Abstract
BACKGROUND
Bowel dysfunction is a known complication of colorectal cancer (CRC) surgery. Poor bowel control has a detrimental impact on survivors’ health-related quality of life (HRQOL). This analysis describes the dietary and behavioral adjustments used by CRC survivors to manage bowel dysfunction and compares adjustments used by survivors with permanent ostomy to those with anastomosis.
METHODS
This mixed-methods analysis included pooled data from several studies that assessed HRQOL in CRC survivors. In all studies, CRC survivors with or without permanent ostomies (N=856) were surveyed using the City of Hope Quality of Life Colorectal Cancer tool. Dietary adjustments were compared by ostomy status and by overall HRQOL score (high versus low). Qualitative data from 13 focus groups and 30 interviews were analyzed to explore specific strategies used by survivors to manage bowel dysfunction.
RESULTS
CRC survivors made substantial, permanent dietary and behavioral adjustments after surgery, regardless of ostomy status. Survivors who took longer after surgery to become comfortable with their diet or regain their appetite were more likely to report worse HRQOL. Adjustments to control bowel function were divided into four major strategies: dietary adjustments, behavioral adjustments, exercise, and medication use.
CONCLUSIONS
CRC survivors struggled with unpredictable bowel function and may fail to find a set of management strategies to achieve regularity. Understanding the myriad adjustments used by CRC survivors may lead to evidence-based interventions to foster positive adjustments after surgery and through long-term survivorship.
Introduction
An estimated 1.2 million colorectal cancer (CRC) survivors are alive in the United States.1,2 Nearly all CRC survivors are treated with transabdominal surgical extirpation. Factors including tumor distance from the anal sphincter and type of procedure are used to guide treatment decision-making, including the need for a temporary or permanent intestinal stoma.3
The impact of bowel dysfunction on health-related quality of life (HRQOL) is well-known; fecal incontinence and urgency significantly affect social and psychological functioning in survivors.4-6 Our previous research suggest that permanent ostomies are associated with substantial HRQOL challenges including leakage, skin complications, dissatisfaction with appearance, and interference with work and activities.7-12 Bowel control may vary more for survivors with an anastomosis, and persistent problems with fecal incontinence and gas are common.5,13
CRC survivors have to adjust psychologically and behaviorally to manage bowel dysfunction following surgery. Strategies include functional self-care, social activity alterations, and use of complementary/alternative approaches.14 Medications, protective pads/diapers, and dietary modifications are common functional self-care strategies. Social activity alterations are used to prevent bowel accidents in public.15 Acupuncture and complementary therapies are less frequently reported.15 Dietary and behavioral adjustments are common functional self-care strategies for managing bowel dysfunction.13,16,17 More than half of CRC survivors report using dietary adjustments and intentional social isolation to prevent bowel accidents in public.18 However, evidence describing the specific types of dietary and behavioral adjustments is lacking. In addition, the effects on HRQOL are not adequately described in the current literature. Therefore, we analyzed data from our studies related to the dietary and behavioral adjustments used by long-term (≥ 5 years) CRC survivors to manage bowel dysfunction. We aimed to answer the following questions: 1) What percentage of long-term CRC survivors adjusted their diets? 2) How long does it take CRC survivors to adjust to dietary changes? 3) Do dietary adjustments affect HRQOL? 4) Do differences exist in adjustments based on ostomy status (permanent versus anastomosis)?
Materials and Methods
Mixed methods data pooled from several studies that assessed HRQOL in CRC survivors were used.19-21 We analyzed quantitative HRQOL data from mailed survey studies at Veterans Affairs centers in Tucson, Arizona, West Los Angeles, and Indianapolis (VA Study)21 and at Kaiser Permanente regions in the Northwest, Northern California, and Hawaii (KP Study).20 Outcomes were compared for cases (survivors who had a major gastrointestinal procedure with a permanent ostomy) or controls (survivors who had a major procedure with an anastomosis). Qualitative data were pooled from focus groups in the VA and KP studies and interviews from one ancillary study with female CRC survivors.19 All studies were approved by the Institutional Review Boards at University of Arizona and other collaborating sites.
