ABSTRACT
Cerebrospinal fluid (CSF) includes conserved factors whose function is largely unexplored. To assess the role of CSF during embryonic development, CSF was repeatedly drained from embryonic zebrafish brain ventricles soon after their inflation. Removal of CSF increased cell death in the diencephalon, indicating a survival function. Factors within the CSF are required for neuroepithelial cell survival as injected mouse CSF but not artificial CSF could prevent cell death after CSF depletion. Mass spectrometry analysis of the CSF identified retinol binding protein 4 (Rbp4), which transports retinol, the precursor to retinoic acid (RA). Consistent with a role for Rbp4 in cell survival, inhibition of Rbp4 or RA synthesis increased neuroepithelial cell death. Conversely, ventricle injection of exogenous human RBP4 plus retinol, or RA alone prevented cell death after CSF depletion. Zebrafish rbp4 is highly expressed in the yolk syncytial layer, suggesting Rbp4 protein and retinol/RA precursors can be transported into the CSF from the yolk. In accord with this suggestion, injection of human RBP4 protein into the yolk prevents neuroepithelial cell death in rbp4 loss‐of‐function embryos. Together, these data support the model that Rbp4 and RA precursors are present within the CSF and used for synthesis of RA, which promotes embryonic neuroepithelial survival. © 2015 Wiley Periodicals, Inc. Develop Neurobiol 76: 75–92, 2016
Keywords: cerebrospinal fluid, retinoic acid, cell survival, brain development, RBP4
INTRODUCTION
Deep within the vertebrate brain lie a series of connected cavities, the brain ventricles, which form from the central lumen of the neural tube and are maintained into adulthood. After the brain ventricles form they fill with a protein rich cerebrospinal fluid (CSF) that circulates, drains and is replenished continuously throughout life. CSF cushions the adult brain, removes waste and transports secreted molecules (Chodobski and Szmydynger‐Chodobska, 2001; Redzic et al., 2005). Compared with adult CSF, embryonic CSF contains three times more protein (Zheng and Chodobski, 2005) and the spectrum of proteins is conserved (Zappaterra et al., 2007) suggesting that CSF components may be crucial for normal brain development.
CSF is an important fluid for the development of the embryonic brain; however, the factors and signaling pathways involved, and the developmental processes affected are just beginning to be identified. The role of CSF during development has been examined in vitro using chick neuroepithelial explants or cultured mouse and chick embryos. These studies suggest that secreted factors within the CSF, hydrostatic pressure, or a combination of the two are required for gene expression, cell proliferation, survival, and neurogenesis (Desmond et al., 2005; Gato et al., 2005; Parada et al., 2005; Mashayekhi and Salehi, 2006a,b; Miyan et al., 2006; Salehi and Mashayekhi, 2006; Lehtinen et al., 2011). Insulin‐like growth factor 2 (IGF2), retinoic acid (RA), fibroblast growth factor 2 (FGF2) and low‐density lipoproteins were identified within mouse and/or chick CSF and, when applied to explanted chick or mouse neuroepithelium, could promote neurogenesis and cell proliferation (Martin et al., 2006; Parada et al., 2008; Parada et al., 2008; Salehi et al., 2009; Alonso et al., 2011; Lehtinen et al., 2011). Together, these studies indicate the importance of CSF during brain development, but also raise several major questions regarding CSF function during development. First, since these studies examined the activity of CSF and CSF factors in culture are these functions conserved in the embryo? Second, what downstream signaling pathways mediate the effects of CSF? Third, what is the effect of chronic removal of CSF and, when is CSF required during development? Fourth, where are CSF factors produced? Finally, are there CSF factors that regulate neuroepithelial cell survival in the embryo and, if so, what are these factors?
RA is a lipophilic vitamin A metabolite that is essential for embryonic development (Sive et al., 1990) and vertebrate brain development (Haskell and LaMantia, 2005; Siegenthaler et al., 2009; Chatzi et al., 2011; Siegenthaler and Pleasure, 2011; Chatzi et al., 2013). The RA precursor, retinol, is transported by RBP4 (Blomhoff et al., 1990) and subsequently metabolized into RA by a series of dehydrogenases (Cunningham and Duester, 2015). Abnormal RA signaling disrupts embryonic patterning, neuronal plasticity, differentiation, regeneration, learning, and memory (Sive et al., 1990; Krezel et al., 1998; Waclaw et al., 2004; Maden, 2007). Several studies suggest that RA promotes survival and differentiation of neurons. In adult mice, RA is required for olfactory neuron survival (Hagglund et al., 2006) and promotes neurogenesis in the dentate gyrus and hippocampus (Jacobs et al., 2006; Bonnet et al., 2008). Retinol and RBP4 were previously isolated from chick CSF (Parada et al., 2008) and, when added to cultured neuroepithelium explants, can increase neurogenesis (Alonso et al., 2011). However, the mechanism by which RA signaling from the CSF promotes brain development in the embryo and whether RA is required for neuroepithelial cell survival has not been previously examined.
The zebrafish is an ideal system to define the mechanisms underlying CSF function during brain development (Lowery and Sive, 2005; Gutzman and Sive, 2009; Chang et al., 2012). Since embryonic development occurs externally CSF withdrawal and factor replacement can be performed in living embryos (Chang and Sive, 2012). This study is the first characterization of CSF function in embryonic zebrafish after chronic removal. Our results connect CSF function, RA signaling, and neuroepithelial cell survival during embryonic brain development.
MATERIALS AND METHODS
Fish Lines and Maintenance
Wild type (AB) Danio rerio fish were raised and bred according to standard methods (Westerfield et al., 2001). Embryos were kept at 28.5°C and staged accordingly (Kimmel et al., 1995). Stages of development are expressed as hours post‐fertilization (hpf).
Brightfield Brain Imaging
During imaging, embryos were anesthetized in 0.1 mg/mL Tricaine (Sigma) dissolved in embryo medium (E3) made according to (Westerfield et al., 2001). Images were taken using a Leica dissecting scope and KT Spot digital camera (RT KE Diagnostic instruments). Images were adjusted for brightness, contrast, and coloring in Photoshop CS5 (Adobe).
Antisense Oligonucleotide Morpholinos
A splice‐site blocking Morpholinos (MO) (rbp4sp: 5′ GTTGACTTACCCTCGTTCTGTTAAA 3′, Gene Tools, LLC) was used to target rbp4 exon2/intron3 as previously described (Nasevicius and Ekker, 2000; Li et al., 2007). Standard control MO (5′‐CCTCTTACCTCAGTTACAATTTATA‐3′) and p53 morpholino (5′‐GCGCCATTGCTTTGCAAGAATTG‐3′) were used (Gene Tools, LLC). MOs were injected at the single cell stage and embryos were analyzed at 24 hpf or 36 hpf. Unless otherwise noted, 2.5 ng rbp4sp plus co‐injection of 3.75 ng p53 MOs (1.5x the target MO) was used. Concentrations used were determined to be the minimum amount of MO that resulted in elevated levels of MO‐specific neuroepithelial cell death. MO‐specific cell death was identified by the ability to rescue cell death with mRNA, which is not recognized by the MO, or with purified protein.
Primers used to detect splicing changes due to rbp4 MO were: rbp4F1 5′ GAGAACGAGGTATCAAGGAAC 3′, rbp4F2 5′ GTAAGTCAACCAGTGTTTCC 3′, rbp4R1 5′ CGCGTCTGTATTTGCCCAGG 3′ as previously demonstrated (Li et al., 2007).
The full‐length zebrafish rbp4 was obtained by PCR and subcloned into pCS2+ with a minimal Kozak consensus sequence adjacent to the initiating ATG. Primers used were: rbp4seqF 5′ TGTGAATTAGCACAGAGGACAGT 3′, rbp4seqR 5′ GGGCAGATTATATCATCAGAAGC 3′. Capped rbp4 mRNAs were obtained by in vitro transcription using the SP6 mMessage mMachine kit (Ambion) after linearization. Embryos were injected at the one‐cell stage with 100 pg of rpb4 mRNA, the minimum amount of mRNA that rescued MO phenotype.
