Abstract
Objective
To review the candidate gene and genome-wide association studies relevant to bronchopulmonary dysplasia, and discuss the emerging understanding of the complexities involved in genetic predisposition to bronchopulmonary dysplasia and its outcomes.
Findings
Genetic factors contribute much of the variance in risk for BPD. Studies to date evaluating single or a few candidate genes have not been successful in yielding results that are replicated in GWAS, perhaps due to more stringent p-value thresholds. GWAS studies have identified only a single gene (SPOCK2) at genome-wide significance in a European White and African cohort, which was not replicated in two North American studies. Pathway gene set analysis in a North American cohort confirmed involvement of known pathways of lung development and repair (e.g. CD44, phosphorus oxygen lyase activity) and indicated novel molecules and pathways (e.g. adenosine deaminase, targets of miR-219) involved in genetic predisposition to BPD. The genetic basis of severe BPD is different from that of mild/moderate BPD, and the variants/pathways associated with BPD vary by race/ethnicity. A pilot study of whole exome sequencing identified hundreds of genes of interest, and indicated the overall feasibility as well as complexity of this approach.
Conclusion
Better phenotyping of BPD by severity and pathophysiology, and careful analysis of race/ethnicity is required to gain a better understanding of the genetic basis of BPD. Future translational studies are required for the identification of potential genetic predispositions (rare variants and dysregulated pathways) by next generation sequencing methods in individual infants (personalized genomics).
Introduction
Extremely preterm infants are at high risk of mortality and morbidity. One of the most common morbidities is bronchopulmonary dysplasia (BPD), affecting more than two-thirds of a recent cohort of extremely preterm infants in the United States.1 BPD also accounts for much of the late (>60 days) mortality in extremely preterm infants.2 Even after controlling for birth weight, gestational age, and sociodemographic characteristics, a diagnosis of BPD is associated with a large increase in direct costs for the initial neonatal intensive care unit hospitalization.3 In a recent large cohort, 28% of extremely preterm infants were discharged home on oxygen,4 suggesting that the morbidity and costs of BPD therapy extend for many infants well beyond the initial hospital stay. The genetics of BPD has been reviewed in Seminars in Perinatology by Bhandari and Gruen in 20065 and subsequently by Shaw and O’Brodovich in 2013.6 This review is therefore an update on recent advances in the field, with a brief overview of the evidence already covered in the previous reviews.
The search for genetic origins of BPD has been complicated by several factors. One issue is that the diagnostic criteria for BPD have changed many times since Northway et al.7 first described BPD in 1967 as a pulmonary disease following mechanical ventilation of infants with respiratory distress syndrome, characterized by airway injury, inflammation, and lung fibrosis. In 1979, William Tooley defined BPD as when an infant at 30 days of age has any radiologic abnormality of the lung parenchyma plus at least one of the following: (1) an O2 tension in arterial blood breathing room air of 60 torr or less: (2) CO2 tension in arterial blood of more than 45 torr; and/or/(3) O2 dependence (i.e., requires an FiO2 of more than 0.21).8 In 1988, Shennan et al.9 observed that the need for oxygen at 28 days became increasingly less useful as gestational age decreased, but irrespective of gestational age at birth, the requirement for additional oxygen at 36 weeks' corrected postnatal gestational age was a better predictor of abnormal outcome. A major limitation of these definitions is the wide-ranging criteria for oxygen “requirement” used by different clinicians. A workshop on BPD organized by the National Institute of Child Health and Human Development (NICHD), the NHLBI, and the Office of Rare Diseases (ORD) developed diagnostic criteria for BPD based on gestational age (< 32 weeks vs. >32 weeks) and severity (Mild, Moderate, or Severe BPD, based on oxygen supplementation at 28 days of age and 36 weeks postmenstrual age).10 Subsequently, Walsh et al.11 described a “physiologic definition” of BPD by a standardized oxygen saturation monitoring at 36 weeks corrected age that was highly reliable and improved the precision of diagnosis of BPD. Currently, the NIH workshop definition and the physiologic definition are the most used. As these multiple definitions have evolved over time, and considering the fact that the infants who develop BPD in the current era (mostly 22–26 weeks gestational age at birth) are much more immature than the infants at highest risk of BPD in the 1970s (30–34 week infants) and 1990s (26–30 week infants), it is safe to state that the infants defined as having “BPD” in the 1970s or 1980s were very different from those with BPD in recent years. The pathology of BPD has also changed over the years, with the lungs in “old” BPD being characterized by alternating areas of atelectasis and overinflation, marked airway epithelial hyperplasia and squamous metaplasia, airway smooth muscle hyperplasia, extensive fibrosis and pulmonary hypertension, and decreased internal surface area and alveoli, while the histology of the current “new” BPD having fewer and larger simplified alveoli, less airway lesions, variable airway smooth muscle hyperplasia and interstitial fibrosis, fewer and dysmorphic capillaries, and less severe arterial remodeling.12,13
Another issue is that the definition of BPD (oxygen requirement) being an operational definition does not indicate the diverse underlying pulmonary pathology or the variable magnitude of pathology between different preterm infants. The magnitude of inhibition of alveolar development,12 the extent of lung fibrosis (and resulting changes in lung compliance),7 the severity of lung vascular remodeling (and resulting pulmonary hypertension),14,15 and the degree of trachea-bronchomalacia16 vary from one infant to another, and perhaps even in the same infant over time, as BPD is a disorder superimposed on normal lung development. Yet another issue that we will discuss subsequently is that severe BPD is different from mild or moderate BPD in its genetic basis, and that biologic pathways associated with BPD risk are very different in infants of different race/ethnicity.17 Therefore, the single diagnosis “BPD” has been applied using varying criteria to infants of varying gestational age and illness severity, varying lung airway/vascular/parenchymal pathology, and of varying genetic background. It is likely that “BPD” is not a single entity, nor even a spectrum of disease resulting from a single pathophysiologic process, but the is the result of multiple pathophysiologic processes leading to varying magnitudes of inhibited alveolar septation, lung fibrosis, and abnormal vascular development and remodeling in preterm infants at the saccular or early alveolar stage of lung development. As may be expected, the genetic basis of each of these sub-phenotypes of BPD is likely to vary, depending upon the underlying clinical variables and pathophysiology.