Mailed surveys included the City of Hope Quality of Life Colorectal Cancer tool (COH-QOL-CRC).22,23 The tool assesses HRQOL in the physical, psychological, social, and spiritual domains.23 It consists of 47 forced-choice and open-ended items and 43 HRQOL items evaluated using 11-point scales. There are multiple items related to diet and behavioral adjustments, including questions on time to comfort with diet, time to appetite return, dietary adjustments following surgery, and specific food categories that were avoided. Validity analysis, including the identification of HRQOL cut-offs for the dietary adjustments, were conducted using content, construct, discriminant, and criterion-related approaches.23
Survivors in both the ostomy and anastomosis groups were identified through medical record searches using ICD-9-CM diagnosis and procedure codes. Eligibility criteria included: 1) Treated in the VA system or current KP membership; 2) Age 18 years or older; 3) Diagnosed with CRC at least 5 years prior to the survey (KP study only); and 4) History of a major procedure that did or did not result in a stoma (those who had a reversed temporary ostomy were excluded). Survivors undergoing treatment at the time of the survey were excluded. Ostomy and anastomosis patients were frequency matched in the VA study by the following: 1) Gender; 2) Years since diagnosis; and 3) Age group in the KP study.
Eligible participants received a mailed packet that included the questionnaires and a postage-paid return envelope. Study implementation took place over 18 months (September 3, 2005 to February 5, 2006) for the VA Study and 12 months (December 5, 2007 to December 6, 2008) for the KP Study. Survivors in the anastomosis group received a modified questionnaire in which ostomy-specific questions were changed to refer to their index CRC surgery. Participants who did not return their completed packets within two weeks of the mailings were contacted by phone (KP Study) or sent a second mailing (VA Study). Overall response rates were 48% for the VA Study and 52% for the KP Study.
Focus groups and interviews were conducted to better characterize postoperative dietary and behavioral adjustments. Survivors from the highest and lowest HRQOL quartile were invited to participate in separate focus groups led by skilled facilitators. A group of female survivors in the KP Study were interviewed individually using a discussion guide with open-ended questions. Focus groups lasted approximately two hours. For individual interviews, women were sent a recruitment letter inviting them to participate in hour-long interviews. Interested participants were contacted to confirm eligibility and schedule an interview. All focus groups and interviews were audio-recorded with the participants’ permission.
Quantitative Analysis
Analysis was performed using the Stata® statistical software release 10 (StataCorp 2007, College Station, TX). Demographic characteristics were compared by ostomy status within each study, using Student’s t-tests for continuous measures and chi-squared tests for categorical measures. Both studies were powered a priori for association of ostomy status with HRQOL outcomes that were not part of the present analysis.
We evaluated the relationship between measures of dietary adjustment and either ostomy status or HRQOL category (score ≥ 7) in both study samples combined. We tested the associations using a multilevel mixed-effects logistic regression model, treating the source study as a random effect and adjusting for tumor site (rectal versus colon), Charlson-Deyo comorbidity score, and the time-since-surgery. Treating the source study as a random effect should account for observed differences between the populations, such as the proportion male or married/partnered, as well as potential unobserved differences. We adjusted for the time-since-surgery because of differences observed by ostomy status as well as different eligibility criteria for time-since-diagnosis between the two studies. We theorized that any observed associations may differ by tumor site (rectal versus colon) and tested for effect modification (interaction) by tumor site prior to adjusting for tumor site.
Qualitative Analysis
Qualitative data derived from the focus groups were transcribed into text format and analyzed using HyperRESEARCH®. Interpretation of data was guided by the direct qualitative content analysis approach. This allows for identifying initial key concepts by expanding on existing theories and research.24 All transcripts were read and individually coded and categorized into themes by investigators experienced in qualitative analysis. A separate secondary analysis of all coded content was undertaken to explore dietary and behavioral adjustments that are commonly used to manage bowel dysfunction. Two investigators (VS and MG) reviewed all coded content related to dietary and behavioral adjustments and categorized the content themes. Investigators who did not participate in the initial review and selection process conducted a final validation of the dietary and behavioral adjustment content and themes. Data that were discordantly coded were discussed with all investigators for refinement and consensus.
