Abstract
Traumatic brain injury (TBI), a brain dysfunction for which there is no present effective treatment, is often caused by a concussive impact to the head and affects an estimated 1.7 million Americans annually. Our laboratory previously demonstrated that exendin-4, a long-lasting glucagon-like peptide 1 receptor (GLP-1R) agonist, has neuroprotective effects in cellular and animal models of TBI. Here, we demonstrate neurotrophic and neuroprotective effects of a different GLP-1R agonist, liraglutide, in neuronal cultures and a mouse model of mild TBI (mTBI). Liraglutide promoted dose-dependent proliferation in SH-SY5Y cells and in a GLP-1R over-expressing cell line at reduced concentrations. Pretreatment with liraglutide rescued neuronal cells from oxidative stress- and glutamate excitotoxicity-induced cell death. Liraglutide produced neurotrophic and neuroprotective effects similar to those of exendin-4 in vitro. The cAMP/PKA/pCREB pathway appears to play an important role in this neuroprotective activity of liraglutide. Furthermore, our findings in cell culture were well-translated in a weight-drop mTBI mouse model. Post-treatment with a clinically relevant dose of liraglutide for 7 days in mice ameliorated memory impairments caused by mTBI when evaluated 7 and 30 days post trauma. These data cross-validate former studies of exendin-4 and suggest that liraglutide holds therapeutic potential for the treatment of mTBI.
Keywords: Liraglutide, traumatic brain injury (TBI), neuroprotection, cAMP-response element binding protein (CREB), apoptosis, glucagon-like peptide-1
Introduction
Traumatic brain injury (TBI) is a common cause of death and long-term disability in the developed world, particularly among young adults. Annually, an estimated 10 million people suffer a TBI event worldwide [Hyder et al, 2007; Ruff et al, 2012]. Projections indicate that TBI will comprise the third largest portion of the total global disease burden by 2020 [Hyder et al, 2007]. Within the US, an estimated 1.7 million people per year sustain a TBI, and approximately 5.3 million people currently live with a TBI-induced disability [Langlois et al, 2006; Prins and Giza, 2012]. In 2000, the estimated US incidence of TBI was 200–558 per 100,000 people, with an estimated overall annual economic cost of US $406 billion [Corso et al, 2006; Leibson et al, 2011]. This figure has undoubtedly increased over the last decade.
80-95% of TBI cases are mild to moderate in nature, with severe TBI comprising the remainder [Tagliaferri et al, 2006], basing the grade of severity on the Glasgow Coma Scale, the loss of consciousness, and the development of post-traumatic amnesia [Mckee and Daneshvar, 2015]. With increases in survival following initial injury, TBI can result in substantial and lifelong cognitive, physical, and behavioral impairments that require long-term access to health care and disability services [Tagliaferri et al, 2006; Shi et al, 2013]. Particularly vulnerable are the elderly, in which the same insult causes greater disability [Susman et al., 2002]. Though TBI occasionally resolves within the first year after injury, some 70-90% of patients continue to manifest prolonged and often permanent neurocognitive dysfunctions that may substantially impact their performance and/or quality of life.
It is now recognized that TBI is a process, not an event. Emerging evidence indicates that this process can initiate cascades of cellular and biological processes that lead to early-onset dementia [Gardner et al, 2014; Barnes et al, 2014], Parkinson’s disease (PD) and development of neuropsychiatric disorders [Chen et al, 2014; Gardiner et al., 2015]. Clinically, TBI is one of the most predictive environmental risk factors for the development of Alzheimer’s disease (AD) and related disorders due to the critical changes impacting brain neurochemistry and cognition that occur over an extended time period after TBI. Recent gene expression studies have described the upregulation of pathways leading to AD and PD, as well as to other chronic neurodegenerative disorders, induced by mild, let alone moderate or severe forms of TBI [Greig et al, 2014; Tweedie et al, 2013a&b; Goldstein et al, 2012]. Given the lack of available therapeutics for the treatment of TBI [Moppett, 2007], it is important to characterize potential intervention points into the known cascades and mechanisms that underlie head injury that may halt or reverse the neuronal dysfunction and loss that ensue in order to develop and test novel hypothesis-driven treatment strategies.
TBI-associated brain damage can be segregated into two key phases. First, an initial primary damage phase occurs at the moment of insult, which may comprise contusion and laceration, as well as diffuse axonal injury and intracranial hemorrhage resulting in immediate (necrotic) cell death [Greig et al, 2014; LaPlaca et al, 2007]. An extended second phase follows, involving cascades of biological processes initiated at the time of injury, which may persist over the subsequent days, weeks and possibly months. These processes result from ischemia, neuroinflammation, glutamate toxicity, altered blood-brain barrier permeability, oxidative stress, astrocyte reactivity, cellular dysfunction and apoptosis initiated in the first phase of injury [Barkhoudarian et al, 2011; Greve and Zink, 2009; Morganti-Kossmann et al, 2002; Schmidt et al, 2005]. A number of studies suggest that secondary brain injury may be reversible, particularly by compounds that can rapidly intervene in one or more of the key biological cascades that drive it [Greig et al, 2014; Moppett, 2007]. In this regard, long-acting analogues of glucagon-like peptide-1 (GLP-1), a small intestine-derived incretin hormone known for its role in blood glucose homeostasis [Campell and Drucker, 2013], have been found to readily enter the brain [Kastin et al, 2002] and exhibit neuroprotection and neurotrophism in animal models of multiple neurological disorders [Salcedo et al., 2012]. GLP-1 and related agonists interact with the G-protein-coupled GLP-1 receptor (GLP-1R), which, in addition to being expressed in pancreatic β-cells, is found on cells throughout the central nervous system, particularly neurons [Salcedo et al. 2012]. Whereas the half-life of endogenous GLP-1 is limited to 1-2 min due to its proteolytic degradation by dipeptidyl peptidase-4 (DPP-4), the GLP-1 analogues exendin-4 (Ex-4) and liraglutide are well-tolerated, long-acting and DPP-4-resistant. Both analogues are clinically available and approved for the treatment of type 2 diabetes mellitus (T2DM). The glucose-dependent activity of both agents ensures that neither readily induces hypoglycemia, supporting their utility in the potential treatment of neurological disorders in euglycemic patients.
In light of our recent demonstration of the efficacy of a clinically-translatable dose of Ex-4 to mitigate cognitive deficits in animal models of both mild and moderate TBI [Rachmany et al., 2013; Eakin et al., 2013], as well as our characterization of the mechanisms through which Ex-4 protects against TBI-induced apoptosis and reverses gene transcriptome changes leading to AD [Tweedie et al., 2013a], we broadened our studies of GLP-1 analogues to evaluate liraglutide. The purpose of the current study was to examine whether a clinically-translatable dose of liraglutide ameliorated cognitive deficits in a rodent model of mTBI in order to further validate the therapeutic potential of GLP-1R agonists for the treatment of mTBI. To evaluate the potential mechanisms involved, the neuroprotective and neurotrophic actions of liraglutide were evaluated in cellular neuronal models pertinent to mTBI, and compared to those of Ex-4.
Materials and Methods
Materials
Ex-4 was obtained from Anaspec (Fremond, CA), and clinical-grade liraglutide from Novo Nordisk. All other reagents were from Sigma (St. Louis, MO), unless otherwise stated.
Cell culture
All cell cultures were maintained at 37°C in a humidified incubator with 5% CO2 and 95% air. SH-SY5Y cells, obtained from American Type Culture Collection (ATCC) (Manassa, VA), and SH-hGLP-1R#9 cells, a human GLP-1R over-expressing cell line derived from SH-SY5Y cells (Li et al., 2010b), were grown in a 1:1 mixture of Eagle’s Minimum Essential Medium and Ham’s F12 Medium supplemented with 10% heat-inactivated fetal bovine serum (FCS) and 100 U/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA). Medium was changed every other day and the cells were split in a 1:3 ratio every 4-5 days (0.25% trypsin, 0.53 mM EDTA solution) or when they reached approximately 80% confluence. Primary cortical neurons were obtained from embryonic day 15 Sprague-Dawley rats and dissociated by mild trypsination, and equal numbers of cells were seeded onto 24-well plates in plating media (DMEM-12 media containing 2% B27 supplement (Invitrogen), 10% HI-FBS, 0.5 mM L-glutamine, and 25 ìM L-glutamate) at a density of approximately 3 × 105 cells/well. From day 3 in vitro (DIV), cultures were maintained in feeding media (Neurobasal medium containing 2% B-27 supplement (Invitrogen) and 0.5mM L-glutamine).