Manual Drainage and Brain Ventricle Injection
Manual drainage technique was performed as previously described (Chang and Sive, 2012). Briefly, a micropipette needle was inserted through the roof plate of the hindbrain ventricle and was positioned either in the hindbrain, midbrain, or forebrain ventricle. CSF was removed from all three brain ventricles, using an Eppendorf CellTram oil microinjector apparatus, every one to two hours from 22–36 hpf (referred to as drained; six times total, [Fig. 1 (A)]). After each drain, the needle was removed and embryos stored at 32°C to speed up development in order to decrease the amount of experimental time required to examine a 14 hour developmental time window. As a control, the micropipette needle was inserted into embryos without removing any CSF (referred to as punctured). Unpunctured and punctured embryos were also stored at 32°C from 22–36 hpf. To introduce factors into drained embryos, 1–2 nL of a factor was injected every 2 hours from 30–36 hpf (three times total, [Fig. 3(A)]) into the brain ventricles as described previously (Gutzman and Sive, 2009). Factors used include: E10.5 mouse CSF (frozen), physiological saline/artificial CSF (118 mM NaCl, 2 mM KCl, 10 mM MgCl2, 10 mM HEPES, 10 mM glucose), Caspase 3 Inhibitor I (Millipore; 500uM), Insulin like growth factor 2 recombinant human (IGF2, US Biologicals, 25ng/mL), Fibroblast growth factor 2 (FGF2, Sigma, 300 μg/mL), all‐trans RA (Sigma, 10−8M), all‐trans retinol (Sigma, 348 nM), recombinant human RBP4 (R&D systems, 2 ng/μL), A1120 (Sigma 30 × 10−8M), Citral (Sigma 250 μM), 4‐Diethylaminobenzaldehyde (DEAB, Sigma, 10 μM). DMSO diluted 1:10‐1:1000 in E3 or E3 alone were used as negative control injections.
Figure 1.
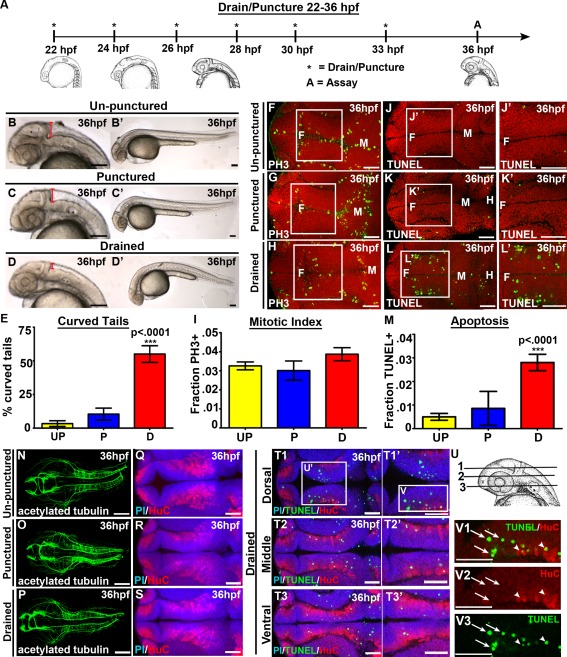
CSF promotes cell survival and tail extension. (A) Experimental design. Drainage/puncture (*) occurred every 2 hours from 22 to 36 hpf. Embryos were assayed (A) at 36 hpf. (B–D) Brightfield lateral view of unpunctured (B), punctured (C), and drained (D) embryos. (B′–D′) Whole embryo phenotype. (E) Percent of embryos with curved tails. (F–H) Dorsal view of PH3 (green) and (J–L) TUNEL (green). Propidium iodide in red. (F‐H; J‐L) white box indicates region quantified. (J′,K′L′) Magnified image of region indicated by white box in J,K,L. (I,M) Quantification of the fraction of PH3 (I) and TUNEL (M) positive cells. (N–P) Dorsal view of acetylated tubulin (green) or (Q–S) HuC (red) and propidium iodide (blue). (T) Dorsal/ventral sections of neural tube (T1‐3) stained with TUNEL (green), HuC (red) and propidium iodide (blue). (U) Representative dorsal/ventral sections of neural tube shown in T. 1‐3 correspond to T1‐T3. (V) Magnified image of TUNEL and HuC (V1), HuC alone (V2) and TUNEL alone (V3) from dorsal section of diencephalon (T1). (N‐S) projection overlaid on single slice of propidium iodide image, all other images are a single 0.6 μm slice. Data represented as mean ± SEM. F= forebrain, M = midbrain. UP = unpunctured, P = punctured, D = drained. Scale bars = 50 μm.
Figure 3.
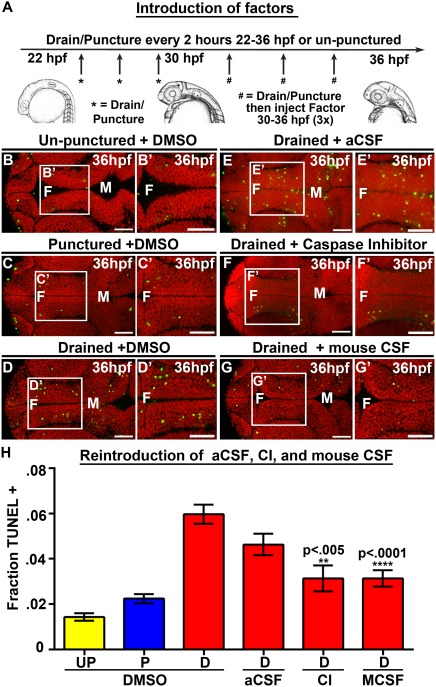
Introduction of Caspase 3 inhibitor and mouse CSF but not aCSF prevents cell death. (A) Experimental design. Drainage/puncture occurred every 2 hours from 22 to 36 hpf. From 30 to 36 hpf a factor was injected every 2 hours into the brain ventricles after drainage/puncture. Embryos were assayed at 36 hpf. (B–G) Dorsal view of TUNEL (green) and propidium iodide (red) in un‐punctured (B) and punctured (C) embryos injected with DMSO or drained embryos injected with DMSO (D) aCSF (E), Caspase 3 inhibitor (F), or E10.5 mouse CSF (G). (H) Quantification of TUNEL. Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained, CI = Caspase 3 inhibitor, MCSF = E10.5 mouse CSF. Scale bars = 50 μm.
To rescue rbp4 morphant phenotype with all‐trans RA, embryos were soaked in RA (10−8 M) from 18–36 hpf and subsequently fixed for TUNEL assay at 36 hpf.
Immunohistochemistry
Whole mount immunohistochemistry was performed using propidium iodide (Invitrogen; 1:1000), PH3 (Millipore 06‐570; 1:800), acetylated tubulin (Sigma, T6793; 1:1000), HuC (Invitrogen A21271: 1:500), GABA (Sigma 2052; 1:500), tyrosine hydroxylase (TH, Millipore MAB318; 1:100) and 5‐HT (serotonin; Sigma, S5545; 1:100). Embryos were fixed with 4% PFA or 2% TCA for two hours at room temperature or overnight at 4°C and blocked overnight at 4°C or for two hours at room temperature. Blocking solutions used were: 2% normal goat serum (NGS) + 1% Triton + 2% BSA + 1% DMSO (PH3), 10% NGS + 0.1% BSA + 1% Triton (acetylated tubulin), 10% NGS + 1% Triton (TH, 5HT, HuC) and 10% NGS + 3% BSA + 1% Triton (GABA). For TH, 5‐HT and GABA, embryos were dehydrated into 100% EtOH and acetone treated at −20°C for 1 hour before proceeding to blocking step. For TUNEL (Apoptag®, Millipore), embryos were fixed overnight in 4% PFA and dehydrated into 100% EtOH. After rehydration, embryos were treated in Proteinase K (2.5 ug/mL, Invitrogen) for 2 minutes and rinsed well. Embryos were subsequently treated using the recommended Apoptag® protocol. TUNEL blocking solution used was 10% BMB (Boehringer Mannheim Blocking reagent) + 10% lamb serum + 80% maleic acid buffer and primary antibody anti‐DIG‐Fluorescein (Roche; 1:100). Propidium iodide was diluted 1:1000 in 1× PBT (PBS + 0.01% Tween) and incubated at room temperature for 45 minutes. Secondary antibodies goat anti‐mouse Alexa Fluor 488, goat anti‐rabbit Alexa Fluor 488 (Sigma), or goat anti‐rabbit Cy5 (Jackson ImmunoResearch) were used at a 1:500 dilution.
Confocal Imaging and Cell Counts
Stained embryos were flat‐mounted as previously described (Cheng et al., 2014) and imaged using the 25× or 63× oil objective on a Zeiss 710 scanning confocal microscope. For quantification of TUNEL or PH3, a single 0.6 μm slice was imaged at 63× in the diencephalon at the dorsal/ventral level where the retina and lens of the eye are in focus and the posterior region of the forebrain ventricle positioned at one edge of the viewing field (example is [Fig. 1(J′)]). This region was chosen since it is an easily reproducible region to image. The number of TUNEL or PH3 positive cells and total number of cells (labeled with propidium iodide) within the diencephalon were manually counted using Image J Cell counter plugin. A 5500 μm2 box surrounding the diencephalon on one side of the ventricular space was quantified and performed for both sides of the diencephalon. Fraction of TUNEL or PH3 positive cells were calculated to account for any alterations in total number of cells in treated versus control groups. GraphPad Prism was used to generate graphs and the unpaired t‐test was used to analyze statistical significance.
