Abstract
Background
Older adults have higher mortality rates after severe traumatic brain injury (TBI) compared to younger adults. Brain derived neurotrophic factor (BDNF) signaling is altered in aging and is important to TBI given its role in neuronal survival/plasticity and autonomic function. Following experimental TBI, acute BDNF administration has not been efficacious. Clinically, genetic variation in BDNF (reduced signaling alleles: rs6265, Met-carriers; rs7124442, C-carriers) were protective in acute mortality. Post-acutely, these genotypes carried lower mortality risk in older adults, and greater mortality risk among younger adults.
Objective
Investigate BDNF levels in mortality/outcome following severe TBI in the context of age and genetic risk.
Methods
CSF and serum BDNF were assessed prospectively during the first week following severe TBI (n=203), and in controls (n=10). Age, BDNF genotype, and BDNF levels were assessed as mortality/outcome predictors.
Results
CSF BDNF levels tended to be higher post-TBI (p=0.061) versus controls and were associated with time until death (p=0.042). In contrast, serum BDNF levels were reduced post-TBI versus controls (p<0.0001). Both gene*BDNF serum and gene*age interactions were mortality predictors post-TBI in the same multivariate model. CSF and serum BDNF tended to be negatively correlated post-TBI (p=0.07).
Conclusions
BDNF levels predicted mortality, in addition to gene*age interactions, suggesting levels capture additional mortality risk. Higher CSF BDNF post-TBI may be detrimental due to injury and age-related increases in pro-apoptotic BDNF target receptors. Negative CSF and serum BDNF correlations post-TBI suggest blood-brain barrier transit alterations. Understanding BDNF signaling in neuronal survival, plasticity, and autonomic function may inform treatment.
Keywords: Traumatic Brain Injury, aging, BDNF, genetics, mortality, Rehabilomics
Introduction
The World Health Organization suggests traumatic brain injury (TBI) will be a leading cause of death and disability by 2020, with about 10 million people affected each year1. Advanced age is a consistent determinant of TBI survival2. Older adults comprise a large segment of the population sustaining TBI, with comparatively worse outcomes and higher mortality rates despite similar injuries3. Recent literature indicates individuals who survive the acute phase following a moderate/severe TBI are still at increased risk for mortality during post-acute phases of recovery, and on average, have a shorter life-span2,4. Systemic and CNS biomarkers can inform acute mortality predictions after moderate/severe TBI5,6. In addition, biomarkers elucidate TBI-specific pathology relevant to the molecular pathways they represent, identifying new targets for neuroprotective therapeutics or indicating important injury-specific phenomena in well-studied pathways. While some studies have evaluated early biomarker patterns predicting mortality and/or global outcomes5–8, less is known about how innate biological factors, like genetics, interact with acute biomarkers to influence mortality and/or global outcomes.
Current studies suggest demographic and clinical variables like age, injury severity, and pupillary reactivity influence mortality prediction9, however our previous work demonstrates genetic variation within the brain derived neurotrophic factor (BDNF) gene informs mortality prognostication beyond that captured by clinical variables10. BDNF, a ubiquitously expressed neurotrophin in the brain, is an intriguing target for TBI intervention research due to its role in neuronal survival, neurogenesis, and plasticity11,12. However, in experimental TBI acute infusions of BDNF did not improve motor or cognitive recovery nor neuronal survival post-injury13.
BDNF is also intriguing for TBI rehabilitation due to its important effects on the autonomic nervous system through hypothalamic metabolic regulation14,15 and brainstem control of the cardiovascular system16–18. Variation within the BDNF gene has been associated with hypothalamus-pituitary-adrenal (HPA) axis reactivity19,20 and autonomic regulation of heart rate21. Major trauma like TBI results in a sustained stress response6,22 and individual variation in stress hormone production and response may lead to different outcomes post-injury7,23. Given BDNF's role in autonomic function and neuronal survival, BDNF may have multiple actions that could impact TBI mortality and global outcomes, making BDNF an attractive biomarker to explore, across recovery where these actions may have dynamic influences.
BDNF is synthesized as pro-BDNF then cleaved to mature BDNF24. Mature BDNF has pro-survival signaling capabilities via the full length TrkB receptor (TrkB.FL), while pro-BDNF can be pro-apoptotic via p75NTR receptor binding25. Age26–28 and experimental TBI29 can shift regional balances in BDNF receptor ratios from pro-survival to pro-apoptotic. In fact, our previous work demonstrated age and injury specific associations with the BDNF gene and mortality post-TBI10. We examined two single nucleotide polymorphisms (SNP) within BDNF, rs6265, with reduced activity-dependent secretion of BDNF30, and rs7124442, which impairs neuronal BDNF mRNA trafficking31. Given the effects on BDNF signaling, rs6265 Met-allele and rs7124442 C-allele were identified as possible risk factors, with the hypothesis that reduced BDNF signaling would be detrimental to recovery. Surprisingly, low signaling genotypes were protective in acute TBI-related mortality. Post-acutely, low signaling genotypes again carried lower mortality risk in older adults, while these same variants were risk factors among younger adults. Given the effects of age and injury on target receptor milieu and our previous findings with BDNF variation in TBI-related mortality, we hypothesize BDNF may be a temporally dynamic, target receptor dependent, and genetically-influenced biomarker for mortality and/or outcome post-TBI.
