Abstract
PURPOSE
We previously identified the R-enantiomer of ketorolac as an inhibitor of the Rho-family GTPases Rac1 and Cdc42. Rac1 and Cdc42 regulate cancer-relevant functions including cytoskeleton remodeling necessary for tumor cell adhesion and migration. This study investigated whether administration of racemic (R,S) ketorolac after ovarian cancer surgery leads to peritoneal distribution of R-ketorolac, target GTPase inhibition in cells retrieved from the peritoneal cavity, and measureable impact on patient outcomes.
EXPERIMENTAL DESIGN
Eligible patients had suspected advanced stage ovarian, fallopian tube or primary peritoneal cancer. Secondary eligibility was met when ovarian cancer was confirmed and optimally debulked, an intraperitoneal port was placed, and there were no contraindications for ketorolac administration. R- and S-ketorolac were measured in serum and peritoneal fluid, and GTPase activity was measured in peritoneal cells. A retrospective study correlated peri-operative ketorolac and ovarian cancer-specific survival in ovarian cancer cases.
RESULTS
Elevated expression and activity of Rac1 and Cdc42 was detected in ovarian cancer patient tissues, confirming target relevance. Ketorolac in peritoneal fluids was enriched in the R-enantiomer and peritoneal cell GTPase activity was inhibited after ketorolac administration when R-ketorolac was at peak levels. After adjusting for age, AJCC stage, completion of chemotherapy, and neo-adjuvant therapy, women given peri-operative ketorolac had a lower hazard of death (Hazard Ratio=0.30 [95%CI 0.11–0.88]).
CONCLUSION
Ketorolac has a novel pharmacologic activity conferred by the R-enantiomer and R-ketorolac achieves sufficient levels in the peritoneal cavity to inhibit Rac1 and Cdc42, potentially contributing to the observed survival benefit in women who received ketorolac.
Keywords: Ovarian cancer, Rac1, Cdc42, ketorolac, Phase 0 trial
INTRODUCTION
Ovarian cancer is the leading cause of death from gynecologic malignancies and the second most common gynecologic cancer (1). Five year patient survival remains less than 50% and the mortality rate hasn’t changed appreciably in two decades (1). The majority of women are diagnosed with metastatic disease, and although a substantial proportion of women respond to initial treatment, recurrence is common (2). Despite concerted efforts, identification of effective targeted therapies has remained elusive in this disease (3). There remains a great need to identify new strategies to treat and manage ovarian cancer.
The Ras-homologous (Rho) family of small GTPases (Rac, Cdc42 and Rho) are key regulators of cancer-relevant cellular functions including actin reorganization, cell motility, cell-cell and cell-extracellular matrix (ECM) adhesion and invasion (4–6). In many human tumors (including colon and breast), there is clinical and experimental evidence that aberrant Rho-family signaling contributes to tumor growth, survival, invasion and metastasis (4, 7–10). Based on these functions, Rac1 and Cdc42 have been recognized as attractive therapeutic targets (11, 12) and inhibitors are effective in experimental systems (13–19), but specific inhibitors of Rac1 or Cdc42 have not been translated to clinical use.
A Cdc42 selective inhibitor effectively blocked migration of two ovarian tumor cell lines (19, 20) suggesting that Rho-family GTPases may be potential therapeutic targets in ovarian cancer. Using findings obtained from a high throughput screen of the Prestwick library of off patent, FDA-approved drugs and cheminformatics approaches, we identified the R-enantiomers of a limited number of non-steroidal anti-inflammatory drugs (NSAIDs) as inhibitors of Rac1 and Cdc42. The corresponding S-enantiomers are considered the active component in racemic drug formulations acting as NSAIDs with selective activity against cyclooxygenases (COX). One candidate, R-ketorolac, inhibited ovarian tumor cell migration and adhesion without causing cytotoxicity (21–24). Our data indicate that the clinically administered racemic ketorolac (Toradol®) has two distinct pharmacologic activities; the well-established inhibition of COX 1 and 2 by S-ketorolac serving as the basis of the FDA-approved indication for pain management, and a previously unrecognized property of Rac1 and Cdc42 inhibition conferred by the R-enantiomer. Cell based measurement of GTPase activity demonstrated that R-ketorolac specifically inhibits epidermal growth factor stimulated Rac1 and Cdc42 activation at low micromolar concentrations. The GTPase inhibitory effects of R-ketorolac in cells mimic those of established Rac1 (NSC23766) and Cdc42 (CID2950007/ML141) specific inhibitors (24).
In this study we report that R-ketorolac achieved an effective concentration in peritoneal fluids and inhibited Rac1 and Cdc42 activity in cells retrieved from the peritoneal compartment of post-surgical ovarian cancer patients following administration of the racemic drug for post-operative pain management. A medical record review to compare the ovarian cancer-specific survival of ovarian cancer patients who did or did not receive ketorolac for post-operative analgesia revealed increased survival of patients receiving ketorolac. This observation is in keeping with reports for improved clinical outcomes associated with ketorolac usage, as compared to other NSAIDs, in breast cancer patients (25–27). Although it has been long recognized that R-enantiomers of NSAIDs are poor inhibitors of cyclooxygenase activity (28–30), potential pharmacologic activities or benefits of the R-enantiomers has remained largely unexplored. Our findings show that Rac1 and Cdc42 are unrealized therapeutic targets in ovarian cancer and use of ketorolac may benefit ovarian cancer patients.
PATIENTS AND METHODS
Immunohistochemical Analyses of GTPase Targets
Immunohistochemical staining was performed using standard procedures (see Supplemental Methods). Rac1 was stained with mAb (clone 102, BD Biosciences, 10155-1-AP) and Cdc42 was stained with rabbit pAb (Protein Tech Group). A Vectastain Ready-to-Use (RTU) ABC-peroxidase kit and ImmPact DAB (SK-4105) were used to visualize primary antibody labeling with hematoxylin nuclear counterstain (H-3401) for tissue staining and samples were mounted in VectaMount (H-5000); (all from Vector Laboratories).
For large scale ovarian tumor profiling, tissue microarrays were purchased from US BioMax, Inc. (Rockville, MD, cat# OV1005 061 and OV8010 009). In total 180 unique tissue samples were included in the evaluation; ranging from stage I-IV and grades 1–3 (see Supplemental Table S1). All tumor types were validated and staining was scored by a pathologist with gynecologic pathology specialty (Dr. Lomo) and evaluated for location (nuclear and cytoplasmic), as well as intensity of positive staining. Scoring was based on the product of the percentage of cells stained and the intensity of the staining in each localization (3+: strong, 2+: intermediate, 1+: weak and 0: no staining), resulting in a minimum of 0 (100% cells×0) and a maximum of 300 (100% cells × 3+).