Mass Spectrometry
Sample Preparation
CSF was collected from 500 embryos, and stored at −80°C in PBS + protease inhibitors (Roche cOmplete Mini tablets EDTA free). Experiment was repeated three times. Proteins were initially separated on a 10–20% SDS‐PAGE gel, and each gel lane to be analyzed was excised and cut into six segments of approximately equal length. Each gel fragment was further cut into smaller pieces, typically 1 mm2 in order to facilitate destaining and SDS removal. In‐gel reduction, alkylation, and trypsin digestion were performed following published procedures (Shevchenko et al., 2007). Trypsin digestion was carried out overnight at room temperature.
Mass Spectrometry
Extracted proteolytic peptides were analyzed by LC‐MS. Peptide separation was carried out with gradient elution (water‐acetonitrile‐0.1% formic acid) from a 75m ID capillary reversed phase C18 column (New Objective) using an Agilent 1100 nano HPLC system (Agilent Technologies); the flow rate was 280 nL/min. In order to optimize peptide separation, a three hour long gradient was used. Peptide molecular weight data as well as peptide fragment ion mass spectra were acquired with an LTQ ion trap mass spectrometer (ThermoFischer Scientific).
Database Searching
Tandem mass spectra were extracted by BioworksBrowser Version 3.3 (ThermoFisher) and submitted to the Mascot database search software version 2.2 (Matrix Science). Charge state deconvolution and deisotoping were not performed. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version Mascot). Mascot was set up to search the NCBInr_081410 database (selected for Danio rerio, unknown version, 44358 entries) assuming the digestion enzyme trypsin. Mascot was searched with a fragment ion mass tolerance of 0.60 Da and a parent ion tolerance of 2.0 Da. Iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification. Deamidation of asparagine and glutamine and oxidation of methionine were specified in Mascot as variable modifications.
Criteria for Protein Identification
Scaffold (version Scaffold_3.3.1, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they exceeded specific database search engine thresholds. Mascot identifications required at least ion scores must be greater than both the associated identity scores and 20, 30, 40, and 40 for singly, doubly, triply, and quadruply charged peptides. Protein identifications were accepted if they contained at least 1 identified peptide in all three experiments performed. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
RESULTS
CSF Is Required for Cell Survival
We began with the hypothesis that CSF is required for proper embryonic brain development. To address this, we used an in vivo technique to manually drain CSF from embryonic zebrafish brain ventricles generating an CSF‐depleted embryo (Chang and Sive, 2012). We drained CSF from 22 hours post fertilization (hpf), when CSF first begins to fill zebrafish embryonic brain ventricles, to 36 hpf spanning a period during which there is a significant amount of cell proliferation, neuronal differentiation, and axonal projection necessary for proper brain morphogenesis and development (Kimmel and Westerfield, 1990; Lowery and Sive, 2005). CSF can be fully replenished after 2–3 hours (Chang and Sive, 2012), therefore, we drained CSF every two hours from 22–36 hpf [Fig. 1(A)]. To ensure that the needle itself was not causing abnormal brain development, we inserted a needle into the embryonic brain without CSF removal (referred to as punctured). Although some CSF does leak out of the needle hole in punctured embryos, we did not observe a difference in gross morphology or brain ventricle size compared with unpunctured embryos [Fig. 1(B,C)]. However, manual drainage from 22–36 hpf reduces brain ventricle size compared with unpunctured and punctured [Fig. 1(D)]. Additionally, 55% of drained embryos had curved tails (n = 60, p < 0.0001) compared with 4% in unpunctured (n = 60), and 11% in punctured (n = 62) embryos [Fig. 1(B–E)] demonstrating that CSF is also necessary for proper tail straightening. At this stage of development, the brain ventricles connect directly to the spinal canal in the tail (Kramer‐Zucker et al., 2005). Therefore, CSF depletion from the brain ventricles may also lead to reduction of CSF within the spinal canal that may result in the curved tail observed in drained embryos.
Since CSF‐depleted embryos had curved tails and CSF is in direct contact with the neuroepithelium, we asked whether there were also abnormalities in brain development. To address this, we analyzed cell proliferation and cell death in our CSF‐depleted system. To label dividing cells, we used phospho‐histone H3 (PH3) and quantified the mitotic index in the diencephalon, the region indicated by the white box [Fig. 1(F–H)]. Drained embryos (n = 15, p = 0.12) did not exhibit a significant change in mitotic index compared with unpunctured (n = 15) or punctured embryos (n = 9) [Fig. 1(F–I)], suggesting that CSF is not required for proliferation at this developmental stage. Next, we assayed for cell death by marking dying cells using TUNEL. We observed a five‐fold increase in cell death in drained embryos (n = 8, p < 0.0001) compared with unpunctured (n = 9) and punctured brains (n = 7) [Fig. 1(J–M)] demonstrating that removal of CSF, and not the insertion of the needle into the brain ventricles, is associated with increased cell death. Although TUNEL positive cells were observed throughout the neuroepithelium after drainage, there was an enrichment of dying cells within two lateral clusters in the dorsal diencephalon [Fig. 1(L, T–U)].
We asked whether we could identify which cells were dying after CSF drainage by analyzing neural progenitor and neuronal markers. Neurogenesis was assayed by immunohistochemistry using acetylated tubulin to label axon tracts. Gross abnormalities in axonal projections were not observed in CSF‐depleted embryos [Fig. 1(N–P)] (unpunctured, n = 10; punctured, n = 14; drained, n = 14). Similarly, drainage (n = 12) did not significantly alter the expression pattern of the pan‐neuronal marker HuC, which labels newly born neurons, compared with punctured (n = 7) or unpunctured embryos (n = 12) [Fig. 1(Q–S)]. Co‐labeling of TUNEL and HuC positive cells showed that a few TUNEL positive‐cells seem to colocalize with HuC positive cells, however, the majority of TUNEL positive‐cells do not colocalize with HuC [Fig. 1(T–V)]. Consistently, we did not observe obvious differences in specific neuronal populations using either NeuroD‐GFP transgenic zebrafish, which label differentiating neurons (Ulitsky et al., 2011), or antibodies detecting serotoninergic, dopaminergic and GABAergic neurons in drained embryos compared with controls, nor did these populations significantly colocalize with TUNEL positive cells [Supporting Information Fig. 1, 2, and data not shown]. Although we cannot rule out the possibility that smaller populations of neurons are affected, repeated depletion of CSF from 22–36 hpf did not significantly alter gross neurogenesis in the developing zebrafish embryo. Together, the data demonstrate that zebrafish CSF is required from 22–36 hpf for tail straightening and neuroepithelial cell survival.
Defining a Sensitive Period for CSF
To identify developmental periods where CSF promotes cell survival, we removed fluid for differing lengths of time. Embryos that were drained only once at 22 hpf (n = 10, p = 0.60), or twice from 22–24 hpf (n = 8, p = 0.76) and allowed to recover until 36 hpf did not show increased cell death compared with controls [Fig. 2(A), Supporting Information Fig. 3]. Draining from 22–26 hpf with subsequent recovery until 36 hpf (n = 10, p = 0.15), resulted in a slight increase in cell death [Fig. 2(A), Supporting Information Fig. 3], while removal of CSF from 22–28 (n = 9, p = 0.04) or 22–30 hpf (n = 6, p = 0.0006) with recovery until 36 hpf was associated with significantly increased levels of cell death compared with unpunctured or punctured embryos [Fig. 2(A), Supporting Information Fig. 3]. Furthermore, we observed a significant increase in cell death in embryos drained from 22–30 hpf (n = 8, p = 0.007) and immediately assayed at 30 hpf [Fig. 2(B), Supporting Information Fig. 3]. Together, the data suggest a requirement for CSF from 25 to 30 hpf to promote cell survival during early stages of embryonic brain development.
Figure 2.
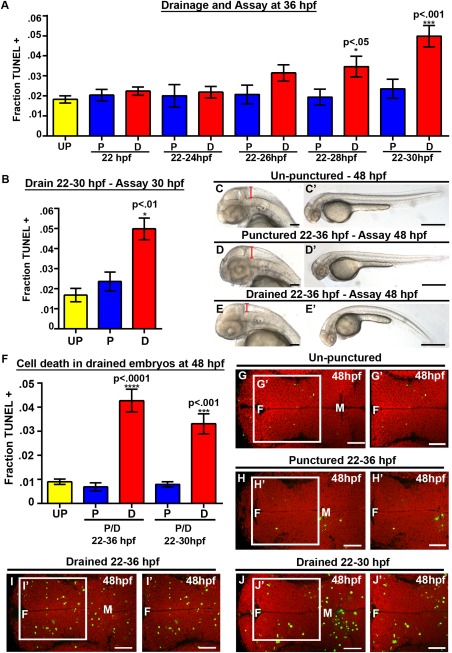
CSF is required from 25 to 30 hpf, while cell death persists at 48 hpf. (A) Quantification of TUNEL. X axis indicates puncture/drainage period. All assayed at 36 hpf. (B) Quantification of TUNEL at 30 hpf after puncture/drainage from 22 to 30 hpf. (C–E) Brightfield dorsal and lateral (C′–E′) images of 48 hpf embryos after puncture/drainage from 22 to 36 hpf. (F) Quantification of TUNEL at 48 hpf. (G–J) Dorsal view of TUNEL (green) and propidium iodide (red) in 48 hpf embryos after puncture/drainage from either 22–36 hpf (H–I) or 22–30 hpf (J) or unpunctured (G). Data represented as mean ± SEM. F = forebrain, M = midbrain. UP = unpunctured, P = punctured, D = drained. Scale bars = 50 μm.
