Summary
13C-pyruvate hyperpolarized magnetic resonance imaging (HP-MRI) is emerging as a viable quantitative biomarker for solid tumor response and normal tissue toxicity following radiation therapy. This technology effectively predicts response related to metabolic agents or alterations in the tumor microenvironment, but challenges remain to be addressed to ensure successful translational implementation.
In this issue of Clinical Cancer Research, Saito and colleagues (1) expanded upon the potential applicability of 13C-pyruvate hyperpolarized magnetic resonance imaging (HP-MRI) to assess the radioresistance of tumors as driven by intrinsic tumor microenvironment and oxygenation characteristics in SCCVII and HT-29 tumor cell line models. Otto Warburg first described that most cancer cells produce energy at a high rate using the glycolytic pathway rather than the oxidative phosphorylation pathway (2), and the ‘Warburg effect’ has become a hallmark of tumor growth. The development of 13C-pyruvate HP-MRI has led to increased interest in the use of lactate, the final metabolite in the glycolysis pathway, as a biomarker of disease progression and treatment response (3). Day et al. reported that HP-MRI could detect radiation-induced changes in tumor lactate levels within 96 hours of irradiation (4). Sandulache and colleagues hypothesized and demonstrated that 13C-pyruvate HP-MRI could be used to detect changes in tumor reducing potential induced by metabolic agents (5) and that HP-MRI could detect radiation-induced effects on tumor lactate levels within hours of irradiation (6). Thind et al. demonstrated that HP-MRI can also detect radiation-induced toxicity to normal tissue within days of irradiation (7, 8). The biochemical model for these effects reflects the parallel and inverse relationship between reducing potential and radiation-induced free radicals or reactive oxygen species. Because the conversion of pyruvate into lactate requires reducing equivalents, 13C-pyruvate HP-MRI can provide an indirect measure of radiation-induced free radical stress.
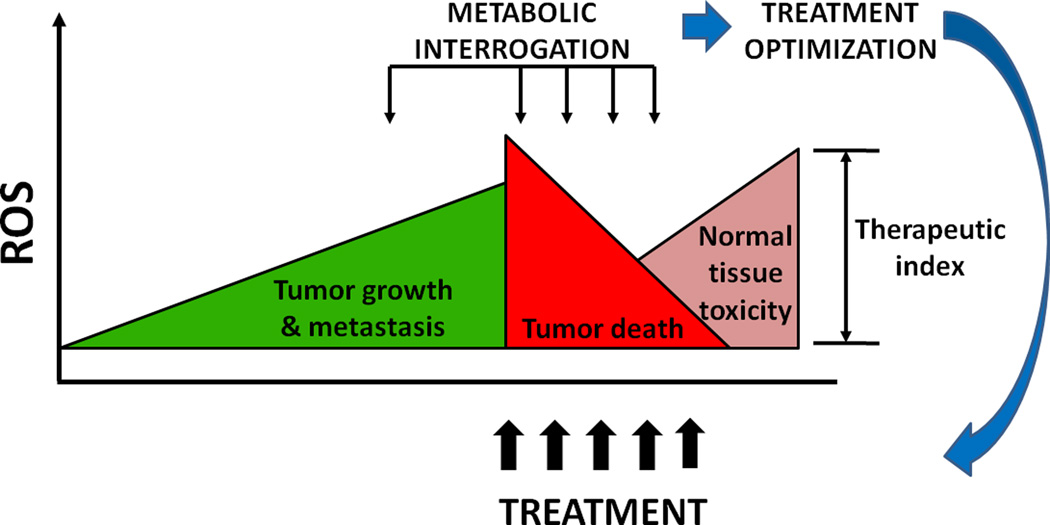
The biochemical model put forth by Sandulache et al., with the pre-clinical data by Saito et al., provides support for continued innovations in the delivery of targeted radiation therapy (Fig. 1). Since radiation is the primary non-surgical treatment modality for the management of solid tumors, elucidating radiation response via HP-MRI has generated enthusiasm for clinical implementation of this technology through adaptive irradiation algorithms and personalized dosimetric planning.
Figure 1. A model of therapeutic optimization using metabolic interrogation.