Quantitative PCR (qPCR) of Ovarian Cancer cDNA Arrays
qPCR analysis of Rho family GTPases was performed using Tissuescan Ovarian Cancer cDNA microarrays from Origene (Rockville, MD, cat# HORT301, HORT302, HORT303) and standard techniques (see Supplemental Methods and Supplemental Table S2). qPCR amplification utilized Qiagen Quantitect primers for Cdc42 (Valencia, CA, QT01674442), Rac1 (QT00065856), and RhoA (QT00044723), and β-actin (Origene), and custom Rac1b primers from Invitrogen (Carlsbad, CA). Primer information for Rac1b, methods for PCR product validation and additional information on the Ovarian Cancer cDNA arrays is presented in Supplemental Methods and Supplemental Table S2. Analysis of serous cancer only is shown in Supplemental Figure S1. CT values obtained using iCycler software. Relative expression levels were determined using ΔΔCT values.
Patients, Study Design and Treatment
A Phase 0 trial investigating the use of postoperative ketorolac was reviewed and approved by the University of New Mexico Health Sciences Center Human Research Review Committee (clinicaltrials.gov - NCT01670799). Patients presenting with a new diagnosis of ovarian, fallopian tube or primary peritoneal cancer were screened for eligibility. Eligible women were at least 18 years old, an ECOG Performance Status <2 and had consented to a planned debulking surgery- Consent was obtained prior to surgery if primary eligibility was met. Secondary eligibility after surgery included confirmed histologic diagnosis of epithelial ovarian, fallopian tube or primary peritoneal cancer; optimal cytoreduction and placement of an intraperitoneal port for planned chemotherapy; adequate renal function and no postoperative complications prohibiting ketorolac use. Patients with known bleeding disorders or other contraindications to NSAID use were excluded.
Subjects received a single IV dose of Toradol® (15 or 30 mg based on the patient age and creatinine clearance) within the first 72 hours of surgery when all clinical safety parameters were met. Use of other NSAIDs during the trial were not permitted; however narcotic regimens were allowed for postoperative pain management. All study protocols were reviewed by an independent data and safety monitoring board. Baseline ascites samples were obtained at surgery. Subsequent peritoneal fluid samples were collected from the intraperitoneal port prior to dosing and at 1, 6, and 24 h after single dose ketorolac administration. Peripheral blood was collected at the same time points. Serum and peritoneal fluid were separated from cellular material via low speed centrifugation. Tumor cells were further purified on Ficoll gradients to remove red blood cells and negative selection with anti-CD45 beads to remove lymphocytes. The resulting tumor cell fractions were analyzed by flow cytometry for EpCAM and MUC16/CA125 (see Supplemental Figure S2 for representative purity).
High Performance Liquid Chromatography
R- and S-enantiomers of ketorolac were analyzed by high performance liquid chromatography (HPLC) using published procedures (31, 32). See Supplemental Methods and Supplemental Figure S3 for additional information. The R-value for the standard curve of total ketorolac was 0.9997 and represented a concentration range that spanned established human serum concentrations [0.092 µg/ml to 6.0 µg/ml).
Analysis of GTPase activity
Two methods were used to assess GTPase activity in cells based on effector binding. Commercial GLISA kits from Cytoskeleton, Inc., analyzed Rac1 (Denver, CO, cat# BK-128), Cdc42 (BK-127) or RhoA (BK-124) per manufacturer’s instructions. Alternatively, Rac1, Cdc42 and RhoA activities were measured using a flow cytometric effector binding assay (24, 33). Briefly, active Rac1 and Cdc42 GTPases in prepared cell lysates were quantified individually based on binding to GST-PAK1-PBD from Millipore (Bellerica, MA, cat#14-864) immobilized on GSH beads and use of specific antibodies for bound GTPase detection. Antibodies used to quantify the amount of active (GTP-bound) GTPases captured on the beads are listed in Supplemental Methods. Fluorescence intensity (mean channel fluorescence, MCF) was measured by flow cytometry (Accuri C6). GTPase activity was calculated by (MCF of sample group – MCF of unstimulated group) / MCF of stimulated group. Equal amounts of protein were used for each assay.
Retrospective Patient Outcomes Review
A medical record review was conducted under institutional review board approval with a waiver of patient consent. Ovarian cancer patients were identified from the New Mexico Tumor Registry [NMTR] a member of the population-based Surveillance, Epidemiology, and End Results [SEER] Program of the National Cancer Institute (34). Inclusion criteria were as follows: invasive, epithelial ovarian cancer (any histology), age 40–79 years at diagnosis, years of diagnosis 2004–2006, and receipt of surgery at an Albuquerque, NM hospital (only three hospitals in the metropolitan area provide this level of surgery). Diagnosis years of 2004–2006 ensured at least 6 years follow-up (mortality followed through Dec 31, 2012) for each patient. We abstracted the surgical medical records for all analgesics and anesthesia medications used before hospital admission, during surgery and hospital stay, and given at discharge. Of the 138 potential cases, 6 women did not undergo surgery because of advanced disease/severe co-morbidities or desired palliative care only, 1 woman had her surgery in another state, 2 women died before surgery, and medical records were not located for 6 women, leaving 123 women in the final analysis.
Statistical analysis
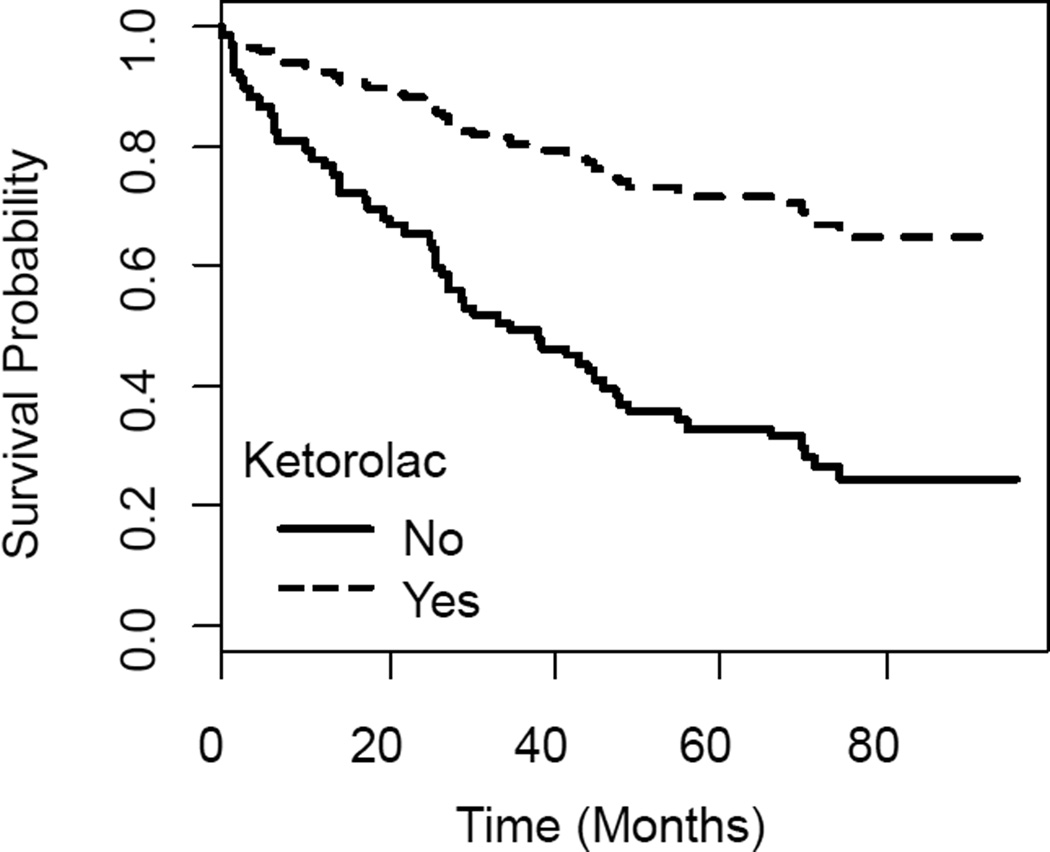
qPCR findings for GTPase expression levels were analyzed using one-way ANOVA followed by Dunnett’s Multiple comparisons test to determine differences between ovarian cancer grade. IHC data was analyzed using one-way ANOVA followed by Tukey's post test to determine significant differences between groups. Data obtained from patient fluid and cell samples was analyzed as a repeated measure ANOVA followed by Dunnett’s multiple comparisons test to determine significant differences between groups. For the retrospective medical record review, information from the medical record was merged with information from the NMTR for final analysis. Clinical and treatment characteristics of patients who did and did not receive peri-operative ketorolac were compared with chi-square tests and t-tests. In a preliminary, crude analysis the Kaplan-Meier method was used to estimate the survival probabilities. The difference in survival based on receipt of peri-operative ketorolac was examined using the stratified log-rank test to adjust the effect of a single categorical factor such as age group, AJCC stage, etc. Because this was an observational study and not a randomized controlled trial, the final analysis was based on a Cox proportional hazards model to adjust for clinical and treatment characteristics that may have differed between those who did and did not receive peri-operative ketorolac. We estimated the hazard ratio (HR) for ovarian cancer-specific mortality comparing those who did and did not receive peri-operative ketorolac while adjusting for age at diagnosis (<50, 50–64, ≥65) AJCC stage (I, II, III, IV), completion of chemotherapy as planned (yes, no), and receipt of neo-adjuvant chemotherapy (yes, no). Based on the Cox proportional hazards regression, an example survival plot is presented in Figure 5. Additional survival plots are presented in Supplemental Figures S4–S7.