BDNF has been extensively evaluated as a biomarker in affective disorders, where lower serum levels are associated with depressive episodes32. Decreased serum BDNF levels have also been associated with mortality in uninjured populations33–35. In uninjured humans and rodent models, serum BDNF levels tend to correlate with CSF and brain BDNF36,37. Studies examining genetic control of BDNF serum levels have been mixed38, but have not been conducted in TBI populations. Following TBI, Kalish and Phillips39 reported that serum BDNF levels are acutely decreased, correlating with injury severity.
Given the evidence for genetic, age, and biosuceptibility (biomarker) relevance to mortality post-TBI, we proposed a multifaceted role for BDNF involvement in mortality and/or outcome post-TBI, which can be operationalized within a Rehabilomics framework40,41. Rehabilomics integrates biological factors, clinical characteristics, and demographics into a multidimensional approach to evaluate outcome and improve individualized rehabilitation strategies post-TBI. In this study, we measured BDNF levels in serum and CSF across the first week after severe TBI in 203 subjects. We evaluated BDNF levels, interactions with BDNF genetic variation, and mortality associations across the first year post-TBI. While this study focused on mortality, we also examined BDNF levels in global outcome following TBI. Both CSF and serum demonstrated significant predictive capacity when assessing mortality over a 1-year recovery period, in addition to genetic risk, suggesting BDNF as a novel and informative TBI biomarker. The findings support further work understanding BDNF pathophysiology as a contributing factor to mortality following TBI.
Methods
Participants
This prospective cohort study was approved by the MASKED Institutional Review Board. Enrollment criteria included age16-74 years and an admission Glasgow Coma Scale (GCS) score ≤8 indicating severe TBI. Exclusion criteria included documented prolonged hypoxia prior to admission or penetrating head injury. Participants were consecutively recruited while receiving inpatient care within the MASKED. Consent was obtained from next-of-kin. All subjects sustained a non-penetrating TBI, and had evidence of intracranial injury on Computed Tomography (CT). Subjects had at least one sample measurement (serum or CSF, at any time-point). These subjects are a subset of a larger study investigating possible biomarkers and genetic factors related to individual recovery following TBI.
Injury severity was defined for analysis as the best GCS obtained within the first 24 hours post-injury. Demographics, including age and sex were collected by chart review and subject or caregiver interviews. Injury severity scores (ISS) were abstracted by trained trauma center registrars. ISS captures the injury severity of the three most injured anatomical regions42. Table 1 contains a detailed description of the study population.
Table 1.
Participant description (n=202).
| Demographic Variable | ||
|---|---|---|
| Age, years | Mean±STD | 37.7±16.3 |
|
| ||
| Sex, # (%) | Men | 163 (80.7) |
| Women | 39 (19.3) | |
|
| ||
| GCS | Median | 7 |
|
| ||
| Mechanism of Injury, # (%) | Automobile/Motorcycle | 132 (71.0) |
| Fall/Jump | 37 (19.9) | |
| Other | 17 (9.1) | |
|
| ||
| Length of Hospital Stay | Days, Mean±STD | 19.7±11.7 |
|
| ||
| Injury Severity Score | Mean±STD | 33.8±9.5 |
|
| ||
| Neurological Injury Type, # (% Present) | SDH | 91 (61.1) |
| DAI | 42 (28.2) | |
| EDH | 23 (15.4) | |
| Contusion | 57 (38.3) | |
| IVH | 42 (28.2) | |
| ICH | 52 (34.9) | |
| SAH | 103 (69.1) | |
|
| ||
| rs6265 | Val/Val | 96 (61.1) |
| Met-carrier | 61 (38.9) | |
|
| ||
| rs7124442 | T/T | 84 (60.9) |
| C-carrier | 54 (39.1) | |
STD, standard deviation; GCS, Glasgow Coma Scale, SDH, subdural hematoma; DAI, diffuse axonal injury; EDH, epidural hematoma; IVH, intraventricular hemorrhage; ICH, intracranial hemorrhage; SAH, subarachnoid hemorrhage.
Healthy control participants were Caucasian with an age range from 18-60 years old, and 40% of participants were women. All samples collected from controls were obtained at ∼7am to match samples from participants with TBI, avoiding significant diurnal variation confounds. In addition, healthy controls had no self-reported history of TBI, other neurological condition, bleeding disorder, or endocrine dysfunction. Women were excluded if pregnant, taking oral contraceptives or hormone replacement therapy.
Sample Collection and Processing
BDNF levels were measured in CSF and serum samples collected for 1 week post-injury. When possible, CSF samples were collected passively up to twice daily via an external ventricular drain placed for clinical care. Serum was collected daily at ∼7am. BDNF values derived from samples were binned by day, and where necessary, an average value was determined for each day post-injury for each subject. Of 203 participants, 149 had CSF samples (n=583), and 141 had serum samples (n=406). A subset had both CSF and serum samples (n=87). For biomarker comparisons, healthy adult control samples were used to establish reference BDNF levels [CSF (n=10) and serum (n=7)].