Identification of the genetic basis of BPD
Familial and genetic susceptibility to BPD (defined as oxygen requirement at 36 weeks PMA with compatible radiographic findings) was evaluated in a multicenter retrospective study of twin pairs born at <32 weeks of gestation by Bhandari et al.18 After controlling for the effects of covariates, the twin data showed that 65.2% (95% CI: 53–79%, p<0.001) of the variance in liability for BPD could be accounted for by genetic and shared environmental factors. The genetic component was estimated from the correlation between monozygotic twins beyond that of dizygotic twins, and the observed concordance in monozygotic twins was significantly higher than the expected concordance. After controlling for covariates, genetic factors were considered to account for as much as 53% (95% CI 16–89%, p=0.004) of the variance in liability for BPD.18 Lavoie et al.19 evaluated the heritability of BPD defined according to the NIH consensus definition using clinical data from 318 twin pairs of known zygosity <30 weeks of gestation. Model-fitting analyses indicated that genetic effects accounted for 79% of the observed variance in moderate to severe BPD susceptibility.19 The genetic factors that contribute to BPD susceptibility (or conversely, to the resistance to development of BPD in an otherwise susceptible extremely preterm infant) have been the subject of much investigation in recent years, when the technology has progressed sufficiently to (a) permit the survival of preterm infants despite severe lung disease and progress to BPD, (b) allow evaluation of single nucleotide polymorphisms (SNPs) in genes at sufficiently low cost and with rapid throughput.
Targeted evaluation of SNPs (candidate gene studies)
Several investigators have attempted to identify associations with BPD of molecules or pathways that are known to be associated with lung development, maturation, inflammation, fibrosis, angiogenesis, oxidative stress, or tissue injury and repair. In general, many of these studies have focused on SNPs in relatively few genes or pathways, and have had limited sample sizes from one or a few centers. A limitation of these approaches is that we currently do not have a good understanding of the regulation of the transition from the saccular to the alveolar stage of lung development. Hence, we do not know all the molecules or pathways of critical importance to distal lung development (and therefore to BPD pathogenesis) that can be targeted for analysis. The SNPs associated with BPD in candidate gene studies have not been corroborated in GWAS, perhaps because most candidate gene studies have used standard thresholds (p<0.05) while GWAS thresholds are generally much stricter p<10−8.
The role of surfactant proteins in surfactant biology is well known, and it is not surprising that multiple surfactant protein loci have been found to be associated with BPD.20 Polymorphisms in surfactant protein B (SP-B) have been associated with BPD in multiple studies, primarily the SP-B i4 deletion variant allele.21,22 Polymorphisms in surfactant protein C (SP-C) have been shown to be associated with RDS and very premature birth, though not with BPD.23 Ryckman et al.24 found associations of surfactant protein D (SFTPD) with RDS and angiotensin-converting enzyme (ACE) with BPD. Polymorphisms in SFTPD have also been found associated with respiratory distress and requirement for respiratory support by Sorensen et al.25 and Hilgendorff et al.26, though not with BPD.
The Toll-like receptor (TLR) family of proteins is important in pathogen recognition and clearance. Sampath et al.27 evaluated a cohort of 289 infants in whom 66 developed BPD and 32 developed severe BPD. Nine TLR pathway single-nucleotide polymorphisms were genotyped, and in regression models that controlled for potential confounders, the TIRAP (g.2054C > T) variant was associated with BPD, and the TLR5 (g.1174C > T) variant that encodes a stop codon was associated with severe BPD.27 Lavoie et al.28 evaluated whether the TLR4 polymorphisms Asp299Gly (rs4986790) and Thr399Ile (rs4986791) are independently associated with BPD, and found that the TLR4-299 heterozygous genotype was significantly under-represented in a Canadian cohort of infants without BPD (1.6% of infants versus 12% in infants with severe BPD) after adjusting for covariates (p = 0.014), but not in a Finnish cohort. TLR6 SNP rs5743827 was associated with a decreased risk for BPD (OR 0.54 [0.31–0.95]).29
As infection and inflammation are considered major contributors to BPD, multiple cytokine SNPs have also been evaluated. Mailaparambil et al.30 assessed 37 polymorphisms within 16 genes in 155 infants <28w gestation, of whom 47 developed moderate/severe BPD. BPD was more strongly associated with clinical factors such as birth weight and earlier gestational age, than with genetic polymorphisms. Polymorphisms in TNF-α (rs1799724), TLR10 (rs11096955) and VEGF (rs699947) were associated with BPD.30 Kazzi et al.31 and Elhawary et al.32 found that the −238G > A polymorphism was associated with risk of BPD. However, Strassberg et al.33 did not find any association between BPD and TNF-α. Huusko et al.34 also evaluated five IL6, nine IL6R, four IL6ST, one IL10, two TNF-α, and 23 NR3C1 SNPs in very preterm infants from Finland and Canada, and did not find any of the analyzed SNPs to be associated with BPD. Huusko et al.35 in a different publication and using more subjects, noted that SNPs in Kit ligand (KITLG) were associated with predisposition to BPD. Floros et al.36 conducted a case-control SNP association study of candidate genes (n=601) or 6,324 SNPs in 1,091 prematurely born infants with gestational age <35 weeks, with or without neonatal lung disease including BPD (O2 at 28 days) and found two significant SNPs, rs3771150 (interleukin 18 receptor accessory protein or IL-18RAP) and rs3771171 (interleukin 18 receptor 1 or IL-18R1), in African Americans but not Caucasians with BPD. On a related note, Krueger et al.37 found that polymorphisms in IL-18 were not associated with BPD.