Cell viability assays
Cell viability was assessed using either 3-(4, 5-dimethylthiazol-2-yl) -5-(3-carboxymethoxyphenyl) -2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay or lactate dehydrogenase (LDH) assay. For the MTS assay, experiments were performed in 96-well plates, and cells were serum-starved (with 0.5% of serum) overnight before pretreatment with various concentrations of liraglutide or Ex-4 for 1 h. Cells were then exposed to different concentrations of glutamate or H2O2 for 24 h, selected from prior studies to achieve a significant but sub-maximal cellular loss within the linear portion of the dose-response curve of each (data not shown). A CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI) was utilized to measure a formazan product, which is directly proportional to the cell viability. For the LDH assay, experiments were performed in 24-well plates, and cells were pretreated with liraglutide or Ex-4 for 1 h before being exposed to glutamate for 24 h. Samples of conditioned media were collected for the measurement of LDH levels (LDH Cytotoxicity Assay Kit, Cayman Chemical), which is an indicator of plasma membrane integrity and cell viability.
Western Blotting
Cells grown in 100 mm plates at a density of approximately 5 × 106 cells/well were used to extract total protein. First, cells were washed twice with cold phosphate-buffered saline (PBS) buffer, and then lysed in Mammalian Protein Extraction Reagent (M-PER) (Thermo Scientific, Rockford, IL) containing Halt Protease Inhibitor Cocktail (Thermo Scientific) for 5 min at room temperature. Total protein was extracted according to the manufacturer’s protocol. For all samples, about 20 μg protein extracts were resolved by NuPage bis-Tris 10% mini gels (Life Technologies, Carlsbad, CA) and transferred onto 0.2 μm polyvinylidene fluoride (PVDF) membrane (Life Technologies). The blots were first blocked in 5% milk in tris-buffered saline and Tweeen 20 (TBST) at room temperature for 1 h, and then incubated in the same blocking solution containing primary antibodies overnight at 4°C. B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) antibodies from Cell Signaling (Danvers, MA) were used at a dilution of 1:1000; AIF antibody from Millipore (Billerica, MA) was used at a dilution of 1:500; α-tubulin antibody from Sigma was used at a dilution of 1: 5000. After sufficient washes with TBST, blots were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. Blots were again washed in TBST and, thereafter, signals were detected by using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). Finally, blots were exposed to HyBlot ES Autoradiography Film (Denville Scientific, Metuchen, NJ) for an appropriate period of time, and densitometric quantification of the protein bands was performed by using a PC version of NIH IMAGE (ImageJ software).
Caspase-3 activity assay
Caspase-3 activity was measured using a colorimetric caspase-3 assay kit (Sigma) as per the manufacturer’s protocol for a 96-well plate microassay. Briefly, cell lysates from approximately 1 × 106 cells were centrifuged at 20,000 g for 10 min at 4°C and the supernatant was used for caspase-3 reaction in a 96-well plate. Specifically, the supernatant was incubated with caspase-3 substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) in the presence or absence of the caspase-3 inhibitor, Ac-DEVD-CHO, overnight at 37 °C; the release of p-nitroaniline (pNA) from caspase-3 hydrolysis was measured by absorbance at 405 nm wavelength. The remaining cell lysate was used to measure total cellular protein concentrations by bicinchoninic (BCA™) protein assay kit (Pierce) with bovine serum albumin (BSA) as standards. Caspase-3 activities were normalized by protein content.
Animals
Male ICR mice weighing 30-40 g were used in our study and maintained under standard conditions, as described previously [Milman et al., 2005]. Mice were bred and then raised within the vivarium of Tel Aviv University, Israel (originally derived from breeding pairs purchased from HSD Jerusalem, Israel). Each mouse was used in a single experiment and for one time point alone. The Ethics Committee of the Sackler Faculty of Medicine approved the experimental protocol (M-14-050), in compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW publication 85-23, revised, 1995). A minimal number of mice were used for each study, determined from prior studies, and all efforts were made to minimize potential suffering.
mTBI model
A previously described weight drop experimental mTBI model [Milman et al., 2005; Rachmany et al., 2013; Tweedie et al., 2013a] was used to induce brain injury. In brief, mice were lightly anesthetized with isoflurane and then placed under the weight drop device with the head carefully aligned in a consistent manner. The device comprised a vertically mounted metal tube (80 cm long with an inner diameter of 13 mm) through which a 30 g metal weight was dropped. This struck the skull of the concisely aligned anaesthetized mouse on the temporal right side, between the corner of the eye and the ear. A sponge positioned below the mouse aided dissipation of the impact. Mice subjected to mTBI recovered rapidly from anesthesia following the procedure and appeared normal and indistinguishable from a previously described sham group of similarly treated animals that were not subjected to weight drop [Milman et al., 2005; Rachmany et al., 2013; Tweedie et al., 2013a].
Drug administration
Liraglutide was administered to mice in a 7 day regimen of subcutaneous injections. Injections were made once a day, with the first injection administered 30 min after the injury. The dose of liraglutide was 246.7μg/kg/day, which is equivalent to the human dose (20μg/kg/day) normalized to body surface area across species. Mice were divided into 4 groups: a control group, an mTBI group, a liraglutide group, and an mTBI+liraglutide group.
Behavioral measurement
Behavioral assessments were conducted at 7 and 30 days after the animals were exposed to mTBI in separate cohorts of mice. Mouse cognition and emotional behavior was assessed using the novel object recognition, Y-maze, and elevated plus maze paradigms. All equipment used for behavioral testing was cleaned with a 70% ethanol solution between testing sessions in order to minimize any olfactory-dependent cognitive influences on mouse behavior.
Novel object recognition paradigm
The novel object recognition task is used to evaluate recognition memory [Messier 1997]. This task is based on the innate tendency of rodents to explore unfamiliar objects within their environment. This behavior is observed in order to assess the mouse’s ability to discriminate between familiar and novel objects.
48 hr prior to the test, mice were individually habituated to an open field Plexiglas box (59×59×20 cm size) for 5 min. At 24 h prior to the test, mice were allowed to explore a set of two identical objects for a 5 min acquisition phase. These objects were suitably heavy and tall to guarantee that mice could neither move them nor climb over them. On the day of the test, the mice were presented with a similar set of objects in the same environment; however, one object was novel to them. They were allowed to freely explore the objects again for a 5 min period. A discrimination preference index was calculated as follows: (time near new object - time near familiar object) / (time near new object + time near familiar object) [Dix and Aggleton 1999].
Y maze paradigm
The Y-maze task was chosen as a means to evaluate spontaneous exploration, responsiveness to novel environments and spatial memory function of the mice. The apparatus used for the Y-maze study was a three-armed black Plexiglas maze with each arm separated by 120° [Baratz et al. 2011]. Each arm measured 8 × 30 × 15 cm and was distinguishable only by spatial cues placed within (i.e. a triangle, a square, or a circle). The starting arm for each experiment was chosen randomly. Each mouse was placed into the Y-maze environment on two occasions separated by a 2 min interval, during which the mouse was returned to its home cage. In the first 5 min trial, one of the two arms was randomly blocked. In the second 2 min trial, all arms were open for exploration, and the total amount of time during which the mouse explored each arm was measured. A discrimination preference index was calculated as follows: (time in new arm – time in familiar arm) / (time in new arm + time in familiar arm) [Dix and Aggleton 1999].
Elevated plus maze paradigm
The elevated plus maze apparatus was used to assess the levels of fear- or anxiety-like behavior in rodents [Zohar et al. 2011]. This assessment is based upon the natural tendency of rodents to prefer exploring enclosed, darkened environments over those that are open, illuminated and elevated. The maze consisted of four arms in a “+” shape: two arms with low walls (30 × 5 × 1 cm) and two arms with high walls (30 × 5 × 15 cm), all arms with an open top. The arms were arranged such that those of the same height were opposite each other. The entire maze was elevated 50 cm above floor level. Each mouse was placed in the center of the elevated plus maze facing one of the open arms. Over a 5 min observation period, the number of entries to the open/closed arms was quantified and the time that each mouse spent in the open arms was measured. Longer duration within the open arms has been associated with a lower anxiety-like behavior [Belzung and Griebel 2001].
