Abstract
Lipid kinases have largely been neglected as targets in cancer, and an increasing number of reports suggest diacylglycerol kinase alpha (DGKα) may be one with promising therapeutic potential. DGKα is one of ten DGK family members that convert diacylglycerol (DAG) to phosphatidic acid (PA), and both DAG and PA are critical lipid second messengers in the plasma membrane. A host of important oncogenic proteins and pathways affect cancer cells in part through DGKα, including the c-Met and VEGF receptors. Others partially mediate the effects of DGKα inhibition in cancer, such as mTOR and HIF-1α. DGKα inhibition can directly impair cancer cell viability, inhibits angiogenesis, and notably may also boost T cell activation and enhance cancer immunotherapies. While two structurally similar inhibitors of DGKα were established decades ago, they have seen minimal in vivo usage and it is unlikely that either of these older DGKα inhibitors will have utility for cancer. An abandoned compound that also inhibits serotonin receptors may have more translational potential as a DGKα inhibitor, but more potent and specific DGKα inhibitors are sorely needed. Other DGK family members may also provide therapeutic targets in cancer, but require further investigation.
Background
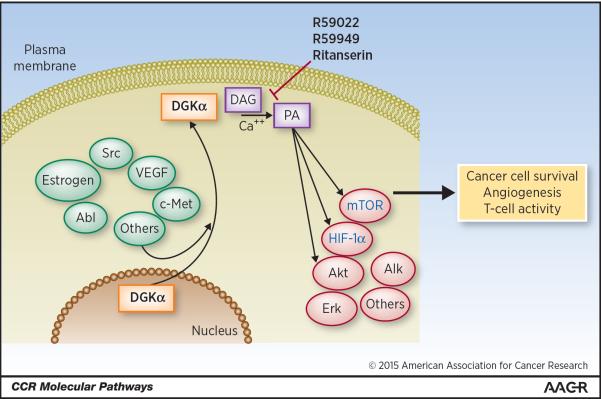
Recent evidence suggests Diacylglycerol kinase alpha (DGKα) as a promising new target in the fight against cancer, with DGKα inhibition exhibiting multiple anti-cancer mechanisms of action. DGKα is one of ten DGK enzymes that convert the membrane lipid diacylglycerol (DAG) into phosphatidic acid (PA), and both DAG and PA play important roles in cellular signaling. Both DAG and PA are found in the plasma membrane, with significantly more DAG than PA present (1). However, both act as important second messengers and can bind directly to and modulate numerous proteins in cancer. DAG is known to bind directly to protein kinase C and protein kinase D family members, as well as to the Ras family and to the DGKs (2, 3). PA has been less well studied than PA, and other than mTOR most of its binding partners remain to be discovered (4). PA has been found to control activity of mTOR, Akt, and Erk, while DGKα has been linked to activation of NF-κB, HIF-1α, c-met, ALK, and VEGF (Fig. 1) (5–13). Despite the association of DGKα and PA to a plethora of oncogenic pathways, they are little-studied in the context of cancer.
Figure 1. DGKa regulation and activity.
DGKa is located in the nucleus till activated by regulators such as Src, at which point it translocates to the inner leaflet of the plasma membrane. There it converts diacylglycerol to phosphatidic acid, acting as a regulator or mediator of numerous oncogenic pathways.