Figure 5. Example Survival among Ovarian Cancer Patients with and without Peri-operative Ketorolac.
Cox proportional hazards regression was used to estimate ovarian cancer specific survival probabilities for women who did (dashed line, 17 women) and did not (solid line, 92 women) receive ketorolac among ovarian cancer cases with AJCC Stage III cancer, 50–60 years of age at diagnosis, no neoadjuvant chemotherapy and completed chemotherapy as planned (overall adjusted Hazard Ratio = 0.30, 95%CI = 0.11 – 0.88, likelihood ratio test p-value = 0.013).
RESULTS
Expression of Rac1 and Cdc42 in ovarian cancer
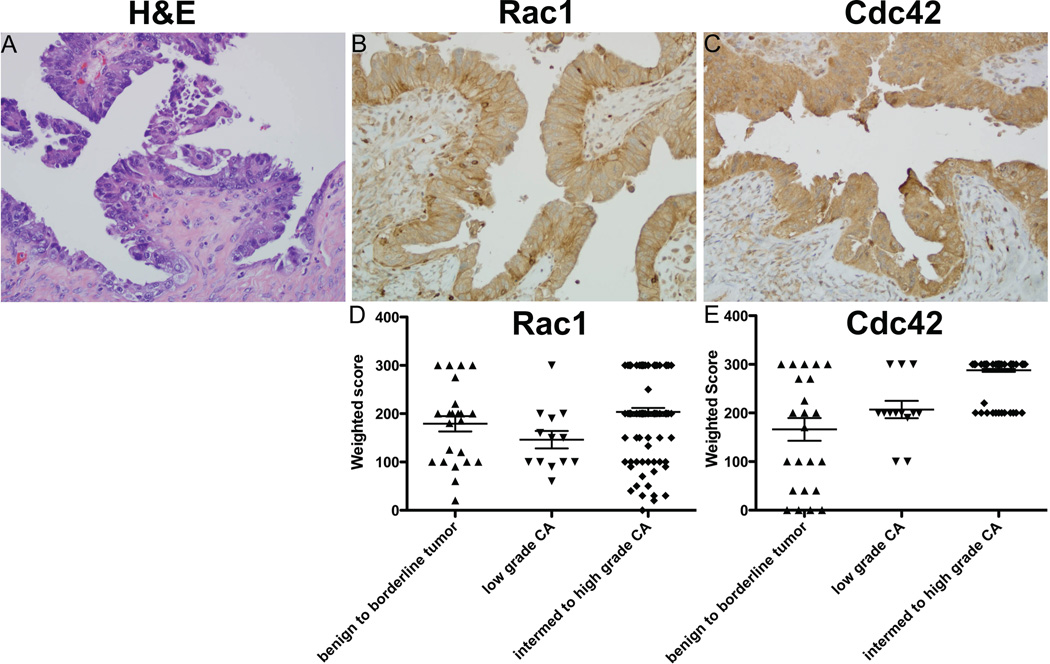
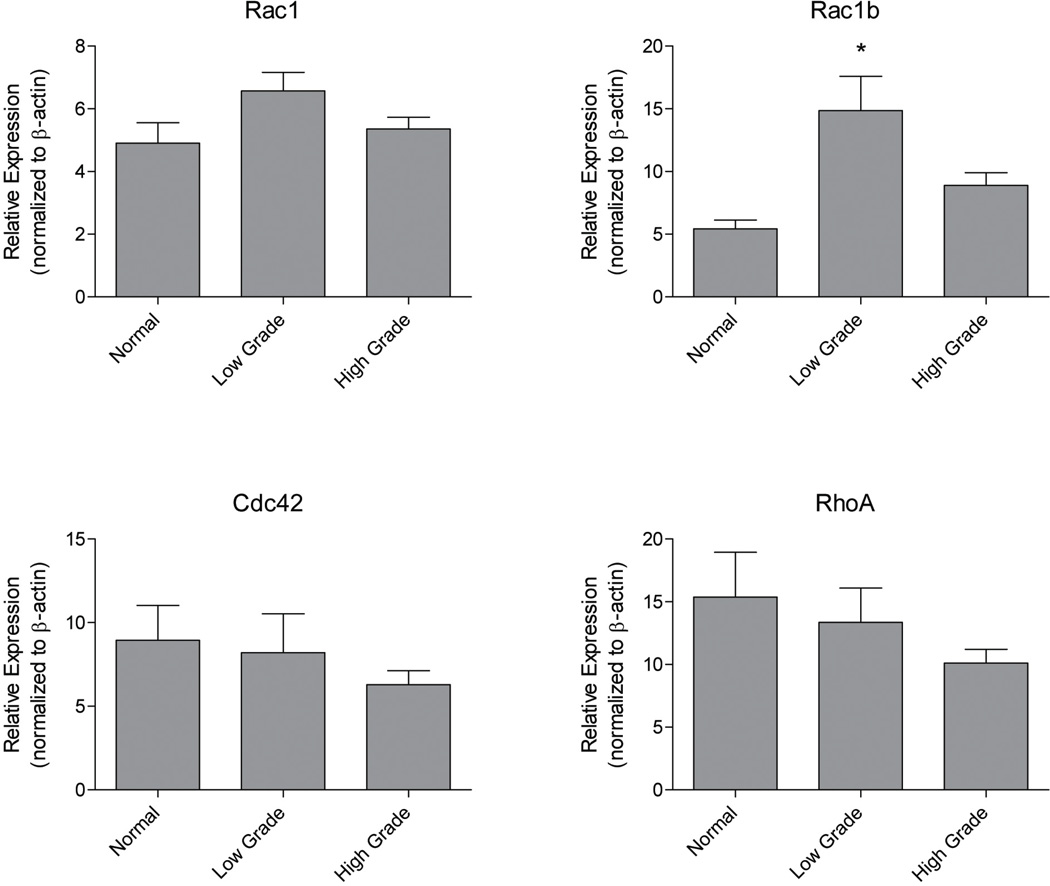
Based on the established functions of Rho-family GTPases, ovarian cancer metastasis is predicted to be strongly dependent on Rac1/Cdc42-regulated pathways for exfoliation, formation of multicellular aggregates, mesothelial adhesion, and localized invasion into the interstitial collagen-rich submesothelial matrix (2, 3, 35). To test if these GTPases are dysregulated in ovarian cancer, we examined grade dependent expression of Cdc42 and Rac1 protein by immunohistochemical staining of human tumor samples (Figure 1) and GTPase mRNA using quantitative PCR analysis of ovarian cancer tissue cDNA arrays (Figure 2 and Supplemental Figure S1). Cdc42 protein overexpression levels were highly significant for malignant, high-grade tumors (p<0.001) compared to lower grade tumors without an apparent increase in mRNA levels. In contrast, there was little evidence of increased expression of Rac1 protein with increasing grade (Figure 1). However, significantly elevated expression of a constitutively active splice variant Rac1b (36–38), was detected in ovarian tumors (Figure 2). These findings provide evidence for aberrant expression of Rac1 and Cdc42 in ovarian cancer and suggest that they may represent therapeutic targets for this disease.