Statistical Analysis
Data are presented as mean ± standard error mean (SEM) and analyzed by Prism or SPSS V 19 software. One-way analysis of variance (ANOVA) tests are used for comparison of multiple samples, followed by Dunnett’s post-test post hoc tests. Additionally, for behavioral experiments fisher’s LSD post hoc analysis was used. *P ≤0.05, **P ≤0.01, ***P ≤0.001.
Results
Liraglutide promotes cell proliferation in SH-SY5Y cells
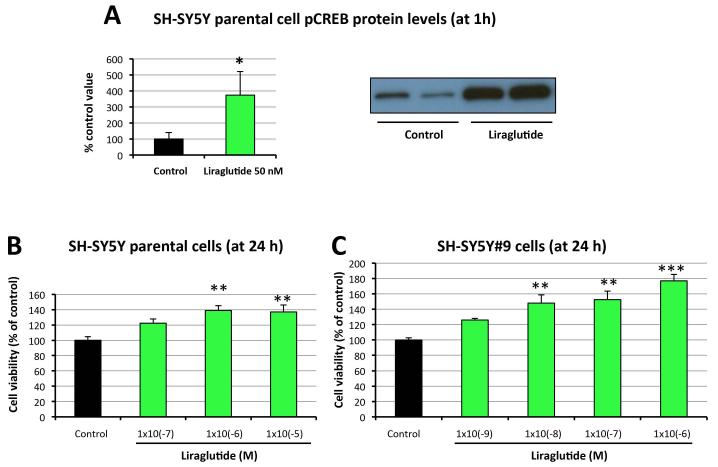
We previously established a human GLP-1R-overexpressing cell line named SH-SY5Y#9, which expresses elevated (>2-fold) GLP-1R protein levels compared to parental SH-SY5Y cells (Li et al., 2010b). We demonstrated in SH-SY5Y cells that stimulation of GLP-1R signaling pathway by Ex-4 can quickly activate adenylyl cyclase and transiently induce intracellular cAMP production [Li et al, 2010a&b]. Increased cAMP levels then activate PKA, which can activate transcription factor CREB by phosphorylation at serine-133. Here we show that liraglutide treatment for 1 h significantly increased pCREB levels to more than 3-fold of controls (Fig. 1A), indicating that liraglutide as a GLP-1R agonist may stimulate the same signaling pathways as GLP-1 and Ex-4. To examine the neurotrophic effects of liraglutide, both cell lines were treated with liraglutide in a concentration-dependent manner for 24 h, and cell viabilities were subsequently measured via MTS assay. Figure 1 shows that liraglutide induced significant cell proliferation of both SH-SY5Y and SH-SY5Y#9 cells. Due to the disparity in GLP-1R density between the two cell lines, induction of cell proliferation was achieved at different concentrations. Cell viability was augmented in parental SH-SY5Y cells at a liraglutide concentration of 10−6 M or higher (Figure 1B), whereas in more sensitive SH-SY5Y#9 cells a 100-fold lower concentration (10−8 M) of liraglutide proved capable of inducing a significant increase (Figure 1C). Nevertheless, liraglutide elevated cell viability in a dose-dependent manner in both cell lines. These results demonstrate that liraglutide is neurotrophic in neuronal cell cultures, and that enhanced GLP-1R signaling further promotes cell proliferation.
Figure 1. Liraglutide treatment promotes cell proliferation in neuroblastoma cells in a dose-dependent manner via CREB phosphorylation.
(A) Liraglutide administration (50 nM) stimulated early (1 h) induction (3.7-fold, P<0.05) of CREB phosphorylation in SH-SY5Y neuroblastoma cells. (B) Liraglutide treatment dose-dependently increased cell viabilities in SH-SY5Y neuroblastoma cells. SH-SY5Y cells were treated with different concentrations (0, 10−7, 10−6 or 10−5M) of liraglutide for 24h. Cell viabilities were subsequently assessed by MTS assay. (C) Liraglutide treatment further increased cell viabilities in GLP-1R-overexpressing SH-SY5Y#9 neuroblastoma cells. SH-SY5Y#9 cells were treated with different concentrations (0, 10−9, 10−8, 10−7, 10−6 M) of liraglutide for 24h. Cell viabilities were subsequently assessed by MTS assay (*P < 0.05; **P <0.01; ***P < 0.001).
Liraglutide treatment protects neural cells from H2O2-induced oxidative stress
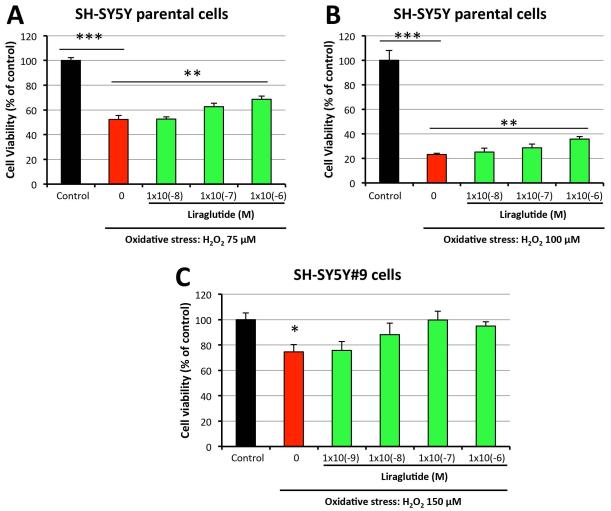
To evaluate whether or not liraglutide protects neuronal cells from oxidative stress, an insult relevant to all forms of TBI [Barkhoudarian et al., 2011; Moppett et al., 2007], SH-SY5Y cells were challenged with H2O2 (75 μM [Figure 2A] or 100 μM [Figure 2B] for 24 h) with concurrent concentration-dependent treatment (0, 10−8, 10−7, 10−6 M) of liraglutide for 1 h. 75 μM and 100 μM H2O2 challenges induced 48% and 77% cell death of SH-SY5Y cells, respectively. As compared to vehicle-treated controls, pretreatment with 1μM liraglutide significantly protected SH-SY5Y cells from both H2O2 concentrations. Lower (less than 1 μM) liraglutide levels lacked significant action. Cell viabilities were increased by 31% and 52% in 1 μM liraglutide pretreated cells exposed to 75 μM and100 μM H2O2 challenge, as compared to cells exposed to H2O2 alone (Figure 2). These results indicate that a relatively high dose of liraglutide can protect SH-SY5Y cells from oxidative stress-induced cell death. Evaluation of SH-SY5Y#9 cells demonstrated that a100-fold lower dose of liraglutide (10 nM) was able to significantly rescue cells from a greater H2O2 challenge (150 μM) (Figure 2C). In this experiment, 100nM liraglutide completely rescued cells from H2O2-induced injury, providing a cell viability equivalent to unchallenged control cells (Figure 2C). Due to the increased sensitivity of the SH-SY5Y#9 cells to treatment with liraglutide, further studies were undertaken on these cells in order to characterize the neuroprotective actions of liraglutide and involved cell signaling pathways.
Figure 2. Liraglutide treatment protects neural cells from H2O2-induced cell death.
SH-SY5Y or SH-SY5Y#9 cells were pretreated with a series concentrations (0, 10−9, 10−8, 10−7, 10−6 M) of liraglutide for 1h, then challenged with either 75μM (A), 100 μM (B) or 150 μM (C) H2O2 for 24h before performing an MTS assay to assess cell viabilities. In (A) and (B), H2O2 challenge in the absence of liraglutide resulted in a significant loss of cell viability vs. control cells, which was significantly mitigated by liraglutide (1×10−6 M). In (C), in the absence of liraglutide resulted in a significant loss of cell viability vs. control cells, with all liraglutide + H2O2 challenge groups being no different from the control (unchallenged) group, (*P < 0.05; **P <0.01; ***P < 0.001).