CSF and serum samples obtained from the TBI and control cohort were stored at -80°C before batch BDNF analysis using an ELISA kit (RayBiotech). To maintain sample integrity for BDNF assessments in TBI and control samples, we used samples not previously thawed for other analyses. Briefly, standards and samples were pipetted onto a 96-well plate pre-coated with human BDNF antibody. Following shaking for 2.5hrs at room-temperature, plates were washed and incubated with biotinylated BDNF antibody for 1hr. HRP-conjugated streptavidin was then added for an incubation of 45 minutes. The addition of a tetramethylbenzidine substrate allowed for a color reaction. Concentrations were calculated using mean absorbance of each sample at 460 nm to correlate with sample BDNF concentrations present. Samples were diluted within the range of the ELISA kit (no dilution for CSF, 1:250 for serum), with a kit sensitivity of 80pg/ml, an intra-assay variation of <10%, and an inter-assay variation of <12%.
Mortality and Outcome
Time until death (TUD) was recorded in days post-injury, up to one year post-injury, using the Social Security Death Index43. Consistent with previous work10, mortality was evaluated over two time-epochs, 0-7d post-injury (acute) and 8d-365d post-injury (post-acute), and then across the entire recovery span of 0-365. For 0-7d, survivorship was right censored at 7d post-injury. For 8-365d, subjects were included if they survived >7d, and survivorship was right censored at 365d. Mortality was also examined as a binary outcome at 365d post-injury. Acutely, 11.62% of participants died (TUD: median=3d, min=0d, max=7d, Q1=2d, Q3=6d). An additional 14.16% died post-acutely (TUD: median=19d, min=8d, max=301d, Q1=11.5d, Q3=31d).
We evaluated whether BDNF levels were indicative of mortality or graded across a range of outcomes using the Glasgow Outcome Scale (GOS) (1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, 5=good recovery) at 6 and 12 months44. Research-trained neuropsychometrists, blinded to genetic and biomarker information, obtained GOS scores. GOS was sub-divided as favorable (4-5) and unfavorable (2-3) outcome for analysis with BDNF levels. For this study, follow-up rates were >85% for 6 months and >80% at 12 months post-injury.
Genotyping and SNP Selection
DNA was isolated from blood using a salting out procedure45 or from CSF using the Qiamp protocol from Qiagen. BDNF rs6269 and rs7124442 were genotyped by TaqMan allele discrimination assay using Assay on Demand reagents (Applied Biosystems). This assay utilized fluorescent labeled probes to detect allele(s) present for DNA sample.
Selected SNPs (rs6265, rs7124442) had a minor allele frequency >20%. Each SNP represents a different haplotype block of BDNF covering variation corresponding to “isoform a” of the translated protein. A cumulative BDNF gene risk score (GRS) was used as previously published10, where rs6265 Met (Val/Met or Met/Met) and rs7124442 C (T/C or C/C) carrier status were hypothesized risk alleles due to reportedly reduced BDNF signaling30,31. Thus, a GRS=0 was the hypothesized no risk group (Val/Val, T/T); GRS=1 included carriers for 1 risk allele (Val/Val, C-carriers or Met-carriers, T/T); and GRS=2 included carriers of both risk alleles (Met-carriers, C-carriers).
Statistical Analysis
Data analysis was conducted using Statistical Analysis Software (SAS) 9.3. Descriptive analyses included mean and standard deviation and/or median for continuous and ordinal variables and frequencies for categorical variables. Demographic and clinical relationships to BDNF levels were assessed using Mann-Whitney and Kruskal-Wallis tests, due to skewedness of BDNF level data. Genetic analysis utilized categorizations based on allele carrier status using Chi-square or Fishers Exact test where appropriate, and the BDNF GRS was used to examine cumulative genetic risk associations with mortality as previously described10. Age was dichotomized at 45yrs similar to previous TBI mortality analyses10. Due to possible genetic stratification effects46, genetic associations are reported in Caucasians only (n=181). Similarly, due to racial differences in BDNF levels47, and analysis with BDNF, analysis was limited to Caucasians only (n=181). One subject was removed due to sample processing issues.
For consistency with previous work10, mortality was examined across 0-7d post-injury (acute), 8d-365d post-injury (post-acute), and across the entire recovery span of 0-365d. Mortality associations with BDNF levels were examined using TUD in Cox proportional hazards models48. The proportionality of hazards assumption was tested and confirmed for all final Cox model variables. Interaction terms were identified by previous studies (in the case of age*GRS) or existing hypotheses (in the case of GRS*serum BDNF) and tested in Cox models. All models were developed using a backwards-stepwise approach, with variables remaining in the final models if p≤0.2. Final models were then tested to determine the effect of removal of the BDNF levels variable on the remaining associations in the model. A p-value<0.05 was considered statistically significant.
Results
Description of CSF and serum BDNF levels 0-6d post-TBI
As depicted in Figure 1A, average CSF BDNF levels tended to be higher for subjects with TBI compared to controls (0.20±0.01ng/mL versus 0.14±0.02ng/mL, p=0.061), with similar trends noted for d1-5 (p<0.10). Average serum BDNF levels in TBI subjects (150.05±4.90ng/mL) were reduced versus controls (277.86±28.11ng/mL, p<0.0001) beginning d0 (p=0.007) and remained below controls for all time-points (p<0.0001 for remaining comparisons, Figure 1B). For subjects with TBI, average CSF and serum BDNF levels tended to be negatively correlated (r=-0.209, p=0.070, n=76). In controls, serum and CSF BDNF levels were positively correlated but this association was not significant (r=0.67, p=0.219, n=5).