Figure 1. Overexpression of Rac1 and Cdc42 protein in ovarian cancer specimens.
(A–C) Representative images of ovarian serous cancer tissue are shown. Clinical characteristics of the samples in the array are provided in Supplemental Table S1. Magnification 200×. Scale bar 20µm. (A) Hematoxylin/Eosin staining (H&E). (B–C) Samples were stained with antibodies against Rac1 or Cdc42 and avidin/biotin horse radish peroxidase enzyme complex. Controls and tissue samples were developed for identical times. (D–E) Tissue pathology and staining evaluated by board certified pathologist with gynecologic pathology specialization (Lesley Lomo, MD) and statistical analyses by statistician (Ed Bedrick, PhD). For Rac1 one way non-parametric ANOVA (p=0.0087) and Tukey's post-test shows normal stroma vs. intermediate to high grade carcinoma p<0.05 with all other comparisons non-significant. For Cdc42 one way non-parametric ANOVA (p=0.0001) and Tukey's post-test shows normal stroma and benign to borderline tumor vs. intermediate to high grade carcinoma p<0.05 with all other comparisons non-significant.
Figure 2. Expression of constitutively active Rac1b mRNA is elevated in ovarian cancer specimens.
Tissuescan ovarian cancer cDNA microarrays (Origene) were amplified using primers against Rac1, Rac1b, Cdc42, RhoA, and β-actin as described in methods. As per the manufacturer's description, patients with endometriosis, leiomyoma of myometrium, follicular cysts, abscesses, or secretory endometrium, but otherwise healthy ovarian tissue, were considered normal (n=19). Tissues defined as low grade have a FIGO score of 1 (n=19) or 2 (n=32). Tissues considered high grade have a FIGO score of 3 (n=60) or 4 (n=9). The cDNAs of 10 patients were excluded due to a lack of grade information. Clinical characteristics of the samples in the array are provided in Supplemental Table S2. Groups were compared to normal using a two-tailed t-test, and significant increase in Rac1b was detected. * indicates significance is p≤0.05. Serous only, analysis by grade is reported in Supplemental Figure S1.
Study design and patient population
In previous work, we identified R-ketorolac as an inhibitor of Rac1 and Cdc42 at low micromolar concentrations (21–24). R-ketorolac is inactive against the enzyme targets of S-ketorolac, COX-1 and COX-2 (28–30), and the inhibitory concentration (IC)50 values for inhibition of Rac1 and Cdc42 by S-ketorolac were more than 100-fold greater than R-ketorolac (24). Thus, the racemic [R,S] ketorolac possesses two distinct pharmacologic activities and our findings identify R-ketorolac as a novel inhibitor of Rac1 and Cdc42.
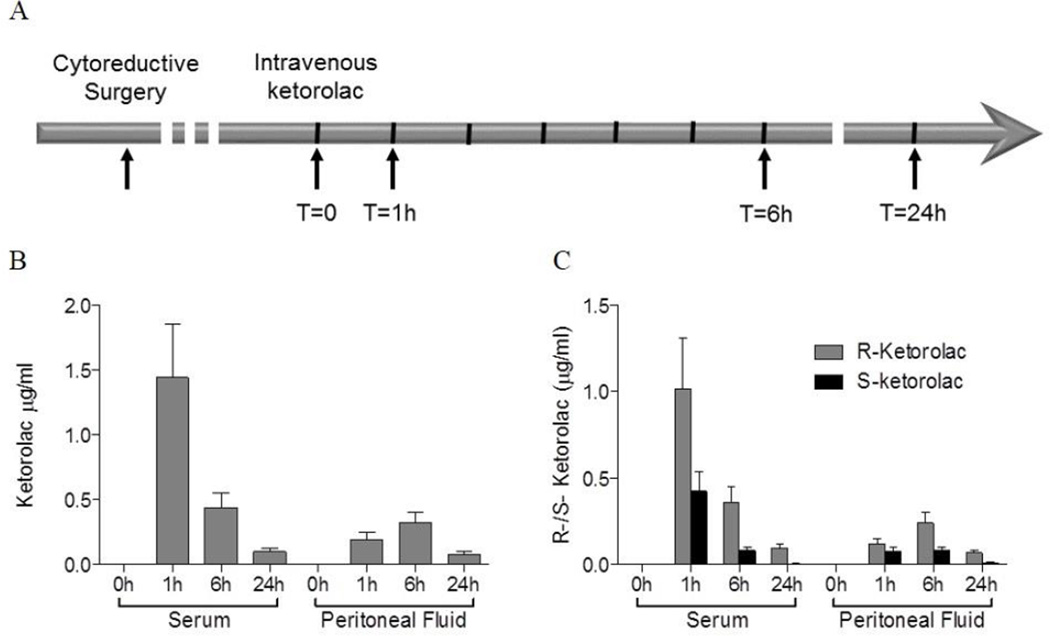
In this “Phase 0” feasibility study, ketorolac was administered for its FDA-approved indication for post-operative pain management. Eligible patients had suspected advanced stage ovarian, fallopian tube or primary peritoneal cancer with planned optimal cytoreductive efforts. Secondary eligibility was met if the patient had confirmed ovarian cancer, was optimally cytoreduced and had an intraperitoneal (IP) port placed for planned peritoneal chemotherapy. The patients had no active post-operative bleeding, did not require therapeutic anticoagulation and had good post-operative organ function. Forty-two patients met primary eligibility, and considering secondary eligibility requirements, twenty patient samples were collected at surgery. Samples of blood and peritoneal fluids after ketorolac administration were obtained from thirteen patients. Pathologic analysis of the twenty surgical samples confirmed fifteen patients (75%) had stage III or IV disease. Histologically, 100% had high-grade carcinoma; one was a carcinosarcoma, 16/19 were pure serous carcinomas, one was primary peritoneal and one fallopian tube primary, and the rest ovarian primary tumors. The average age was 60.8 (Range 33–81), race and ethnic distribution was 85% Caucasian (29.4% Hispanic), 5% American Indian and 10% Black or African American. Ketorolac dosages administered based on clinical indications were 15 mg (33% of patients) or 30 mg (77% of patients). As illustrated in Figure 3A, blood and peritoneal fluid were obtained at T=0, 1 h, 6 h and 24 h after administration of the recommended dose of ketorolac.