Liraglutide treatment protects neural cells from glutamate-induced excitotoxicity
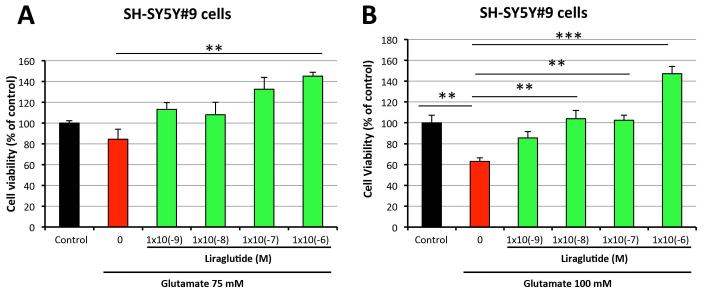
Excess glutamate can induce neuronal excitotoxicity and ensuing cell death, and has been reported to occur in TBI [Barkhoudarian et al., 2011; Khatri and Man 2013]. To evaluate whether liraglutide is neuroprotective against glutamate-induced excitotoxicity, SH-SY5Y#9 cells were treated with liraglutide (0, 10−9, 10−8, 10−7, 10−6 M) for 1 h. These cells were then challenged with glutamate (75 mM or 100 mM) for a further 24 h, and cell viability was assessed by MTS assay. Figure 3 demonstrates that 75 mM and 100mM glutamate induced 16% and 37% cell death of SH-SY5Y#9 cells, respectively, as compared to unchallenged control cells. Liraglutide treatment provided significant protection from both glutamate doses, even at the lowest liraglutide concentration (10−9 M) evaluated. Higher liraglutide amounts not only fully protected cells from excitotoxicity, but also promoted cellular proliferation. Specifically for 75 mM glutamate challenge, liraglutide 10−7 M and 10−6 M augmented cell viability to 133% and 145% of unchallenged control values (Figure 3A). Furthermore, 10−6 M liraglutide treatment improved cell viability to 147% of control levels in cells exposed to 100 mM glutamate (Figure 3B). These results confirm the neurotrophic and neuroprotective actions of liraglutide in a neuronal cellular model relevant to TBI.
Figure 3. Liraglutide treatment protects neural cells from glutamate-induced cell death.
SH-SY5Y#9 cells were pretreated with a series concentrations (0, 10−9 , 10−8, 10−7, 10−6 M) of liraglutide for 1h, then challenged with either 75 mM (A) or 100 mM glutamate (B) for 24h before performing an MTS assay to assess cell viabilities. In (A), cell viability of liraglutide (1×10−6 M) + glutamate challenge was significantly greater than + glutamate challenge alone. In (B), cell viability following glutamate challenge alone was significantly reduced compared to all groups with the exception of liraglutide (1×10−9 M) (**P <0.01; ***P < 0.001).
Liraglutide treatment reduces apoptosis in neural cells
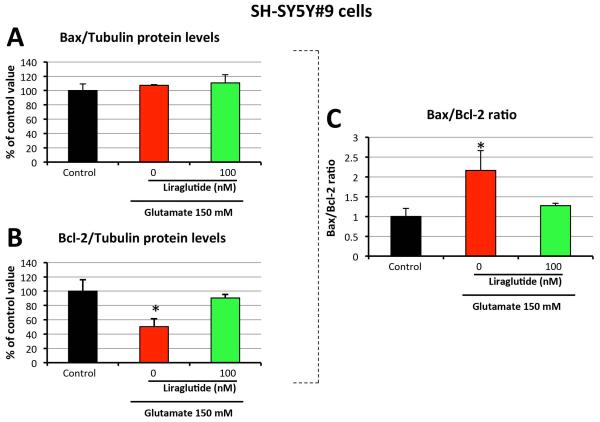
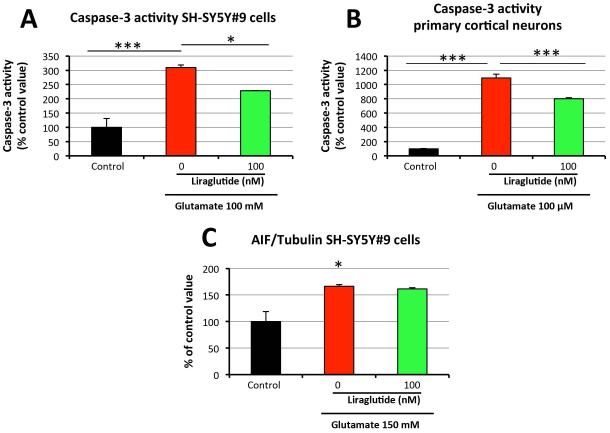
As glutamate-induced excitotoxicity causes cell death via apoptosis, key markers of the apoptotic pathway were evaluated to define liraglutide’s effect on their expression. Using Western Blot analysis, protein levels of pro-apoptotic Bax and anti-apoptotic Bcl-2 were quantified in SH-SY5Y#9 cells. As illustrated in Figure 4, challenge with 150 mM glutamate did not alter Bax expression but significantly lowered Bcl-2 expression to 50.3% of control levels. 100 nM liraglutide treatment mitigated this decline (90.4% of the control value). The ratio of Bax to Bcl-2 is a key checkpoint within the apoptotic pathway that predicts cell death/survival. 150 mM glutamate challenge elevated the Bax/Bcl-2 ratio by 2.2-fold, with 100 nM liraglutide mitigating this rise (1.3-fold the unchallenged control value). An increased Bax/Bcl-2 ratio can up-regulate caspase-3 activity, thus inducing cell death via the caspase-dependent pathway. Indeed, for SH-SY5Y#9 cells, 100 mM glutamate challenge provoked a 3-fold elevation in caspase-3 activity, which was significantly decreased (~30%) by 100 nM liraglutide treatment (Figure 5A). In order to ensure translation to primary cortical neuron cultures, a 100 μM glutamate challenge was assayed in these cells. The glutamate challenge induced a more than 10-fold increase in caspase-3 activity. Significantly, this effect of glutamate excitotoxicity was mitigated by 100 nM liraglutide, which reduced caspase-3 activity by ~30% (Figure 5B).
Figure 4. Liraglutide reduces glutamate-induced apoptosis in neural cells.
The effect of liraglutide treatment on: Bax (A), Bcl-2 protein levels (B) and the ratio of Bax/Bcl-2 protein levels (C) in SH-SY5Y#9 cells (Western blot) (*P < 0.05).
Figure 5. Liraglutide’s anti-apoptotic actions in response to glutamate excitotxicity are mediated via a caspase-dependent pathway, rather than via AIF.
Following simultaneous glutamate challenge and liraglutide/vehicle treatment, the following measures were evaluated in liraglutide-treated and -untreated cells at 6h: (A) Caspase-3 activities in SH-SY5Y#9 cells (Caspase-3 activity assay); (B) Caspase-3 activities in primary cortical neurons (Caspase-3 activity assay). (C) AIF protein levels in SH-SY5Y#9 cells (Western blot) (*P < 0.05; ***P < 0.001).
Together, these data indicate that liraglutide’s anti-apoptotic actions in the face of glutamate-induced excitotoxicity are mediated, at least in part, through the caspase-dependent cell death program. Accumulating evidence supports a substantial role for a caspase-independent pathway in the neuronal death observed in neurodegenerative disorders [Mattson 2000]. The mitochondrial release of apoptosis-inducing factor (AIF) triggers the caspase-independent cell death process [Culmsee and Landshamer 2006; Polster 2013], and is therefore a key marker in this neuronal apoptosis cascade worth evaluating. As illustrated in Figure 5C, 150 mM glutamate challenge of SH-SY5Y#9 cells elevated AIF protein levels to 166.5% of control values, which 100 nM liraglutide treatment proved unable to alter. This suggests that liraglutide’s neuronal anti-apoptotic action is mediated via the caspase-dependent pathway, rather than via the caspase-independent one.