An increasing number of reports are indicating key roles for DGKα in cancer. While normally DGKα is significantly expressed only in brain, kidney, and T cells (14), it appears to be relevant in numerous malignancies. One of the earliest studies on DGKα in cancer notes DGKα over-expression and promotion of NF-κB signaling in melanoma cells (13). A few reports have linked DGKα to cancer cell motility; one report implicates DGKα in cancer cell invasion through α5β1 integrin recycling (RCP) (15). Dominguez and colleagues studied DGKα as a cancer target in vitro and in vivo (16). DGKα was identified as a potential cancer target through the study of tumor-suppressive microRNAs. After observing that microRNA-297 had tumor-suppressive function and was cytotoxic to glioblastoma cells, it was noted that its top predicted targets in online databases did not include established oncogenes (17). However, the kinase DGKα was predicted to be strongly targeted, and there were suggestions in the literature that DGKα and its product PA might play major roles in cancer. The possibility that DGKα could be a signaling hub in cancer led to testing the effects of its knockdown and inhibition in cancer cells (16). Induction of apoptosis in human glioblastoma lines was noted, including resistant glioblastoma stem cell-like lines, with both DGKA knockdown and with treatment with established inhibitors R59022 and R59949. Normal human cells proved insensitive to knockdown/inhibition. Importantly, these effects were specific, as glioblastoma cells were rescued by exogenous PA. Over-expression of DGKα increased glioblastoma cell numbers in vitro. Studies of downstream pathways supported the role of DGKα as a key signaling node in cancer, with its inhibition leading to decreased expression and/or activation of mTOR, Akt, HIF-1α, c-myc, and the SREBP cholesterol synthesis pathway. Rescue experiments indicated mTOR and HIF-1α suppression to be key mediators of DGKα inhibition in glioblastoma and melanoma cells, and a unique role of DGKα in regulating mTOR transcription via a novel cAMP-dependent pathway was described. The report also showed for the first time in mouse models the anti-cancer efficacy of DGKα knockdown and inhibition, and demonstrated potent antiangiogenic activity. In vivo efficacy of the small-molecule DGKα inhibitor R59022 was observed despite unfavorable pharmacokinetics(16).
Downstream effects of DGKα in cancer may be due largely to modulation of total PA, or specific PA molecules, or PA in specific cellular locations. There are numerous PA (and DAG) species that differ in their two hydrocarbon side chains, but whether different PA molecules functionally diverge has yet to be determined. Modulating PA levels likely mediates DGKα effects through direct binding of PA to oncogenes, as has been demonstrated for mTOR (4). Effects of DGKα on oncogenes can also be indirect, with one example being the regulation of HIF-1α via modulating the interaction of the degradative von Hippel Lindau (vHL) protein with HIF-1α; the role of PA in this interaction is not established (12, 18). DGKα effects in cancer might also stem from affecting DAG levels (19)—though this seems less likely given the high concentration of DAG in the membrane, the numerous DGK family members, and the existence of other DAG-modulating pathways; DAG can be generated by lipase action on triacylglycerols, phospholipase action on phospholipids, phosphatase action on PA, and acyltransferase action on monoacylglycerols (20).
It is unknown whether there is functional redundancy of DGK family members, and whether other DGK family members or PA-synthesizing enzymes can compensate for DGKα knockdown or inhibition. In addition to the DGKs, the lysophosphatidic acid acyltransferases (LPAATs) and phospholipase D (PLD) enzymes also generate PA. LPAAT and PLD enzymes have also been linked to cancer, further supporting roles for PA in malignant cells (21–24). Though all DGKs convert DAG to PA, they may still play unique roles in cancer given their different cellular localizations. For example, DGKα and a few other DGKs are generally thought to translocate from the nucleus to the plasma membrane when active (Fig. 1) (25–27), while DGKβ is localized on actin fibers (28). Different DGKs may also have varying specificity against different DAG species (29), potentially contributing to diverse roles for the DGK family members in cancer. Notably, a recent report indicates that DGKζ may also play a prominent role in cancer (30). DGKδ has been shown to regulate expression of the epidermal growth factor receptor (EGFR) (31), and DGKη may be a downstream mediator of EGFR signaling in cancer (32). DGKι increases activity of Ras/Rap signaling in cancer (33).
While roles for most of the DGKs in cancer have not yet been explored, TCGA data (accessed through the cBioPortal) show frequent mutations and amplifications of some of the DGKs in a number of cancers (34)—suggesting that they may have oncogenic functions in certain settings. Over 40% of melanomas have at least one mutation in a DGK family member, with cases of clustered and repetitive mutations suggesting oncogenic function (34). Further studies need to be performed to dissect the roles of other DGK family members in cancer and determine any overlap; it is very possible that other DGKs may be cancer targets in their own right.
Some insight has been gained into upstream activators of DGKα. The oncogenic Src kinase has been found to phosphorylate DGKα to promote its activity (6, 35), but whether Src inhibitors such as dasatinib have a significant effect on DGKα activity remains to be tested. Src may also lie downstream of DGKα, with Src and DGKα comprising a positive feedback loop (36). The Abl oncogene product also modulates DGKα, through regulation of its export from the nucleus (37). It is possible that DGKα mediates a number of oncogenic stimuli. DGKα is activated by estrogen signaling in endometrial CA (38), and it promotes cell invasiveness downstream of SDF-1α and HGF (39). Calcium plays a well-established and important role in activation of DGKα. DGKα, DGKβ, and DGKγ are all Type I DGKs that contain a calcium-binding region important for activation, which is not the case for the other seven DGK family members (40). Intracellular calcium and DGKα might in fact act in a positive feedback loop, as DGKα inhibitors have been found to reduce intracellular calcium levels (Fig. 1) (41, 42).
