Structured Abstract
Background
Conventional cytogenetics (CC) and interphase fluorescence in situ hybridization (FISH) identify a high-risk multiple myeloma population characterized by poor response and shorter survival.
Patients and Methods
We compared outcomes between high-risk and standard-risk myeloma patients who underwent autologous hematopoietic stem cell transplantation (auto-HCT) at our institution between January 2005 and December 2009. High-risk myeloma was defined as –13/del(13q) or hypodiploidy in at least two metaphases of CC, or –17/del(17p), t(4;14), t(14;16), t(14;20), hypodiploidy (<45 chromosomes excluding –Y), or chromosome 1 abnormalities (+1q, –1p, t(1;x)) on FISH or CC.
Results
Of 670 myeloma patients, 74 (11%) had high-risk myeloma. These high-risk patients had significantly lower overall response rates (74% vs. 85%; p<0.01), shorter median progression-free survival (PFS; 10.3 vs. 32.4 months; p<0.001), and shorter overall survival (OS; 28 months vs. not reached; p<0.001) than the standard-risk patients. Having only one high-risk cytogenetic abnormality or achieving at least very good partial remission after auto-HCT independently predicted improved PFS and OS (p<0.05) in the high-risk patients.
Conclusion
Even in an era of novel therapies, cytogenetically identified high-risk myeloma patients have worse prognoses than standard-risk myeloma patients after auto-HCT, and having > one high-risk cytogenetic abnormality further reduces survival.
Keywords: Stem cell transplantation, Multiple Myeloma, Risk stratification, Cytogenetics, Prognosis
Introduction
Structural chromosomal alterations in clonal myeloma cells, recognized by conventional metaphase cytogenetics and interphase fluorescence in situ hybridization (FISH), are used to identify a subgroup (15–25%) of high-risk multiple myeloma (MM) patients with poor prognosis. 1–4
A consensus statement from the International Myeloma Working Group recommends classifying MM as high-risk if cytogenetic analysis of bone marrow samples reveals monosomy 13 (–13) or del(13q), del(17p), t(4;14), or t(4;16); or if interphase FISH identifies t(4;14), t(14;16), or del(17p) in MM cells. Identification of –13/del(13q) by FISH alone does not confer high-risk status.5 A classification system proposed by Mayo Clinic group defines high-risk MM as having t(4;14), t(14;16), t(14;20), or del(17p13) on FISH analysis of clonal plasma cells or having –13/del(13q) or aneuploidy on metaphase cytogenetic analysis and a plasma cell labeling index of >3.6 Other high-risk chromosomal abnormalities include: chromosome 1 aberrations, which are complex and may involve deletions in 1p, amplifications in 1q, and unbalanced translocations,7,8 and the presence of a hypodiploid karyotype, which are independently associated with short survival.9
Approaches for treating high-risk MM are evolving. Induction regimens containing novel anti-myeloma agents such as bortezomib, thalidomide, and lenalidomide show promise; however, these agents typically cannot completely overcome the resistance of high-risk MM.10 Bianchi et al. reported that the adverse risk of t(4;14) was abated by the use of bortezomib in induction and maintenance therapy in association with tandem auto-HCT.11 Other evidence suggests that allogeneic hematopoietic stem cell transplantation (allo-HCT) can overcome the negative prognostic effects of del(17p13) and/or t(4;14) and that the achievement of molecular remission after allo-HCT can result in longer relapse-free survival; however, only a minority of patients are eligible for allo-HCT.12
Auto-HCT is available broadly, improves survival in MM patients, and is currently considered a standard of care for transplant-eligible patients.13 Data about the benefits of auto-HCT in high-risk MM, however, are limited. Thus, to elucidate the benefits and characterize in greater detail the role of auto-HCT in patients with high-risk MM we compared the patient characteristics and outcomes between high-risk and standard-risk myeloma patients who underwent auto-HCT at our institute.