In order to determine whether the early requirement for fluid has long term effects on embryonic development, embryos were assayed at 48 hpf after drainage from 22–36 hpf. At 48 hpf, drained embryos had reduced brain ventricle size and curved tails indicating that brain ventricle size, inflation and tail straightening are affected by CSF depletion and do not fully recover [Fig. 2(C–E)] (drained [75% curved tails; n = 20] compared with punctured [30% curved tails; n = 17] and unpunctured embryos [0% curved tails; n = 19]). A five‐fold increase in cell death was observed in embryos drained from 22–36 hpf and assayed at 48 hpf (n=20, p<.0001) compared with punctured (n = 17) or unpunctured (n = 18) [Fig. 2(F–I)]. Although cell death was quantified in the diencephalon at 48 hpf, we also observed an increase in neuroepithelial cell death throughout the embryonic brain, thus it is unlikely that a specific neuronal cell population is dying. Consistently, no change was observed in HuC‐positive, serotonergic, dopaminergic or GABAergic populations of cells at 48 hpf after drainage from 22–36 hpf [data not shown]. Similarly, drainage or puncture from 22 to 30 hpf with recovery to 48 hpf, increased cell death in drained (n = 16, p = 0.0006) compared with punctured (n = 14) consistent with a critical period between 25 and 30 hpf [Fig. 2(F, J)]. Together these data demonstrate that CSF is required from 25 to 30 hpf to promote cell survival and the increase in cell death is first present at 30 hpf and persists through 48 hpf. These data highlight a sensitive period for neuroepithelial cell survival mediated by the CSF.
Cell Survival Is Not Dependent on Ionic Concentration or Presence of Fluid
The increase in cell death after CSF removal suggests a requirement for the presence of fluid or for specific factors within the fluid. We tested the hypothesis that the presence of fluid was necessary by injecting physiological saline/artificial CSF (aCSF) into the brain ventricle lumen of CSF‐depleted embryos. We also addressed whether cell death occurred by apoptosis using a Caspase 3 inhibitor (Millipore). DMSO, aCSF, or the Caspase 3 inhibitor were injected three times from 30 to 36 hpf into the brain ventricles of drained, punctured (22–36 hpf) and unpunctured embryos [Fig. 3(A)]. An increase in cell death was observed in drained embryos injected with DMSO (n = 32, p < 0.0001) compared with unpunctured or punctured brains plus DMSO [Fig. 3(B–D,H)]. However, injection of aCSF into drained embryonic brain ventricles, ensuring inflation of ventricles comparable to wild type brain ventricle size, did not prevent the increase in cell death seen after CSF drainage (n = 7, p = 0.16) [Fig. 3(E,H), Supporting Information Fig. 4]. These findings indicate that the fluid component of CSF and ionic concentration are not sufficient to restore cell survival. Injection of the Caspase 3 inhibitor significantly reduced levels of cell death in drained embryos compared with those injected with DMSO (n = 9, p = 0.0014) [Fig. 3(F,H), Supporting Information Fig. 4) indicating that cell death occurs via apoptosis.
Since introduction of aCSF could not prevent cell death in CSF‐depleted embryos, we hypothesized that factors in the CSF regulate cell survival. As an initial test, we asked whether introduction of mouse CSF promoted cell survival after endogenous CSF withdrawal. E10.5 mouse CSF was used as relatively large amounts could be obtained and that time point during mouse brain development is similar to zebrafish at 25 hpf. Mouse CSF was injected into drained, punctured and un‐punctured embryonic zebrafish brain ventricles from 30 to 36 hpf [Fig. 3(A)]. Drained embryos injected with mouse CSF showed significantly less cell death (n = 16, p < 0.0001) [Fig. 3(G,H), Supporting Information Fig. 4] suggesting that a CSF factor provides the cell survival signal. In contrast, we did not observe a rescue of tail straightening by mouse CSF [data not shown]. Together, the data suggest that the presence of fluid is not sufficient to promote cell survival and rather, that the CSF contains at least one conserved neuroepithelial cell survival factor. This is the first demonstration that vertebrate CSF function is conserved and suggests that CSF likely plays a similar role during the development of the nervous system across vertebrate species.
Retinoic Acid Can Substitute for CSF to Promote Neuroepithelial Cell Survival
In order to identify candidate survival factors within zebrafish CSF, we performed mass spectrometry analysis of CSF collected from 500 zebrafish embryos at 25–30 hpf, when CSF is necessary for cell survival [Fig. 2(A)]. Fractions of CSF were subjected to trypsin digestion and subsequent LC‐MS/MS analysis. We identified 378 unique proteins within the zebrafish CSF of which 163 proteins were conserved in either human or rat CSF while 73 were identified in both human and rat CSF (Zappaterra et al., 2007) demonstrating that CSF content is highly conserved [Supporting Information Table 1].
We hypothesized that among the CSF factors identified one or more was required for neuroepithelial cell survival. One interesting candidate was Rbp4 [Supporting Information Table 1], which has also been identified within human, rat, and chick CSF (Zappaterra et al., 2007; Parada et al., 2008). RBP4 is a plasma protein that transports retinol, the precursor for RA. We therefore hypothesized that RA would mediate cell survival through the CSF. To test this, RA or DMSO was injected from 30 to 36 hpf into embryos drained or punctured from 22 to 36 hpf, or unpunctured embryos [Fig. 3(A), Fig. 4(A–D), Supporting Information Fig. 5]. After RA injection into drained brain ventricles, cell death was reduced (n = 16, p < 0.0001) compared with DMSO controls (n = 39) [Fig. 4(C–E)] to levels comparable to punctured or unpunctured embryos [Fig. 4(A, B, E), Supporting Information Fig. 5] (unpunctured + DMSO [n = 41], punctured + DMSO [n = 38]). Exogenous RA injected into un‐punctured or punctured embryos did not change cell death levels compared with DMSO injection demonstrating that RA cannot block “normal” cell death observed during development [Supporting Information Fig. 5]. Again, the curved tail phenotype observed in drained embryos was not prevented by RA injection [Supporting Information Fig. 5] suggesting a separate function or timing of CSF in this phenotype. Similar results were obtained when embryos were assayed at 48 hpf after CSF drainage from 22 to 36 hpf. Levels of cell death were significantly reduced in drained embryos injected with RA embryos (n = 16, p = 0.0031) compared with DMSO (n = 20) [Fig. 4(F)] (unpunctured + DMSO, n = 13; punctured + DMSO, n = 17). These data suggest that the increase in neuroepithelial cell death is first observed at 30 hpf, persists to 48 hpf, and can be reversed by application of exogenous RA.
Figure 4.
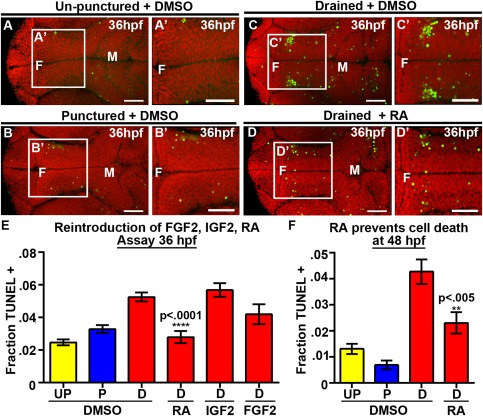
RA prevents neuroepithelial cell death at 36 and 48 hpf. (A–D) Dorsal view of TUNEL (green) and propidum iodide (red) of un‐punctured (A), punctured (B) or drained embryos (C) injected with DMSO and drained embryos injected with RA (D). (E–F) Quantification of cell death at 36 hpf after introduction of FGF2, IGF2, or RA (E) or at 48 hpf after introduction of RA (F). Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained. Scale bars = 50 μm.