Intriguingly, DGKα and DGKζ limit T cell activation, and a steadily increasing number of reports are showing that DGK inhibition enhances the T-cell anti-tumor response and immunotherapies such as chimeric antigen receptor-modified T (CART) cells (43–48). DGKα and DGKζ may be more important than other DGK family members in T cells due to higher expression. Several years ago, initial reports appeared indicating that DGKα played a role in T cell anergy, and that its inhibition or knockdown could rescue T cell activation (46, 47). DGKA-knockout mice have hyperactive T cells resistant to anergy (47). A few mechanisms have been posited to explain this. In T cells, DGKα inhibition elevates Ras/Erk pathway activity, which is well-known to promote T cell activity (49). T-cell receptor engagement drives movement of the Ras partner Sos to the cell membrane (50), fostering Ras activation, and Ras activation drives IL-2 receptor expression and T cell proliferation (51). Ras activation by DGKα inhibition in T cells stands in contradistinction to reports of Ras pathway suppression by DGKα inhibition in cancer (52), and suggests that DGKα inhibition may have context-dependent effects that vary across different cells and tissues. Other mechanisms for the effect on T cells have been suggested as well, including regulation of the immunologic synapse (53). DGKα inhibition has also been reported to boost the activity of NK cells, providing another potential immunologic anti-cancer benefit (54). From a speculative viewpoint, DGKα inhibition might have another mechanism for boosting the anti-tumor immune response in vivo through its antiangiogenic activity; this should generate areas of necrosis within tumors, and necrotic cell death is more immunogenic than structured cell death via apoptosis. A few reports have suggested that DGKζ may be stronger than DGKα in modulating T cells. However, DGKζ may be a less appealing target than DGKα for increasing the anti-tumor immune response, as one report indicates that DGKζ may be especially critical in restraining immunosuppressive Treg cells (55). DGKζ has also been found to suppress the oncogenic NF-κB pathway, providing another potential drawback to targeting it for cancer therapy (56).
Clinical-Translational Advances
The prospect that DGKα inhibition may directly attack cancer cells, suppress angiogenesis, and boost immunotherapies makes it an attractive target in cancer. The ability of DGKα inhibition to affect multiple cancer pathways, and in particular mTOR and HIF-1α, broadens its potential applicability. However, the lack of adequate small-molecule inhibitors is a clear barrier to its clinical translation. The only known DGKα inhibitors are R59022 and R59949, which share highly similar structures. They have modest inhibitory potency against DGKα and have only been tested in vivo against cancer in one report (16). A limited intraperitoneal course of R59022 increased median mouse survival in an orthotopic xenograft GBM model by approximately 20% (p=0.01). However, the half-life in mice of R59022 appears to be very short, on the order of one to two hours (16), while R59949 has not been tested in vivo. It would take years and substantial resources to optimize these compounds for potential clinical usage, with the need for SAR (structure-activity relationship) studies to better understand their interaction with DGKα. It may be possible to attack cancer with DGKA knockdown by siRNA or shRNA, as shown in one report (16), but this of course presents the tremendous challenge of efficient delivery to cancers in patients.
Recently it has been noted that the known DGKα inhibitor R59022 differs structurally only by a single fluorine from the established serotonin receptor inhibitor ritanserin (57). Ritanserin has been tested for applications including schizophrenia, alcoholism, and insomnia. While it was bypassed by other schizophrenia medications and never put forward for FDA approval, it was shown to be safe in human trials. Ritanserin is orally bioavailable, has a 40-hour half-life in humans, and has some degree of blood-brain barrier penetrability. Our group has now found that ritanserin inhibits DGKα activity more potently than R59022, and we are further testing its potential as a DGKα inhibitor that may be translated to the clinic with relative rapidity. Early experiments with single-agent ritanserin in intracranial glioblastoma and melanoma xenograft models suggest increases in median mouse survival of up to 30% (p<0.05; B. Purow; unpublished data). However, ritanserin only has potency in the low micromolar range and is not very specific, with much more potent inhibition of serotonin 5HT2A and 5HT2B receptors and possibly with effects on dopamine signaling as well. That being said, some of the non-DGKα-mediated effects of ritanserin may be beneficial in cancer patients, such as having a stimulating effect when awake but also improving sleep. Ultimately there is a clear need for new small-molecule inhibitors of DGKα with greater potency and specificity, and efforts are ongoing to identify candidate compounds.