Bokodi et al.38 found that the IFN-γ (+874) A allele was over-represented in LBW infants, while carriers of the IFN-γ (+874) T allele were protected against BPD, suggesting that carrier state of the IFN-γ (+874) A allele may be an increased risk for premature birth and BPD.38 Prencipe et al.39 found that macrophage migration inhibitory factor (MIF) expression was increased in lung and serum of preterm infants and the high producing MIF −173*C allele was associated with a lower incidence of BPD (OR 0.2). Polymorphisms in mannose binding lectin (MBL) have been associated with risk of BPD in studies by Cakmak et al.40, Hilgendorff et al.41 and Capoluongo et al.42
Oxidative stress is considered to contribute to lung injury and BPD. Therefore, polymorphisms in antioxidant enzymes have been evaluated by many investigators. Poggi et al.43 and Giusti et al.44 showed that SNPs in superoxide dismutase (SOD) 2 and SOD3 were associated with BPD risk. Sampath et al.45 genotyped variants in the SOD2, NFE2L2, GCLC, GSTP1, HMOX1, and NQO1 genes in a cohort of 659 infants, of whom 284 infants had BPD and 135 had severe BPD. The hypomorphic NQO1 SNP (rs1800566) in homozygous state was associated with increased BPD, while NFE2L2 (nuclear factor erythroid-2 related factor-2) SNP (rs6721961) was associated with decreased severe BPD.45 The null genotypes of Glutathione S-transferases GSTM1 and GSTT1 have been associated with BPD in the Chinese Han population.46
Vitamin D and its receptor (VDR) are important in lung development, and VDR Fok 1 polymorphisms have been associated with increased risk of BPD.47 Many peptide growth factors, such as the fibroblast growth factor (FGF), are also key players in normal lung development. A SNP (rs1966265) in FGF receptor 4 (FGFR4) has been associated with both RDS and BPD.48 Vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-β) signaling are necessary for normal lung development. Kwinta et al.49 evaluated selected polymorphisms in VEGF, TGF-β, IGF-1, and MTHFR. Multivariate analysis indicated that the T allele in the VEGF −460T>C polymorphism increased the risk of BPD by 9% (95%CI: 2–14%) above the baseline risk established for given gestational age, length of oxygen therapy, and sex.49 Fujioka found that the VEGF −634C > G polymorphism was also an independent risk factor for BPD.50
Extracellular matrix remodeling requires the activity of matrix metalloproteinases (MMPs), and Hadchouel et al. evaluated SNPs in MMP2, MMP14, and MMP16. After adjustment for birth weight and ethnicity, the TT genotype of MMP16 C/T (rs2664352) and the GG genotype of MMP16 A/G (rs2664349) were protective against BPD.51
Genome-wide association studies
To date, there have been three genome-wide association studies (GWAS) for BPD – the ones by Hadchouel et al.52, Wang et al.53, and Ambalavanan et al.17.
Hadchouel et al.52 conducted a GWAS of a multicenter population, combining pooling-based genome-wide case-control analysis, fine-scale mapping of gene, and animal experiments. 418 infants with a gestational age <28 weeks were prospectively included from three French NICUs, and BPD was defined using the physiologic BPD definition. The population was divided into two discovery series and an independent replication series. A GWAS was done using a DNA pooling strategy in the two discovery series. SNPs associated with BPD were validated by individual genotyping and the validated SNPs were then genotyped in two different replication populations: the internal replication population and an external population composed of 213 Finnish neonates < 30 weeks of gestation. In this study, the 418 French neonates had a mean gestational age of 26.4+0.1w and birth weight of 845+9g, and 22% were diagnosed with BPD. Birth weight, male sex, need for a second dose of surfactant, PDA, and postnatal sepsis were significantly associated with BPD, and were used as adjustment covariates in genetic analyses. Two methods (allelic frequency differences and combined Z-score analysis) were used to select SNPs for further individual genotyping. SPOCK2 (Entrez Gene ID 9806) was the only gene identified in both populations using both selection methods. rs1245560 (p=1.66× 10−7) was the only SNP showing similar MAF in white and African control pools (0.463 and 0.497, respectively), and was validated in the two discovery series and in the independent replication set. The C allele was significantly associated with the risk of BPD in both white and African series, and in the replication sample as well. The CC genotype was a risk factor for BPD even after adjustment for perinatal factors with OR 2.96 (95% CI 1.37–6.40) for white subjects and 4.87 (95% CI 1.88–12.63) for African subjects, respectively.52 The investigators also evaluated SPOCK2 during alveolarization in the newborn rat, after air or hyperoxia exposure, and found that SPOCK2 mRNA levels increased during alveolarization. After exposure to hyperoxia, SPOCK2 expression increased relative to air-exposed controls. Immunofluorescence studies localized SPOCK2 to the extracellular matrix of the lung.