Comparison of liraglutide and Ex-4 neurotrophic and neuroprotective effects
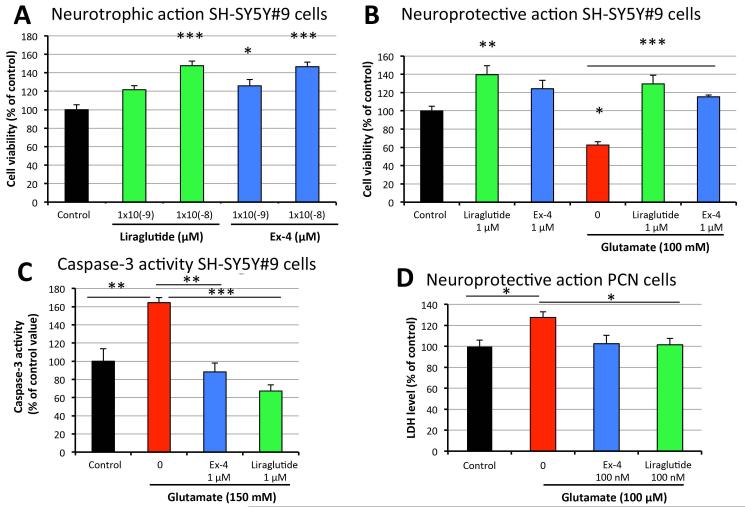
Our previous studies demonstrated that the GLP-1R agonist Ex-4 has neurotrophic and neuroprotective effects on human immortal neuronal cells as well as rodent primary neural cultures [Li et al., 2009, 2010a&b, 2012]. These in vitro effects appear to translate into protective actions across a number of animal models of acute and chronic neurodegeneration [Salcedo et al., 2012; Holscher 2014], including TBI [Greig et al., 2014]. In light of the neurotrophic and neuroprotective actions of Ex-4 and the lack of information currently available on liraglutide’s actions on cultured neurons, we compared the neurotrophic/neuroprotective properties of liraglutide to those of Ex-4 in a side-by-side experiment. Equimolar concentrations (10−9 M and 10−8M) of liraglutide and Ex-4 had similar actions on SH-SY5Y#9 cells (Figure 6A), increasing the viability of unchallenged cells. Evaluated at a higher concentration (10−6M), both GLP-1R agonists not only fully protected cells from 100 mM glutamate-induced cell death, but also elevated cell viability to ~120% of control cell levels, simultaneously providing neurotrophic and neuroprotective actions (Figure 6B). Likewise, both liraglutide and Ex-4 mitigated glutamate-induced elevation of caspase-3 activity (Figure 6C). Furthermore, the neuroprotective effect of GLP-1R agonists observed in neuroblastoma cells was confirmed in primary cortical neurons. 100 nM liraglutide and 100 nM Ex-4 both fully protected neurons from glutamate-induced disruption of plasma membrane integrity (Figure 6D).
Figure 6. Comparison of neurotrophic and neuroprotective effects of liraglutide and exendin-4 in neuroblastoma cells.
(A) Neurotrophic effects of liraglutide and Ex-4 in SH-SY5Y#9 cells; (B) Neuroprotective effects of liraglutide and Ex-4 in SH-SY5Y#9 cells; (C) Caspase-3 activities in SH-SY5Y#9 cells; (D) Neuroprotective effects of liraglutide and Ex-4 in primary cortical neurons (*P < 0.05; **P <0.01; ***P < 0.001).
Liraglutide treatment reverses mTBI-induced behavioral impairment in mice
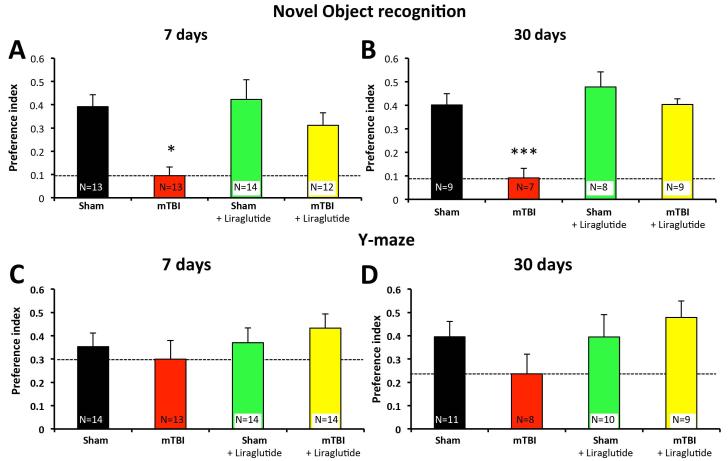
In light of liraglutide’s neurotrophic and protective actions in neural cultures, its equivalent potency to that achieved by Ex-4 in these cultures, and the efficacy of Ex-4 in the amelioration of TBI-induced cognitive impairments in rodents [Greig et al., 2014], we evaluated the protective actions of liraglutide in a murine close-head model of mTBI in order to cross-validate the potential therapeutic utility of GLP-1R agonists in head trauma. As reported previously, mice subjected to mTBI were indistinguishable from those subjected to the sham procedure when evaluated at 1 or 24 h later in relation to a range of measures associated with health and wellbeing (combining subjective measures, such as grooming and appearance, righting skills, ambulation, and blinking reflex, with objective ones encompassing parameters such as weight, body temperature, anxiety-like behavior, and motor skills) [Zohar et al., 2003; Baratz et al., 2015]. As illustrated in Figure 7A; however, mice subjected to a single 30 g weight drop injury demonstrated impaired novel object recognition as compared to sham control animals when evaluated in separate cohorts of mice at 7 and 30 days post-trauma. Specifically, untreated TBI-challenged animals demonstrated a significantly lower object discriminatory preference. This impairment was substantially ameliorated by liraglutide at both appraisal times. Evaluation of the same animals in the Y-maze paradigm demonstrated a trend towards impairment in mice subjected mTBI as compared to sham control animals at both 7 and 30 days post-trauma. This trend was not evident in animals administered liraglutide (Figure 7C,D). Anxiety-like behavior was assessed by use of the elevated plus maze paradigm across all group of mice, and in accord with prior studies [Baratz et al., 2001; Rachmany et al., 2013] was unaffected by TBI challenge. It was likewise unaffected by liraglutide in the presence and absence of mTBI (not shown). In conclusion, the early administration of liraglutide (starting 30 min post trauma and administered once daily × 7 days) inhibited behavioral impairments induced by mTBI.
Figure 7. Liraglutide treatment leads to behavioral improvement in a mouse model of mTBI at both 7 and 30 days post trauma.
(A) and (B): Novel object recognition paradigm at 7 (A) and 30 (B) days post injury. Preference index is used to represent the relative time that animals spend on novel object, which reflects visual memory. The formula used to calculate the preference index is: (time near the new object – time near the familiar object) / (time near the new object + time near the familiar object). One-way ANOVA revealed that mTBI animals had a deficit in visual memory compared to all other groups. For 7 days: F(3,48) = 5.981, p = 0.002 Fisher’s LSD post hoc, p<0.05, n = 12-14 /group; For 30 days: F(3,29) = 12.329, p = 0.000, Fisher’s LSD post hoc, p<0.001, n = 7-9/group. (C) and (D): Y maze paradigm at 7 (E) and 30 (F) days post injury. Performance was quantitatively assessed by Y maze paradigm, which reflects spatial learning abilities. Preference index is calculated as (time in new arm – time in familiar arm)/(time in new arm + time in familiar arm). All values are presented as mean ± SEM, *P < 0.05; **P <0.01; ***P < 0.001.
Discussion
The present study demonstrates that the long-acting GLP-1R agonist liraglutide, a well-tolerated drug that is FDA approved for the treatment of T2DM [Scott 2014], possesses neurotrophic/neuroprotective actions in human neuroblastoma SH-SY5Y cells, properties of relevance to the treatment of TBI. Indeed, glutamate excitotoxicity and oxidative stress have been implicated in the pathogenesis of a wide number of neurological disorders that develop both acutely, as in TBI and stroke, and chronically, as in AD and PD [Barger 2004; Wang and Michaelis 2010; Doyle et al., 2011]. The current study confirms that liraglutide has anti-apoptotic effects in immortal neuronal cultures [Sharma et al., 2014b] and, importantly, extend this to primary cortical neurons. Such effects proved to be equipotent to those of the other widely used long-acting GLP-1R agonist Ex-4, whose neurotrophic/neuroprotective effects have been well characterized [Li et al., 2009, 2010a&b, 2012]. Notably, the neurotrophic/neuroprotective actions of liraglutide translated into the amelioration of cognitive impairment in a murine model of concussive mTBI, thus cross-validating the activity of Ex-4 in this same paradigm [Rachmany et al., 2013; Tweedie et al., 2013a] as well as other TBI models [Eakin et al., 2013; Greig et al., 2014]. Together, these results underline the potential to reverse the secondary phase of brain injury induced by TBI using clinically available GLP-1R agonists.