It is vital to consider potential side effects of DGKα inhibition as it is developed further at the preclinical stage and hopefully moved on to the clinic. Early mouse studies have not revealed any major side effects of DGKα inhibition, in keeping with the findings in DGKα knockout mice mentioned above. The effects on T cells may result in increased autoimmunity with DGKα inhibition, but this is speculative. Drugs such as ritanserin with effects on serotonin receptors might affect serotonin-related processes such as coagulation, but this has not been shown previously. Mice with other DGK family members knocked out have displayed significant pathologic phenotypes—including glucose intolerance in DGKδ knockout mice and neurologic disorders in DGKβ knockout mice (58, 59)—but these concerns do not seem to cross over to DGKα inhibition, perhaps due to tissue-specific expression. It might be expected that downstream targets observed in cancer cells such as the Ras and mTOR pathways would result in substantial side effects of DGKα inhibition in vivo, but these may not arise because of context-dependent differential effects on various cell types.
It is clear that much further study is needed on the effects of DGKα on downstream oncogenic pathways, immunity, angiogenesis, and combinatorial effects with other anti-cancer agents. Ongoing work is investigating the combination of DGKα inhibition with standard therapies such as radiation and chemotherapy, as well as with other targeted therapies and immunotherapies. With the recent dramatic successes of cancer immunotherapies, the potential for DGKα inhibition to boost them is especially intriguing. DGKα inhibition may be most useful in enhancing T cell or NK cell immunotherapies, though this requires further exploration, It is also tempting to speculate that a more complete attack on PA synthesis might have even greater efficacy against cancer, through combining DGKα inhibition with blockade of other DGKs or other PA-synthesizing enzymes LPAATs or PLDs. At present there are no known inhibitors of other DGKs—besides possible non-specific and limited effects by the known DGKα inhibitors—but inhibitors of LPAAT and PLD already exist. Numerous therapeutic opportunities such as this remain to be explored. While study of the DGKs remains a field in its infancy, it appears increasingly likely to yield important biological and therapeutic advances.
Acknowledgments
Grant Support B. Purow was supported by the NIH under award numbers R01CA180699 and R01CA189524.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–46. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–42. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, et al. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–203. [PubMed] [Google Scholar]
- 4.Avila-Flores A, Santos T, Rincon E, Merida I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J Biol Chem. 2005;280:10091–9. doi: 10.1074/jbc.M412296200. [DOI] [PubMed] [Google Scholar]
- 5.Bacchiocchi R, Baldanzi G, Carbonari D, Capomagi C, Colombo E, van Blitterswijk WJ, et al. Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood. 2005;106:2175–82. doi: 10.1182/blood-2005-01-0316. [DOI] [PubMed] [Google Scholar]
- 6.Baldanzi G, Cutrupi S, Chianale F, Gnocchi V, Rainero E, Porporato P, et al. Diacylglycerol kinase-alpha phosphorylation by Src on Y335 is required for activation, membrane recruitment and Hgf-induced cell motility. Oncogene. 2008;27:942–56. doi: 10.1038/sj.onc.1210717. [DOI] [PubMed] [Google Scholar]
- 7.Baldanzi G, Mitola S, Cutrupi S, Filigheddu N, van Blitterswijk WJ, Sinigaglia F, et al. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene. 2004;23:4828–38. doi: 10.1038/sj.onc.1207633. [DOI] [PubMed] [Google Scholar]
- 8.Baldanzi G, Pietronave S, Locarno D, Merlin S, Porporato P, Chianale F, et al. Diacylglycerol kinases are essential for hepatocyte growth factor-dependent proliferation and motility of Kaposi's sarcoma cells. Cancer Sci. 2011;102:1329–36. doi: 10.1111/j.1349-7006.2011.01953.x. [DOI] [PubMed] [Google Scholar]