Results
Demographic Characteristics
A total of 856 CRC survivors were included in the quantitative analysis (185 for VA study; 671 for KP study), with 345 ostomy and 511 anastomosis participants. The VA study consisted primarily of male veterans, and more than half of the KP study sample was male (Table 1). Overall, the mean age was 72, and the majority of survivors were Non-Hispanic White. The VA subjects were less likely to be married or partnered prior to surgery (both ostomy and anastomosis) and at time of study (ostomy group only), compared to KP subjects. VA subjects had a lower mean time since surgery (both ostomy and anastomosis), compared to KP subjects. For tumor sites, the difference in proportion with rectal tumors is significantly higher in ostomates for both studies (p<0.001). No significant demographic or clinical differences were observed by group (ostomy versus anastomosis).
Table 1. Subject Characteristics.
| VA Study | KP Study | |||
|---|---|---|---|---|
|
| ||||
| Item |
Ostomy (n=66) |
Anastomosis (n=119) |
Ostomy (n=279) |
Anastomosis (n=392) |
|
| ||||
| Male (%) | 95.4 a | 94.9 a | 58.8 | 58.6 |
|
| ||||
| Age, mean (SD) (yrs) | 69.2 (11.7) | 70.5 (10.5) | 72.2 (10.3) | 71.0 (11.2) |
|
| ||||
| Race/Ethnicity (%) | ||||
|
| ||||
| Non-Hispanic White | 84.8 | 80.7 | 74.5 | 78.8 |
|
| ||||
| Hispanic | 6.1 | 2.5 | 7.5 | 3.6 |
|
| ||||
| Black/African-American | 6.1 | 10.1 | 3.6 | 3.6 |
|
| ||||
| Asian | 0 | 1.7 | 9.0 | 8.2 |
|
| ||||
| Other/unknown | 3.0 | 5.0 | 5.4 | 5.9 |
|
| ||||
| Married/Partnered Prior (%) | 54.5 a | 61.9 a | 74.7 | 77.4 |
|
| ||||
| Married/Partnered Now (%) | 48.5 b | 58.5 | 62.5 | 65.0 |
|
| ||||
| Years since surgery, mean (SD) |
4.7 (5.2)a | 3.6 (2.6)a | 11.7 (7.2) | 10.9 (5.5) |
|
| ||||
| Tumor site | ||||
| Rectalc | 78.8d | 16.0 | 88.1d | 62.5 |
| Colon | 21.2 | 81.3 | 11.7 | 37.5 |
p=<0.001 compared to KP study, ostomy and anastomosis respectively
p = 0.04 compared to KP study, ostomy only
includes recto-sigmoid junction
p<0.001 compared to anastomosis, VA and KP study respectively
Dietary Adjustments by Ostomy Status
We did not observe significant differences in dietary adjustments, specifically time-to-comfort with diet and time for appetite to return by ostomy status (Table 2). An isolated significant interaction was observed between tumor site (rectal vs. colon) and time-to-appetite return (p = .03), suggesting that in subjects with rectal tumors, having an ostomy may be associated with greater likelihood to need 1 to 12 months for appetite to return, whereas in subjects with colon tumors, having an ostomy may be associated with lower likelihood to need 1 to 12 months for appetite to return (referenced to less than 1 month). However, since no significant interaction was observed for the longer categories of appetite adjustment, and the associations were not significant when we modeled tumor site groups separately, we report the findings for appetite to return adjusted for tumor site, rather than separately by tumor site. No other interactions with tumor site were observed. Subjects with an ostomy were more likely to report avoidance of carbonated beverages and vegetables.
Table 2. Dietary Adjustments by Ostomy Status: Ostomy versus Anastomosisa.