Wang et al.53 conducted a discovery GWAS on 1726 very low birth weight infants (birth weight <1500g, gestational age 25w to 29w 6d) who had a minimum of 3 days of mechanical ventilation and were in the hospital at 36 weeks’ PMA. Moderate/severe BPD (n=899) were those who required continuous supplemental oxygen, while controls (n=827) were those on air. 795 comparable infants (371 cases and 424 controls) served as a replication population. DNA isolated from newborn screening bloodspots was used for the GWAS. In this study, no SNPs were associated with BPD at the genome-wide significance level (5 × 10−8) and none of the SNPs evaluated in previous studies reached replication-wide significance. Pathway analyses that were done were also not informative.53 Of interest, the investigators further evaluated the GWAS discovery cohort and identified 259 “super-controls” (infants breathing air at both 28 days’ age and 36w PMA) and 568 infants with mild BPD (O2 at 28 days but air at 36w PMA). However, no SNPs were found at the genome-wide significance threshold associated with moderate-severe BPD relative to either of these two groups (“super-controls” or mild BPD).53 15 SNPs were identified with p<10−5 in either the discovery stage (e.g. rs8089528, rs118078182, rs12571250) or joint analyses (e.g. rs556493, rs12356475). This study suggests that the heritability of BPD is probably not attributable to one or a few variants in genes. A possible reason that the findings from previous studies could not be replicated is because the majority of infants in this study were of Mexican-Hispanic origin, and Caucasian or African-American infants were in relatively small numbers (while Caucasian infants made up the majority of the previous studies).
More recently, Ambalavanan et al.17 used GWAS combined with pathway-based approaches, and integrated these results with gene expression comparing BPD to controls, and a newborn mouse model of hyperoxia exposure simulating BPD. In this study, 751 extremely preterm infants were analyzed, of whom 428 developed BPD or died. A genome-wide scan was conducted on 1.2 million genotyped SNPs, and an additional 7 million imputed SNPs. Genome-wide association and gene set analysis was performed for BPD or death, severe BPD or death, and severe BPD in survivors. No SNPs reached genome-wide significance (p<10−8) although multiple SNPs in adenosine deaminase (ADARB2), CD44, and other genes were just below p<10−6. In the pathway/gene set enrichment analysis, of the approximately 7650 gene sets evaluated, 77 pathways were identified with a false discovery rate (FDR) <0.1 (suggesting about 10% of the pathways are false positives) for pathways associated with BPD or death vs. survivors without BPD. Of these 77, only 3 were shared with Severe BPD or death, or severe BPD in survivors. The top pathway was MIR-219 (http://www.broadinstitute.org/gsea/msigdb/cards/GACAATC,MIR-219.html). The gene set analysis for severe BPD or death vs. survivors without severe BPD identified 123 pathways at FDR <0.1, of which only 3 where shared with BPD/death, but 108 (including the 3 shared with BPD/death) were shared with those involved with severe BPD in survivors, including the top 43 pathways. The top pathway associated with severe BPD or death and survivors with severe BPD was Phosphorus Oxygen Lyase Activity (http://www.broadinstitute.org/gsea/msigdb/cards/PHOSPHORUS_OXYGEN_LYASE_ACTIVITY.html), which consists of adenylate and guanylate cyclases. The gene expression of components of these gene sets were validated using a data set of gene expression in BPD versus controls.54 Evaluation of MIR-219 and CD44 was performed in mouse models, and it was found that expression of MIR-219 and CD44 decreased over the course of alveolar septation and hyperoxia exposure (a murine BPD model) was associated with an increase in MIR-219 and CD44 expression. Expression of MIR-219 and CD44 were also noted to be increased in autopsy sections of human lung, suggesting pathophysiological significance to the gene set and GWAS findings.
One of the main findings of this study is that there is significant overlap in the genetic basis for severe BPD or death and severe BPD in survivors, but mild/moderate BPD probably has a different genetic basis. The major pathway associated with severe BPD/death and in survivors with severe BPD was Phosphorus Oxygen Lyase Activity. This result suggests that modulation of the cGMP pathway (e.g. with inhaled nitric oxide or sildenafil) and cAMP pathways (e.g. with caffeine or prostacyclin) may be specifically relevant to severe BPD, and perhaps less important in mild/moderate BPD. Clinical studies have shown benefit regarding BPD with use of caffeine,55,56 but inhaled nitric oxide has not been shown to reduce BPD.57,58 It may be important to analyze specifically for severe BPD, as the lack of observed effect may be due to “any BPD” being evaluated, which does not seem to be dependent upon Phosphorus Oxygen Lyase Activity.
The major finding was of marked differences in pathways by race/ethnicity indicates that although the clinical phenotype of BPD may be similar, the underlying genetic predisposition may differ significantly. In this study, for Severe BPD/death, the p-values/FDR were 4.29E-07/ 0.000917 for Black, 1.34E-06/ 2.44E-06 for Hispanic, and 0.08125/ 0.09776 for White. The differences in race/ethnicity of the population being studied may be one reason that the results of the three GWAS studies17,52,53 are not similar. This finding also indicates that therapies may need to be targeted at pathways found to be dysregulated, and therefore suggests a role of “personalized genomics” in BPD.
