%) |
pT1c (n=709) No(%) |
|||
| Age, years | |||||
| Median | 54 | 49 | 51 | 55 | |
| Range | (26–81) | (30–70) | (28–76) | (26–81) | 0.002 |
|
| |||||
| Race | |||||
| White | 799(77.5) | 26(74.3) | 226(79) | 546(77) | |
| Hispanic | 129(12.5) | 5(14.3) | 29(10.1) | 95(13.4) | |
| Black | 50(4.8) | 0 | 1(<1) | 6(<1) | |
| Asian | 46(4.5) | 4(11.4) | 16(5.6) | 26(3.7) | |
| Other | 7(<1) | 0 | 1(<1) | 6(<1) | 0.165† |
|
| |||||
| Menopausal status | |||||
| Premenopausal | 364(35.5) | 14(40) | 111(39.1) | 239(33.9) | |
| Postmenopausal | 661(64.5) | 21(60) | 173(60.9) | 467(66.1) | |
| Unknown | 6 | 0 | 2 | 3 | 0.245 |
|
| |||||
| Pathological stage | |||||
| IA | 984(95.4) | 28(80) | 253(88.5) | 626(88.3) | |
| IB | 47(4.6) | 7(20) | 33(11.5) | 83(11.7) | 1.000 |
|
| |||||
| Histological grade | |||||
| 1 | 254(29.6) | 14(50) | 82(33.5) | 157(26.9) | |
| 2 | 460(53.6) | 11(39.3) | 131(53.5) | 318(54.5) | |
| 3 | 144(16.8) | 3(10.7) | 32(13.1) | 109(18.7) | |
| Unknown | 173 | 7 | 41 | 125 | 0.024 |
|
| |||||
| Nuclear grade | |||||
| 1 | 151(16.8) | 10(31.3) | 38(15.2) | 103(16.7) | |
| 2 | 580(64.5) | 18(56.2) | 165(66.3) | 396(64.2) | |
| 3 | 168(18.7) | 4(12.5) | 46(18.5) | 118(19.1) | |
| Unknown | 132 | 3 | 37 | 92 | 0.293 |
|
| |||||
| LVI | |||||
| Negative | 858(87.1) | 30(100) | 238(88.1) | 589(86.1) | |
| Positive | 127(12.9) | 0 | 32(11.9) | 95(13.9) | |
| Unknown | 46 | 5 | 16 | 25 | 0.044 |
|
| |||||
| Proliferation index (Ki67 expression) | |||||
| Median | 10 | 10 | 10.5 | 10 | |
| Range | (0–91) | (1–62) | (1–81) | (0–91) | 0.603 |
|
| |||||
| ER (%) | |||||
| Median | 95 | 95 | 94 | 95 | |
| Range | (10–100) | (20–100) | (10–100) | (10–100) | 0.344 |
|
| |||||
| PR (%) | |||||
| Median | 80 | 88 | 80 | 80 | |
| Range | (0–100) | (0–100) | (0–100) | (0–100) | 0.989 |
Abbreviations: ER, estrogen receptor; LVI, lymphovascular invasion; PR, progesterone receptor.
P-value when comparing stages (T1a, T1b, T1c) among asian, black, spanish and white only.
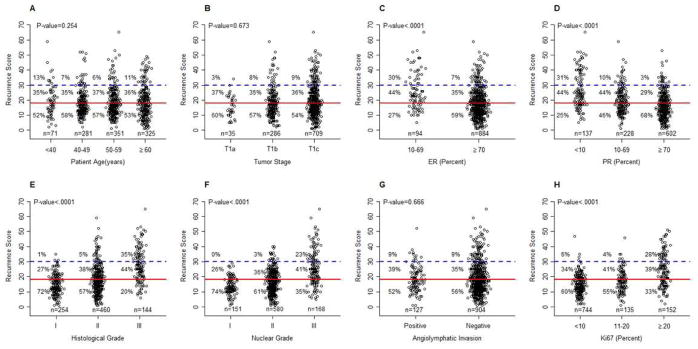
Of the 1030 patients in the study, 571 (55.5%) had low RS, 370 (35.9%) had intermediate RS, and 89 (8.6%) had high RS. Interestingly, the distributions of low, intermediate, and high RS were not significantly different among pT1a, pT1b and pT1c subgroups (P=.673). In each of these 3 subgroups, fewer than 10% of the patients had high RS, and more than half of the patients had low RS (Figure 2B).