| Item |
Ostomy (n=345) |
Anastomosis (n=511) |
Odds Ratio | 95% CI |
p- value |
|---|---|---|---|---|---|
|
Time-to-comfort-
with-diet |
% | % | |||
| < 1 mo | 43.2 | 47.3 | REF | ||
| 1– 12 months | 38.3 | 33.3 | 1.2 | .84-1.7 | .31 |
| >12 months | 7.1 | 8.5 | .69 | .37-1.3 | .22 |
| Never | 11.3 | 10.9 | .89 | .53-1.5 | .66 |
|
Time-to-appetite-
return |
% | % | |||
| < 1 mo | 57.1 | 61.3 | REF | ||
| 1– 12 months | 34.4 | 31.0 | 1.1 | .82-1.64 | .43 |
| >12 months | 5.0 | 3.2 | 1.8 | .82-3.9 | .14 |
| Never | 3.5 | 4.5 | .80 | .37-1.7 | .58 |
|
Adjusted diet because
of ostomy/surgery |
44.7 | 43.0 | 1.0 | .73-1.3 | .96 |
|
Avoided dietary
components |
% | % | |||
| Carbonated beverages | 27.2 | 13.3 | 2.4 | 1.6-3.7 | <.001 |
| Dairy products | 11.1 | 7.9 | 1.3 | .76-2.1 | .36 |
| Fruits | 3.6 | 3.6 | .78 | .35-1.7 | .55 |
| Snacks (any foods that were eaten between the regular three meals) |
8.5 | 10.7 | .77 | .45-1.3 | .33 |
| Vegetables | 9.1 | 3.6 | 2.3 | 1.2-4.4 | .01 |
| Special diets (vegetarian, vegan, macrobiotic, Dean Ornish, Atkin’s) |
10.3 | 16.1 | .644 | .41-1.0 | .06 |
| Special diets helpfulb | 68.7 | 75.0 | .77 | .30-2.0 | .61 |
Mixed-effects logistic regression adjusted for tumor site, Charlson-Deyo comorbidity score, and years-since-surgery, study site as random effect
Question was not asked in the VA study
CI = confidence interval
Dietary Adjustments by HRQOL Score
Subjects who took longer to regain comfort with diet were significantly more likely to report lower HRQOL, across all categories of time relative to less than 1 month (Table 3). Odds ratios were greater as the time category increased. Similarly, subjects who took longer to regain appetite were significantly more likely to report lower HRQOL, across all categories of time relative to less than 1 month, with those taking over 1 year having an odds ratio more than twice that of those taking 1 – 12 months (relative to less than 1 month). Subjects who adjusted diet because of surgery or to prevent gas after surgery were significantly more likely to report low HRQOL. No interactions with tumor site were observed.
Table 3. Dietary Adjustments by Overall HRQOL Scoresa,b.
|
Total HRQOL < 7 (n=309) |
Total HRQOL ≥ 7 (n=547) |
Odds Ratio | 95% CI |
p- value |
|
|---|---|---|---|---|---|
|
Time-to-comfort-with-
diet |
% | % | |||
| < 1 mo | 30.4 | 54.4 | REF | ||
| 1– 12 months | 40.1 | 32.6 | 2.3 | 1.6-3.2 | <.001 |
| >12 months | 10.7 | 6.3 | 3.3 | 1.9-5.8 | <.001 |
| Never | 18.7 | 6.9 | 5.0 | 3.0-8.3 | <.001 |
| Time-to-appetite-return | % | % | |||
| < 1 mo | 46.8 | 66.7 | REF | ||
| 1– 12 months | 39.8 | 28.3 | 2.0 | 1.4-2.7 | <.001 |
| >12 months | 7.0 | 2.2 | 4.3 | 2.0-9.0 | <.001 |
| Never | 6.3 | 2.8 | 3.0 | 1.5-6.1 | .002 |
|
Adjusted diet because of
ostomy/surgery |
49.0 | 40.1 | 1.4 | 1.1-1.9 | .01 |
|
Adjusted diet to prevent
gas c |
43.4 | 31.7 | 1.7 | 1.1-2.6 | .02 |
0-10 scale; higher score = better HRQOL
Mixed-effects logistic regression adjusted for tumor site, Charlson-Deyo comorbidity score, and years-since-surgery, study site as random effect
Asked only in the ostomy group
: CI=Confidence Interval
Strategies to Regulate Bowel Function
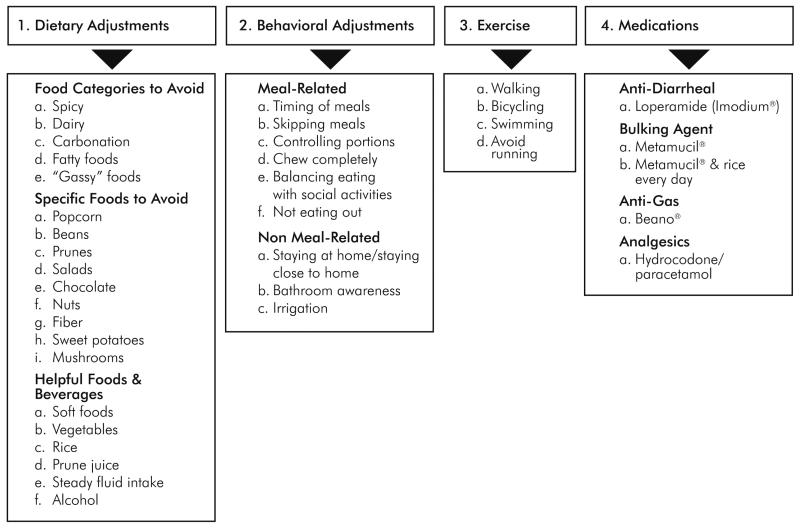
A total of 63 survivors participated in 13 focus groups across both studies (VA study = 29; KP study = 34; range = 2-8 survivors per focus group). Thirty female CRC survivors participated in the individual interviews. Strategies for regulating bowel function are depicted in Figure 1. The first strategy involved dietary adjustments to improve bowel control, and comprised the majority of modifications. Three food classes were identified: food categories to avoid, specific foods to avoid, and helpful foods. A second strategy for bowel regulation involved behavioral adjustments, and included those directly related to meals and eating, and those that were non-meal related. The third strategy involved the use of exercise for bowel regulation. The fourth strategy involved medications, including anti-diarrheal agents, bulking agents, and pain medications.
Figure 1.
Framework of Strategies for Regulating Bowel Function and Coping with Bowel Dysfunction