IGF2 and FGF2 were identified in the CSF of mice and chick respectively, and can promote cell proliferation and neurogenesis while having no direct influence on cell survival in explants (Martin et al., 2006; Lehtinen et al., 2011). Consistently, neither of these factors prevented cell death nor facilitated tail straightening in zebrafish [Fig. 4(E), Supporting Information Fig. 5] (drained + IGF2 n = 15, p = 0.40, drained + FGF2 n‐13, p = 0.09). These data support the hypothesis that RA signaling from the CSF promotes neuroepithelial cell survival in zebrafish embryos during early brain development.
Rbp4 and Retinol Promote Neuroepithelial Cell Survival
The ability of RA to prevent cell death is consistent with a role for Rbp4 in normal neuroepithelial cell survival. Rbp4 was identified by our mass spectrometry analysis with ∼40% coverage [Fig. 5(A)] suggesting that it may be a CSF signaling factor. Rbp4 is highly expressed in the yolk syncytial layer and to lower levels in the epidermis surrounding the neural tube (Tingaud‐Sequeira et al., 2006; Li et al., 2007) [Supporting Information Fig. 6]. We hypothesized that Rbp4 is bound to retinol in the CSF, promoting RA synthesis in the neuroepithelium and therefore cell survival. To test this, we used a previously published morpholino (modified antisense oligonucleotide; MO), which targets the exon 2/intron 3 splice site of rbp4 (Li et al., 2007) and asked whether inhibition of rbp4 increased cell death. While MO technology is useful, stringent controls must be performed to ascertain specificity. As will be described in the following sections, we performed such controls, including rescue of a MO phenotype with injected zebrafish mRNA or human protein in addition to use of chemical agonists and inhibitors, that together solidify the conclusions we are able to draw.
Figure 5.
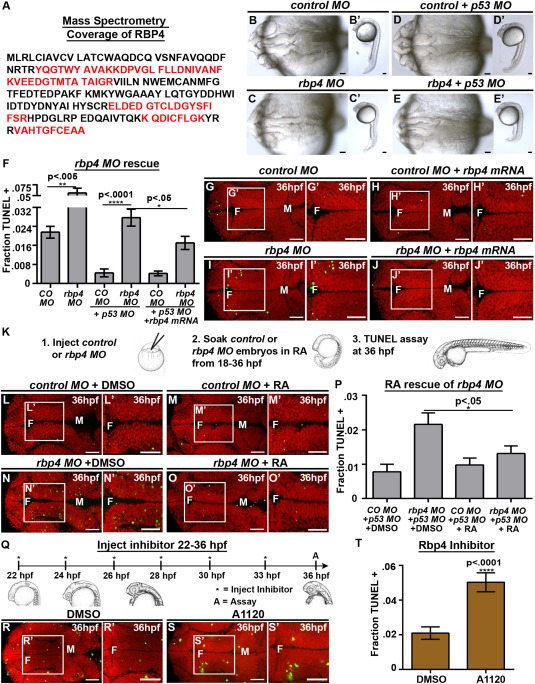
Loss of Rbp4 increases cell death. (A) Rbp4 sequence coverage from mass spectrometry (red). (B–E) Brightfield dorsal and lateral (B′–E′) view of control MO (B), control MO + p53 MO (C), rbp4 MO (D) and rbp4 MO + p53 MO (E) embryos. (F) Quantification of TUNEL after rbp4 MO and mRNA rescue. (G–J, L–O, R–S) Dorsal view of TUNEL (green) and propidium iodide (red). (G–J) TUNEL staining in control MO + p53 MO (G), control MO + rbp4 mRNA+ p53 MO (H), rbp4 MO+ p53 MO (I), rbp4 MO + rbp4 mRNA + p53 MO (J). (K) Method for RA rescue of rbp4 MO. (L–O) TUNEL staining in control MO + p53 MO + DMSO (L) control MO + p53 MO + RA (M), rbp4 MO+ p53 MO + DMSO (N), rbp4 MO + p53 MO + RA (O). (P) Quantification of TUNEL in RA rescue of rbp4 MO. (Q) Method of injection of Rbp4 inhibitor, A1120. (R‐S) TUNEL staining in DMSO (R) or A1120 (S) injected embryos (T) Quantification of TUNEL in A1120 or DMSO injected embryos. Data represented as mean ± SEM. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 μm.
RT‐PCR analysis identified that the rbp4 MO resulted in an intron inclusion, predicted to generate a premature stop codon [Supporting Information Fig. 6] (Li et al., 2007). At the concentration used, rbp4 morphants (embryos injected with MO) were normal at the gross morphological level [Fig. 5(B–E)], however, we observed a significant increase in cell death in the brain of rpb4 morphants (p = 0.0041) [Fig. 5(F), Supporting Information Fig. 6] (control, n = 108, 2.5 ng rbp4 MO, n = 67). Since several MOs have been reported to cause non‐specific cell death, p53 MO is commonly co‐injected with a target MO to prevent nonspecific MO‐mediated cell death and to reveal the specific loss‐of‐function phenotype (Robu et al., 2007). Although co‐injection of p53 MO with rbp4 MO reduced cell death, rbp4 morphants retained elevated levels of cell death (p < 0.0001) compared with controls [Fig. 5(D–G,I), Supporting Information Fig. 6] (control + p53, n = 116, 2.5 ng rbp4 MO + p53, n = 55) indicating that increased cell death is specific to loss of rbp4. p53 MO was co‐injected with rbp4 MO for all further analyses.
Specificity of the rbp4 MO was further demonstrated since cell death in rbp4 morphants was prevented by co‐injection of rbp4 mRNA (n = 20, p = 0.02), which does not bind the MO [Fig. 5(F–J)] (rbp4 MO + p53 + control mRNA, n = 21, control MO + p53 + control mRNA, n = 20; control MO + p53 + rbp4 mRNA, n = 18). Additionally, rbp4 morphants soaked in RA from 18 to 36 hpf [Fig. 5(K)] had reduced levels of cell death (n = 18, p = 0.03) compared with DMSO treated animals while, RA had no effect on control morphants [Fig 5(L–P)] (control MO + DMSO, n = 10; control MO + RA n = 17, rbp4 MO + DMSO, n = 16).
To further test the requirement for Rbp4, we prevented Rbp4 function using the A1120 inhibitor, which competes with retinol for binding to RBP4 (Motani et al., 2009) [Fig. 7(M)]. A1120 was injected into the brain ventricles of wild‐type embryos every two hours from 22 to 36 hpf [Fig. 5(Q)] and a significant increase in cell death (n = 6 p < 0.0001) compared with DMSO injected embryos (n = 18) was observed [Fig. 5(R–T)]. Together, these data support the hypothesis that Rbp4 is required for neuroepithelial cell survival.
Figure 7.
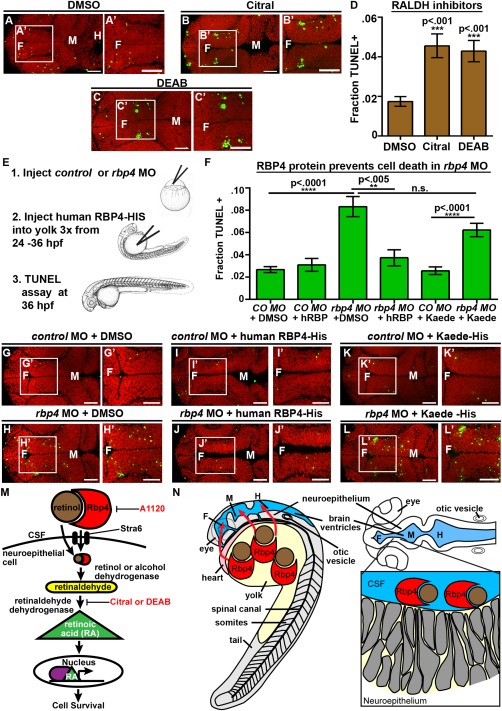
RA synthesis is required for cell survival and Rbp4 can be transported from the yolk to the CSF. (A–C) Dorsal view of TUNEL (green) and propidium iodide (red) in embryos injected with DMSO (A), Citral (B) or DEAB (C). (D) Quantification of TUNEL after injection of inhibitors into wild‐type embryos every two hours from 22 to 36 hpf. (E) Experimental design for injection of human RBP4 protein into yolk of rbp4 morphants. (F) Quantification of TUNEL positive cells in rbp4 morphants after injection of RBP4 or Kaede protein. (G–L) Dorsal view of TUNEL (green) and propidium iodide (red) in control MO + p53 MO (G,I,K) or rbp4 MO+ p53 MO (H,J,L) embryos injected with DMSO (G,H), human RBP4‐His protein (I,J) or Kaede‐His protein (K,L). (M) Model of retinoid signaling pathway in the embryonic brain. Inhibitors used in red. (N) Model of Rbp4 + RA precursor transport. Rbp4 bound to retinol/RA precursors can be transported from the yolk to CSF (left, red arrows) where it is taken up from the CSF by a RA metabolizing cell, oxidized into RA and promotes cell survival (right). Data represented as mean ± SEM. F = forebrain, M = midbrain, UP = unpunctured, P = punctured, D = drained. Scale bars = 50 μm.