Figure 2.
Distribution of RS and standard prognostic factors:: (A), Age; (B), pathological tumor stage; (C), ER IHC expression; (D), PR IHC expression; (E), histological grade; (F), nuclear grade; (G), angiolymphatic invasion; (H), Ki67 proliferation index. The cut-offs used were based on the literature: ER, 70%16; PR, 10%, 70%16; Ki67, 10%, 20%17,18. Percentages were calculated for each RS groups. P-values are from chi-squared test or Fisher’s exact test.
Relationship between RS and clinical prognostic factors
Only 7% of patients with high ER expression and 3% of patients with high PR expression (both defined as ≥ 70% cells stained by IHC) had high RS (Figure 2C–D). Only 1% of patients with histologic grade I and none with nuclear grade I tumors had high RS. Additionally, only 6% of patients with low Ki67 expression (≤ 10% of cells stained for Ki67) had high RS (Figure 2G). If these clinical factors were all correlated to RS, age at diagnosis and lymphovascular invasion did not correlate with RS (Figure 2A–H).
Prognostic value of RS
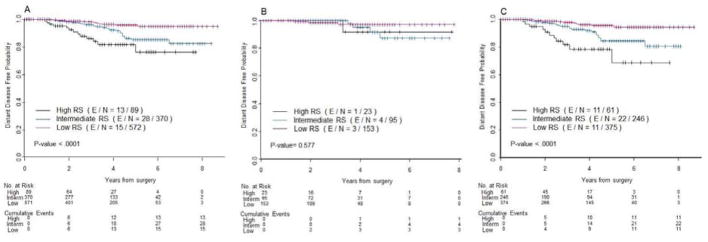
The median follow-up time for the cohort was 3.2 years (95% CI: 3 – 3.4 years). A total of 56 distant metastases or death, whichever happens first, have been observed. In the overall study population and in the subset of patients with pT1c disease, RS was a predictor of DDFS (P<.0001 for both), with high RS being the worst (5-year DDFS of 76.4%, 95% confidence interval (CI): (59.2%, 87.1%) in stage I; 70.3%, 95% CI: (48.2%, 84.3%) in T1c subgroup), and low RS being the best (95.9%, 95% CI: (93.0%, 97.6%) in stage I; 95.6%, 95% CI: (91.9%, 97.7%) in T1c subgroup). However, RS was not a predictor of DDFS in the patients with pT1b breast cancer (P=.577) (Figure 3B).
Figure 3.
Kaplan-Meier plots for distant distant-free survival (DDFS) by RS in (A) overall, (B) pT1b and (C) pT1c patients. Red, low RS; blue, intermediate RS; black, high RS. The number of patients at risk and the number of events (in parentheses) are provided below each part of the figures.
In a univariate analyses, the following were reported to be prognostic factors of DDFS: RS (P<.0001), histologic grade (P<.0001), nuclear grade (P<.0001), Ki67 expression (P=.0002), PR positivity by IHC (P=.002), ER expression level by IHC, (P=.017), and PR expression level by IHC (P=0.018).
However, in a multivariate Cox regression model including RS, only histologic grade remained an independent predictor of DDFS and RS trended toward significant (P=.083, high vs low; P=.066, intermediate vs low) (Table 2).
Table 2.
Multivariate Cox regression analysis of predictors of distant disease-free survival.a
| Covariate | Subgroup | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| Histologic grade | 1 vs 3 | 0.115 (0.033–0.403) | .0007 |
| 2 vs 3 | 0.509 (0.271–0.955) | .035 | |
| 1 vs 2 | 0.226 (0.068–0.751) | .015 | |
| Recurrence Score | High vs low | 2.197 (0.901–5.356) | .083 |
| Intermediate vs low | 1.880 (0.960–3.683) | .066 | |
| High vs intermediate | 1.169 (0.543–2.514) | .690 |
Only significant results are presented (except for RS).
Impact of RS on choice of adjuvant therapy and value of RS as a predictor of benefit from chemotherapy
Majority of patients received either HT-alone or CH-T adjuvant treatment. When only considering these two treatments we found the following. Only 1 patient with pT1a disease had a high RS. Of the 13 patients with pT1a disease and an intermediate RS, 12 (92%) received hormonal therapy alone. More than half of T1b and T1c patients with intermediate RS received HT alone (Table 3).