Methods
The charts of 670 patients with MM who underwent auto-HCT and had follow-up at The University of Texas MD Anderson Cancer Center between January 2005 and December 2009 were identified and retrospectively reviewed. Results of metaphase cytogenetic and FISH studies of bone marrow aspiration samples were obtained for all the patients from the clinical chart. Patients who lacked metaphase cytogenetic analysis and FISH analysis results, had received tandem auto-HCT, or had undergone allo-HCT were excluded from the study. Patients were defined as having high-risk MM if conventional cytogenetics in at least two metaphases performed at diagnosis or any time before auto-HCT revealed –13/del(13q), –17/del(17p), t(4;14), t(14;16), t(14;20), hypodiploidy (<45 chromosomes excluding –Y), or a chromosome 1 aberration (+1, –1, t(1;x)) or if FISH or conventional cytogenetics showed del(17p13), t(4;14), t(14;16), t(14;20), or chromosome 1 abnormalities at any time prior to auto-HCT. CD138 enrichment was done if the plasma cell percentages in bone marrow aspirate differential revealed 3-15 percent plasma cells. Enrichment was not done if plasma cells percentage was <3% (percentage too low) or >15% (no enrichment needed). Patients were included if the data was available for conventional cytogenetics, FISH, or both. Patients who showed no high-risk cytogenetic features were defined as having standard-risk MM; this group included patients in whom –13/del(13q) was identified only through FISH.
Descriptive statistics such as mean, standard deviation, median, and range were used for continuous variables, and frequency counts and percentages were used for categorical variables. Fisher exact tests or chi-square tests were used to compare variables between the groups. Wilcoxon rank sum tests or Kruskal-Wallis tests were used to compare continuous variables between the groups. The primary endpoints were OS and PFS in the high-risk MM and standard-risk MM groups. The secondary endpoints were overall response rate and treatment-related mortality (TRM) in each group. Response and progression were defined according to International Myeloma Working Group criteria.14 The overall response rate was assessed at 3 months after auto-HCT and included patients who had achieved partial remission (PR), very good PR (VGPR), complete remission (CR), or stringent CR (sCR). Kaplan-Meier method was used to calculate PFS from the date of transplantation to the date of progression or death from any cause and OS from the date of transplantation to the date of death. Log-rank tests were used to compare PFS and OS between the high-risk and standard-risk groups. Cox proportional hazards models were fitted for multivariate analysis to assess the effects of significant prognostic factors on survival in the high-risk MM patients. SPSS version 20 was used for all the analyses. The Institutional Review Board at The MD Ander Cancer Center approved the study.
Results
Patient and disease characteristics
During the study period, 670 MM patients with a median age of 58 (standard deviation, 31–80) years, of whom 388 (58%) were male, underwent auto-HCT at MD Anderson Cancer Center. Seventy-four (11%) patients were classified as having high-risk MM, and 596 (89%) patients had standard-risk MM. Chromosome 1 aberrations, –13/del(13q), hypodiploid cytogenetics, del(17p13), t(4;14), and t(14;16) were observed in 53 (72%), 48 (65%), 27 (36%), 16 (22%), five (7%), and none of the high-risk MM patients, respectively. Of the 74 high-risk MM patients, 31 patients had only one high-risk abnormality, and 43 (58%) had multiple, concurrent high-risk abnormalities. Of the 31 patients with only one high-risk abnormality, 15 had chromosome 1 aberrations, seven had –13/del(13q), six had hypodiploidy, two had del(17p), and one had t(4;14).
The baseline characteristics of the high-risk and standard-risk MM patients are summarized in Table I. Compared with the standard-risk MM patients, the high-risk MM patients were more frequently male (72% vs. 56%; p=0.003), had higher Durie-Salmon stage (p=0.03), more frequently showed IgA isotype on bone marrow specimen (p=0.03), had a lower median hemoglobin level at presentation (10.0 vs. 11.4 g/dl; p<0.003), and had a higher median percentage of clonal plasma cells in bone marrow aspirates at diagnosis (52% vs. 29%; p<0.001). In the high-risk group, all except two patients received induction chemotherapy containing at least one novel anti-myeloma agent (bortezomib, lenalidomide, or thalidomide). Fifty-three of the 74 high-risk MM patients (72%) received at least one cycle of bortezomib during the induction phase. Most high-risk MM patients received more than one novel agent during induction chemotherapy before auto-HCT; 27 (36%), 37 (50%), and eight (11%) patients received one, two, and three novel agents during induction chemotherapy, respectively.
Table I.