Overall, while the twin studies indicate that the majority of the variance in risk of BPD is genetically determined, the GWAS studies explain only a small proportion of the heritability. The use of pathway analysis enables the detection of variants across multiple genes in the same pathway. Pathway-based approaches consider multiple variants in the same biological pathway and complement the single marker approach and provide understanding of GWAS data in many human diseases.59,60 However, GWAS methods may miss other heritable processes, such as epigenetic processes (e.g DNA methylation), copy number variation, interactions among many loci, uncommon variants (that may not be included on the chip) etc. It is possible that rare variants with relatively large effects on risk may contribute to BPD.61
Exome sequencing
Next generation sequencing (NGS) technology has become increasingly affordable, and enables the rapid identification of new or rare variants of potential functional and pathological relevance. NGS may be either whole genome sequencing (WGS) or whole exome sequencing (WES). WGS allows evaluation of single nucleotide variants, indels, structural variants, and copy number variants in both protein coding sequences as well as non-coding sequences. WES on the other hand is targeted to protein coding regions, which represent only 1–2% of the genome. WES is therefore cheaper than WGS, although the cost differential is not as much as might be anticipated.
Carrera et al.62 performed whole exome sequencing on 26 Italian infants of Caucasian origin with severe BPD, selected from a larger prospective cohort of 366 premature infants from 12 NICUs. Overall, 3369 novel variants were identified (1524 potentially damaging), with a median of 400 variations per sample (100 with a strong impact on protein structure and function). 745 potentially interesting genes were identified. To narrow down the list of genes, data analysis was focused on genes previously associated with BPD susceptibility and to new candidates in related pathways. The top candidate genes were NOS2 (inducible nitric oxide synthase), MMP1, CRP (C-reactive protein), LBP (lipopolysaccharide binding protein), and the TLR family. Novel mis-sense mutations in ATP-binding cassette 3 (ABCA3) gene were also found in 3 patients, suggesting that ABCA3 may require further investigation in BPD.62 This pilot study is encouraging, as it suggests that NGS techniques may help investigate the genetic background of BPD. Studies of this type identify multiple potential candidate genes that will require additional functional studies as well as genetic analysis to determine their relevance and importance to lung development and pathogenesis of BPD.
In conclusion
Twin studies indicate genetic factors contribute much of the variance in risk for BPD. Most studies to date evaluating single or a few candidate genes have generally not been successful in yielding results that are replicated in other cohorts. GWAS studies have identified only a single gene (SPOCK2) at genome-wide significance in a European White and African cohort,52 which was not replicated in two North American studies.17,53 Pathway gene set analysis in a North American cohort confirmed involvement of known pathways of lung development and repair (e.g. CD44, phosphorus oxygen lyase activity) and indicated novel molecules and pathways (e.g. adenosine deaminase, targets of miR-219) involved in genetic predisposition to BPD.17 A pilot study of whole exome sequencing identified hundreds of genes of interest, and indicated the overall feasibility as well as complexity of this approach.
Better phenotyping of BPD by severity (severe vs. moderate; gradations within severe e.g. on mechanical ventilation at 36w PMA vs. nasal cannula or CPAP at 36w PMA) and pathophysiology (e.g. airway malacia vs. pulmonary hypertension vs. parenchymal cystic lesions vs. parenchymal fibrosis without cysts), and careful analysis of race/ethnicity (not self-reported but by analysis of genetic markers) is required to gain a better understanding of the genetic basis of BPD. Future translational studies are required for the identification of potential genetic predispositions (rare variants and dysregulated pathways) by NGS methods in individual infants (personalized genomics), and determine techniques for intervention (selective pathway modulation using small molecule agonists or inhibitors) for preventing or treating BPD.
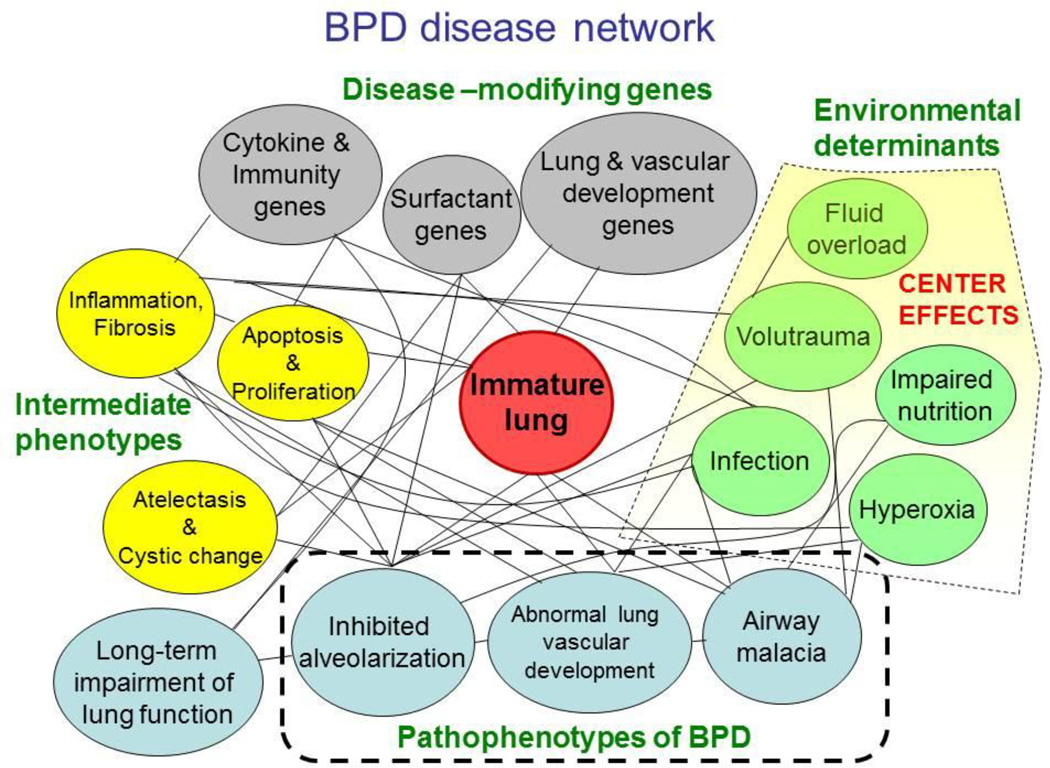
It is however important to remember that despite the strong genetic basis of BPD, environmental influences (mechanical ventilation, hyperoxia, infection, fluid and nutrition status) interact with the underlying genetic basis to either predispose to BPD or prevent it. These environmental influences are probably more easily measured and more readily modifiable. Intercenter variations in BPD rate are large,63,64 and are of greater magnitude than the effect of existing successful therapies (caffeine,56 vitamin A65) for the prevention of BPD. Prediction of BPD risk using just a limited set of clinical variables (gestational age, birth weight, race and ethnicity, sex, respiratory support, and FiO2) is good, increasing from a C statistic of 0.79 on day 1 to a maximum of 0.85 on day 28.66 Addition of genetic data to these models is likely to provide only incremental improvement in prediction. The disease phenotype of BPD is likely a reflection of various pathophysiological processes that interact in a complex network, and the BPD disease network should include not only the genetic factors but environmental factors that interact to induce pathology in the immature lung, leading to intermediate phenotypes and subsequent varied outcomes (Figure). Therefore, while efforts are underway to determine the genetic basis of BPD and develop personalized genomic management, it is important to optimize routine clinical management of very preterm infants in order to reduce the potential for lung injury, improve lung development, and reduce BPD risk.