Table 3.
Type of treatment by pathologic T category and Recurrence Score (RS) a
| Type of Treatment | All | Low RS | Intermediate RS | High RS | P Value |
|---|---|---|---|---|---|
| All T categories | |||||
|
| |||||
| HT alone | 722 (71.7) | 513 (91.4) | 201 (56.2) | 8 (9.1) | <.0001 |
| CT alone | 2 (0.2) | 0 | 0 | 2 (2.3) | |
| CT-HT | 266 (26.4) | 36 (6.4) | 153 (42.7) | 77 (87.5) | |
| No treatment | 17 (1.7) | 12 (2.2) | 4 (1.1) | 1 (1.1) | |
| Missing treatment data | 23 | 10 | 12 | 1 | |
| Total | 1030 | 571 | 370 | 89 | |
|
| |||||
| T1a | |||||
|
| |||||
| HT alone | 33 (94) | 21 (100) | 12 (92) | 0 | .0219 |
| CT-HT | 2 (6) | 0 | 1 (8) | 1 (100) | |
| Total | 35 | 21 | 13 | 1 | |
|
| |||||
| T1b | |||||
|
| |||||
| HT alone | 204 (75.3) | 147 (96.1) | 56 (58.9) | 1 (4.3) | <.0001 |
| CT-HT | 67 (24.7) | 6 (3.9) | 39 (41.1) | 22 (95.7) | |
| Total | 271 | 153 | 95 | 23 | |
|
| |||||
| T1c | |||||
|
| |||||
| HT alone | 485 (71.1) | 345 (92.0) | 133 (54.1) | 7 (11.5) | <.0001 |
| CT-HT | 197 (28.9) | 30 (8.0) | 113 (45.9) | 54 (88.5) | |
| Total | 682 | 375 | 246 | 61 | |
Abbreviations: CT, chemotherapy; HT, hormonal therapy; CT-HT, CT followed by HT.
Values in table are number of patients (%) unless otherwise specified. Percentages of patients who received HT alone and CT-HT were calculated after exclusion of patients who received non-guideline-recommended treatments (CT alone, therapeutic abstention) or had missing data.
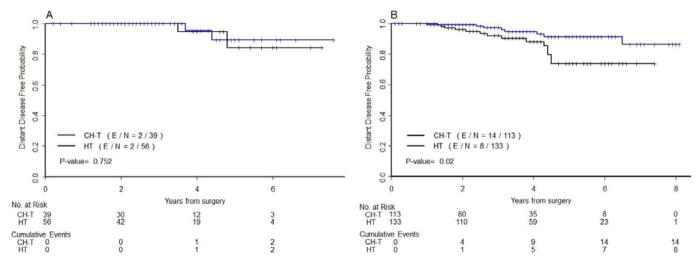
Among the 95 patients with pT1b disease and an intermediate RS, DDFS did not differ significantly between the 56 patients (58,9%) who received hormonal therapy alone and the 39 patients (41,1%) who received both chemotherapy and hormonal therapy after having RS done (P=.752) (Figure 4A). The 2 populations were well balanced except for age (median, 56 years for hormonal therapy alone vs 49 years for combined therapy, P=.002). However, among the 246 patients with pT1c disease and an intermediate RS, the 113 patients (45.9%) treated with both chemotherapy and hormonal therapy had worse DDFS than the 133 patients (54.1%) treated with hormonal therapy alone (P=.020) (Figure 4B). In addition, although all patients in these 2 subgroups had an intermediate RS, the subgroups differed significantly with respect to clinical prognostic factors: compared to the patients treated with hormonal therapy alone, the patients treated with both chemotherapy and hormonal therapy were younger (P<.0001) and had higher histologic grade (P=.0002), higher nuclear grade (P=.001), and higher Ki67 expression (proliferation index) (P=.006).
Figure 4.
Kaplan-Meier plots for distant disease-free survival (DDFS) by adjuvant treatment received in patients with (A) pT1b disease with intermediate RS and (B) pT1c disease with intermediate RS. Blue, hormonal therapy alone; black, hormonal therapy plus chemotherapy. The number of patients at risk and the number of events (in parentheses) are provided below each part of the figures.