Based on our previous studies, which focused on the neurotrophic/protective effects of Ex-4 in cellular and animal models of neurodegenerative disorders [Perry et al., 2002a&b; Perry et al., 2003; Perry et al., 2007;Li et al, 2009, 2010a&b, 2012], we here examined the neurotrophic/protective effects of another long-lasting GLP-1R agonist, liraglutide, in both neuronal cultures and a mouse model of mTBI. 24 h liraglutide treatment of SH-SY5Y neuroblastoma cells increased cell viability in a dose-dependent manner, thereby demonstrating the drug’s neurotrophic action. This proved more robust in GLP-1R-overexpressing SH-SY5Y#9 cells, as significantly greater increases in SH-SY5Y#9 cell viabilities were observed as compared to SH-SY5Y cells treated with the same liraglutide concentration. Indeed, liraglutide enhanced SH-SY5Y#9 cell viability at concentrations 100-fold lower than those required to augment SH-SY5Y cell viability, indicating that these liraglutide-induced neurotrophic effects are mediated by the GLP-1R pathway. Furthermore, liraglutide treatment proved neuroprotective against H2O2-induced oxidative stress and glutamate-induced excitotoxicity in SH-SY5Y cells. Whereas liraglutide significantly protected parental SH-SY5Y cells from H2O2-induced oxidative stress, this protection was only partially effective and it required a high dose (1 uM) of liraglutide. In contrast, liraglutide treatment was more effective in sensitive SH-SY5Y#9 cells, inducing effects at a dose 1000 times lower (1 nM) than the dose required for efficacy in SH-SY5Y cells and completely rescuing cells from oxidative stress and glutamate-induced excitotoxicity at concentration of 10 nM. Interestingly, a higher liraglutide dose (1 uM) showed combined effects of neuroproliferation and neuroprotection against glutamate insult, and as a consequence, cell viabilities that reached over 140% of controls were measured. These results further confirmed that the neuroprotective effect of liraglutide is dependent on the GLP-1R.
The mechanism of post-TBI secondary brain injury includes oxidative stress and glutamate toxicity, which are known to be triggers of apoptosis [Barkhoudarian et al, 2011; Greve and Zink, 2009; Morganti-Kossmann et al, 2002; Schmidt et al, 2005]. As with Ex-4, the neuroprotective effect of liraglutide may involve modulation of apoptotic pathways. This hypothesis is supported by our results showing that liraglutide significantly reduced levels of key apoptotic markers, represented by a reduced Bax/Bcl-2 ratio. In addition, our results suggest that the anti-apoptotic action of liraglutide is achieved through modification of the caspase-dependent pathway but not the caspase-independent pathway, as liraglutide treatment significantly reduced caspase-3 activity but did not modulate AIF levels. Importantly, this result was observed in both neuroblastoma cells and primary cortical neuron cultures.
Both GLP-1R agonists, liraglutide (Victoza) and the synthetic product of Ex-4 (Byetta/Bydurion), are FDA-approved drugs prescribed for the treatment of T2DM. As such, they both effectively reduce glycemia and induce weight loss in diabetic patients [Campbell and Drucker 2013]. Several studies have revealed differences between the two. In the Liraglutide Effect and Action in Diabetes (LEAD)-6 trial, 1.8 mg, once-a-day subcutaneous liraglutide was compared to Ex-4 10 μg twice a day (in the form of Byetta). The results suggested that liraglutide provided significantly greater improvement in glycemic control than Ex-4 [Buse et al., 2009]. Such advantage was maintained in a more recent head-to head clinical evaluation of 1.8 mg, once-a-day subcutaneous liraglutide comparison to once-weekly subcutaneous Ex-4 (2 mg) [Buse et al., 2013] (in the form of Bydurion), albeit the latter was better tolerated. A recent study reported divergent effects of liraglutide and Ex-4 on β-cell mass. Long-term treatment with liraglutide caused a sharp reduction in β-cell mass in both normal diet and high-fat diet mice, whereas Ex-4 exerted no effect [Mondragon et al., 2014]. It is important to keep in mind the fact that the difference found in these studies is limited to specific concentrations of these two drugs used in a particular experimental design. To date, there is no knowledge of whether liraglutide and Ex-4 perform differently in terms of neuroprotection. We are the first to demonstrate that equimolar concentrations of liraglutide and Ex-4 have similar neurotrophic, neuroprotective and anti-apoptotic effects against glutamate in neuronal cultures, including primary cortical neuron (Figure 6). It has been demonstrated that Ex-4 and liraglutide can also promote neurogenesis. In animal models, both these GLP-1 agonists increased the number of neural stem/progenitor cells in the subventricular zone as revealed by BrdU staining [Bertilsson et al., 2008; Parthsarathy and Hölscher, 2013]. Whether liraglutide induces a similar effect in our mouse model of TBI is an interesting question to address in future studies.
Like GLP-1, liraglutide exerts its action by binding to the GLP-1R, a 7-transmembrane G-protein-coupled receptor, which is expressed on the cell membrane of both pancreatic β-cells and several neuronal cell types within the nervous system. Activation of GLP-1R stimulates the adenylyl cyclase pathway, leading to an increase of intracellular cAMP levels. Increased cAMP can then activate protein kinase A (PKA). cAMP response element binding protein (CREB) is an important transcription factor that is phosphorylated and activated by PKA. It has been demonstrated that stimulation of GLP-1R pathway activates CREB by phosphorylation at serine-133 and plays an important role in β-cell growth and survival [Jhala et al., 2003; van de Velde et al., 2011; Velmurugan et al., 2012]. On one hand, studies have shown that CREB is critical to GLP-1 activity in β-cells due to its mediation of the insulinotrophic, proliferative and anti-apoptotic effects of GLP-1 [Shin et al., 2014]. On the other hand, and of interest to us, many studies have demonstrated that CREB is also pivotal in nerve cell survival as well as neuronal proliferation, differentiation and plasticity due to its mediation of gene expression [Dragunow 2004; Lonze and Ginty, 2002; Sakamoto et al., 2011). Using animal models, it has been demonstrated that stimulation of the GLP-1R pathway by either Ex-4 or liraglutide induces CREB activation in rodent brain [DellaValle et al., 2014; Ohtake et al., 2014]. Indeed, in SH-SY5Y neuroblastoma cells, one hour liraglutide treatment induced a more than 3-fold increase in pCREB protein levels (Figure 1). This result suggests that the cAMP/PKA/CREB pathway is rapidly activated by liraglutide in neuronal cultures. This acute activation is similar to findings in INS-1 cells (a rat insulinoma β-cell line) treated with Ex-4 [van de Velde et al., 2011), suggesting similar signaling pathways are shared by β-cells and neurons after stimulation of the GLP-1R pathway. A number of CREB-mediated genes such as insulin, IRS2, bcl-2 and cyclin D1 are essential for β-cell growth and survival [Jhala et al., 2003; Kim et al., 2006; Velmurugan et al., 2012). Here we show that anti-apoptotic protein bcl-2 is down-regulated in SH-SY5Y cells exposed to glutamate-induced excitotoxicity. Bcl-2 is up-regulated, however, by liraglutide treatment (Figure 5), indicating that stimulation of GLP-1R signaling may induce common pathways in both β-cells and neurons. This result is consistent with other studies demonstrating that an induction of bcl-2 expression through CREB activation is an important part of anti-apoptotic action in neurons [Wilson et al., 1996; Velmurugan et al., 2012). In addition, CREB is known to play a significant role in learning, memory, and synaptic plasticity [Lonze and Ginty, 2002]. Drugs that promote CREB-mediated neuroprotection may also enhance cognition, while disruption of CREB expression may lead to neuronal death and neurodegeneration [Dragunow 2004]. In our study, liraglutide-induced behavioral improvements in mice subjected to mild TBI indicate enhanced cognition, an effect likely mediated, at least in part, through the cAMP/PKA/CREB pathway and CREB-regulated genes (Figure 7). Interestingly, DellaValle et al. [2014] recently found that CREB is activated in the brain of mice subjected to a cold lesion-induced severe brain trauma model, and that liraglutide treatment further promoted CREB activation. The activation of CREB by liraglutide was, however, pathology-dependent as liraglutide-induced CREB activation was only seen in lesioned mice, not in sham mice [DellaValle et al., 2014]. It remains to be determined whether liraglutide treatment can affect CREB activation in vivo in our model of mTBI, and if so, whether the effect is pathology-dependent.