A wide array of foods and food categories were reported to be harmful to bowel function (Table 4). Survivors avoided spicy, fatty, and dairy products, because they resulted in diarrhea, constipation, and gas. Specific foods, such as corn, beans, peanuts, popcorn, onions, and lettuce, were avoided. Helpful foods included adequate fluids, fibrous foods, and prune juices. One form of fluid intake that was found to be harmful was carbonated beverages. Some food categories, including vegetables and fruits, were reported as being harmful by some survivors but helpful for others.
Table 4. Qualitative Findings: Strategies for Bowel Function Regulation.
| Theme/Strategy | Exemplar: Dietary Adjustments |
|---|---|
|
Food Categories
and Specific Foods to Avoid |
I eat everything except fatty foods. And I can eat fatty foods, but then I pay a terrible price. |
| I can’t eat peanuts or anything with a hard shell on it. I think anything with a hard shell on it like peas, if I eat too many of those.… I don’t eat a lot of apples, but I usually take the skin off. | |
| I can’t eat corn, I can’t eat salads, I can’t eat baked beans and there’s several others I can’t think of off hand, that I don’t eat. I like a lot of fruit, you know, oranges and grapefruits, and I can’t eat them. It plugs up. Your body doesn’t dissolve grapefruit. I can drink the juice, but it doesn’t dissolve the pulp in there. | |
| I don’t eat beans, I don’t eat onions, raw onions. I’m kind of careful on greens, cause they just don’t digest well. | |
| An overdose of coleslaw put me out of commission for three months. I lost a job over this, so it is important what you eat. | |
| Helpful Foods | I’ve gotten a lot smarter, and I drink water constantly. And it makes a big difference. |
| I eat lots of fruit. I eat lots of green vegetables and lots of salad. Lots of roughage. Oatmeal and things like that, because my problem is if I take and say eat nuts or popcorn, my colon just simply contracts, wham, and then there’s spasms. Hot and cold flashes. | |
| I find that if you eat like you should and you have enough fiber and enough fluid, and everybody is gonna take a different amount of water. I happen to take quite a bit. It’s like 5 cups of water, kind of warm, and basically I can keep the thing running smoothly. |
| Exemplar: Behavioral Adjustments | |
|---|---|
| Meal-Related | And you learn, over the years, what you can and can’t do. And you can’t overeat, either. That’s why it’s good to graze. You just eat all the time, and that’s not a problem. But if you just sat down and ate a huge meal, like a farmhand, it would be a bad thing. The pouch fills, and you get uncomfortable, things back up. |
| I find that if I eat smaller meals – because if you eat a whole lot, it goes right through – it doesn’t take much time for everything to eliminate. And you don’t want to eat a huge meal. | |
| I try to chew my food slowly. And I don’t ever drink until after I eat. I chew my food and then I drink. | |
| I just try to balance when I eat things versus what my schedule is gonna be.… you just plan out your schedule and figure out when you’re gonna eat what when. I still eat everything that I want. I just don’t necessarily get to eat it when I want to eat it. | |
| Non Meal-Related | I don’t…choose to be around groups of people I do not know. I suppose there’s times when I’ve cut myself off from something that might be an enjoyable activity. |
| I try not to drive as much as I used to. When I drive, I want to make sure that I know where I’m going. | |
| If I have to change my pouch or I have other things to do, I get as much things done in the morning that I need to do and so even stretch it out a little longer, so by the time I’m ready to change the pouch, I’m ten or twelve hours have gone by between eating. |
| Exemplar: Exercise | |
|---|---|
| You have to really watch your diet and exercise. You know, walking, exercycle, swimming. |
| Exemplar: Medications | |
|---|---|
| I’ve always had to take Imodium and it still doesn’t really do a whole bunch of good. | |
| I live on stool softeners in the morning and a laxative at night…and that’s how I get through. And even then, sometimes it all blocks up. | |