Among the patients with pT1b disease and an intermediate RS, patients who received both chemotherapy and hormonal therapy tend to have higher RSs than patients who received hormonal therapy alone (median, 23.6 [range, 18–30] vs 21.4 [range, 18–27], P=.005). Same observation was highlighted for patients with pT1c disease and an intermediate RS (median, 23.5 [range, 18–30] vs 21.3 [range, 18–30], P<.0001) (Figure 5A). T1c patients with Ki67 superior to 15% and/or nuclear grade III tend to receive combined CH-T more than HT alone. For T1b patients, we were not able to observe such trend (Figure 5B–C).
Figure 5.
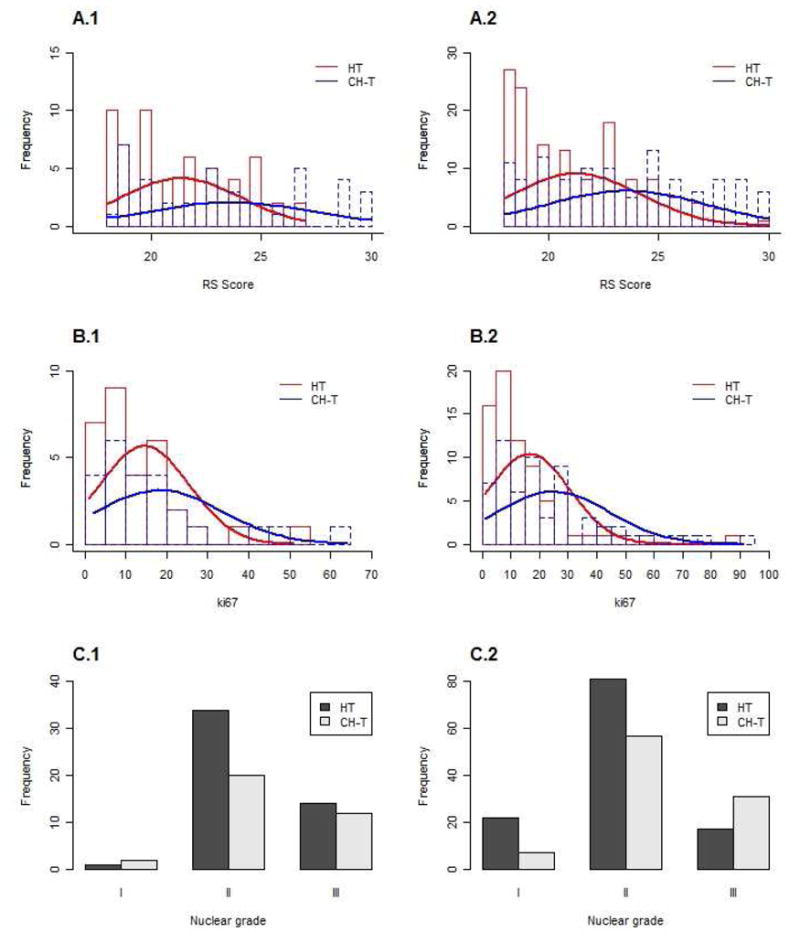
Treatment administered based on (A) RS absolute number, (B) Ki67 proliferation index and (C) nuclear grade to patients with (1) pT1b disease with intermediate RS and (2) pT1c disease with intermediate RS.
Conclusion
In our retrospective chart review of patients with stage I disease, fewer than of 10% of the patients had a high RS, and more than half of the patients had a low RS. Overall, approximately, 6% of patients with low RS, 43% of patients with intermediate RS, and 88% of patients with high RS received chemotherapy. Intermediate RS did not predict benefit from chemotherapy in patients with pT1b disease. RS was not an independent prognostic factor of DDFS in our population.
The 21-gene RS was performed for 35 patients with pT1a disease. The very low percentage of pT1a disease with high RS and the anecdotal percentage of patients with pT1a breast cancer and intermediate RS receiving combined treatment indicate that the test does not seem appropriate for pT1a patients, even if our number of patients is quite small.
Among patients with pT1b breast cancer, 21-gene RS groups did not significantly differ in term of DDFS. For patients with pT1b disease and intermediate RS, no significant difference in DDFS was observed between patients treated with hormonal therapy alone and those treated with both chemotherapy and hormonal therapy. However, the sample size and the number of events for this group were too small to permit conclusions regarding the performance of the assay in this group.