Figure 3. Ketorolac distributes to peritoneal fluids and is enriched in the R-enantiomer.
(A) Ascites samples were obtained at cytoreductive surgery and one to three days after surgery patients received a single dose of either 15 mg or 30 mg of clinical racemic ketorolac. Blood and peritoneal fluid from patients were collected prior to dosing (T=0), and at 1 hour, 6 hours, and 24 hours after dosing as depicted by the arrows. (B–C) Ketorolac enantiomers (R and S) were measured in blood and peritoneal fluids using HPLC. (B) Total ketorolac levels in sera and peritoneal fluids. (C) The levels of each ketorolac enantiomer (R or S) at each time point in sera and peritoneal fluids were measured. Concentration conversion to micromolar in serum and peritoneal fluids is provided in Supplemental Table S3. Administered drug is a 1:1 ratio of R to S (Supplemental Figure S3), but S-ketorolac is eliminated more rapidly than R-ketorolac leading to a ratio favoring the R-enantiomer in both serum and peritoneal fluids. The R-value for the standard curve used to calculate the ketorolac concentrations was 0.9997 and represented a concentration range that spanned established human serum concentrations [0.092 µg/ml to 4.0 µg/ml; 3.0 µg/ml= 10 µM).
Distribution of R- and S-ketorolac in peritoneal fluids
Serum and peritoneal fluid samples were analyzed by HPLC to resolve and quantify R- and S-ketorolac enantiomers in order to determine enantiomeric ratios and distribution over time (Figure 3B,C). Clinical-grade ketorolac tromethamine is a 1:1 mixture of R- and S-enantiomers (Supplemental Figure S3); however, the racemic distribution favors the R-form in both serum and peritoneal fluids at each time point in keeping with the established shorter half-life of S-ketorolac in human serum based on differences in pharmacokinetic parameters for each enantiomer (28, 30, 39). Ketorolac distributes to the peritoneum within 1h after IV administration, and ketorolac levels in the peritoneal fluids are nearly equivalent to those present in the serum at 6 h and decline dramatically by 24 h in both serum and peritoneal fluids. Our results represent the first evidence of ketorolac distribution to peritoneal fluids.
The half-maximal inhibitory concentration (IC50) for Rac1 and Cdc42 by R-ketorolac are 0.57 and 1.07 µM, respectively whereas the IC50 values for S-ketorolac for these targets was >100 µM (24). The concentrations of R- and S-ketorolac in the peritoneal fluids were 0.98 µM and 0.32 µM respectively, 6 h after IV ketorolac administration. Thus, R-ketorolac achieved concentrations in the peritoneal fluids at or above the IC50 values for Rac1 and Cdc42 and is predicted to inhibit these GTPase targets in cells obtained from this compartment.
Analysis of patient-derived cells
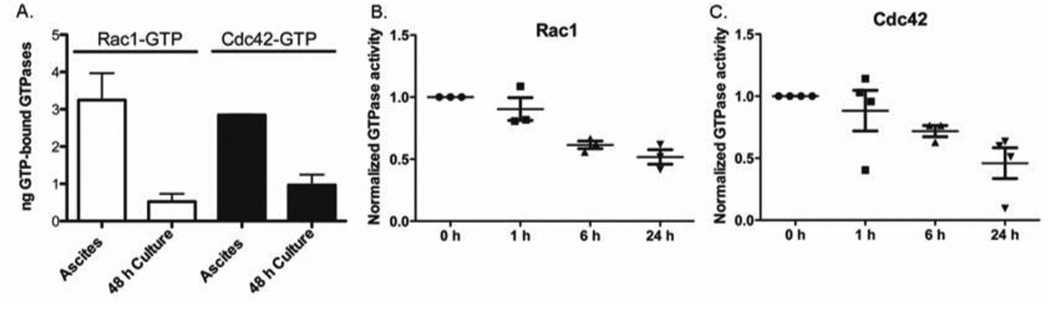
Tumor cell enriched fractions were prepared from ascites samples obtained at the time of cytoreductive surgery from ovarian cancer patients and post-surgery immediately prior and 1 h, 6 h, and 24 h post IV ketorolac administration (Figure 3A). Both Rac1 and Cdc42 were highly activated in freshly isolated tumor cells from ascites and the activity level declined within 48 h in culture medium (Figure 4A) suggesting that the ovarian tumor environment fosters Rac1 and Cdc42 GTPase activation. Post-surgery, we observed a statistically significant decrease in Rac1 and Cdc42 activity with time after ketorolac administration (Figure 4B,C). In contrast, RhoA activity was insensitive to ketorolac (Supplemental Figure S8), further affirming the selectivity of the drug. R-ketorolac predominates in the peritoneal fluids at the S-enantiomer is virtually undetectable at 24 h (Figure 3C) indicating that the R-enantiomer is bioactive and accounts for the observed inhibition of the GTPases in vivo.
Figure 4. GTPases are activated in patient ascites and inhibited by ketorolac administration in vivo.
(A) GTPase activity and target inhibition in patient derived cells. GLISA PAK-effector binding was used to individually detect activated Rac1-GTP or Cdc42-GTP in tumor cells isolated from ovarian cancer patient ascites. Purified GTP-loaded GTPases were used to calculate ng GTP-bound GTPase in the patient sample. Unpaired, two-tailed t-tests showed samples in culture for 48 h were statistically different from fresh ascites samples for both Rac1 and Cdc42 (p=0.0109). The levels of active GTPase declined sharply with 48 h in culture indicating that soluble factors in the ascites serve to upregulate Rac1 and Cdc42 GTPase activities. (B–C) Rac1 GTPase target inhibition following administration of racemic ketorolac to ovarian cancer patients post-surgery. Cells isolated from patient ascites samples post-surgery were assayed for active Rac1 or Cdc42 using a flow based effector binding assay. Patient diagnoses were all stage III, high grade ovarian serous or papillary serous carcinoma, with one mixed serous endometrioid carcinoma and one suspected primary peritoneal carcinoma (Pt 20, 24, 35, 39, 43). Fluorescence readings were normalized to the 0 h time point drawn immediately prior to ketorolac administration. For Rac1, one way non-parametric ANOVA (p=0.0009) and Bonferroni multiple comparison test p<0.05 for: 0 h vs 6 h (**); 0 h vs 24 h (**); 1 h vs 6 h (*); 1 h vs 24 h (**). Differences between 0 h vs. 1 h and 6 h vs. 24 h were non-significant. For Cdc42, one way non-parametric ANOVA (p=0.0250) and Bonferroni multiple comparison test p<0.05 for 0 h vs 24 h (*) was significant and all others were non-significant. RhoA was not responsive to ketorolac (Supplemental Figure S8).