| He gave me Metamucil to try and that just kind of makes a sludge. | |
| And even though I have the Imodium, that doesn’t help immediately. |
The majority of behavioral adjustments were meal-related, such as not overeating, eating smaller meals, eating fewer meals, chewing food slowly, and planning meals around social activities. Some survivors skipped dining out altogether to avoid potentially embarrassing situations in public. Survivors changed their meal patterns or the quantities of foods they consumed based on known effects on bowel function (e.g., frequency and urgency) or other events in their lives.
Non-meal-related adjustments included staying at home or close to home/familiar bathrooms and knowing public bathroom locations. These adjustments reduced their fears about leakages or embarrassing situations. Some ostomy survivors used irrigation to control bowel habits. Survivors also described using physical activity to help regulate bowel patterns. Participation in more strenuous exercises, such as running, increased the frequency of bowel movements.
Medications and supplements were also frequently discussed as a remedy for irregular bowel patterns. As a group, survivors did not take a uniform approach with medications, but experimented with a variety of agents to control their bowel function.
Discussion
Our findings revealed that although many long-term CRC survivors adjust to bowel changes after surgery, a substantial number continue to struggle with maintaining bowel control. Specifically, the results revealed that for long-term CRC survivors, dietary adjustments occur regardless of ostomy status. With the current analysis we were unable to determine and delineate similarities and differences in strategies used to manage bowel dysfunction based on ostomy status. Our findings also confirmed the association between bowel dysfunction and HRQOL, and we observed that long-term survivors who report difficulties in dietary adjustments experience worse HRQOL. This finding suggests that interventions focused on providing CRC survivors with dietary and behavioral strategies to manage bowel dysfunction in the postoperative setting have the potential to improve long-term HRQOL. Our previous research found that excessive and uncontrollable gas significantly impacted CRC survivors’ psychological and social well-being postoperatively.7,9,10,25-27 This detrimental effect may result in lower overall HRQOL and increased social isolation. Finally, although we did not identify any significant differences in dietary adjustments by gender, our previous research suggests that gender differences in adjustments may exist.28 Further research is needed to delineate the influence of gender on dietary and behavioral adjustments and HRQOL.
Our qualitative content analysis revealed heterogeneity in the strategies that CRC survivors used to manage bowel dysfunction. The exact dietary modifications varied, and the current analysis could not identify which modifications were the most important. Behavioral modifications such as changing the number and timing of meals and staying close to home were common. Social well-being is a unique feature in CRC survivorship; therefore these behavioral changes have particular relevance.29 Intentional isolation from daily and social activities may result in lower HRQOL related to bowel dysfunction postoperatively.
Several limitations of the analysis warrant discussion. First, the analysis was unable to address potential confounding factors related to the primary clinical course following surgery, such as adjuvant treatments and complications. For colon cancer survivors included in the analysis, we were unable to include distinction of right or sided tumor location. Second, our findings may not be generalizable to non-VA and non-HMO populations. Additionally, the results should be confirmed with CRC survivors in other health care systems. Our sample population was predominantly white and male. Future research should focus on examining differences in dietary and behavioral adjustments by including a more diverse sample population.