Comparison of baseline patient characteristics between high-risk and standard-risk MM patients.
| Characteristic | High-risk MM*, n (%) or median (range) | Standard-risk MM, n (%) or median (range) | p-value |
|---|---|---|---|
| Total number of patients | 74 (11) | 596 (89) | |
|
| |||
| Age, years | 58 (40–73) | 58 (31–80) | 0.88 |
|
| |||
| Sex | |||
| Male | 53 (72) | 335 (56) | 0.003 |
|
| |||
| Race | |||
| White | 52 (70.3) | 394 (66.1) | |
| Black | 9 (12.2) | 99 (16.6) | 0.61 |
| Other | 12 (16.2) | 99 (16.6) | |
| Missing Data | 1 (1.4) | 4 (0.7) | |
|
| |||
| Histologic features | |||
| Immunoglobulin G | 37 (50) | 339 (57) | |
| Immunoglobulin A | 17 (23) | 123 (21) | 0.03 |
| Light chain only | 10 (13.5) | 96 (16) | |
| Other | 10 (13.5) | 38 (6) | |
|
| |||
| Durie-Salmon stage | |||
| I | 5 (7) | 111 (18.6) | |
| II | 35 (47) | 237 (39.8) | 0.03 |
| III | 32 (43) | 225 (37.8) | |
| Missing data | 2 (3) | 23 (3.9) | |
|
| |||
| Bone marrow plasma cell percentage | 52 (0–95) | 29 (0–98) | <0.001 |
| Hemoglobin, g/dl | 10 (6–15) | 11.4 (6–17) | 0.003 |
| Lactate dehydrogenase, unit/l | 344 (44–1365) | 369 (51–1950) | 0.04 |
| Calcium, mg/dl | 9.5 (7–17) | 9.4 (7–18) | 0.06 |
| Creatinine, mg/dl | 1.2 (1–15) | 1.1 (0–21) | 0.08 |
| Beta-2-microgloobulin, mg/l | 3.9 (2–35) | 3.1 (1–47) | 0.21 |
| Immunoglobin G, mg/dl | 839 (168–10310) | 1634 (10–14946) | 0.15 |
| Immunoglobin A, mg/dl | 50 (6–8089) | 65 (2–9818) | 0.93 |
| Immunoglobin M, mg/dl | 21 (3–8050) | 21 (1–10400) | 0.19 |
| Bence Jones protein, mg/dl | 1.01 (0–20) | 0.25 (0–37) | 0.08 |
| Serum M-protein (g/dL) | 3 (0–8) | 2.60 (0–13) | 0.5 |
Based on cytogenetics or FISH at any time before autologous hematopoietic stem cell transplantation.
Table II lists auto-HCT–related characteristics and outcomes in the high-risk and standard-risk MM patients. At the time of auto-HCT, the high-risk group had a higher proportion of patients with refractory or relapsed MM (34% vs. 22%, respectively; p=0.01) and had a higher median percentage of clonal plasma cells in bone marrow aspirates obtained prior to auto-HCT (5% vs. 2%, respectively; p<0.001 compared with the standard-risk group. The median time from diagnosis to auto-HCT and the choice of conditioning regimens used did not significantly differ between the groups. Maintenance treatment was used in 15 high-risk MM patients (20% of the high-risk group), with thalidomide in seven patients, lenalidomide in seven patients and interferon in 1 patient.
Table II.
Comparison of auto-HCT–related patient characteristics and outcomes between high-risk and standard-risk MM patients.