- 9.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo MA, Shome K, Vasudevan C, Stolz DB, Sung TC, Frohman MA, et al. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J Biol Chem. 1999;274:1131–9. doi: 10.1074/jbc.274.2.1131. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo MA, Shome K, Watkins SC, Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J Biol Chem. 2000;275:23911–8. doi: 10.1074/jbc.M001553200. [DOI] [PubMed] [Google Scholar]
- 12.Temes E, Martin-Puig S, Acosta-Iborra B, Castellanos MC, Feijoo-Cuaresma M, Olmos G, et al. Activation of HIF-prolyl hydroxylases by R59949, an inhibitor of the diacylglycerol kinase. J Biol Chem. 2005;280:24238–44. doi: 10.1074/jbc.M414694200. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa K, Yasuda S, Kai M, Imai S, Yamada K, Yamashita T, et al. Diacylglycerol kinase alpha suppresses tumor necrosis factor-alpha-induced apoptosis of human melanoma cells through NF-kappaB activation. Biochim Biophys Acta. 2007;1771:462–74. doi: 10.1016/j.bbalip.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Goto K, Kondo H. Diacylglycerol kinase in the central nervous system--molecular heterogeneity and gene expression. Chem Phys Lipids. 1999;98:109–17. doi: 10.1016/s0009-3084(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 15.Rainero E, Caswell PT, Muller PA, Grindlay J, McCaffrey MW, Zhang Q, et al. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J Cell Biol. 2012;196:277–95. doi: 10.1083/jcb.201109112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez CL, Floyd DH, Xiao A, Mullins GR, Kefas BA, Xin W, et al. Diacylglycerol kinase alpha is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov. 2013;3:782–97. doi: 10.1158/2159-8290.CD-12-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kefas B, Floyd DH, Comeau L, Frisbee A, Dominguez C, Dipierro CG, et al. A miR-297/hypoxia/DGK-alpha axis regulating glioblastoma survival. Neuro Oncol. 2013;15:1652–63. doi: 10.1093/neuonc/not118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temes E, Martin-Puig S, Aragones J, Jones DR, Olmos G, Merida I, et al. Role of diacylglycerol induced by hypoxia in the regulation of HIF-1alpha activity. Biochem Biophys Res Commun. 2004;315:44–50. doi: 10.1016/j.bbrc.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Merida I, Andrada E, Gharbi SI, Avila-Flores A. Redundant and specialized roles for diacylglycerol kinases alpha and zeta in the control of T cell functions. Sci Signal. 2015;8:re6. doi: 10.1126/scisignal.aaa0974. [DOI] [PubMed] [Google Scholar]
- 20.Eichmann TO, Lass A. DAG tales: the multiple faces of diacylglycerol-stereochemistry, metabolism, and signaling. Cell Mol Life Sci. 2015;72:3931–52. doi: 10.1007/s00018-015-1982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Rosee P, Jia T, Demehri S, Hartel N, de Vries P, Bonham L, et al. Antileukemic activity of lysophosphatidic acid acyltransferase-beta inhibitor CT32228 in chronic myelogenous leukemia sensitive and resistant to imatinib. Clin Cancer Res. 2006;12:6540–6. doi: 10.1158/1078-0432.CCR-06-0140. [DOI] [PubMed] [Google Scholar]
- 22.Diefenbach CS, Soslow RA, Iasonos A, Linkov I, Hedvat C, Bonham L, et al. Lysophosphatidic acid acyltransferase-beta (LPAAT-beta) is highly expressed in advanced ovarian cancer and is associated with aggressive histology and poor survival. Cancer. 2006;107:1511–9. doi: 10.1002/cncr.22184. [DOI] [PubMed] [Google Scholar]
- 23.Hatton N, Lintz E, Mahankali M, Henkels KM, Gomez-Cambronero J. Phosphatidic acid increases epidermal growth factor receptor expression by stabilizing mRNA decay and by inhibiting lysosomal and proteasomal degradation of the internalized receptor. Mol Cell Biol. 2015;35:3131–44. doi: 10.1128/MCB.00286-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Frohman MA. Cellular and physiological roles for phospholipase D1 in cancer. J Biol Chem. 2014;289:22567–74. doi: 10.1074/jbc.R114.576876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA, et al. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–83. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 26.Santos T, Carrasco S, Jones DR, Merida I, Eguinoa A. Dynamics of diacylglycerol kinase zeta translocation in living T-cells. Study of the structural domain requirements for translocation and activity. J Biol Chem. 2002;277:30300–9. doi: 10.1074/jbc.M200999200. [DOI] [PubMed] [Google Scholar]