Retrospective Patient Outcomes Review
Peri-operative ketorolac was used in 14% of the 123 women in the study. Younger women (<50 years) were more likely than older women to receive peri-operative ketorolac (p<0.05); all other clinical and treatment characteristics were similar between the two groups. At 60 months of follow-up, 3/17 ketorolac treated patients (18%) and 40/92 non-treated patients (43%) had died of ovarian cancer. Stratified log-rank tests for categorical factors such as age group, AJCC stage, completion of chemotherapy as planned, and receipt of neo-adjuvant chemotherapy as coded in Table 1, showed a consistent ketorolac survival benefit in each strata (see Supplemental Figures S4–S7). The better survival in women treated with ketorolac consistently found in the stratified analysis was also evident in the proportional hazards analysis when we adjusted for age at diagnosis, AJCC stage, completion of chemotherapy as planned, and receipt of neo-adjuvant chemotherapy: the adjusted hazard ratio for ovarian cancer-specific mortality associated with perioperative ketorolac (yes vs. no) was 0.30 (95% CI 0.11–0.88) (Table 1). Based on the proportional hazards model, an example survival plot is shown in Figure 5 for women who had AJCC stage III cancer, were 50–60 years at diagnosis, did not receive neoadjuvant therapy, and completed post-surgery chemotherapy as planned. Based on the proportional hazards model, an example survival plot is shown in Figure 5 for women who had AJCC stage III cancer, were 50–60 years at diagnosis, did not receive neoadjuvant therapy, and completed post-surgery chemotherapy as planned. Other survival plots are shown in Supplemental Figures S4–S7 and while these plots highlight the results for women who completed their post-surgery chemotherapy as planned, all combinations of women defined by stage of disease, age at diagnosis, neoadjuvant therapy, and post-surgery chemotherapy showed a consistently better survival with ketorolac versus without. These preliminary findings are suggestive that perioperative ketorolac reduces ovarian cancer-specific mortality.
Table 1.
Hazard ratios for ovarian cancer specific mortality for each characteristic adjusted for the other characteristics in the table.
| Characteristic | Number | Hazard Ratio (95% Confidence Interval) |
p-value** |
|---|---|---|---|
| Peri-operative ketorolac | .011 | ||
| No | 106 | 1.00 (reference) | |
| Yes | 17 | 0.30 (0.11 – 0.88) | |
| AJCC* stage | .050 | ||
| I | 26 | 1.00 (reference) | |
| II | 11 | 0.52 (0.11 – 2.47) | |
| III | 57 | 2.17 (0.97 – 4.86) | |
| IV | 29 | 1.56 (0.65 – 3.74) | |
| Age (years) | .045 | ||
| < 50 | 36 | 1.00 (reference) | |
| 50 – 60 | 55 | 2.50 (1.17 – 5.35) | |
| 60 + | 32 | 1.85 (0.80 – 4.29) | |
| Completion of post-surgery chemotherapy as planned | .557 | ||
| No | 78 | 1.00 (reference) | |
| Yes | 45 | 1.19 (0.67 – 2.14) | |
| Neoadjuvant chemotherapy | .050 | ||
| No | 101 | 1.00 (reference) | |
| Yes | 22 | 1.94 (1.03 – 3.66) |
AJCC = American Joint Committee on Cancer
Likelihood Ratio Test p-value
DISCUSSION
In many human cancers, aberrant Rho-family GTPase activity or downstream signaling pathways are associated with increased aggressiveness and poor patient prognosis (4, 6,8, 9, 11, 12). The specific mechanisms by which Rho-family GTPases modulate tumor development and progression remain under investigation (4, 5,7–9, 14); however, experimental evidence places Rac1 and Cdc42 within the metastatic cascade. Little is known regarding Rac1 and Cdc42 expression in ovarian cancer. We demonstrated elevated expression of Rac1 and Cdc42 in human ovarian cancer specimens and high activity of these GTPases in freshly isolated tumor cells from ascites obtained at surgery (Figs. 1 and 4). In a recent study paralleling our own work, high Rac1 protein expression in ovarian cancer was associated with early recurrence and poor prognosis (40). Furthermore, partial silencing of Rac1 by shRNA decreased tumor cell proliferation, migration and invasion in culture, and decreased growth of subcutaneous ovarian cancer xenografts in vivo (40). We find that R-ketorolac inhibits adhesion and invasion of primary human ovarian tumor cells from patient ascites (Guo et al, in preparation) thereby indicating that pharmacologic inhibition of Rac1 and Cdc42 also blocks these tumor-relevant functions. The cumulative observations in conjunction with inhibition of ovarian tumor cell migration by a Cdc42 specific inhibitor (19) indicate the potential value of targeting Rac1 and Cdc42 in ovarian cancer.
In the present study, we show evidence that Rac1 and Cdc42 inhibition can be achieved in ovarian cancer patients following administration of racemic ketorolac (Toradol®). Ketorolac is a 1:1 racemic mix of the R- and S-enantiomers. The S-form inhibits COX enzymes which confers the drug’s anti-inflammatory activities. The COX inhibitory action of S-ketorolac supports its indication for post-operative pain management, but also limits its long term use due to COX-related toxicity (28–30). R-ketorolac has little activity against COX (28–30) and therefore is not functional as an NSAID, but is bioactive and inhibits Rac1 and Cdc42 (24). Importantly, the levels of R-ketorolac within the peritoneal fluids were sufficient to inhibit Rac1 and Cdc42 activity in cells obtained from the peritoneal cavity following ketorolac administration. The innovative Phase 0 clinical trial design enabled real time sampling of fluids and cells from the peritoneal cavity. Direct demonstration of the difference in racemic distribution of ketorolac enantiomers illustrates the value of a study design that allows direct testing of drug and cell activities within peritoneal fluids rather than extrapolation from serum drug levels.