In summary, CRC survivors undertake substantial dietary and behavioral adjustments to maintain bowel function, and these adjustments have implications for long-term HRQOL following surgery. Wide arrays of strategies were used by survivors to attempt to regulate bowel dysfunction. Surgeons should be aware of the myriad strategies that CRC survivors use to adjust to post-operative changes in bowel function. These strategies can be incorporated into empirically-based recommendations on coping with impaired bowel function during patient-centered treatment decision-making discussions.
Synopsis.
Colorectal cancer survivors may continue to suffer from unpredictable bowel function, and many long-term survivors never find effective strategies to achieve regularity. Evidence-based interventions are needed to support positive adjustments following surgery.
Acknowledgments
This research was supported by VA HSR&D grant IIR-02-221, R01 CA106912, CA106912-02S1, Sun Capital Partners Foundation, and Arizona Cancer Center Support Grant CA023074. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the NIH, or any other funders.
Footnotes
Disclosures: Dr. Herrinton: Research funding support from Centocor, P & G, Genentech, and Medimmune. The other authors have no financial disclosures to report.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015 Jan;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014 Mar;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Johnston CF, Tomlinson G, Temple LK, Baxter NN. The management of patients with T1 adenocarcinoma of the low rectum: a decision analysis. Dis Colon Rectum. 2013 Apr;56(4):400–407. doi: 10.1097/DCR.0b013e3182805eb8. [DOI] [PubMed] [Google Scholar]
- 4.Vironen JH, Kairaluoma M, Aalto AM, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum. 2006 May;49(5):568–578. doi: 10.1007/s10350-006-0513-6. [DOI] [PubMed] [Google Scholar]
- 5.Pucciarelli S, Del Bianco P, Efficace F, et al. Health-related quality of life, faecal continence and bowel function in rectal cancer patients after chemoradiotherapy followed by radical surgery. Support Care Cancer. 2010 May;18(5):601–608. doi: 10.1007/s00520-009-0699-y. [DOI] [PubMed] [Google Scholar]
- 6.Emmertsen KJ, Laurberg S, Rectal Cancer Function Study G Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. The British journal of surgery. 2013 Sep;100(10):1377–1387. doi: 10.1002/bjs.9223. [DOI] [PubMed] [Google Scholar]
- 7.Pittman J, Rawl SM, Schmidt CM, et al. Demographic and Clinical Factors Related to Ostomy Complications and Quality of Life in Veterans With an Ostomy. Journal of Wound, Ostomy & Continence Nursing September/October. 2008;35(5):493–503. doi: 10.1097/01.WON.0000335961.68113.cb. [DOI] [PubMed] [Google Scholar]
- 8.McMullen CK, Hornbrook MC, Grant M, et al. The greatest challenges reported by long-term colorectal cancer survivors with stomas. The journal of supportive oncology. 2008 Apr;6(4):175–182. [PubMed] [Google Scholar]
- 9.Krouse RS, Grant M, Wendel CS, et al. A mixed-methods evaluation of health-related quality of life for male veterans with and without intestinal stomas. Dis Colon Rectum. 2007 Dec;50(12):2054–2066. doi: 10.1007/s10350-007-9004-7. [DOI] [PubMed] [Google Scholar]
- 10.Krouse R, Grant M, Ferrell B, Dean G, Nelson R, Chu D. Quality of life outcomes in 599 cancer and non-cancer patients with colostomies. The Journal of surgical research. 2007 Mar;138(1):79–87. doi: 10.1016/j.jss.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Sun V, Grant M, McMullen CK, et al. Surviving colorectal cancer: long-term, persistent ostomy-specific concerns and adaptations. J Wound Ostomy Continence Nurs. 2013 Jan-Feb;40(1):61–72. doi: 10.1097/WON.0b013e3182750143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horner DJ, Wendel CS, Skeps R, et al. Positive correlation of employment and psychological well-being for veterans with major abdominal surgery. American journal of surgery. 2010 Nov;200(5):585–590. doi: 10.1016/j.amjsurg.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Knowles G, Haigh R, McLean C, Phillips HA, Dunlop MG, Din FV. Long term effect of surgery and radiotherapy for colorectal cancer on defecatory function and quality of life. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2013 Oct;17(5):570–577. doi: 10.1016/j.ejon.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Landers M, Savage E, McCarthy G, Fitzpatrick JJ. Self-care strategies for the management of bowel symptoms following sphincter-saving surgery for rectal cancer. Clinical journal of oncology nursing. 2011 Dec;15(6):E105–113. doi: 10.1188/11.CJON.E105-E113. [DOI] [PubMed] [Google Scholar]