- 27.van Baal J, de Widt J, Divecha N, van Blitterswijk WJ. Translocation of diacylglycerol kinase theta from cytosol to plasma membrane in response to activation of G protein-coupled receptors and protein kinase C. J Biol Chem. 2005;280:9870–8. doi: 10.1074/jbc.M409301200. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi N, Hozumi Y, Ito T, Hosoya T, Kondo H, Goto K. Differential subcellular targeting and activity-dependent subcellular localization of diacylglycerol kinase isozymes in transfected cells. Eur J Cell Biol. 2007;86:433–44. doi: 10.1016/j.ejcb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Epand RM, Kam A, Bridgelal N, Saiga A, Topham MK. The alpha isoform of diacylglycerol kinase exhibits arachidonoyl specificity with alkylacylglycerol. Biochemistry. 2004;43:14778–83. doi: 10.1021/bi0484724. [DOI] [PubMed] [Google Scholar]
- 30.Torres-Ayuso P, Tello-Lafoz M, Merida I, Avila-Flores A. Diacylglycerol kinase-zeta regulates mTORC1 and lipogenic metabolism in cancer cells through SREBP-1. Oncogenesis. 2015;4:e164. doi: 10.1038/oncsis.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai J, Crotty TM, Reichert E, Carraway KL, 3rd, Stafforini DM, Topham MK. Diacylglycerol kinase delta and protein kinase C(alpha) modulate epidermal growth factor receptor abundance and degradation through ubiquitin-specific protease 8. J Biol Chem. 2010;285:6952–9. doi: 10.1074/jbc.M109.055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano T, Iravani A, Kim M, Hozumi Y, Lohse M, Reichert E, et al. Diacylglycerol kinase eta modulates oncogenic properties of lung cancer cells. Clin Transl Oncol. 2014;16:29–35. doi: 10.1007/s12094-013-1036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regier DS, Higbee J, Lund KM, Sakane F, Prescott SM, Topham MK. Diacylglycerol kinase iota regulates Ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc Natl Acad Sci U S A. 2005;102:7595–600. doi: 10.1073/pnas.0500663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutrupi S, Baldanzi G, Gramaglia D, Maffe A, Schaap D, Giraudo E, et al. Src-mediated activation of alpha-diacylglycerol kinase is required for hepatocyte growth factor-induced cell motility. EMBO J. 2000;19:4614–22. doi: 10.1093/emboj/19.17.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres-Ayuso P, Daza-Martin M, Martin-Perez J, Avila-Flores A, Merida I. Diacylglycerol kinase alpha promotes 3D cancer cell growth and limits drug sensitivity through functional interaction with Src. Oncotarget. 2014;5:9710–26. doi: 10.18632/oncotarget.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsubara T, Ikeda M, Kiso Y, Sakuma M, Yoshino K, Sakane F, et al. c-Abl tyrosine kinase regulates serum-induced nuclear export of diacylglycerol kinase alpha by phosphorylation at Tyr-218. J Biol Chem. 2012;287:5507–17. doi: 10.1074/jbc.M111.296897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filigheddu N, Sampietro S, Chianale F, Porporato PE, Gaggianesi M, Gregnanin I, et al. Diacylglycerol kinase alpha mediates 17-beta-estradiol-induced proliferation, motility, and anchorage-independent growth of Hec-1A endometrial cancer cell line through the G protein-coupled estrogen receptor GPR30. Cell Signal. 2011;23:1988–96. doi: 10.1016/j.cellsig.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Rainero E, Cianflone C, Porporato PE, Chianale F, Malacarne V, Bettio V, et al. The diacylglycerol kinase alpha/atypical PKC/beta1 integrin pathway in SDF-1alpha mammary carcinoma invasiveness. PLoS One. 2014;9:e97144. doi: 10.1371/journal.pone.0097144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada K, Sakane F, Matsushima N, Kanoh H. EF-hand motifs of alpha, beta and gamma isoforms of diacylglycerol kinase bind calcium with different affinities and conformational changes. Biochem J. 1997;321(Pt 1):59–64. doi: 10.1042/bj3210059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nobe K, Ohata H, Momose K. Effect of diacylglycerol kinase inhibitor, R59022 on cytosolic free calcium level and force development in guinea pig taenia coli. Res Commun Chem Pathol Pharmacol. 1993;81:331–43. [PubMed] [Google Scholar]