| High-risk MM n (%), median (range) |
Standard-risk MM n (%) or median (range) |
p-value | |
|---|---|---|---|
|
| |||
| Characteristic or outcome | |||
| Bone marrow plasma cell percentage before auto-HCT, % | 5 (0–92) | 2 (0–88) | <0.001 |
|
| |||
| Overall response rate after induction chemotherapy (≥ PR) | 56 (77) | 499 (85) | 0.06 |
|
| |||
| Disease status at the time of auto-HCT | |||
| First remission | 48 (66) | 461 (78) | 0.01 |
| Relapsed/refractory disease | 25 (34) | 127 (22) | |
|
| |||
| Interval to auto-HCT (months) | 7 (2–159) | 7 (1–262) | 0.77 |
|
| |||
| MM related laboratory values before auto-HCT | |||
| Hemoglobin (g/dl) | 10.4 (8–15) | 11 (7–17) | 0.08 |
| Lactate dehydrogenase, unit/l | 585 (301–1768) | 535 (7–2748) | 0.12 |
| Calcium, mg/dl | 8.9 (7–11) | 9 (7–11) | 0.07 |
| Creatinine, mg/dl | 0.9 (0.4–5) | 0.9 (0.4–13) | 0.37 |
| Beta-2-microgloobulin, mg/l | 2.9 (1–23) | 2.5 (1–40) | 0.24 |
| Immunoglobin G, mg/dl | 524 (132–6970) | 789 (98–7700) | 0.84 |
| Immunoglobin A, mg/dl | 34.5 (6–5238) | 69 (6–3620) | 0.53 |
| Immunoglobin M, mg/dl | 21.5 (4–1190) | 38 (4–3440) | 0.49 |
| Bence-Jones protein, mg/dl | 0.09 (0–7) | 0.04 (0–6) | 0.002 |
| Serum M-protein, g/dl | 0.5 (0–5) | 0.6 (0–6) | 0.38 |
|
| |||
| Conditioning regimen | |||
| Melphalan 200mg/m2 | 47 (64) | 354 (59) | 0.5 |
| Others1 | 27 (36) | 242 (41) | |
|
| |||
| Engraftment (days) | |||
| Neutrophil count >0.5 x 109/l, days | 10 (9–12) | 10 (0–20) | 0.57 |
| Platelet count >20 x 109/l, days | 11 (0–20) | 11 (0–70) | 0.42 |
|
| |||
| Response to auto-HCT1 | |||
| Stringent complete remission | 5 (7) | 70 (11.8) | |
| Complete remission | 6 (8) | 110 (18.6) | |
| Very good partial remission | 18 (24) | 163 (27.6) | <0.001 |
| Partial remission | 26 (35) | 154 (26) | |
| Stable disease | 8 (11) | 72 (12) | |
| Progressive disease | 11 (15) | 22 (4) | |
Other conditioning regimens: busulfan and melphalan; melphalan, arsenic trioxide and bortezomib; melphalan and arsenic trioxide; and melphalan and holmium.
Engraftment
The median numbers of infused CD34+ cells/kg body weight were 4.46 x 106 cells/kg in the high-risk group and 4.50 x 106 cells/kg in the standard-risk group (p=0.57). The median times to neutrophil and platelet engraftment were 10 (9–12) days and 11 (0–20) days, respectively (p=0.57), in the high-risk group and 10 (0–20) days and 11 (0–70) days, respectively, in the standard-risk group (p=0.42).
Response to auto-HCT and treatment-related mortality
Response rate was assessed at 3 months after the auto-HCT. The overall response rate for high-risk MM patients was 74% (sCR in 7%, CR in 8%, VGPR in 24%, and PR in 35%), which was significantly lower than in the standard-risk group’s response rate of 85% (sCR in 11.8%, CR in 18.6%, VGPR in 27.6%, and PR in 26%); p<0.001 (Table II). 100-day TRM was similar in both groups, with one death (1.4%) in the high-risk group and eight deaths (1.3%) in the standard-risk group. In the high-risk group, univariate analysis showed that the factors of undergoing auto-HCT during the first remission, a pre-transplant plasma cell percentage of <10% in the bone marrow aspirate, and achieving at least a PR at the time of auto-HCT were each significantly associated with a response of ≥VGPR after auto-HCT. However, on multivariate analysis, undergoing auto-HCT during the first remission was the only factor independently associated with ≥VGPR after auto-HCT in the high-risk patients. For the standard-risk patients, all the above-mentioned factors remained independent, significant predictors of ≥VGPR.