To extend these data, we asked whether injection of exogenous human RBP4 and/or retinol into CSF was sufficient to prevent neuroepithelial cell death in drained embryos. Exogenous human RBP4, retinol or a combination was injected every two hours from 30 to 36 hpf, into brain ventricles after drainage from 22 to 36 hpf [Fig. 3(A)]. Injection of human RBP4, retinol, or both retinol and RBP4 did not significantly affect cell death in un‐punctured or punctured embryos compared with DMSO treatment [Fig. 6(A, B), Supporting Information Fig. 7] (unpunctured + DMSO, n = 10, punctured + DMSO, n = 14). Drained embryos injected with DMSO showed elevated levels of cell death relative to un‐punctured and punctured controls (n = 14) [Fig. 6(C, G)]. Injection of human RBP4 alone into brain ventricles of drained embryos had no effect on cell death (n = 18, p > 0.05) [Fig. 6(D, G), Supporting Information Fig. 7]. Drained embryos injected with retinol alone had slightly reduced cell death (n = 14, p < 0.05) compared with injection with DMSO [Fig. 6(E, G), Supporting Information Fig. 7]. However, combined injection of RBP4 + retinol decreased cell death (n = 15, p < 0.0001) to control levels [Fig. 6(F–G), Supporting Information Fig. 7].
Figure 6.
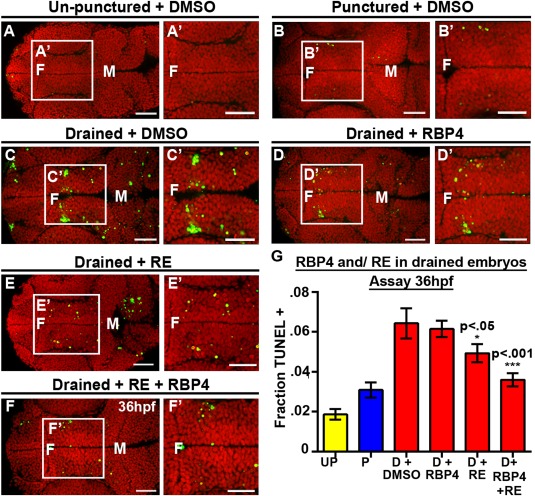
Rbp4 is required for cell survival. (A–F) Dorsal view of TUNEL (green) and propidium iodide (red) in unpunctured (A), punctured (B) and drained (C) embryos injected with DMSO or drained embryos injected with RBP4 only (D), retinol (RE) only (E), or combination of retinol (RE) and Rbp4 (F). (G) Quantification of TUNEL after RBP4/retinol injection. Data represented as mean ± SEM. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 μm.
Together, the data show that retinol is able to partially substitute for CSF, and its ability to do so is enhanced by the presence of RBP4, supporting the hypothesis that Rbp4 and retinol within the CSF are normally required for neuroepithelial cell survival.
Requirement for RA Synthesis in Neuroepithelial Cell Survival
Since RA and its precursor retinol in conjunction with RBP4 could prevent neuroepithelial cell death after CSF drainage, we hypothesized that retinol is converted to RA in the neuroepithelium. Once retinol has entered a cell, it is oxidized into retinaldehyde by alcohol dehydrogenases and to RA by retinaldehyde dehydrogenases (RALDH) (Cunningham and Duester, 2015). To determine whether RA synthesis is required for neuroepithelial cell survival, we asked whether inhibition of RA synthesis affects neuroepithelial cell survival. Citral and DEAB inhibit RALDH, the enzyme necessary for converting retinaldehyde to RA [Fig. 7(M)] (Marsh‐Armstrong et al., 1994; Perz‐Edwards et al., 2001). Injection of DEAB or Citral into the wild‐type brain ventricles every two hours from 22 to 36 hpf [Fig. 5(Q)] resulted in a significant increase in neuroepithelial cell death (n = 11, n = 9 respectively, p < 0.0001) compared with DMSO treated embryos (n = 11) [Fig. 7(A–D)]. Thus, inhibiting RA production increases cell death and supports the hypothesis that RA synthesis is required for cell survival in the developing brain.
RBP4 can Prevent Neuroepithelial Cell Death after Injection into the Yolk
Zebrafish rbp4 is highly expressed at 24 hpf in the yolk syncytial layer, a transient extraembryonic syncytial tissue (Li et al., 2007) but not significantly in the neuroepithelium. The yolk contains carotenoids and maternally deposited retinaldehydes, which can be used as precursors for RA synthesis and are necessary for development of embryonic tissues including eye, jaw, and pectoral fin (Lampert et al., 2003). We therefore hypothesized that Rbp4 binds to retinol or other RA precursors in the yolk and is transported into the CSF. To test this, we injected human RBP4 protein into the yolk of rbp4 morphants and asked whether this could prevent cell death in the neuroepithelium. Human RBP4 or DMSO was injected three times into the yolk from 24 to 36 hpf and cell death assayed at 36 hpf [Fig. 7(E)]. rbp4 morphants injected with DMSO had elevated levels of cell death compared with controls [Fig. 7(F–H)], (control MO + p53 MO + DMSO, n = 39; rbp4 MO + p53 MO + DMSO, n = 32, p < 0.0001). While injection of DMSO or RBP4 had no effect on cell death in control morphants (control MO + p53 MO + hRBP4, n = 20, p = 0.45)[Fig. 7(F, G, I)], RBP4 significantly lowered levels of cell death in rbp‐4 morphants (n = 20; p = 0.001) compared with DMSO [(Fig. 7(F–J)]. As a control for specificity, we injected Kaede, a protein of similar molecular weight as RBP4, into the yolk of control or rpb‐4 morphants [Fig. 7(E)] and did not observe a significant difference in cell death in controls or rbp‐4 morphants upon Kaede injection [Fig. 7(F, K–L)], (control MO + p53 MO + Kaede, n = 18, p = 0.80 compared with control MO + p53 MO + DMSO; rbp4 MO + p53 MO + Kaede, n = 14, p = 0.22).
These data show that RBP4 originating in the yolk can act within the brain to prevent cell death. The data support the hypothesis that endogenous Rbp4 produced by the yolk syncytial layer, binds to retinol and/or its precursors in the yolk, and is transported to the CSF where the complex supports neuroepithelial cell survival [Fig. 7(M, N)].
DISCUSSION
This study has examined the role of CSF during early brain development using the zebrafish system. There are four conclusions: (1) CSF is required in vivo for neuroepithelial cell survival, (2) RA synthesis is required for neuroepithelial cell survival, (3) Rbp4 is present within the CSF and promotes neuroepithelial cell survival in combination with retinol and (4) Rbp4 can be transported into the CSF. The study identifies a novel role for RA signaling acting through the CSF to promote neuroepithelial cell survival in vivo.
CSF Promotes Cell Survival during Embryonic Development
The reduction in cell survival after chronic CSF depletion in the intact zebrafish is consistent with reports of increased cell death after a single CSF drainage from cultured chick embryos (Salehi and Mashayekhi, 2006) or in explanted chick neuroepithelium cultured in minimal growth media (Gato et al., 2005). The ability of mouse CSF to prevent zebrafish brain cell death indicates conservation of CSF activity across species. This is consistent with in vitro studies where application of CSF to human neural progenitor cell cultures or explanted chick neuroepithelium increased cell survival (Gato et al., 2005; Buddensiek et al., 2009).
In contrast with our observations where increased cell death was the overarching outcome of CSF drainage, application of CSF to chick mesencephalic and mouse cortical explants increased neurogenesis, cell proliferation and cell survival (Gato et al., 2005; Lehtinen et al., 2011). The differences between our zebrafish analyses and those in chick and mouse may reflect the stage at which assays were performed or the effects of culture conditions in amniotes. Furthermore, compared with previous in vitro studies that added CSF to explanted tissue, we repeatedly removed CSF over the course of 14 hours of development and this method may alter levels of factors in ways that are not mimicked in culture. There may also be species‐specific functions of CSF during brain development however; Rbp4 is present in the CSF of all vertebrate species.
The Role of the Retinoic Acid Pathway in CSF Function
Several studies have identified factors from the CSF that promote cell proliferation and neurogenesis, including FGF2 and IGF2, however, these factors did not promote cell survival in the assays performed (Martin et al., 2006; Lehtinen et al., 2011). This study is the first to identify the retinoid signaling pathway from the CSF as promoting neuroepithelial cell survival. While previous studies have identified a role for RA in neurogenesis and proliferation in chick explanted neuroepithelia (Alonso et al., 2011), analysis of the effect of RA on cell survival was not performed and we cannot comment on the role of RA during cell survival in the chick.