In contrast, among patients with pT1c breast cancer, treatment after RS, impacted the outcomes. Among patients with pT1c disease, the Kaplan-Meier DDFS curve for patients with intermediate RS treated with hormonal therapy alone was similar to the curve for patients with T1c disease and low RS. In contrast, the Kaplan-Meier curve for patients with pT1c tumor and intermediate RS treated with chemotherapy and hormonal therapy was more like the curve for patients with high RS treated with chemotherapy and hormonal therapy. However, these results could be data-driven due to significant differences in clinical prognostic factors between patients treated with hormonal therapy alone and those who received combined treatment. In fact, patients in the combined treatment group had worse clinical prognostic factors (younger age, higher histological and nuclear grade, and higher proliferation index) that could worsen their prognosis. Consequently, it is quite likely that chemotherapy did not contribute to the bad outcome in this group and that the poor prognostic factors explain the discrepancy between the treatment groups. The other explanation could be that chemotherapy is not effective in such population – pT1c tumors with intermediate RS - because of the relatively good prognosis of patients treated with hormonal therapy alone.
Because of our observations in patients with pT1b and pT1c disease and intermediate RS, we used the Martingale residual Cox model with DDFS as an end point to try to identify a new cut-off in the intermediate RS population to define groups with different outcomes; however, we were unable to find such a cut-off. However, we observed a trend in patients with pT1b and pT1c disease: patients with RS less than 25 were more likely to receive hormonal therapy alone than were patients with RS of 25 or greater (Figure 4A).
Discussion
Nowadays, the NCCN guidelines recommend that patients with T1c stage I ER-positive breast cancer receive chemotherapy followed by hormonal therapy sequentially [CH-T]) for T1c stage I BC. For patients with T1b stage I breast cancer, adding chemotherapy to hormonal therapy is an option, whereas for patients with T1a stage I breast cancer, hormonal therapy alone is recommended 10. However, RS could impact these guidelines. Scientific societies including the American Society of Clinical Oncology 11, the National Comprehensive Cancer Network (NCCN) 10, and the European Society for Medical Oncology 12, have recently included the 21-gene RT-PCR assay in their guidelines. The NCCN guidelines “[consider] the 21-gene RT-PCR assay as an option when evaluating patients with primary tumors characterized as 0.6 to 1.0 cm (T1b) with unfavorable features or >1 cm (T1c) and node-negative, ER-positive, and HER2-negative. In this circumstance, the RS may be determined to assist in estimating likelihood of recurrence and benefit from chemotherapy” 10.
One strength of our study is that whereas most of the recent retrospective studies on the impact of the 21-gene RS evaluated only the impact on treatment recommendations 5–8, we sought to evaluate the impact of the 21-gene RS assay on both treatment and outcomes. Previous studies reported higher rates of high RS among patients with T1c disease, ranging from 26% to 32% 1,3,13,14. Clinicians ordering the 21-gene RS for stage I breast cancer may hope for a low RS indicating that patients can be spared chemotherapy and thus may be more likely to offer the test to breast cancer patients with favorable features. However, our proportion of patients with low RS is consistent with those in previous reports. In these hallmark studies, 63% to 75% of enrolled patients had stage I breast cancer, but only 18% to 30% had T1a or T1b disease 1,3,13. Another strengths of our retrospective study are the large number of patients with T1b disease.
However, our study has several limitations. Our median follow-up time was only 3.2 years, which is too short for evaluation of distant recurrences or overall survival for patients with stage I breast cancer. The small number of events resulted in insufficient power in our RS subgroups, especially the pT1b subgroup. This fact should be considered in the interpretation of these results. Moreover, the lack of significant difference between patients treated with hormonal therapy alone and those treated with both chemotherapy and hormonal therapy in the low-RS (P=.677) and high-RS (P=.763) pT1b subgroups should be analyzed with extreme caution due to the small number of patients in each treatment arm. Since it was a retrospective analysis, there is no assurance that treatment allocation was based solely on the RS; in fact, the significantly higher rate of combined chemotherapy and hormonal therapy in the patients with less favorable prognostic factors suggests that such factors might have contributed significantly to the choice of therapy.
Current prospective TAILORx study (NCT00310180) randomize node-negative breast cancer patients with RS from 11 to 25 between HT alone or CH-T 15. The TAILORx study randomized more than 11,000 patients with node-negative breast cancer and more than 4 years after completing recruitment has insufficient events to answer the question. In our retrospective study, 65 patients were enrolled in the TAILORx study: 12 T1b and 53 T1c patients. Only 17 patients were considered as intermediate RS.