Figure 1.
Proposed BPD disease network indicating potential disease-modifying genes and environmental factors (which probably account for much of the center effects) that act on the immature lung, leading to intermediate phenotypes of inflammation, fibrosis, apoptosis or proliferation, atelectasis and cystic change. These in turn lead to the pathophenotypes with varying components of inhibited alveolarization, abnormal lung vascular development and pulmonary hypertension, and airway malacia (tracheo- or broncho-malacia). These pathophenotypes in turn result in long-term impairment of lung function, which may also be modified by genetic factors directly.
Acknowledgments
Funding
Supported in part by funding from the National Institutes of Health (NIH) to Dr. Ambalavanan (U01 HL122626; R01 HD067126; R01 HD066982; U10 HD34216)
Disclosure: Dr. Ambalavanan: recent funding from Pfizer, Ikaria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010 Sep;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015 Jan 22;372(4):331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. 2013 Feb;162(2):243–249. e241. doi: 10.1016/j.jpeds.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagatta JM, Clark RH, Brousseau DC, Hoffmann RG, Spitzer AR. Varying patterns of home oxygen use in infants at 23–43 weeks' gestation discharged from United States neonatal intensive care units. J Pediatr. 2013 Oct;163(4):976–982. e972. doi: 10.1016/j.jpeds.2013.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari V, Gruen JR. The genetics of bronchopulmonary dysplasia. Semin Perinatol. 2006 Aug;30(4):185–191. doi: 10.1053/j.semperi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Shaw GM, O'Brodovich HM. Progress in understanding the genetics of bronchopulmonary dysplasia. Semin Perinatol. 2013 Apr;37(2):85–93. doi: 10.1053/j.semperi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967 Feb 16;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 8.Tooley WH. Epidemiology of bronchopulmonary dysplasia. J Pediatr. 1979 Nov;95(5 Pt 2):851–858. doi: 10.1016/s0022-3476(79)80451-5. [DOI] [PubMed] [Google Scholar]
- 9.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988 Oct;82(4):527–532. [PubMed] [Google Scholar]
- 10.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001 Jun;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 11.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003 Sep;23(6):451–456. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 12.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003 Feb;8(1):73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 13.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006 Aug;30(4):179–184. doi: 10.1053/j.semperi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012 Mar;129(3):e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambalavanan N, Mourani P. Pulmonary hypertension in bronchopulmonary dysplasia. Birth defects research. Part A, Clinical and molecular teratology. 2014 Mar;100(3):240–246. doi: 10.1002/bdra.23241. [DOI] [PubMed] [Google Scholar]
- 16.Doull IJ, Mok Q, Tasker RC. Tracheobronchomalacia in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 1997 May;76(3):F203–F205. doi: 10.1136/fn.76.3.f203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambalavanan N, Cotten CM, Page GP, et al. Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr. 2015 Mar;166(3):531–537. e513. doi: 10.1016/j.jpeds.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari V, Bizzarro MJ, Shetty A, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006 Jun;117(6):1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008 Sep;122(3):479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlovic J, Papagaroufalis C, Xanthou M, et al. Genetic variants of surfactant proteins A, B, C, and D in bronchopulmonary dysplasia. Disease markers. 2006;22(5–6):277–291. doi: 10.1155/2006/817805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai BH, Chang LW, Li WB, et al. Association of surfactant protein B gene polymorphisms (C/A-18, C/T1580, intron 4 and A/G9306) and haplotypes with bronchopulmonary dysplasia in chinese han population. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 2013 Jun;33(3):323–328. doi: 10.1007/s11596-013-1118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallman M, Haataja R. Surfactant protein polymorphisms and neonatal lung disease. Semin Perinatol. 2006 Dec;30(6):350–361. doi: 10.1053/j.semperi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Lahti M, Marttila R, Hallman M. Surfactant protein C gene variation in the Finnish population - association with perinatal respiratory disease. European journal of human genetics : EJHG. 2004 Apr;12(4):312–320. doi: 10.1038/sj.ejhg.5201137. [DOI] [PubMed] [Google Scholar]