Furthermore, the peritoneal bioactivity of ketorolac is shown to have benefit for ovarian cancer patient outcomes. We found that perioperative use of ketorolac reduces ovarian cancer specific mortality (Fig. 5). There is precedence in the literature that ketorolac usage in the perioperative period is associated with improved cancer outcomes. The first observation was made for breast cancer patients in 2010 (25). In this study, ketorolac use was associated with a decrease risk of breast cancer relapse (HR=0.37, 95%CI=0.0–0.79). Follow-up papers noted that this relapse reduction was most pronounced in the first 24 months post-surgery (26, 27). No change in breast cancer recurrence was noted in patients who received sufentanil, clonidine, ketamine, or other intraoperative analgesics. Lung cancer patients receiving ketorolac displayed improved overall survival as well (41). The authors hypothesize that the benefit is due to the anti-inflammatory actions of ketorolac, particularly on the extravasation of circulating tumor cells in the transient inflammatory environment stimulated by surgery (42). Ketorolac appears to have more pronounced positive outcomes than other NSAIDs (42), and this may be based on the combined impact of anti-inflammatory activity by the S-enantiomer and R-enantiomer effects on Rac1 and Cdc42 leading to decreased adhesion and implantation of circulating or residual tumor cells. Ketorolac is not cytotoxic to ovarian tumor cells (24), but predicted decreases in establishment or further development of micrometastases due to Rac1 and Cdc42 inhibition would be expected to improve response to subsequent chemotherapy, which cannot be initiated until patients have recovered from cytoreductive surgery, approximately 4 weeks.
Collectively, our findings support the potential repositioning of ketorolac as an addition to current ovarian cancer therapy. Our work demonstrates that the R-enantiomer of ketorolac acts as a first-in-class drug for inhibition of the cancer-relevant targets Rac1 and Cdc42 (23, 24) and provides the first evidence that these therapeutic targets can be inhibited in humans using an approved drug. There is precedence for pharmacologic activities dictated by R-enantiomers of specific NSAIDs against novel (non-COX) targets (43–45). For example, R-etodolac and its analogs SDX-301 and SDX-308 display anti-tumor activity in chronic lymphocytic leukemia and activity against multiple myeloma in cell and animal models (44, 46–49). R-etodolac also significantly suppressed tumors in a colitis-related mouse model colon cancer (43) and retarded tumor development and metastasis in a transgenic mouse model of prostate cancer (45). These examples and others demonstrate that R-enantiomers of NSAIDs can possess unanticipated anti-cancer activities based on interactions with non-COX targets. Further evidence that targeting Rac1 may provide therapeutic benefit in ovarian cancer was recently reported (50). Zoledronic acid is a nitrogen containing bisphosphonate that inhibits prenylation of small GTPases. Administration of this drug inhibited growth of ovarian cancer peritoneal xenografts through inhibition of angiogenesis driven by a Rac1 mediated pathway (50). Collectively, our results suggest that racemic ketorolac may provide a survival benefit to ovarian cancer patients through inhibition of COX enzymes by the S-enantiomer and inhibition of the small GTPases Rac1 and Cdc42 by the R-enantiomer. Additional studies to determine whether clinical benefit can be observed in ovarian cancer patients through perioperative administration of ketorolac in a placebo-controlled clinical trial are in process.
Supplementary Material
Statement of translational significance.
Clinically administered racemic ketorolac has two distinct pharmacologic properties; inhibition of cyclooxygenases by S-ketorolac and a previously unknown inhibitory activity against the small GTPases Rac1 and Cdc42 by R-ketorolac. Rac1 and Cdc42 are highly relevant tumor targets lacking clinically available selective inhibitors. In this study we provide the first demonstration that ketorolac administration inhibits Rac1 and Cdc42 in cells retrieved from the peritoneal cavity of post-surgical ovarian cancer patients. A novel Phase 0 trial design allowed real time sampling of fluids and cells from the peritoneal cavity. Measurement of each enantiomer within the peritoneal fluids revealed greater concentration of the R- versus S-enantiomer thereby accounting for the GTPase inhibition. Furthermore, a retrospective study identified improved ovarian cancer specific survival in patients who received ketorolac. This work provides evidence that targeting the tumor-relevant Rho-family GTPases Rac1 and Cdc42 through repositioning of ketorolac may benefit ovarian cancer patients.
Acknowledgements
This work is dedicated to memory of Austin Hudson-LaPore.
Financial Support: DOD OC110514 W81XWH-11-OCRP-TEA, UNM Science and Technology Corporation Gap Fund, Core Facility support (Flow Cytometry Shared Resource, Fluorescence Microscopy Shared Resource and Human Tissue Repository) from the University of New Mexico Cancer Research and Treatment Center (P30 CA118100). Focus Interactive Group grants from UNM Cancer Center (0990MD; 0990Q8 to AWN and LGH; 1146 to LC). Contract HHSN261201300010I from the National Cancer Institute. Center for Molecular Discovery grants from the NIH Molecular Libraries Program (MH074425 and MH084690 to LAS). NCI R25CA153825 (predoctoral fellowship to YG); INBRE NCRR 5P20RR016480 (predoctoral fellowship to SRK).
Footnotes
Conflicts of interest:
The authors declare no major competing financial interests.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Lederman JA. Ovarian Cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek J, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 5.Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. 2014;7:5. doi: 10.4161/sgtp.27958. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 9:690–701. doi: 10.1038/nrm2476. 2008. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23:1415–1423. doi: 10.1016/j.cellsig.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle. 2011;10:1571–1581. doi: 10.4161/cc.10.10.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Bid HK, Roberts RD, Manchanda PK, Houghton PJ. RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther. 2013;12:1925–1934. doi: 10.1158/1535-7163.MCT-13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mardilovich K, Olson MF, Baugh M. Targeting Rho GTPase signaling for cancer therapy. Future Oncol. 2012;8:165–177. doi: 10.2217/fon.11.143. [DOI] [PubMed] [Google Scholar]
- 13.Zins K, Gunawardhana S, Lucas T, Abraham D, Aharinejad S. Targeting Cdc42 with the small molecule drug AZA197 suppresses primary colon cancer growth and prolongs survival in a preclinical mouse xenograft model by downregulation of PAK1 activity. J Transl Med. 2013;11:295. doi: 10.1186/1479-5876-11-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS One. 2013;11:e74924. doi: 10.1371/journal.pone.0074924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friesland A, Zhao Y, Chen YH, Wang L, Zhou H, Lu Q. Small molecule targeting Cdc42-intersectin interaction disrupts Golgi organization and suppresses cell motility. Proc Natl Acad Sci U S A. 2013;110:1261–1266. doi: 10.1073/pnas.1116051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montalvo-Ortiz BL, Castillo-Pichardo L, Hernández E, Humphries-Bickley T, De La Mota-Peynado A, Cubano LA, et al. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012;287:13228–13238. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surviladze Z, Waller A, Wu Y, Romero E, Edwards BS, Wandinger-Ness A, et al. Identification of a small GTPase inhibitor using a high-throughput flow cytometry bead-based multiplex assay. J Biomol Screen. 2010;15:10–20. doi: 10.1177/1087057109352240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 2008;439:111–129. doi: 10.1016/S0076-6879(07)00409-0. [DOI] [PubMed] [Google Scholar]