The behavioral data obtained in this study are consistent with our findings in previous studies that utilized the same closed head mTBI model [Rachmany 2013; Tweedie 2013a]. Using this mTBI model, we reliably found that mice display substantial deficits in the novel object recognition paradigm after TBI when compared to sham. This defect in visual memory is significant and detectable at both 7 and 30 days post-mTBI. In this study, we additionally demonstrated that post-treatment with liraglutide successfully prevented mTBI-induced visual memory loss in mice, with liraglutide alone having no effect on this behavior. Complete protection afforded by liraglutide in novel object recognition was observed not only at 7 days post-mTBI, when mice have just finished a 7-day treatment regimen, but also at 30 days post-mTBI, when treatment has been discontinued for 3 weeks, and is suggestive of a long-term rather than interim symptomatic effect. These results are in line with our previous findings that Ex-4, administered either pre- or post-treatment, completely ameliorated mTBI-induced deficits in visual memory [Rachmany 2013]. Such action was associated with broad reversal of gene transcriptome pathways up- and down-regulated by mTBI [Tweedie 2013a]. In the current study, similar to our prior ones with Ex-4, a clinically relevant liraglutide subcutaneous dose was appraised (246.7μg/kg/day) that translates to a human subcutaneous dose of 20 μg/kg/day following normalization to body surface area between species, which is in line with a clinical dose of 1.2 to 1.8 mg liraglutide daily in a 60 to 90 kg human. In order to assess the effects of liraglutide on spatial memory and anxiety, we performed Y-maze and elevated plus maze tests. Although there were trends showing mTBI-induced behavioral deficits in the Y-maze test, particularly at 30 days post-mTBI in which preference index decreased from 0.4 in sham to 0.24 in the mTBI group, these did not reach statistical significance. Such trends were not evident in the mTBI + liraglutide group. No differences in anxiety-like behavior were apparent between the groups at either time point evaluated.
There are controversial opinions in the field about the relationship between GLP-1R agonism and stress. Kinzig et al. [2003] reported that GLP-1 may induce anxiety and suggested that reduction of GLP-1R signaling in the CNS may help treat stress-related diseases [Kinzig et al., 2003]. However, a number of recent studies have demonstrated anti- anxiety and anti-depressive actions of GLP-1R agonists, including liraglutide, in different rodent models such as a T2DM mice model [Komsuoglu Celikyurt et al, 2014] and an alcohol-related rat model [Sharma et al., 2014a]. Since our elevated plus maze data did not show a statistically significant difference between groups, we remain undecided as to the effects of GLP-1R agonism on anxiety and stress.
Perry et al. [2002a&b] and During et al. [2003] initially reported that stimulation of the GLP-1R pathway in the brain is neuroprotective [Perry et al., 2002a&b] and can enhance both associative and spatial learning, as well as memory [During et al., 2003]. To date, numerous studies have replicated neuroprotection and memory improvement in various animal models of T2DM and neurodegenerative disorders induced both by GLP-1R agonists, including liraglutide, and DPP4 inhibitors [Salcedo et al., 2012; Holscher, 2014). For example, our laboratory demonstrated that Ex-4 treatment protected neurons from Aβ toxicity and significantly reduced brain levels of Aβ protein precursor and Aβ in 3xTg-AD mice [Li et al., 2010a; Perry et al., 2003]. McClean et al. [2001] reported that liraglutide treatment enhanced memory formation and synaptic plasticity, while reducing amyloid plaque and inflammation, in the brains of APP/PS1 mice [McClean et al, 2011]. Parallel GLP-1-mediated improvements have been described in rodent models of PD, amyotrophic lateral sclerosis and stroke [Bertilsson et al., 2008; Li et al., 2009, Li et al., 2011]. Interestingly, not only do many TBI survivors experience prolonged or even permanent neurological dysfunction, with lasting changes in cognition, motor function and personality [Comper et al., 2005), a growing body of evidence indicates that TBI triggers a process that leads to early dementia onset [Gardner et al., 2014] and is one of the most powerful environmental risk factors for the development of AD [Felminger et al., 2003] and PD [Gardner et al., 2015]. Using the same mTBI model evaluated herein, our group has shown that mTBI induced changes in gene expression in the hippocampus associated with the development of AD and PD [Tweedie et al., 2013a&b], and that Ex-4 reversed these changes in gene expressions and cognitive impairments [Tweedie et al., 2013a]. We predict that liraglutide, like Ex-4, induces similar changes in gene expression, which may translate into behavioral improvements beneficial to patients with secondary impairment due to TBI. Clearly, further study is needed to test this hypothesis. Interestingly, a recent publication by Hakon et al., [2015] reports beneficial effects of liraglutide in a moderate to severe rat experimental TBI model involving controlled cortical impact, in which the agent reduced cerebral edema, blood-brain barrier opening and early lesion size following TBI.
Due the growing body of evidence on the neuroprotective effect of GLP-1R agonists in cultured neurons and across animal models of neurodegenerative diseases, several clinical trials are currently evaluating translational possibilities of this new class of drugs as a neuroprotective strategy for the treatment of neurological disorders. For example, the National Institute on Aging is currently recruiting patients for a pilot clinical trial of Ex-4 in the treatment of early AD (NCT01255163). Another pilot study on Ex-4, led by University College, London, has shown improved motor and cognitive symptoms in Parkinson’s patients in an open-label trial and a 12-month follow-up study (NCT01174810) [Aviles-Olmos et al., 2013 & 2014). A double-blind placebo-controlled trial is currently ongoing (NCT01971242). Particularly pertinent, the University of North Carolina recently initiated a study to assess the feasibility of exenatide infusion for the treatment of hyperglycemia following acute brain injury (NCT02058940). This is the first trial of an incretin-based drug used for the treatment of brain injury. We look forward to future clinical trials that will evaluate the feasibility of repositioning GLP-1R agonist-based drugs for the treatment of TBI.
Acknowledgements
This research was supported in part by (i) the Intramural Research Program of the National Institute on Aging, National Institutes of Health, (ii) the Ari and Regine Aprijaskis Fund, at Tel-Aviv University, and (iii) a grant from the Israel Science Foundation, grant number 108/09.