Reactive oxygen species (ROS) have important roles in tumor growth and metastasis. Although low levels of ROS can have oncogenic effects, high levels of ROS, such as those resulting from ionizing radiation or chemotherapy are cytotoxic, and thus are crucial role for treatment effectiveness. Treatment-related ROS are also responsible for some of the toxicity to normal tissue associated with treatment of solid tumors. Metabolic interrogation, whether invasive (biochemical analysis) or noninvasive (metabolic imaging) could be used to devise treatment regimens that generate tumoricidal levels of ROS while maximally sparing normal tissues.
However, several important caveats should be considered. Imaging irradiation of solid tumors must take into account spatial as well as temporal resolution. Since tumors are heterogeneous, spatial resolution is essential for appropriate targeting; moreover, as tumors undergo clonal regression based on relative radiosensitivity during radiation treatment, spatial resolution becomes even more important. As demonstrated by Sandulache et al, radiated tumors exhibit significant spatial heterogeneity with respect to viability. Consequently, imaging pyruvate metabolism in irradiated tumors requires a detailed voxel by voxel map with which clonal regression can be tracked over time. This issue is also a significant concern in the current report, because clinical implementation of HP-MRI in adaptive irradiation algorithms cannot proceed without adequate spatial resolution of changes in the pyruvate-lactate conversion rate. Since the conversion of pyruvate into lactate is a dynamic process, temporal resolution is also essential. Golman and colleagues demonstrated the heterogeneity of pyruvate-to-lactate conversion in tumors by using timed snapshots. Progress in the development of algorithms with adequate spatial and temporal resolution should allow for continued improvements leading to clinical translation.
HP-MRI can provides “real-time” imaging of tumor metabolism and overcomes several limitations of metabolic imaging with positron emission tomography (PET), such as poor temporal-spatial resolution or dynamic PET assessments that can require greater than 20 minutes to obtain in clinical settings. Furthermore, serial HP-MRI measurements are safe and feasible to obtain without additional exposure to radiation, facilitating high-frequency radiation response assessment in patients undergoing therapy.
The first-in-human imaging studies using HP-MRI in men with prostate cancer have demonstrated its safety and efficacy for non-invasively characterizing tumor metabolism (9). The current study further supports the ability of HP-MRI to monitor energy metabolism in both tumors and normal tissue and provides useful findings along the temporal continuum of radiation-induced effects on tumor lactate levels. These findings may provide a better understanding of early response to radiation therapy in the definitive, recurrent and palliative settings.
However, we would caution that additional work is required before HP-MRI can be implemented in the clinic. First, as detailed above, future studies must employ imaging algorithms with sufficient spatial and temporal resolution. Spatial resolution is particularly important because of normal tissue toxicity (7, 8). Second, preclinical studies must reproduce clinically relevant radiation doses. The most commonly accepted biological model for radiation effects in solid tumors involves a free radical mechanism that triggers aberrant DNA repair and programmed cell death. As the authors acknowledge, the 10-Gy doses used by Saito et al. or the 15-Gy doses used by Day et al. are far higher than the radiation doses typically used in radiation therapy (indeed, they approach the doses used in stereotactic ablative radiation therapy) and carry significant risks such as direct DNA and protein damage. Third, future studies must fully investigate temporal effects. Sandulache et al showed lactate perturbations within hours of irradiation. Saito et al. and others showed lactate perturbations within days of irradiation. Future studies should combine measurements during the acute, delayed and chronic phases following irradiation to provide a more comprehensive understanding of radiation-driven lactate perturbations in solid tumors. Lastly, other hyperpolarized biomarkers interrogating pH imaging, ROS pathway and the tricarboxylic acid cycle may provide insight into chemoresistance and changes in energy metabolism due to therapy (10).
The translational implications of HP-MRI for precision radiotherapy approaches are exciting. Not only can HP-MRI be coupled with anatomic MRI, but it can also be merged with functional multi-parametric MRI, providing simultaneous assessment of perfusion or cell density through combination with same-visit dynamic contrast-enhanced or diffusion-weighted MRI sequences. Thus, “perfusion-weighted” metabolic imaging, already done in animal models (11), may be a reality in the near-intermediate term. The advent of MRI-guided radiotherapy, as commercial vendors transition to MR-only treatment planning, coupled with adaptive strategies for tumor volume reduction, underscore the intriguing possibility of combining serial HP-MRI with adaptive MRI-based radiation planning (12). As the current body of literature has shown, 13C-pyruvate HP-MRI has great promise as a quantitative imaging-based biomarker of early radiation response. Immediate clinical applications in tumors currently treated with high radiation doses per fraction (e.g., 5–20 Gy) within the brain, lung, liver, kidney, prostate, or spine may provide an opportunity to monitor the immediate response to radiation therapy, providing clinical evidence for the use of concurrent radiosensitizing targeted agents or surgical resection of radioresistant tumors. HP-MRI thus warrants further investigation as a promising imaging biomarker for future clinical trials and personalized treatment approaches, especially within the context of translational radiotherapy.