Figure 1.
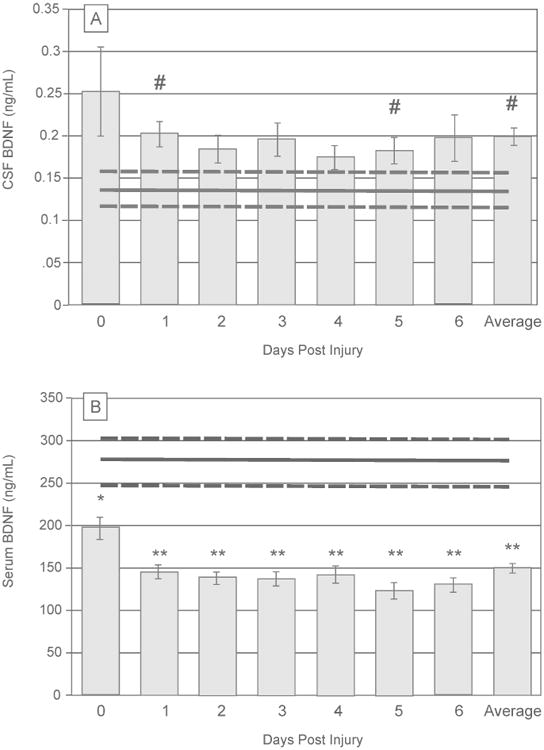
Daily brain derived neurotrophic factor (BDNF) levels over the first 7 days post-injury, compared to healthy controls (mean represented by gray horizontal line, ±standard error in dashed gray horizontal lines). (A) Daily mean CSF BDNF levels tend to be higher than control levels (trending at days 1 and 5, p<0.1 for both). Average CSF levels across the first week post-injury are show trends elevated from control levels (p=0.061). (B) Daily mean serum BDNF levels fall below control levels immediately following injury (day 0, p=0.007) and remain reduced through day 6 (p<0.0001 for all comparisons). (# p<0.1; *p<0.05, **p<0.001)
Associations of CSF and serum BDNF levels with demographic and clinical variables
Average (d0-6) CSF and serum BDNF levels were examined for associations with demographic and clinical variables. Table 2 reports relationships between serum BDNF, CSF BDNF, demographics, clinical variables, and genetic variants (BDNF rs6265 and rs7124442). All genotype frequencies were in Hardy-Weinberg equilibrium. There were no significant associations between genetic variants and BDNF levels. Average CSF levels positively correlated with age (r=0.17, p=0.045), while serum levels were not correlate with age. Correlations between serum and CSF BDNF levels were examined by age category. There was a negative correlation among subjects <45 yrs. (r=-0.307, p=0.029, n=51), but this was not significant for subjects >45yrs. (r=-0.113, p=0.590, n=25).
Table 2.
BDNF CSF and serum level associations with demographic and injury variables.
| Demographic Variable | CSF Weekly Average, ng/mL (n=149) | Serum Weekly Average, ng/mL, (n=141) | |
|---|---|---|---|
| rs6265 | Val/Val (n=96) | 0.20±0.11 | 159.05 ±106.59 |
| Met-carrier (n=61) | 0.19±0.13 | 159.47±60.57 | |
| p value | 0.145 | 0.155 | |
|
| |||
| rs7124442 | T/T (n=84) | 0.19±0.10 | 163.13 ±113.62 |
| C-carrier (n=54) | 0.19±0.14 | 153.35 ±65.49 | |
| p value | 0.238 | 0.311 | |
|
| |||
| Age | R | 0.17 | -0.11 |
| p value | 0.045 | 0.244 | |
|
| |||
| Sex | Males | 0.20±0.13 | 147.28±51.81 |
| Females | 0.19±0.10 | 162.09±64.25 | |
| p value | 0.438 | 0.099 | |
|
| |||
| GCS | R | -0.08 | -0.01 |
| p value | 0.343 | 0.927 | |
|
| |||
| Injury Severity | R | -0.05 | -0.05 |
| Score | p value | 0.568 | 0.618 |
CSF and serum BDNF Associations with Mortality
Consistent with previous work10, CSF and serum BDNF were examined for associations within two mortality time-epochs and the total survival period. Mortality associations are presented in Table 3. Age and GCS were associated with mortality during both time epochs and across the total survival period. Average CSF BDNF levels were higher in those who died within the post-acute time epoch. Serum BDNF levels tended to be associated with acute mortality; participants who died 0-7d post-injury had lower serum levels (130.75±17.26ng/mL) versus survivors (159.47±60.57ng/mL).
Table 3.