The value of adding 21-gene RS to the usual clinical prognostic factors appears limited for patients with T1a stage I breast cancer because of the high rate of low RS and the high rate of administration of hormonal therapy alone. For patients with T1b breast cancer, given the lack of difference in survival between patients with intermediate RS treated with hormonal therapy alone and those treated with chemotherapy plus hormonal therapy, the role of the 21-gene RS assay will not be known until the TAILORx study results are available.
Clinical Practice Points.
The NCCN recommend (Category 2A recommendation) considering the 21-gene RT-PCR assay for patients with early-stage node-negative, ER-positive, and HER2 normal breast cancer. However, data are limited on the prognostic and predictive value of RS in patients with stage I breast cancer.
The purpose of the current analysis was to investigate the potential impact of RS on choice of adjuvant therapy and whether intermediate RS predicts benefit from chemotherapy and whether RS is an independent prognostic factor for patients with stage I breast cancer.
The reported data indicate that fewer than of 10% of stage 1 patients had high RS. Among patients with intermediate RS, for pT1b disease, DDFS did not differ between HT alone and CT+HT however; for pT1c, DDFS was superior for CT+HT.
These hypothesis-generating data strongly question about cost-benefit ratio of 21-gene assay for T1b patients; however, our 3.5 years of median of follow-up do not allow us to be conclusive. We await final results of the prospective study.
Acknowledgments
We thank the MD Anderson Moon Shots program for support. We also thank the Department of Scientific Publications at MD Anderson for manuscript editing.
Research Support
This work was supported in part by a grant from the Eugène Marquis Cancer Center, Rennes, France (to F. Le Du); the National Institutes of Health/National Cancer Institute under award P30CA016672, which supports the Biostatistics Shared Resource at MD Anderson Cancer Center; and grant R01CA07466 from the National Cancer Institute (to D.L.).
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conception and design: all authors; Provision of study materials or patients: Limin Shu; Collection and assembly of data: Fanny Le Du, Limin Shu; Data analysis and interpretation: Fanny Le Du, Minjeong Park, Diane D. Liu, Naoto T. Ueno; Manuscript writing and final approval: All authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 2.Paik S. Expression of the 21 genes in the Recurrence Score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer [abstract 510]. Presented at the 2005 ASCO Annual Meeting. [Google Scholar]
- 3.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albanell J, González A, Ruiz-Borrego M, et al. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol. 2012;23(3):625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 6.Eiermann W, Rezai M, Kümmel S, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013;24(3):618–624. doi: 10.1093/annonc/mds512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geffen DB, Abu-Ghanem S, Sion-Vardy N, et al. The impact of the 21-gene recurrence score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22(11):2381–2386. doi: 10.1093/annonc/mdq769. [DOI] [PubMed] [Google Scholar]
- 8.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(10):1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 9.Oratz R, Kim B, Chao C, et al. Physician Survey of the Effect of the 21-Gene Recurrence Score Assay Results on Treatment Recommendations for Patients With Lymph Node–Positive, Estrogen Receptor–Positive Breast Cancer. J Oncol Pract. 2011;7(2):94–99. doi: 10.1200/JOP.2010.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Practice Guidelines in Oncology. Breast Cancer (version v3.2012) http://www.nccn.org.
- 11.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 Update of Recommendations for the Use of Tumor Markers in Breast Cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 12.Aebi S, Davidson T, Gruber G, Castiglione M. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v9–v14. doi: 10.1093/annonc/mdq159. [DOI] [PubMed] [Google Scholar]
- 13.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacirca JL, Tsai ML, Brufsky A, et al. Initial results from the 21-gene breast cancer assay registry: A prospective observational study in patients (pts) with ER+, early-stage invasive breast cancer (EBC) [Accessed June 16, 2014];J Clin Oncol. 2013 31(suppl):abstr 565. http://meetinglibrary.asco.org/content/113096-132. [Google Scholar]
- 15.Sparano JA, Paik S. Development of the 21-Gene Assay and Its Application in Clinical Practice and Clinical Trials. J Clin Oncol. 2008;26(5):721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 16.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen Receptor Status by Immunohistochemistry Is Superior to the Ligand-Binding Assay for Predicting Response to Adjuvant Endocrine Therapy in Breast Cancer. J Clin Oncol. 1999;17(5):1474–1474. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Azambuja E, Cardoso F, de Castro G, Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96(10):1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]