Acknowledgments
The authors thank Vlad C. Sandulache, MD, PhD, for scientific and editorial input, including contribution to figure creation, and Christine F. Wogan for editorial input.
C.D. Fuller reports receiving commercial research grants from General Electric and Elekta AB, and is a consultant/advisory board member for Elekta AB. S.J. Frank reports receiving a commercial research grant from Elekta AB and is founder of, is on the Board of Directors for, and has ownership interest (including patents) in C4 Imaging.
Grant Support
S.Y. Laiis supported by the NCI of the NIH under award number R21CA178450. C.D. Fuller is supported by the NCI of the NIH under award number K12 CA088084 and the NIH under award number L30CA136381.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Saito K, Matsumoto S, Takakusagi Y, Matsuo M, Morris HD, Lizak MJ, et al. 13C-MR spectroscopic imaging with hyperpolarized [1-13C]pyruvate detects early response to radiotherapy in SCC tumors and HT-29 tumors. Clin Cancer Res. 2015 Feb 11; doi: 10.1158/1078-0432.CCR-14-1717. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006;66:10855–10860. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 4.Day SE, Kettunen MI, Cherukuri MK, Mitchell JB, Lizak MJ, Morris HD, et al. Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1-13C]pyruvate and 13C magnetic resonance spectroscopic imaging. Magn Resonance Med. 2011;65:557–563. doi: 10.1002/mrm.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandulache VC, Skinner HD, Wang Y, Chen Y, Dodge CT, Ow TJ, et al. Glycolytic inhibition alters anaplastic thyroid carcinoma tumor metabolism and improves response to conventional chemotherapy and radiation. Mol Cancer Ther. 2012;11:1373–1380. doi: 10.1158/1535-7163.MCT-12-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandulache VC, Chen Y, Lee J, Rubinstein A, Ramirez MS, Skinner HD, et al. Evaluation of hyperpolarized [1-(13)C]-pyruvate by magnetic resonance to detect ionizing radiation effects in real time. PLoS One. 2014;9:e87031. doi: 10.1371/journal.pone.0087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thind K, Chen A, Friesen-Waldner L, Ouriadov A, Scholl TJ, Fox M, et al. Detection of radiation-induced lung injury using hyperpolarized (13)C magnetic resonance spectroscopy and imaging. Magn Resonance Med. 2013;70:601–609. doi: 10.1002/mrm.24525. [DOI] [PubMed] [Google Scholar]
- 8.Thind K, Jensen MD, Hegarty E, Chen AP, Lim H, Martinez-Santiesteban F, et al. Mapping metabolic changes associated with early radiation induced lung injury post conformal radiotherapy using hyperpolarized (13)C-pyruvate magnetic resonance spectroscopic imaging. Radiother Oncol. 2014;110:317–322. doi: 10.1016/j.radonc.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(13)C]pyruvate. Sci Transl Med. 2013;5:198ra08. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, Zacharias NM, Piwnica-Worms D, Bhattacharya PK. Chemical reaction-induced multi-molecular polarization (CRIMP) Chem Commun (Camb) 2014;50:13030–13033. doi: 10.1039/c4cc06199c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohndiek SE, Kettunen MI, Hu DE, Brindle KM. Hyperpolarized (13)C spectroscopy detects early changes in tumor vasculature and metabolism after VEGF neutralization. Cancer Res. 2012;72:854–864. doi: 10.1158/0008-5472.CAN-11-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontaxis C, Bol GH, Lagendijk JJ, Raaymakers BW. Towards adaptive IMRT sequencing for the MR-linac. Phys Med Biol. 2015;60:2493–2509. doi: 10.1088/0031-9155/60/6/2493. [DOI] [PubMed] [Google Scholar]