Survival
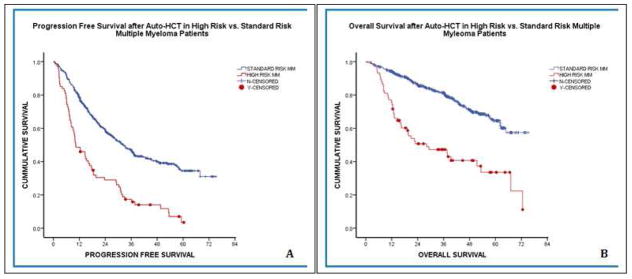
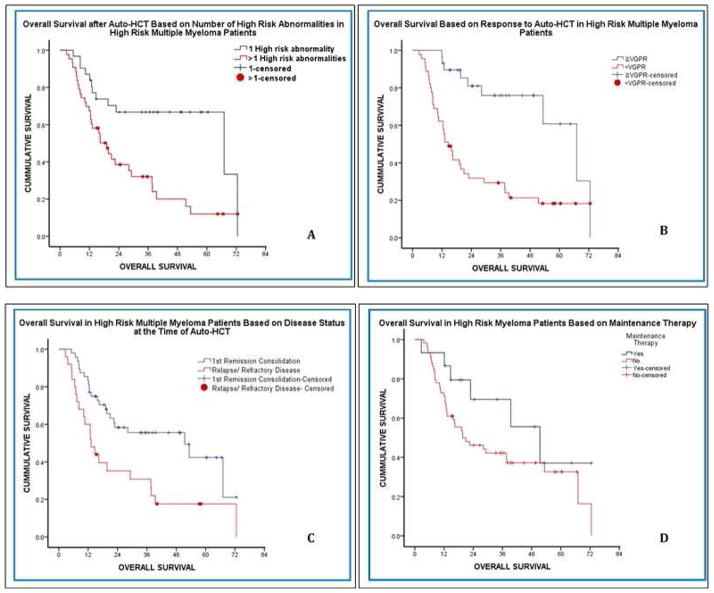
The median follow-up was 39.8 months after auto-HCT for all patients, and the high-risk and standard-risk groups significantly differed in median PFS (10.3 vs. 32.4 months, respectively; p<0.001) and OS (28 months vs. not reached, respectively; p<0.001) after auto-HCT (Figure 1). In the high-risk MM patients, univariate analysis and multivariate Cox regression analysis were performed to identify independent variables associated with improved PFS and OS (Table III). The univariate analysis revealed that having only one high-risk abnormality and attaining ≥VGPR after auto-HCT were each significantly associated with prolonged PFS and OS. In addition, ≥PR with induction therapy and performing auto-HCT during the first remission were each significantly associated with longer OS (but not PFS) (Figure 2). On multivariate analysis, having only one high-risk abnormality (p=0.006) and achieving ≥VGPR after auto-HCT (p=0.003) were independently associated with longer PFS. The variables also maintained independent significance for longer OS; having only one high-risk abnormality (p=0.001), and achieving ≥VGPR after auto-HCT (p=0.004).
Figure 1.
PFS (A) and OS (B) in high-risk versus standard-risk myeloma patients after auto-HCT.
Table III.
Factors influencing outcomes after auto-HCT among high-risk MM patients on univariate analysis.
| Factor | n (%) | Median PFS, (months) | p-value | Median OS (months) | p-value |
|---|---|---|---|---|---|
| Number of high-risk Abnormalities | |||||
| 1 | 30 (42) | 17.73 | 0.001 | 67.07 | 0.003 |
| >1 | 44 (58) | 7.67 | 19.13 | ||
|
| |||||
| Induction with bortezomib | |||||
| Yes | 53 (72) | 10.07 | 0.217 | 22.9 | 0.518 |
| No | 21 (28) | 15.4 | 37.73 | ||
|
| |||||
| Response before auto-HCT | |||||
| ≥PR | 56 (76) | 12.37 | 0.226 | 37.6 | 0.01 |
| <PR | 17 (23) | 8.23 | 12.87 | ||
|
| |||||
| Age at auto-HCT | |||||
| <65 | 61 (82) | 12.37 | 0.343 | 28.17 | 0.342 |
| >65 | 13 (18) | 9.3 | 13.13 | ||
|
| |||||
| Disease Status at Time of auto-HCT | |||||
| First remission | 48 (65) | 12.43 | 0.064 | 51.43 | 0.004 |
| Relapsed/refractory | 25 (34) | 9.3 | 13.13 | ||
|
| |||||
| Bone marrow plasma cell percentage before auto-HCT | |||||
| <10% | 41 (55) | 10.37 | 0.371 | 28.16 | 0.316 |
| >10% | 24 (32) | 8.23 | 14.67 | ||
|
| |||||
| Response to auto-HCT | |||||
| ≥VGPR | 29 (39) | 29 | 0.001 | 67.07 | <0.001 |
| <VGPR | 45 (61) | 7.67 | 14.67 | ||
|
| |||||
| Maintenance therapy after auto-HCT | |||||
| Yes | 15 (20) | 17.67 | 0.364 | 51.43 | 0.121 |
| No | 59 (80) | 9.8 | 19.7 | ||
Figure 2.