- 24.Ryckman KK, Dagle JM, Kelsey K, Momany AM, Murray JC. Genetic associations of surfactant protein D and angiotensin-converting enzyme with lung disease in preterm neonates. J Perinatol. 2012 May;32(5):349–355. doi: 10.1038/jp.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen GL, Dahl M, Tan Q, Bendixen C, Holmskov U, Husby S. Surfactant protein-D-encoding gene variant polymorphisms are linked to respiratory outcome in premature infants. J Pediatr. 2014 Oct;165(4):683–689. doi: 10.1016/j.jpeds.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Hilgendorff A, Heidinger K, Bohnert A, et al. Association of polymorphisms in the human surfactant protein-D (SFTPD) gene and postnatal pulmonary adaptation in the preterm infant. Acta Paediatr. 2009 Jan;98(1):112–117. doi: 10.1111/j.1651-2227.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 27.Sampath V, Garland JS, Le M, et al. A TLR5 (g.1174C >T) variant that encodes a stop codon (R392X) is associated with bronchopulmonary dysplasia. Pediatr Pulmonol. 2012 May;47(5):460–468. doi: 10.1002/ppul.21568. [DOI] [PubMed] [Google Scholar]
- 28.Lavoie PM, Ladd M, Hirschfeld AF, et al. Influence of common non-synonymous Toll-like receptor 4 polymorphisms on bronchopulmonary dysplasia and prematurity in human infants. PLoS One. 2012;7(2):e31351. doi: 10.1371/journal.pone.0031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winters AH, Levan TD, Vogel SN, Chesko KL, Pollin TI, Viscardi RM. Single nucleotide polymorphism in toll-like receptor 6 is associated with a decreased risk for ureaplasma respiratory tract colonization and bronchopulmonary dysplasia in preterm infants. The Pediatric infectious disease journal. 2013 Aug;32(8):898–904. doi: 10.1097/INF.0b013e31828fc693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mailaparambil B, Krueger M, Heizmann U, Schlegel K, Heinze J, Heinzmann A. Genetic and epidemiological risk factors in the development of bronchopulmonary dysplasia. Disease markers. 2010;29(1):1–9. doi: 10.3233/DMA-2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazzi SN, Kim UO, Quasney MW, Buhimschi I. Polymorphism of tumor necrosis factor-alpha and risk and severity of bronchopulmonary dysplasia among very low birth weight infants. Pediatrics. 2004 Aug;114(2):e243–e248. doi: 10.1542/peds.114.2.e243. [DOI] [PubMed] [Google Scholar]
- 32.Elhawary NA, Tayeb MT, Abdel-Ghafar S, Rashad M, Alkhotani AA. TNF-238 polymorphism may predict bronchopulmonary dysplasia among preterm infants in the Egyptian population. Pediatr Pulmonol. 2013 Jul;48(7):699–706. doi: 10.1002/ppul.22748. [DOI] [PubMed] [Google Scholar]
- 33.Strassberg SS, Cristea IA, Qian D, Parton LA. Single nucleotide polymorphisms of tumor necrosis factor-alpha and the susceptibility to bronchopulmonary dysplasia. Pediatr Pulmonol. 2007 Jan;42(1):29–36. doi: 10.1002/ppul.20526. [DOI] [PubMed] [Google Scholar]
- 34.Huusko JM, Karjalainen MK, Mahlman M, et al. A study of genes encoding cytokines (IL6, IL10, TNF), cytokine receptors (IL6R, IL6ST), and glucocorticoid receptor (NR3C1) and susceptibility to bronchopulmonary dysplasia. BMC medical genetics. 2014;15:120. doi: 10.1186/s12881-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huusko JM, Mahlman M, Karjalainen MK, et al. Polymorphisms of the gene encoding Kit ligand are associated with bronchopulmonary dysplasia. Pediatr Pulmonol. 2014 Mar 9; doi: 10.1002/ppul.23018. [DOI] [PubMed] [Google Scholar]
- 36.Floros J, Londono D, Gordon D, et al. IL-18R1 and IL-18RAP SNPs may be associated with bronchopulmonary dysplasia in African-American infants. Pediatr Res. 2012 Jan;71(1):107–114. doi: 10.1038/pr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krueger M, Heinzmann A, Mailaparambil B, Hartel C, Gopel W. Polymorphisms of interleukin 18 in the genetics of preterm birth and bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 2011 Jul;96(4):F299–F300. doi: 10.1136/adc.2009.174862. [DOI] [PubMed] [Google Scholar]
- 38.Bokodi G, Derzbach L, Banyasz I, Tulassay T, Vasarhelyi B. Association of interferon gamma T+874A and interleukin 12 p40 promoter CTCTAA/GC polymorphism with the need for respiratory support and perinatal complications in low birth weight neonates. Arch Dis Child Fetal Neonatal Ed. 2007 Jan;92(1):F25–F29. doi: 10.1136/adc.2005.086421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prencipe G, Auriti C, Inglese R, et al. A polymorphism in the macrophage migration inhibitory factor promoter is associated with bronchopulmonary dysplasia. Pediatr Res. 2011 Feb;69(2):142–147. doi: 10.1203/PDR.0b013e3182042496. [DOI] [PubMed] [Google Scholar]
- 40.Cakmak BC, Calkavur S, Ozkinay F, et al. Association between bronchopulmonary dysplasia and MBL2 and IL1-RN polymorphisms. Pediatrics international : official journal of the Japan Pediatric Society. 2012 Dec;54(6):863–868. doi: 10.1111/j.1442-200X.2012.03714.x. [DOI] [PubMed] [Google Scholar]
- 41.Hilgendorff A, Heidinger K, Pfeiffer A, et al. Association of polymorphisms in the mannose-binding lectin gene and pulmonary morbidity in preterm infants. Genes Immun. 2007 Dec;8(8):671–677. doi: 10.1038/sj.gene.6364432. [DOI] [PubMed] [Google Scholar]