Identification of Rbp4 in CSF and the demonstration that it is necessary and sufficient to prevent cell death may provide a mechanism to regulate the neural progenitor population. RBP4 null mice are viable, fertile, have reduced levels of retinol and impaired retina function, however, brain tissue was not studied and the effects on cell survival were not examined (Quadro et al., 1999). RA also plays a major role in cell differentiation and patterning of the brain (Maden, 2007). It is unclear whether the pathway for cell survival and differentiation are governed by similar receptors and other modulators.
Seven different protein‐based, molecular and chemical approaches were employed in this study to reach the conclusion that RA signaling from the CSF promotes neuroepithelial cell survival. These were (1) a repeated drainage assay demonstrating the requirement for CSF in cell survival; (2) mass spectrometry that identified Rbp4 as a conserved CSF protein in zebrafish; (3) the ability of RA or human RBP4 protein in combination with retinol to prevent cell death when injected into brain ventricles drained of CSF; (4) the rescue of a rbp4 loss of function phenotype after injection of zebrafish rbp4 mRNA or (5) after injection of human RBP4 protein into the yolk; (6) increased cell death after injection of the RBP4 antagonist, A1120, into the brain ventricles and (7) increased cell death after inhibition of RA synthesis (retinaldehyde dehydrogenase) by injection of Citral or DEAB into the brain ventricles. While antisense MO approaches may include some non‐specific effects (Robu et al., 2007; Kok et al., 2014), the MO‐based experiments performed in this study were well controlled and formed only a part of the methodology that allow us to conclude that RA signaling contributes to neuroepithelial cell survival through the CSF.
The Origin and Action of Rbp4 and RA Precursors in the CSF
While Rbp4 and retinol have previously been identified within the CSF (Zappaterra et al., 2007; Parada et al., 2008; Alonso et al., 2011), the source has remained unknown. In amniotes, vitamin A is supplied to the fetus by the mother via the circulation (Blomhoff et al., 1990). Additionally, in chick, proteomic analysis of CSF determined that many CSF proteins are transferred from the blood serum (Parvas et al., 2008) suggesting that the circulatory system may be crucial for movement of proteins into and out of the CSF. Similarly, the zebrafish circulatory system may transport Rbp4 from the yolk to the CSF, and consistently, the timing of the CSF requirement for neuroepithelial cell survival coincides with start of heartbeat. Thus, transport of vitamin A from the nutrient source, the yolk in zebrafish or mother in amniotes, to the embryo via the circulatory system may be conserved.
STRA6 has previously been identified as the receptor that binds RBP4 and transports retinol into the cell for conversion to RA in mice (Kawaguchi et al., 2007; Amengual et al., 2014). It is not known whether Stra6 acts as a transporter in zebrafish, however, similar to Stra6‐null mice, loss of stra6 in zebrafish results in reduction of RA precursors and phenotypes characteristic of RA deficiency suggesting that Stra6 may be the receptor for retinol (Isken et al., 2008; Ruiz et al., 2012; Amengual et al., 2014).
While the role of RA signaling in patterning the brain is well described (Holder and Hill, 1991; Begemann et al., 2001; Grandel et al., 2002; Glover et al., 2006), the role we describe in this study occurs after initial brain patterning. The embryonic brain expresses multiple retinol and RALDH that could potentially metabolize retinol found in the CSF and provide a source of RA that is used for cell survival (Begemann et al., 2001; Grandel et al., 2002; Dobbs‐McAuliffe et al., 2004; Glover et al., 2006). RA synthesizing cells are likely to be apically localized as these cells are in direct contact with the CSF. It is also possible that the Rbp4/retinol complex may diffuse deeper into the neuroepithelium to RA synthesizing cells.
Studies in adult mice have suggested that the source of RA is from the meninges, which are the protective layers surrounding the brain (Siegenthaler et al., 2009; Siegenthaler and Pleasure, 2011), the choroid plexus (Yamamoto et al., 1996; Lun et al., 2015) or the ventricular cells adjacent to the CSF (Chatzi et al., 2011; Chatzi et al., 2013). Our work in the embryonic zebrafish supports the hypothesis that RA is produced by neuroepithelial cells in contact with the CSF. However, as the embryonic fish does not have fully developed meninges or choroid plexus at this stage, we cannot comment on whether these tissues could also be a possible source of RA for later stages of development and adulthood.
CONCLUSION
This study demonstrates the importance of CSF for embryonic brain development. An intriguing observation from this study is that after repeated drainage over 14 hours of development the brain ventricles fail to reinflate. One hypothesis is that during and after CSF drainage, the absence of fluid pressure allows the growing brain to occlude the collapsed lumen. Thus, although there is a major increase in cell death after CSF drainage in some parts of the developing brain, there is also much cell division in other parts. This “overgrowth” has been observed in children and adolescents where the excess CSF present in hydrocephalic syndromes was over‐drained by shunting, resulting in slit ventricle syndrome (Foltz, 1993). The drainage paradigm we have set up phenocopies a slit‐brain phenotype and will allow further analyses of the role played by the CSF.
The composition of CSF is abnormal in neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS) and Alzheimer's disease and several reports demonstrate that levels of RBP and RA signaling are disrupted (Maury and Teppo, 1987; Corcoran et al., 2002; Mashayekhi and Salehi, 2006a,b). These data and our observations suggest that not only is the RA pathway employed for embryonic neuroepithelial health, but may also maintain brain health in the older animal.
Supporting information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Figure 5.
Supporting Information Figure 6.
Supporting Information Figure 7.
ACKNOWLEDGMENTS
JTC was a NSF graduate research fellow. Special thanks to Dr. Jen Gutzman, Dr. Amanda Dickinson, Dr. Ryann Fame, Dr. Christian Cortes‐Campos, Dr. Joey Davis, and other Sive lab members for many useful discussions and constructive criticism, Ioannis Papayannopolous (Koch Institute Biopolymers and Proteomics) and Eric Spooner for help with mass spectrometry and analysis and to Olivier Paugois for expert fish husbandry.
REFERENCES
- Alonso MI, Martin C, Carnicero E, Bueno D, Gato A. 2011. Cerebrospinal fluid control of neurogenesis induced by retinoic acid during early brain development. Dev Dyn 240:1650–1659. [DOI] [PubMed] [Google Scholar]
- Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, Von Lintig J. 2014. Stra6 is critical for cellular vitamin a uptake and homeostasis. Hum Mol Genet 23:5402–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. 2001. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 128:3081–3094. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Green MH, Berg T, Norum KR. 1990. Transport and storage of vitamin A. Science 250:399–404. [DOI] [PubMed] [Google Scholar]
- Bonnet E, Touyarot K, Alfos S, Pallet V, Higueret P, Abrous DN. 2008. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS One 3:e3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddensiek J, Dressel A, Kowalski M, Storch A, Sabolek M. 2009. Adult cerebrospinal fluid inhibits neurogenesis but facilitates gliogenesis from fetal rat neural stem cells. J Neurosci Res 87:3054–3066. [DOI] [PubMed] [Google Scholar]
- Chang JT, Lowery LA, Sive H. 2012. Multiple roles for the Na,K‐ATPase subunits, Atp1a1 and Fxyd1, during brain ventricle development. Dev Biol 368:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Sive H. 2012. Manual drainage of the zebrafish embryonic brain ventricles. J Vis Exp 70:e4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Brade T, Duester G. 2011. Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. Plos Biology 9:e1000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Cunningham TJ, Duester G. 2013. Investigation of retinoic acid function during embryonic brain development using retinaldehyde‐rescued rdh10 knockout mice. Dev Dyn 242:1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CN, Li Y, Marra AN, Verdun V, Wingert RA. 2014. Flat mount preparation for observation and analysis of zebrafish embryo specimens stained by whole mount in situ hybridization. J Vis Exp 89:e51604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodobski A, Szmydynger‐Chodobska J. 2001. Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech 52:65–82. [DOI] [PubMed] [Google Scholar]
- Corcoran J, So PL, Maden M. 2002. Absence of retinoids can induce motoneuron disease in the adult rat and a retinoid defect is present in motoneuron disease patients. Journal of Cell Science 115:4735–4741. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Duester G. 2015. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol 16:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond ME, Levitan ML, Haas AR. 2005. Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat Rec a Discov Mol Cell Evol Biol 285:737–747. [DOI] [PubMed] [Google Scholar]
- Dobbs‐McAuliffe B, Zhao Q, Linney E. 2004. Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo. Mech Dev 121:339–350. [DOI] [PubMed] [Google Scholar]
- Foltz EL. 1993. Hydrocephalus: slit ventricles, shunt obstructions, and third ventricle shunts: a clinical study. Surg Neurol 40:119–124. [DOI] [PubMed] [Google Scholar]