Abbreviations
- (TBI)
Traumatic brain injury
- (mTBI)
mild traumatic brain injury
- (GLP-1)
glucagon-like peptide-1
- (GLP-1R)
glucagon-like peptide-1 receptor
- (CREB)
cAMP-response element binding protein
- (AD)
Alzheimer’s disease
- (PD)
Parkinson’s disease
- (DPP-4)
dipeptidyl peptidase-4
- (Ex-4)
exendin-4
- (T2DM)
type 2 diabetes mellitus
- (ATCC)
American Type Culture Collection
- (FBS)
fetal bovine serum
- (DIV)
days in vitro
- (MTS)
3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- (LDH)
lactate dehydrogenase
- (M-PER)
Mammalian Protein Extraction Reagent
- (PBS)
phosphate-buffered saline
- (PVDF)
polyvinylidene fluoride
- (TBST)
tris-buffered saline and Tween 20
- (Bcl-2)
B-cell lymphoma 2
- (Bax)
Bcl-2-associated X protein
- (HRP)
horseradish peroxidase
- (Ac-DEVD-pNA)
acetyl-Asp-Glu-Val-Asp p-nitroanilide
- (pNA)
p-nitroaniline
- (BSA)
bovine serum albumin
- (AIF)
apoptosis-inducing factor
- (LEAD)
Liraglutide Effect and Action in Diabetes
- (PKA)
protein kinase A
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4:337–344. doi: 10.3233/JPD-140364. [DOI] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, Greig NH, Pick CG. Tumor necrosis factor-alpha synthesis inhibitor, 3,6 ′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Wang JY, Rubovitch V, Luo W, Hoffer BJ, Greig NH, Pick CG. Transiently lowering tumor necrosis factor-á synthesis ameliorates neuronal cell loss and cognitive impairments induced by minimal traumatic brain injury in mice. J Neuroinflammation. 2015;12:45. doi: 10.1186/s12974-015-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SW. An unconventional hypothesis of oxidation in Alzheimer’s disease: intersections with excitotoxicity. Front Biosci. 2004;9:3286–3295. doi: 10.2741/1481. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011a;30:33–48. vii–iii. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83:312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, Group L-S. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Garcia GE, Huang W, Constantini S. The involvement of secondary neuronal damage in the development of neuropsychiatric disorders following brain insults. Front Neurol. 2014;5:22. doi: 10.3389/fneur.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper P, Bisschop SM, Carnide N, Tricco A. A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005;19:863–880. doi: 10.1080/02699050400025042. [DOI] [PubMed] [Google Scholar]
- Corso P, Finkelstein E, Miller T, Fiebelkorn I, Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj Prev. 2006;12:212–218. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res. 2006;3:269–283. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- DellaValle B, Hempel C, Johansen FF, Kurtzhals JA. GLP-1 improves neuropathology after murine cold lesion brain trauma. Ann Clin Transl Neurol. 2014;1:721–732. doi: 10.1002/acn3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, Samali A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med. 2011;15:2025–2039. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M. CREB and neurodegeneration. Front Biosci. 2004;9:100–103. doi: 10.2741/1197. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Eakin K, Li Y, Chiang YH, Hoffer BJ, Rosenheim H, Greig NH, Miller JP. Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS One. 2013;8:e82016. doi: 10.1371/journal.pone.0082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015 doi: 10.1002/ana.24396. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig NH, Tweedie D, Rachmany L, Li Y, Rubovitch V, Schreiber S, Chiang YH, Hoffer BJ, Miller J, Lahiri DK, Sambamurti K, Becker RE, Pick CG. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014;10:S62–75. doi: 10.1016/j.jalz.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- Hakon J, Ruscher K, Romner B, Tomasevic G. Preservation of the blood brain barrier and cortical neuronal tissue by liraglutide, a long acting glucagon-like-1 analogue, after experimental traumatic brain injury. PLoS One. 2015;10(3):e0120074. doi: 10.1371/journal.pone.0120074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimers Dement. 2014;10:S47–54. doi: 10.1016/j.jalz.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- Khatri N, Man HY. Synaptic activity and bioenergy homeostasis: implications in brain trauma and neurodegenerative diseases. Front Neurol. 2013;4:199. doi: 10.3389/fneur.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kang JH, Park YG, Ryu GR, Ko SH, Jeong IK, Koh KH, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, Jo YH. Exendin-4 induction of cyclin D1 expression in INS-1 beta-cells: involvement of cAMP-responsive element. J Endocrinol. 2006;188:623–633. doi: 10.1677/joe.1.06480. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ, Figueredo HF. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komsuoglu Celikyurt I, Mutlu O, Ulak G, Uyar E, Bektaş E, Yildiz Akar F, Erden F, Tarkun I. Exenatide treatment exerts anxiolytic- and antidepressant-like effects and reverses neuropathy in a mouse model of type-2 diabetes. Med Sci Monit Basic Res. 2014;20:112–117. doi: 10.12659/MSMBR.891168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Simon CM, Prado GR, Cullen DK. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- Leibson CL, Brown AW, Hall Long K, Ransom JE, Mandrekar J, Osler TM, Malec JF. Medical care costs associated with traumatic brain injury over the full spectrum of disease: a controlled population-based study. J Neurotrauma. 2012;29:2038–2049. doi: 10.1089/neu.2010.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J, Malec JF. Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology. 2011;22:836–844. doi: 10.1097/EDE.0b013e318231d535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, Mattson MP, Wang Y, Harvey BK, Ray B, Lahiri DK, Greig NH. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7:e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK, Greig NH. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010a;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 2010b;113:1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J, Malec JF. Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology. 2011;22:836–844. doi: 10.1097/EDE.0b013e318231d535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C. Object recognition in mice: improvement of memory by glucose. Neurobiol Learn Mem. 1997;67:172–175. doi: 10.1006/nlme.1996.3755. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Mondragon A, Davidsson D, Kyriakoudi S, Bertling A, Gomes-Faria R, Cohen P, Rothery S, Chabosseau P, Rutter GA, da Silva Xavier G. Divergent effects of liraglutide, exendin-4, and sitagliptin on beta-cell mass and indicators of pancreatitis in a mouse model of hyperglycaemia. PLoS One. 2014;9:e104873. doi: 10.1371/journal.pone.0104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Ohtake N, Saito M, Eto M, Seki K. Exendin-4 promotes the membrane trafficking of the AMPA receptor GluR1 subunit and ADAM10 in the mouse neocortex. Regul Pept. 2014:190–191. 1–11. doi: 10.1016/j.regpep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Parthsarathy V, Hölscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One. 2013;8:e58784. doi: 10.1371/journal.pone.0058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T, Haughey N, Mattson M, Egan J, Greig N. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002a;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri D, Chen D, Zhou J, Shaw K, Egan J, Greig N. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002b;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-β peptide (Aβ) levels and protects hippocampal neurons from death induced by Aβ and iron. Journal of Neuroscience Research. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, Greig NH. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Experimental Neurology. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM. AIF, reactive oxygen species, and neurodegeneration: a “complex” problem. Neurochem Int. 2013;62:695–702. doi: 10.1016/j.neuint.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Giza CC. Repeat traumatic brain injury in the developing brain. Int J Dev Neurosci. 2012;30:185–190. doi: 10.1016/j.ijdevneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr) 2013;35:1621–1636. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL, Riechers RG. Effective treatment of traumatic brain injury: learning from experience. JAMA. 2012;308:2032–2033. doi: 10.1001/jama.2012.14008. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res Brain Res Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Scott LJ. Liraglutide: a review of its use in adult patients with type 2 diabetes mellitus. Drugs. 2014;74:2161–2174. doi: 10.1007/s40265-014-0321-6. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Pise A, Sharma JN, Shukla P. Glucagon-like peptide-1 (GLP-1) receptor agonist prevents development of tolerance to anti-anxiety effect of ethanol and withdrawal-induced anxiety in rats. Metab Brain Dis. 2014a doi: 10.1007/s11011-014-9627-z. [DOI] [PubMed] [Google Scholar]
- Sharma MK, Jalewa J, Hölscher C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J Neurochem. 2014b;128:459–471. doi: 10.1111/jnc.12469. [DOI] [PubMed] [Google Scholar]
- Shi HY, Hwang SL, Lee KT, Lin CL. Temporal trends and volume-outcome associations after traumatic brain injury: a 12-year study in Taiwan. J Neurosurg. 2013;118:732–738. doi: 10.3171/2012.12.JNS12693. [DOI] [PubMed] [Google Scholar]
- Shin S, Le Lay J, Everett LJ, Gupta R, Rafiq K, Kaestner KH. CREB mediates the insulinotropic and anti-apoptotic effects of GLP-1 signaling in adult mouse β-cells. Mol Metab. 2014;3:803–812. doi: 10.1016/j.molmet.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien) 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013a;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Zhang Y, Becker KG, Perez E, Hoffer BJ, Pick CG, Greig NH. Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol Dis. 2013b;54:1–11. doi: 10.1016/j.nbd.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde S, Hogan MF, Montminy M. mTOR links incretin signaling to HIF induction in pancreatic beta cells. Proc Natl Acad Sci U S A. 2011;108:16876–16882. doi: 10.1073/pnas.1114228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan K, Balamurugan AN, Loganathan G, Ahmad A, Hering BJ, Pugazhenthi S. Antiapoptotic actions of exendin-4 against hypoxia and cytokines are augmented by CREB. Endocrinology. 2012;153:1116–1128. doi: 10.1210/en.2011-1895. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp (Wars) 2011;71:36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]