OS among high-risk MM patients by (A) number of high-risk abnormalities, (B) depth of response to auto-HCT, (C) response prior to auto-HCT, and (D) maintenance treatment after auto-HCT.
The use of bortezomib as part of induction treatment did not significantly affect PFS or OS after auto-HCT. In contrast, the use of maintenance chemotherapy compared with no maintenance chemotherapy after auto-HCT in high-risk MM patients showed a trend toward improving PFS (18 vs. 10 months; p=0.36) and OS (51 vs. 20 months; p=0.12), although these differences were not statistically significant (Figure 2).
Discussion
Our study confirms previous reports that high-risk MM patients respond poorly to induction chemotherapy treatment and to auto-HCT, leading to worse PFS and OS than in standard-risk MM patients.1–7 In addition, our multivariate analysis showed that survival outcomes in high-risk MM patients are superior in those with only one high-risk chromosomal abnormality and those who achieve at least a very good partial response with auto-HCT.
Several individual high-risk chromosomal abnormalities have been identified in the literature, and MM patients often harbor more than one high-risk abnormality.2,8,15 Neben et al. found that del(13q14) and +1(q21) were strongly correlated with the presence of t(4;14) or del(17p13) in myeloma cells.15 The distribution of high-risk abnormalities in our high-risk MM population also showed that these frequently occurred concurrently and, furthermore, that concurrent abnormalities were associated with worse survival. Indeed, Chang et al. stratified MM into low-risk (52%), intermediate-risk (33%), and high-risk (15%) categories according to the number of FISH-identified high-risk genetic abnormalities and reported inferior survival for the latter two groups, similar to the findings in our population.2
The survival after auto-HCT was improved if patients achieved ≥VGPR after auto-HCT. In our high-risk MM patients, VGPR after auto-HCT was achieved more frequently when auto-HCT was performed in the first remission. Achieving VGPR was also a major prognostic indicator of better survival in the IFM 2005-01 Phase 3 Trial.16 As has been seen in several studies, our data show that the maximum benefit from auto-HCT is derived when the transplantation is performed in the first remission rather than when the disease is relapsed or refractory.
The use of bortezomib in induction and maintenance therapy has been shown in some studies to decrease the adverse risk of t(4;14) as well as del 17, with conflicting results among studies.11,17–20 We did not notice a substantial benefit from bortezomib in our high-risk group, but only five patients in our cohort had t(4;14), precluding any definitive conclusions about that mutation. Another reason for this apparent lack of benefit from novel agents may be that the high-risk patients need ongoing therapy in the form of induction chemotherapy, high-dose therapy, and consolidation chemotherapy, followed by long-term maintenance therapy. Maintenance therapy with lenalidomide after auto-HCT has also been shown to prolong PFS in multiple myeloma patients including those with high-risk cytogenetic abnormalities. 21 In our high-risk group, most patients (80%) did not receive maintenance therapy after auto-HCT, probably because our data go back to 2005, when post–auto-HCT maintenance therapy was not a standard approach. Despite this, we observed that the 15 high-risk patients who received maintenance therapy had longer median OS than the other high-risk patients (51.4 vs. 19.7 months; p=0.121), although this difference was not statistically significant, possibly owing to the low number of patients who received maintenance treatment. Nevertheless, it seems appropriate that eligible high-risk MM patients be aggressively treated with induction and high-dose therapy followed by long-term maintenance therapy to reduce the risk of relapse.
The frequency of high-risk patients in our cohort was less than that reported in other studies.11 A possible explanation could be that some patients lacked FISH results; however, all patients had cytogenetic data available for analysis, and at least two metaphases had to be abnormal to confer high-risk status.
Conclusions
We acknowledge the inherent limitations of our retrospective review, including missing data, a lack of appropriate controls, and patient heterogeneity. Furthermore, the definition of high-risk MM based on chromosomal abnormalities continues to evolve as more sensitive detection techniques and more effective therapeutic agents become available. However, our data does emphasize that the high-risk MM patients are characterized by a higher bone marrow plasma cell burden, concurrence of high-risk chromosomal abnormalities, and poor responses to high-dose chemotherapy and auto-HCT as compared with standard-risk myeloma MM patients. In high-risk MM patients, the presence of only one high-risk cytogenetic abnormality and achieving ≥VGPR after auto-HCT are each independently associated with improved survival. Prospective clinical trials are needed to optimize the timing, sequence, and duration of therapy with novel agents, auto-HCT, and possibly allo-HCT in this high-risk patient population.