- Gato A, Moro JA, Alonso MI, Bueno D, De La Mano A, Martin C. 2005. Embryonic cerebrospinal fluid regulates neuroepithelial survival, proliferation, and neurogenesis in chick embryos. Anat Rec a Discov Mol Cell Evol Biol 284:475–484. [DOI] [PubMed] [Google Scholar]
- Glover JC, Renaud JS, Rijli FM. 2006. Retinoic acid and hindbrain patterning. J Neurobiol 66:705–725. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Kuchler AM, Schulte‐Merker S, Geisler R, Holder N, Wilson SW, Brand M. 2002. Retinoic acid signalling in the zebrafish embryo is necessary during pre‐segmentation stages to pattern the anterior‐posterior axis of the CNS and to induce a pectoral fin bud. Development 129:2851–2865. [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Sive H. 2009. Zebrafish brain ventricle injection. J Vis Exp 26:e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagglund M, Berghard A, Strotmann J, Bohm S. 2006. Retinoic acid receptor‐dependent survival of olfactory sensory neurons in postnatal and adult mice. J Neurosci 26:3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell GT, LaMantia AS. 2005. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci 25:7636–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder N, Hill J. 1991. Retinoic acid modifies development of the midbrain‐hindbrain border and affects cranial ganglion formation in zebrafish embryos. Development 113:1159–1170. [DOI] [PubMed] [Google Scholar]
- Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. 2008. Rbp4 disrupts vitamin a uptake homeostasis in a STRA6‐deficient animal model for Matthew‐wood syndrome. Cell Metab 7:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. 2006. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A 103:3902–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315:820–825. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Westerfield M. 1990. Primary neurons of the zebrafish. New York: Wiley. [Google Scholar]
- Kok FO, Shin M, Ni C, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson‐Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard‐Tindell S, Ebarasi L, Betsholtz C, Schulte‐Merker S, Wolfe SA, Lawson ND. 2014. Reverse genetic screening reveals poor correlation between Morpholino‐induced and mutant phenotypes in zebrafish. Dev Cell 32:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer‐Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. 2005. Cilia‐driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132:1907–1921. [DOI] [PubMed] [Google Scholar]
- Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, Chambon P. 1998. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science 279:863–867. [DOI] [PubMed] [Google Scholar]
- Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, von Lintig J. 2003. Provitamin A conversion to retinal via the beta,beta‐carotene‐15,15'‐oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 130:2173–2186. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D'Ercole AJ, Wong ET, LaMantia AS, Walsh CA. 2011. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Korzh V, Gong Z. 2007. Localized rbp4 expression in the yolk syncytial layer plays a role in yolk cell extension and early liver development. BMC Dev Biol 7:117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Sive H. 2005. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development 132:2057–2067. [DOI] [PubMed] [Google Scholar]
- Lun MP, Johnson MB, Broadbelt KG, Watanabe M, Kang YJ, Chau KF, Springel MW, Malesz A, Sousa AM, Pletikos M, Adelita T, Calicchio ML, Zhang Y, Holtzman MJ, Lidov HG, Sestan N, Steen H, Monuki ES, Lehtinen MK. 2015. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J Neurosci 35:4903–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. 2007. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8:755–765. [DOI] [PubMed] [Google Scholar]
- Marsh‐Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC. 1994. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci U S A 91:7286–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Bueno D, Alonso MI, Moro JA, Callejo S, Parada C, Martin P, Carnicero E, Gato A. 2006. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev Biol 297:402–416. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F, Salehi Z. 2006a. Cerebrospinal fluid nerve growth factor levels in patients with Alzheimer's disease. Ann Saudi Med 26:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi F, Salehi Z. 2006b. The importance of cerebrospinal fluid on neural cell proliferation in developing chick cerebral cortex. Eur J Neurol 13:266–272. [DOI] [PubMed] [Google Scholar]
- Maury CP, Teppo AM. 1987. Immunodetection of protein composition in cerebral amyloid extracts in Alzheimer's disease: enrichment of retinol‐binding protein. J Neurol Sci 80:221–228. [DOI] [PubMed] [Google Scholar]
- Miyan JA, Zendah M, Mashayekhi F, Owen‐Lynch PJ. 2006. Cerebrospinal fluid supports viability and proliferation of cortical cells in vitro, mirroring in vivo development. Cerebrospinal Fluid Res 3:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani A, Wang Z, Conn M, Siegler K, Zhang Y, Liu Q, Johnstone S, Xu H, Thibault S, Wang Y, Fan P, Connors R, Le H, Xu G, Walker N, Shan B, Coward P. 2009. Identification and characterization of a non‐retinoid ligand for retinol‐binding protein 4 which lowers serum retinol‐binding protein 4 levels in vivo. J Biol Chem 284:7673–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. 2000. Effective targeted gene 'knockdown' in zebrafish. Nat Genet 26:216–220. [DOI] [PubMed] [Google Scholar]
- Parada C, Escola‐Gil JC, Bueno D. 2008. Low‐density lipoproteins from embryonic cerebrospinal fluid are required for neural differentiation. J Neurosci Res 86:2674–2684. [DOI] [PubMed] [Google Scholar]
- Parada C, Gato A, Bueno D. 2008. All‐trans retinol and retinol‐binding protein from embryonic cerebrospinal fluid exhibit dynamic behaviour during early central nervous system development. Neuroreport 19:945–950. [DOI] [PubMed] [Google Scholar]
- Parada C, Martin C, Alonso MI, Moro JA, Bueno D, Gato A. 2005. Embryonic cerebrospinal fluid collaborates with the isthmic organizer to regulate mesencephalic gene expression. J Neurosci Res 82:333–345. [DOI] [PubMed] [Google Scholar]
- Parvas M, Parada C, Bueno D. 2008. A blood‐CSF barrier function controls embryonic CSF protein composition and homeostasis during early CNS development. Dev Biol 321:51–63. [DOI] [PubMed] [Google Scholar]
- Perz‐Edwards A, Hardison NL, Linney E. 2001. Retinoic acid‐mediated gene expression in transgenic reporter zebrafish. Dev Biol 229:89–101. [DOI] [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. 1999. Impaired retinal function and vitamin A availability in mice lacking retinol‐binding protein. EMBO J 18:4633–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger‐Chodobska J. 2005. The choroid plexus‐cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. 2007. P53 activation by knockdown technologies. PLoS Genet 3:e78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, Nusinowitz S, Bok D. 2012. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol‐binding protein receptor, STRA6. Invest Ophthalmol Vis Sci 53:3027–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi Z, Mashayekhi F. 2006. The role of cerebrospinal fluid on neural cell survival in the developing chick cerebral cortex: an in vivo study. Eur J Neurol 13:760–764. [DOI] [PubMed] [Google Scholar]
- Salehi Z, Mashayekhi F, Naji M, Pandamooz S. 2009. Insulin‐like growth factor‐1 and insulin‐like growth factor binding proteins in cerebrospinal fluid during the development of mouse embryos. J Clin Neurosci 16:950–953. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. 2007. In‐gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protocols 1:2856–2860. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. 2009. Retinoic acid from the meninges regulates cortical neuron generation. Cell 139:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Pleasure SJ. 2011. We have got you 'covered': how the meninges control brain development. Curr Opin Genet Dev 21:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Draper BW, Harland RM, Weintraub H. 1990. Identification of a retinoic acid‐sensitive period during primary axis formation in Xenopus laevis. Genes Dev 4:932–942. [DOI] [PubMed] [Google Scholar]
- Tingaud‐Sequeira A, Forgue J, Andre M, Babin PJ. 2006. Epidermal transient down‐regulation of retinol‐binding protein 4 and mirror expression of apolipoprotein Eb and estrogen receptor 2a during zebrafish fin and scale development. Dev Dyn 235:3071–3079. [DOI] [PubMed] [Google Scholar]
- Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. 2011. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 147:1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Campbell K. 2004. The homeobox gene Gsh2 is required for retinoid production in the embryonic mouse telencephalon. Development 131:4013–4020. [DOI] [PubMed] [Google Scholar]
- Westerfield M, Sprague J, Doerry E, Douglas S, Grp Z. 2001. The zebrafish information network (ZFIN): a resource for genetic, genomic and developmental research. Nucleic Acids Research 29:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, McCaffery P, Drager UC. 1996. Influence of the choroid plexus on cerebellar development: analysis of retinoic acid synthesis. Brain Res Dev Brain Res 93:182–190. [DOI] [PubMed] [Google Scholar]
- Zappaterra MD, Lisgo SN, Lindsay S, Gygi SP, Walsh CA, Ballif BA. 2007. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res 6:3537–3548. [DOI] [PubMed] [Google Scholar]
- Zheng W, Chodobski A. 2005. The Blood‐Cerebrospinal Fluid Barrier. Boca Raton, FL: Taylor and; Francis [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Figure 5.
Supporting Information Figure 6.
Supporting Information Figure 7.