Demographic associations with mortality time epochs.
| Acute Mortality (0-7 days) | Post-Acute Mortality (8-365 days) | Total Mortality (0-365 days) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Died (n=23) | Survived (n=157) | p value | Died (n=32) | Survived (n=125) | p value | Died (n=55) | Survived (n=125) | p value | |
| Age, mean±STD | 45.3±17.2 | 36.4±15.6 | 0.010 | 49.8±16.1 | 32.9±13.6 | <0.001 | 47±16.6 | 32.9±13.6 | <0.001 |
| GCS, median | 6 | 7 | 0.004 | 6 | 7 | 0.007 | 6 | 7 | <0.001 |
| Male, # (%) | 15 (65.2) | 129 (82.2) | 0.073 | 26 (81.3) | 103 (82.4) | 0.880 | 41 (74.6) | 103 (82.4) | 0.232 |
| CSF Weekly Average, ng/mL (n=133) | 0.25±0.17 | 0.19±0.12 | 0.214 | 0.26±0.17 | 0.18±0.10 | 0.013 | 0.25±0.17 | 0.18±0.10 | 0.012 |
| Serum Weekly Average, ng/mL (n=124) | 130.75 ±17.26 | 159.47 ±60.57 | 0.054 | 147.04 ±44.46 | 155.23 ±51.84 | 0.353 | 140.06 ±58.30 | 155.23 ±51.84 | 0.107 |
STD=standard deviation; GCS=Glasgow Coma Scale; CSF=cerebrospinal fluid
Associations between CSF BDNF levels and TUD were examined across the first year (0-365) (Table 4A). In this model, average CSF BDNF levels predicted TUD (p=0.042, HR=10.973) even when including age*GRS interaction (p=0.0561, HR=0.968) and GCS (p=0.004, HR=0.728) as covariates. Importantly, when CSF BDNF was excluded from this model, age*GRS was significant (p=0.034, HR=0.971), where similar to our previous report10, older individuals were at higher risk for mortality if they carried fewer of the hypothesized risk (i.e. low BDNF secretion) alleles.
Table 4.
Modeling time until death across recovery (0-365 days).
| Parameter | Parameter Estimate | Standard Error | Chi-Square | p value | Hazard Ratio | 95% Hazard Ratio Confidence Limits |
|---|---|---|---|---|---|---|
| A. CSF Model | ||||||
|
| ||||||
| Age | 0.11680 | 0.03343 | 12.2106 | 0.0005 | 1.124 | (1.053 - 1.200) |
| GCS | -0.31807 | 0.11010 | 8.3462 | 0.0039 | 0.728 | (0.586 - 0.903) |
| GRS=1* | 1.69271 | 1.04421 | 2.6278 | 0.1050 | 5.434 | (0.702 - 42.069) |
| GRS=2 | 3.29990 | 1.74153 | 3.5904 | 0.0581 | 27.110 | (0.893 - 823.231) |
| Weekly average CSF (ng/mL) | 2.39545 | 1.17526 | 4.1544 | 0.0415 | 10.973 | (1.096 - 109.828) |
| GRS × Age | -0.03228 | 0.01690 | 3.6485 | 0.0561 | 0.968 | (0.937 - 1.001) |
|
| ||||||
| B. Serum Model | ||||||
|
| ||||||
| Age | 0.16690 | 0.04778 | 12.2032 | 0.0005 | 1.182 | (1.076-1.298) |
| GCS | -0.24855 | 0.09676 | 6.5977 | 0.0102 | 0.780 | (0.645-0.943) |
| GRS=1 | 5.38067 | 1.74053 | 9.5567 | 0.0020 | 217.168 | (7.166-6581.739) |
| GRS=2 | 9.97054 | 3.06393 | 10.5896 | 0.0011 | 21387.04 | (52.739-8673014) |
| Weekly Average Serum (ng/mL) | 0.02089 | 0.01435 | 2.1189 | 0.1455 | 1.021 | (0.993-1.050) |
| GRS × Age | -0.05840 | 0.02180 | 7.1755 | 0.0074 | 0.943 | (0.904-0.984) |
| GRS × Weekly Average Serum (ng/mL) | -0.01261 | 0.00634 | 3.9496 | 0.0469 | 0.987 | (0.975-1.000) |
Reference Group, GRS=0; GCS=Glasgow Coma Score, GRS=Gene Risk Score
Associations between average serum BDNF and TUD were examined across the first year of recovery (d0-365). There was a significant serum BDNF*GRS interaction (p=0.047, HR=0.987), even when age*GRS (p=0.007, HR=0.943) and GCS (p=0.010, HR=0.780) were included as covariates (Table 4B).
CSF and serum BDNF Associations with GOS
In a secondary analysis, we evaluated CSF and serum BDNF associations with global outcome (GOS) to determine if BDNF levels were indicative of mortality or graded across a range of outcomes. Average CSF BDNF levels differed at 6 months between subjects who died versus survived (with both favorable and unfavorable outcome groups, Figure 2A). At 12 months, those with unfavorable outcome had CSF levels between those with favorable outcomes and those that died, but were not significantly different from either group. However, CSF BDNF levels were higher in those who died by 12 months vs. those with favorable outcome. Serum BDNF was not associated with 6-month GOS scores. Serum BDNF tended to be lower in subjects who died by 12 months (140.06±8.99 ng/mL) versus those with favorable outcomes (167.97±8.23 ng/mL, p=0.051, Figure 2B).
Figure 2.