- 42.Capoluongo E, Vento G, Rocchetti S, et al. Mannose-binding lectin polymorphisms and pulmonary outcome in premature neonates: a pilot study. Intensive care medicine. 2007 Oct;33(10):1787–1794. doi: 10.1007/s00134-007-0793-x. [DOI] [PubMed] [Google Scholar]
- 43.Poggi C, Giusti B, Vestri A, Pasquini E, Abbate R, Dani C. Genetic polymorphisms of antioxidant enzymes in preterm infants. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012 Oct;25(Suppl 4):131–134. doi: 10.3109/14767058.2012.714976. [DOI] [PubMed] [Google Scholar]
- 44.Giusti B, Vestrini A, Poggi C, et al. Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic Res. 2012 Sep;46(9):1130–1139. doi: 10.3109/10715762.2012.692787. [DOI] [PubMed] [Google Scholar]
- 45.Sampath V, Garland JS, Helbling D, et al. Antioxidant response genes sequence variants and BPD susceptibility in VLBW infants. Pediatr Res. 2015 Mar;77(3):477–483. doi: 10.1038/pr.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Li W, Liu W, et al. GSTM1 and GSTT1 gene polymorphisms as major risk factors for bronchopulmonary dysplasia in a Chinese Han population. Gene. 2014 Jan 1;533(1):48–51. doi: 10.1016/j.gene.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Koroglu OA, Onay H, Cakmak B, et al. Association of vitamin D receptor gene polymorphisms and bronchopulmonary dysplasia. Pediatr Res. 2014 Aug;76(2):171–176. doi: 10.1038/pr.2014.63. [DOI] [PubMed] [Google Scholar]
- 48.Rezvani M, Wilde J, Vitt P, et al. Association of a FGFR-4 gene polymorphism with bronchopulmonary dysplasia and neonatal respiratory distress. Disease markers. 2013;35(6):633–640. doi: 10.1155/2013/932356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwinta P, Bik-Multanowski M, Mitkowska Z, Tomasik T, Legutko M, Pietrzyk JJ. Genetic risk factors of bronchopulmonary dysplasia. Pediatr Res. 2008 Dec;64(6):682–688. doi: 10.1203/PDR.0b013e318184edeb. [DOI] [PubMed] [Google Scholar]
- 50.Fujioka K, Shibata A, Yokota T, et al. Association of a vascular endothelial growth factor polymorphism with the development of bronchopulmonary dysplasia in Japanese premature newborns. Scientific reports. 2014;4:4459. doi: 10.1038/srep04459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadchouel A, Decobert F, Franco-Montoya ML, et al. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PLoS One. 2008;3(9):e3188. doi: 10.1371/journal.pone.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hadchouel A, Durrmeyer X, Bouzigon E, et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1164–1170. doi: 10.1164/rccm.201103-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, St Julien KR, Stevenson DK, et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013 Aug;132(2):290–297. doi: 10.1542/peds.2013-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharya S, Go D, Krenitsky DL, et al. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012 Aug 15;186(4):349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodha A, Seshia M, McMillan DD, et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA pediatrics. 2015 Jan;169(1):33–38. doi: 10.1001/jamapediatrics.2014.2223. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006 May 18;354(20):2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 57.Kumar P Committee on F, Newborn, American Academy of P. Use of inhaled nitric oxide in preterm infants. Pediatrics. 2014 Jan;133(1):164–170. doi: 10.1542/peds.2013-3444. [DOI] [PubMed] [Google Scholar]
- 58.Askie LM, Ballard RA, Cutter GR, et al. Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics. 2011 Oct;128(4):729–739. doi: 10.1542/peds.2010-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K, Li M, Bucan M. Pathway-based approaches for analysis of genome wide association studies. American journal of human genetics. 2007 Dec;81(6):1278–1283. doi: 10.1086/522374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nature reviews. Genetics. 2010 Dec;11(12):843–854. doi: 10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- 61.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nature reviews. Genetics. 2010 Jun;11(6):415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 62.Carrera P, Di Resta C, Volonteri C, et al. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: A pilot study. Clinica chimica acta; international journal of clinical chemistry. 2015 Jan 8; doi: 10.1016/j.cca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Ambalavanan N, Walsh M, Bobashev G, et al. Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics. 2011 Jan;127(1):e106–e116. doi: 10.1542/peds.2010-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walsh M, Laptook A, Kazzi SN, et al. A cluster-randomized trial of benchmarking and multimodal quality improvement to improve rates of survival free of bronchopulmonary dysplasia for infants with birth weights of less than 1250 grams. Pediatrics. 2007 May;119(5):876–890. doi: 10.1542/peds.2006-2656. [DOI] [PubMed] [Google Scholar]
- 65.Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2011;(10) doi: 10.1002/14651858.CD000501.pub4. CD000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011 Jun 15;183(12):1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]