- 19.Hong L, Kenney SR, Phillips GK, Simpson D, Schroeder CE, Nöth, et al. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. J Biol Chem. 2014;288:8531–8543. doi: 10.1074/jbc.M112.435941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surviladze Z, Young SM, Sklar LA. High-throughput flow cytometry bead-based multiplex assay for identification of Rho GTPase inhibitors. Methods Mol Biol. 2012;827:253–270. doi: 10.1007/978-1-61779-442-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenney SR, Roxby J, Romero E, Ursu O, Oprea T, Sklar L, et al. Enantiomer specific inhibition of Rac1 and Cdc42 in ovarian cancer. Molec. Biol. Cell. 2011;22 abstr 1363. [Google Scholar]
- 22.Muller C, Hudson LG, Kenney SR, Guo Y, Gaede M, Adams SF, et al. R-ketorolac as a GTPase inhibitor: Phase 0 intraperitoneal pharmacokinetic and biologic activity in ovarian cancer patients. Gynec. Oncol. 2014;133:56S. [Google Scholar]
- 23.Oprea TI, Bauman JE, Bologa CG, Buranda T, Chigaev A, Edwards BS, et al. Drug Repurposing from an Academic Perspective. Drug Discov Today Ther Strateg. 2011;8:61–69. doi: 10.1016/j.ddstr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Kenney SR, Romero E, Oprea T, Adams S, Muller C, Sklar L, Hudson L, Wandinger-Ness A. Selected NSAIDs Target GTPases for Ovarian Cancer Therapy. AACR Advances in Ovarian Cancer Research: From Concept to Clinic; Sept. 18–21, 2013; Miami, FL. [Google Scholar]
- 25.Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110:1630–1635. doi: 10.1213/ANE.0b013e3181d2ad07. [DOI] [PubMed] [Google Scholar]
- 26.Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Cock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth. 2014;113(Suppl 1):i82–i87. doi: 10.1093/bja/aet464. [DOI] [PubMed] [Google Scholar]
- 27.Retsky M, Demicheli R, Hrushesky WJ, Forget P, De Kock M, Gukas I, et al. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem. 2013;20:4163–4176. doi: 10.2174/09298673113209990250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mroszczak E, Combs D, Chaplin M, Tsina I, Tarnowski T, Rocha C, et al. Chiral kinetics and dynamics of ketorolac. J Clin Pharmacol. 1996;36:521–539. doi: 10.1002/j.1552-4604.1996.tb05042.x. [DOI] [PubMed] [Google Scholar]
- 29.Jett MF, Ramesha CS, Brown CD, Chiu S, Emmett C, Voronin T, et al. Characterization of the analgesic and anti-inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther. 1999;288:1288–1297. [PubMed] [Google Scholar]
- 30.Handley DA, Cervoni P, McCray JE, McCullough JR. Preclinical enantioselective pharmacology of (R)- and (S)-ketorolac. J Clin Pharmacol. 1998;38:25S–35S. doi: 10.1002/j.1552-4604.1998.tb04414.x. [DOI] [PubMed] [Google Scholar]
- 31.Phenomenex, Phenomenex Chiral Column Protocols-online, App ID: 20367
- 32.Vakily M, Corrigan B, Jamali F. The problem of racemization in the stereospecific assay and pharmacokinetic evaluation of ketorolac in humans and rats. Pharm Res. 1995;12:1652–1657. doi: 10.1023/a:1016245101389. [DOI] [PubMed] [Google Scholar]
- 33.Buranda T, BasuRay S, Swanson S, Agola J, Bondu V, Wandinger-Ness A. Rapid parallel flow cytometry assays of active GTPases using effector beads. Anal Biochem. 2013;442:149–157. doi: 10.1016/j.ab.2013.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hankey BF, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results Program: A national resource. Cancer Epidemiology Biomarkers and Prevention. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 35.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene. 2004;23:9369–9380. doi: 10.1038/sj.onc.1208182. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, Chen QK, Lui C, Chicon MA, Radisky DC, Nelson CM. Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:4097–4108. doi: 10.1091/mbc.E12-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Lee W, Jiang Z, Chen Z, Jhunjhunwala S, Haverty PM, et al. Genome and transcriptome sequencing of lung cancers reveal diverse mutational and splicing events. Genome Res. 2012;22:2315–2327. doi: 10.1101/gr.140988.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayball PJ, Wrobel J, Tamblyn JG, Nation RL. The pharmacokinetics of ketorolac enantiomers following intramuscular administration of the racemate. Br J Clin Pharmacol. 1994;37:75–78. doi: 10.1111/j.1365-2125.1994.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leng R, Liao G, Wang H, Kuang J, Tang L. Rac1 expression in epithelial ovarian cancer: effect on cell EMT and cancer outcome. Med Oncol. 2015;32 doi: 10.1007/s12032-014-0329-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20(Suppl 3):S654–S660. doi: 10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- 42.Forget P, De Kock M. Perspectives in anaesthesia for cancer surgery. J Cancer Res Clin Oncol. 2014;140:353–359. doi: 10.1007/s00432-013-1522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue T, Murano M, Yoda Y, Kuramoto T, Kakimoto K, Ishida K, et al. R-etodolac induces E-cadherin and suppresses colitis-related mouse colon tumorigenesis. Oncol Rep. 2010;24:1487–1492. doi: 10.3892/or_00001009. [DOI] [PubMed] [Google Scholar]
- 44.Yasui H, Hideshima T, Hamasaki M, Roccaro AM, Shiraishi N, Kumar S, et al. SDX-101, the R-enantiomer of etodolac, induces cytotoxicity, overcomes drug resistance, and enhances the activity of dexamethasone in multiple myeloma. Blood. 2005;106:706–712. doi: 10.1182/blood-2005-02-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolluri SK, Corr M, James SY, Bernasconi M, Lu D, Liu W, et al. The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proc Natl Acad Sci U S A. 2005;102:2525–2530. doi: 10.1073/pnas.0409721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng R, Lentzsch S. Treatment of multiple myeloma with SDX-308. Drug News Perspect. 2007;20:431–435. doi: 10.1358/dnp.2007.20.7.1149631. [DOI] [PubMed] [Google Scholar]
- 47.Lindhagen E, Nissle S, Leoni L, Elliott G, Chao Q, Larsson R, et al. R-etodolac (SDX-101) and the related indole-pyran analogues SDX-308 and SDX-309 potentiate the antileukemic activity of standard cytotoxic agents in primary chronic lymphocytic leukaemia cells. Cancer Chemother Pharmacol. 2007;60:545–553. doi: 10.1007/s00280-006-0400-9. [DOI] [PubMed] [Google Scholar]
- 48.Robak P, Smolewski P, Robak T. The role of non-steroidal anti-inflammatory drugs in the risk of development and treatment of hematologic malignancies. Leuk Lymphoma. 2008;49:1452–1462. doi: 10.1080/10428190802108854. [DOI] [PubMed] [Google Scholar]
- 49.Yasui H, Hideshima T, Ikeda H, Ocio EM, Kiziltepe T, Vallet S, et al. Novel etodolac analog SDX-308 (CEP-18082) induces cytotoxicity in multiple myeloma cells associated with inhibition of beta-catenin/TCF pathway. Leukemia. 2007;21:535–540. doi: 10.1038/sj.leu.2404561. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Villasana V, Fuentes-Mattei E, Ivan C, Dalton HJ, Rodriquez-Aguayo C, Fernandez-de Thomas RJ, et al. Rac1/Pak1/p38/MMp-2 axis regulates angiogenesis in ovarian cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2279. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.