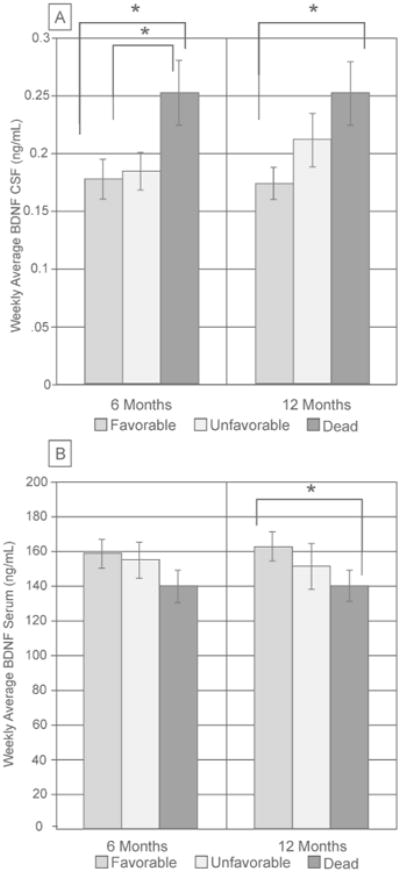
GOS was sub-divided as favorable (4-5) and unfavorable (2-3) for analyses with BDNF levels. (A) At 6 months, weekly averages of CSF were significantly higher in subjects who died compared to subjects who survived (both favorable and unfavorable outcomes, p<0.05). At 12 months, GOS levels were significantly different between subjects who died and subjects with favorable outcomes (p<0.05). (B) Weekly serum levels tended to be lower in subjects who died by 12 months compared to subjects who had favorable outcomes (p=0.051), but there was no significant association at 6 months.
Discussion
This study investigated BDNF as a dynamic and genetically-modifiable biomarker for mortality and global outcome following TBI. Compared to controls, serum BDNF levels were reduced and CSF BDNF levels were modestly increased one week post-TBI. Additionally, relationships between age, BDNF genetic variation, and BDNF levels post-TBI showed two important interactions in mortality predictions, gene*age and gene*serum. This work suggests BDNF is a biomarker for mortality post-TBI, possibly due to BDNF's role in neuronal survival/apoptotic signaling as well as autonomic nervous system reactivity.
Clinical TBI biomarker studies evaluating BDNF are limited, and to our knowledge, none have investigated BDNF levels and TBI outcome, especially in the context of genetic variation. One study in pediatric TBI showed increases in CSF BDNF levels immediately following injury (2hrs post-injury) that remained higher than controls throughout the first 24hrs49. Kalish and Phillips39 found serum BDNF was correlated with injury severity post-TBI, such that subjects with mild injuries had the highest levels. Yet, BDNF levels have never been examined as a marker for TBI-related mortality.
BDNF is an intriguing biomarker for TBI-related mortality and outcome. There is evidence of BDNF upregulation immediately following experimental TBI29,50, findings which were thought to aid neuroprotection, given BDNF's role in neuronal survival51,52. Yet, subsequent studies demonstrated that acute infusions of BDNF following injury do not attenuate tissue loss or improve motor or cognitive recovery13. Additionally, BDNF may be relevant in TBI due to its relationship to autonomic nervous system functioning14,15. HPA axis dysregulation is common following TBI22,53 and thus variation in plasticity and HPA axis reactivity associated with BDNF signaling19 could affect mortality and/or outcomes post-injury.
Additional support for BDNF as a biomarker comes from our previous research which demonstrated important BDNF gene variation interactions with age that influenced mortality predictions post-TBI10. We have previously suggested age-specific risk profiles could be due to a known balance shift in BDNF receptor ratios with aging, from pro-survival to pro-apoptotic26–28. Injury can also shift receptor balance. Experimental TBI induces transient increases in hippocampal TrkB.FL followed by delayed increases in TrkB.T (truncated, inactive form) with regionally-specific p75NTR increases up to 8 weeks post-TBI29. Relevant to acute TBI mortality, TrkB.FL/Trk.T expression ratios may support cell survival during excitotoxic injury54,55. With a shift in BDNF receptor ratios occurring with injury and aging, our work indicates that understanding the effects of both is likely necessary to generate sensitive TBI-related mortality prediction models. Importantly, this shifting receptor profiles suggest BDNF could be both beneficial and detrimental following neurological injury depending on receptor balances.
Our current study examined how BDNF levels in CSF and serum predicted mortality and outcome post-TBI, especially in concert with BDNF genetic variation and age. Interestingly, elevated CSF levels were associated with increased mortality risk. We suggest mortality risk associated with higher BDNF signaling could be due to the age or injury related changes in BDNF target receptor expression that favor apoptotic (p75) pathways, effectively making BDNF exposure detrimental, especially early after injury.