- 15.Lai X, Wong FK, Ching SS. Review of bowel dysfunction of rectal cancer patients during the first five years after sphincter-preserving surgery: a population in need of nursing attention. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2013 Oct;17(5):681–692. doi: 10.1016/j.ejon.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Thaysen HV, Jess P, Laurberg S. Health-related quality of life after surgery for primary advanced rectal cancer and recurrent rectal cancer: a review. Colorectal Dis. 2012 Jul;14(7):797–803. doi: 10.1111/j.1463-1318.2011.02668.x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor C, Morgan L. Quality of life following reversal of temporary stoma after rectal cancer treatment. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2011 Feb;15(1):59–66. doi: 10.1016/j.ejon.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Hida J, Yoshifuji T, Tokoro T, et al. Long-term functional outcome of low anterior resection with colonic J-pouch reconstruction for rectal cancer in the elderly. Dis Colon Rectum. 2004 Sep;47(9):1448–1454. doi: 10.1007/s10350-004-0622-z. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez M, McMullen C, Grant M, Altschuler A, Hornbrook MC, Krouse RS. Figuring out sex in a reconfigured body: experiences of female colorectal cancer survivors with ostomies. Women Health. 2009 Dec;49(8):608–624. doi: 10.1080/03630240903496093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohler MJ, Coons SJ, Hornbrook MC, et al. The health-related quality of life in long-term colorectal cancer survivors study: objectives, methods and patient sample. Current medical research and opinion. 2008 Jul;24(7):2059–2070. doi: 10.1185/03007990802118360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krouse RS, Mohler MJ, Wendel CS, et al. The VA Ostomy Health-Related Quality of Life Study: objectives, methods, and patient sample. Current medical research and opinion. 2006 Apr;22(4):781–791. doi: 10.1185/030079906X96380. [DOI] [PubMed] [Google Scholar]
- 22.McHorney CA, Ware JE, Jr., Rogers W, Raczek AE, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Medical care. 1992 May;30(5 Suppl):MS253–265. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 23.Grant M, Ferrell B, Dean G, Uman G, Chu D, Krouse R. Revision and psychometric testing of the City of Hope Quality of Life-Ostomy Questionnaire. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2004 Oct;13(8):1445–1457. doi: 10.1023/B:QURE.0000040784.65830.9f. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative health research. 2005 Nov;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Herrinton LJ, Hornbrook MC, Wendel CS, Grant M, Krouse RS. Early and late complications among long-term colorectal cancer survivors with ostomy or anastomosis. Dis Colon Rectum. 2010 Feb;53(2):200–212. doi: 10.1007/DCR.0b013e3181bdc408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krouse RS, Herrinton LJ, Grant M, et al. Health-related quality of life among long-term rectal cancer survivors with an ostomy: manifestations by sex. J Clin Oncol. 2009 Oct 1;27(28):4664–4670. doi: 10.1200/JCO.2008.20.9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krouse RS, Grant M, Rawl SM, et al. Coping and acceptance: the greatest challenge for veterans with intestinal stomas. J Psychosom Res. 2009 Mar;66(3):227–233. doi: 10.1016/j.jpsychores.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Grant M, McMullen CK, Altschuler A, et al. Gender Differences in Quality of Life Among Long-Term Colorectal Cancer Survivors With Ostomies. Oncology Nursing Forum. 2011 Sep;38(5):587–596. doi: 10.1188/11.ONF.587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun V, Borneman T, Koczywas M, et al. Quality of life and barriers to symptom management in colon cancer. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2012 Jul;16(3):276–280. doi: 10.1016/j.ejon.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]