Clinical Practice Points.
Outcomes among cytogenetically defined high-risk myeloma patients remain poor even in the era of novel agents.
Among high-risk MM patients, survival outcomes are superior in those with only one high-risk chromosomal abnormality compared to the patients with more then one high-risk chromosomal abnormalities.
Among high-risk MM patients, achievement of at least very good partial response with auto-HCT is associated with superior survival.
Maintenance therapy after auto-HCT may prolong survival among high-risk MM patients and its role should be further researched in larger scale studies.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672.
Footnotes
Conflict of Interest statement: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007 Apr 15;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 2.Chang H, Qi XY, Samiee S, et al. Genetic risk identifies multiple myeloma patients who do not benefit from autologous stem cell transplantation. Bone marrow transplantation. 2005 Nov;36(9):793–796. doi: 10.1038/sj.bmt.1705131. [DOI] [PubMed] [Google Scholar]
- 3.Inamoto Y, Kurahashi S, Imahashi N, et al. Combinations of cytogenetics and international scoring system can predict poor prognosis in multiple myeloma after high-dose chemotherapy and autologous stem cell transplantation. American journal of hematology. 2009 May;84(5):283–286. doi: 10.1002/ajh.21390. [DOI] [PubMed] [Google Scholar]
- 4.Lonial S. Presentation and risk stratification--improving prognosis for patients with multiple myeloma. Cancer treatment reviews. 2010 May;36( Suppl 2):S12–17. doi: 10.1016/S0305-7372(10)70007-4. [DOI] [PubMed] [Google Scholar]
- 5.Munshi NC, Anderson KC, Bergsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011 May 5;117(18):4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007 Mar;21(3):529–534. doi: 10.1038/sj.leu.2404516. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer genetics. 2011 Jan;204(1):3–12. doi: 10.1016/j.cancergencyto.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Shaughnessy JD, Jr, Barlogie B. Using genomics to identify high-risk myeloma after autologous stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006 Jan;12(1 Suppl 1):77–80. doi: 10.1016/j.bbmt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Van Wier S, Braggio E, Baker A, et al. Hypodiploid multiple myeloma is characterized by more aggressive molecular markers than non-hyperdiploid multiple myeloma. Haematologica. 2013 Oct;98(10):1586–1592. doi: 10.3324/haematol.2012.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.San-Miguel J, Mateos MV, Gutierrez NC. Risk stratification in the era of novel therapies. Cancer journal. 2009 Nov-Dec;15(6):457–464. doi: 10.1097/PPO.0b013e3181c51efa. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi G, Richardson PG, Anderson KC. Best treatment strategies in high-risk multiple myeloma: navigating a gray area. J Clin Oncol. 2014 Jul 10;32(20):2125–2132. doi: 10.1200/JCO.2014.55.7900. [DOI] [PubMed] [Google Scholar]
- 12.Kroger N, Badbaran A, Zabelina T, et al. Impact of high-risk cytogenetics and achievement of molecular remission on long-term freedom from disease after autologous-allogeneic tandem transplantation in patients with multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Mar;19(3):398–404. doi: 10.1016/j.bbmt.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV. Multiple myeloma: 2013 update on diagnosis, risk-stratification, and management. American journal of hematology. 2013 Mar;88(3):226–235. doi: 10.1002/ajh.23390. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 15.Neben K, Jauch A, Bertsch U, et al. Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica. 2010 Jul;95(7):1150–1157. doi: 10.3324/haematol.2009.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harousseau J-L, Avet-Loiseau H, Attal M, et al. High Complete and Very Good Partial Response Rates with Bortezomib--Dexamethasone as Induction Prior to ASCT in Newly Diagnosed Patients with High-Risk Myeloma: Results of the IFM2005-01 Phase 3 Trial. ASH Annual Meeting Abstracts; November 20, 2009; p. 353. [Google Scholar]
- 17.Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Oct 20;28(30):4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 18.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012 Jan 26;119(4):940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 19.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012 Jul 5;120(1):9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 20.Sonneveld P, Goldschmidt H, Rosinol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Sep 10;31(26):3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 21.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012 May 10;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]