In uninjured populations, serum BDNF levels appear to reflect brain and CSF BDNF levels where BDNF is released into the blood from brain56,57. However, in our study, there tended to be a negative correlation between CSF and serum that differs from healthy populations37 and rodent models36. There may be multiple reasons for this negative correlation between compartments. In addition to the brain, BDNF is also synthesized and secreted from vascular endothelial cells and may have peripheral actions58. BDNF is also stored and released from platelets59, especially in response to injured tissue60. The negative correlation between CSF and serum following TBI could be due to BBB disruption, with platelets dumping BDNF and serum BDNF transit into the CNS acutely after TBI. BDNF transit into the CNS could also effectively represent an unsuccessful compensatory mechanism after TBI. Also, serum BDNF may be attributable to increased autonomic function, as HPA axis activation can diminish BDNF levels61. Thus, the lower serum BDNF associations with mortality may again reflect transit or dumping of BDNF into the CNS and/or reflect autonomic changes in BDNF levels related to HPA axis reactivity. Thus, higher CSF BDNF and lower serum BDNF associations with mortality may represent target receptor expression profiles, BBB disruption, and compensatory BDNF flow regulation.
When examining BDNF levels (either serum or CSF) associations with mortality, the BDNF-GRS*age interaction remained significant or a trend over the one-year monitoring period. In this current study, BDNF levels predicted mortality in addition to age*GRS interactions, suggesting BDNF levels contribute additional information about mortality beyond age*GRS. However, the magnitude and significance of the age*GRS interaction was reduced when accounting for BDNF levels in this study, findings which indicate that the age*GRS interaction may capture some element of aging effects on BDNF secretion in the context of TBI, in addition to aging influences on target receptor milieu associated with normal aging. Notably, BDNF serum levels are reduced in older individuals in studies without TBI62,63, although an age*BDNF levels interaction with mortality was not observed.
Our final mortality model includes GRS*age and GRS*serum BDNF interactions. While additional research is needed to understand the mechanisms of these interactions, we suggest BDNF profiles immediately post-injury could reflect both neuronal survival/apoptotic signaling as well as autonomic nervous system effects on outcome. Given findings regarding BDNF variation in autonomic function19,20, and aging effects on autonomic function/reactivity64, GRS*age interactions may also reflect HPA-axis reactivity. Yet, the GRS*serum interaction likely reflects BDNF gene control over BDNF secretion30,31. There may be the possibility for a three-variable interaction of GRS*age*serum, due to age related increases in BBB permeability65,66, yet this study is underpowered for exploring a three-variable interaction. CSF BDNF levels did not interact with GRS in mortality. Regardless of GRS, high CSF BDNF levels were associated with mortality, suggesting other factors like BBB transport and neuronal BDNF production contribute to CSF BDNF contributions to mortality.
We also examined CSF and serum relationships to 6 and 12 month GOS. GOS includes mortality as an outcome, and thus CSF relationships with GOS were driven by mortality status. Serum levels showed no relationship to 6 month GOS, but there was some capacity for mortality prediction with 12 month GOS. Similar to CSF analyses, the primary significant comparison at 12 months was between those who died versus those with favorable outcomes. This finding suggests acute BDNF is more reflective of mortality status, and this biomarker may be lacking when discriminating global outcome among survivors. However, future BDNF evaluation in the context of other survivor-specific outcome measures should be explored.
BDNF biomeasurements have many important caveats to consider, such as processing time, storage temperature, and inter-assay variation67–70. To combat these issues, we used freshly frozen samples, reduced processing time, and stored samples at -80°C. Long storage times at -80°C could affect BDNF serum measurements, but importantly, TBI and control samples were subject to similar storage times and conditions. Additionally, the BDNF ELISA used here does not differentiate between proBDNF and mature BDNF. Future studies may investigate differences in pro-BDNF versus mature BDNF. Serum BDNF levels can also be affected by circadian rhythms71. However, it is not clear if/how BDNF levels vary daily in the context of acute TBI as studies report a loss of normal circadian rhythms acutely after TBI6. As BDNF levels are associated with depression, participants depressed at the time of injury may have lower serum BDNF initially. Examining pre-injury function and pre-morbidity may better delineate these issues. One study suggests plasma BDNF levels predict mortality in ICU patients without TBI. While associated mechanisms are unclear33, with altered metabolic homeostasis and autonomic dysfunction immediately following TBI, there may be vascular BDNF actions that influence systemic contributors to mortality. Additionally, the cause of death for individuals is not clear as the Social Security Database does not provide cause of death. Larger studies, may generate meaningful information and categorization strategies comparing cause of death and BDNF pathology.