- 42.Marumo M, Nakano T, Takeda Y, Goto K, Wakabayashi I. Inhibition of thrombin-induced Ca(2)(+) influx in platelets by R59949, an inhibitor of diacylglycerol kinase. J Pharm Pharmacol. 2012;64:855–61. doi: 10.1111/j.2042-7158.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 43.Riese MJ, Wang LC, Moon EK, Joshi RP, Ranganathan A, June CH, et al. Enhanced Effector Responses in Activated CD8+ T Cells Deficient in Diacylglycerol Kinases. Cancer Res. 2013;73:3566–77. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol. 2012;188:5990–6000. doi: 10.4049/jimmunol.1103028. [DOI] [PubMed] [Google Scholar]
- 45.Baldanzi G, Pighini A, Bettio V, Rainero E, Traini S, Chianale F, et al. SAP-mediated inhibition of diacylglycerol kinase alpha regulates TCR-induced diacylglycerol signaling. J Immunol. 2011;187:5941–51. doi: 10.4049/jimmunol.1002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–81. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 47.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–73. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 48.Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med. 2012;209:2157–63. doi: 10.1084/jem.20120342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, et al. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A. 2008;105:11909–14. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 1996;15:3923–33. [PMC free article] [PubMed] [Google Scholar]
- 51.Faris M, Kokot N, Lee L, Nel AE. Regulation of interleukin-2 transcription by inducible stable expression of dominant negative and dominant active mitogen-activated protein kinase kinase kinase in jurkat T cells. Evidence for the importance of Ras in a pathway that is controlled by dual receptor stimulation. J Biol Chem. 1996;271:27366–73. doi: 10.1074/jbc.271.44.27366. [DOI] [PubMed] [Google Scholar]
- 52.Takeishi K, Taketomi A, Shirabe K, Toshima T, Motomura T, Ikegami T, et al. Diacylglycerol kinase alpha enhances hepatocellular carcinoma progression by activation of Ras-Raf-MEKERK pathway. J Hepatol. 2012;57:77–83. doi: 10.1016/j.jhep.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Chauveau A, Le Floc'h A, Bantilan NS, Koretzky GA, Huse M. Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci Signal. 2014;7:ra82. doi: 10.1126/scisignal.2005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prinz PU, Mendler AN, Brech D, Masouris I, Oberneder R, Noessner E. NK-cell dysfunction in human renal carcinoma reveals diacylglycerol kinase as key regulator and target for therapeutic intervention. Int J Cancer. 2014;135:1832–41. doi: 10.1002/ijc.28837. [DOI] [PubMed] [Google Scholar]
- 55.Joshi RP, Schmidt AM, Das J, Pytel D, Riese MJ, Lester M, et al. The zeta isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling. Sci Signal. 2013;6:ra102. doi: 10.1126/scisignal.2004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuchiya R, Tanaka T, Hozumi Y, Nakano T, Okada M, Topham MK, et al. Downregulation of diacylglycerol kinase zeta enhances activation of cytokine-induced NF-kappaB signaling pathway. Biochim Biophys Acta. 2015;1853:361–9. doi: 10.1016/j.bbamcr.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Floyd DH, Comeau L, Boroda S, Hayes N, Roller D, Xiao A, et al. Diacylglycerol kinase alpha inhibition prolongs survival of mice with primary and metastatic brain tumors [abstract]. Proceedings from the 19th Annual Scientific Meeting of the Society for Neuro-Oncology; 2014 Nov 13–16; Miami, FL; Houston (TX): Society for Neuro-Oncology; 2014. Abstract nr PM-02. [Google Scholar]
- 58.Chibalin AV, Leng Y, Vieira E, Krook A, Bjornholm M, Long YC, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–86. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 59.Ishisaka M, Kakefuda K, Oyagi A, Ono Y, Tsuruma K, Shimazawa M, et al. Diacylglycerol kinase beta knockout mice exhibit attention-deficit behavior and an abnormal response on methylphenidate-induced hyperactivity. PLoS One. 2012;7:e37058. doi: 10.1371/journal.pone.0037058. [DOI] [PMC free article] [PubMed] [Google Scholar]