While there are important limitations to consider, this study suggests that BDNF pathology is likely an important new target in relationship to individual variation in mortality predictions post-TBI. With the increasingly higher incidence of TBI in elderly populations, the implications of altered BDNF signaling in the context of advanced age could inform geriatric TBI care. Additionally, experimental TBI studies in aging/aged animals could confirm mechanisms that link age and injury alterations on BDNF target receptors to mortality post-TBI. Incorporating this work into a Rehabilomics40,41 framework for further study with neurological outcomes and autonomic function after TBI may allow for more individualized rehabilitation and medical interventions with regard to BDNF pathology, genetics, and age. Still, future studies are needed to examine how BDNF pathology evolves over time, especially in regard to outcomes like depression72 and cognitive functioning73 where higher BDNF levels may contribute to positive outcomes. Additionally, this work may have applications in other acquired brain injuries like stroke, where BDNF signaling is altered under ischemic conditions54,55. Understanding BDNF signaling in relation to other secondary injury phenomena like HPA axis dysfunction, age, and/or target receptor regulation in neurological injury may inform new and more tailored treatments.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- 2.Brooks JC, et al. Long-Term Disability and Survival in Traumatic Brain Injury: Results From the National Institute on Disability and Rehabilitation Research Model Systems. Arch Phys Med Rehabil. 2013;94:2203–2209. doi: 10.1016/j.apmr.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Susman M, et al. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. discussion 223–224. [DOI] [PubMed] [Google Scholar]
- 4.Harrison-Felix C, Whiteneck G, Devivo MJ, Hammond FM, Jha A. Causes of death following 1 year postinjury among individuals with traumatic brain injury. J Head Trauma Rehabil. 2006;21:22–33. doi: 10.1097/00001199-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Goyal A, et al. S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J Neurotrauma. 2013;30:946–957. doi: 10.1089/neu.2012.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner AK, et al. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J Neurotrauma. 2011;28:871–888. doi: 10.1089/neu.2010.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santarsieri M, et al. CSF Cortisol and Progesterone Profiles and Outcomes Prognostication after Severe TBI. J Neurotrauma. 2013 doi: 10.1089/neu.2013.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner AK, et al. CSF Bcl-2 and cytochrome C temporal profiles in outcome prediction for adults with severe TBI. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2011;31:1886–1896. doi: 10.1038/jcbfm.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roozenbeek B, et al. Predicting 14-Day Mortality after Severe Traumatic Brain Injury: Application of the IMPACT Models in the Brain Trauma Foundation TBI-trac ® New York State Database. J Neurotrauma. 2012;29:1306–1312. doi: 10.1089/neu.2011.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Failla MD, et al. Variation in the BDNF Gene Interacts With Age to Predict Mortality in a Prospective, Longitudinal Cohort with Severe TBI. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314542617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ZY, et al. Variant Brain-Derived Neurotrophic Factor (BDNF) (Met66) Alters the Intracellular Trafficking and Activity-Dependent Secretion of Wild-Type BDNF in Neurosecretory Cells and Cortical Neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 13.Blaha GR, Raghupathi R, Saatman KE, McIntosh TK. Brain-derived neurotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficits. Neuroscience. 2000;99:483–493. doi: 10.1016/s0306-4522(00)00214-1. [DOI] [PubMed] [Google Scholar]
- 14.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 15.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Zhou XF. Injection of brain-derived neurotrophic factor in the rostral ventrolateral medulla increases arterial blood pressure in anaesthetized rats. Neuroscience. 2002;112:967–975. doi: 10.1016/s0306-4522(02)00085-4. [DOI] [PubMed] [Google Scholar]
- 17.Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci Off J Soc Neurosci. 1999;19:2131–2142. doi: 10.1523/JNEUROSCI.19-06-02131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan R, et al. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014 doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander N, et al. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology. 2010;35:949–953. doi: 10.1016/j.psyneuen.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Shalev I, et al. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34:382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Yang AC, et al. BDNF Val66Met polymorphism alters sympathovagal balance in healthy subjects. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2010;153B:1024–1030. doi: 10.1002/ajmg.b.31069. [DOI] [PubMed] [Google Scholar]
- 22.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992;20:216–226. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Santarsieri M, Kumar RG, Kochanek PM, Berga S, Wagner AK. Variable neuroendocrine-immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker PA. Whither proBDNF? Nat Neurosci. 2009;12:105–106. doi: 10.1038/nn0209-105. [DOI] [PubMed] [Google Scholar]
- 25.Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61:205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 26.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Romanczyk TB, et al. Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur J Neurosci. 2002;15:269–280. doi: 10.1046/j.0953-816x.2001.01858.x. [DOI] [PubMed] [Google Scholar]
- 28.Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Rostami E, et al. Alteration in BDNF and its receptors, full-length and truncated TrkB and p75NTR following penetrating traumatic brain injury. Brain Res. 2013 doi: 10.1016/j.brainres.2013.10.047. at < http://www.sciencedirect.com/science/article/pii/S0006899313014571>. [DOI] [PubMed]
- 30.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 31.Orefice LL, et al. Distinct roles for somatically and dendritically synthesized brain-derived neurotrophic factor in morphogenesis of dendritic spines. J Neurosci Off J Soc Neurosci. 2013;33:11618–11632. doi: 10.1523/JNEUROSCI.0012-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunoni AR, Lopes M, Fregni F. A Systematic Review and Meta-Analysis of Clinical Studies on Major Depression and BDNF Levels: Implications for the Role of Neuroplasticity in Depression. Int J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 33.Ritter C, et al. Brain-derived neurotrophic factor plasma levels are associated with mortality in critically ill patients even in the absence of brain injury. Crit Care. 2012;16:R234. doi: 10.1186/cc11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krabbe KS, et al. Brain-derived neurotrophic factor predicts mortality risk in older women. J Am Geriatr Soc. 2009;57:1447–1452. doi: 10.1111/j.1532-5415.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 35.Halldén S, et al. Smoking and obesity associated BDNF gene variance predicts total and cardiovascular mortality in smokers. Heart Br Card Soc. 2013;99:949–953. doi: 10.1136/heartjnl-2013-303634. [DOI] [PubMed] [Google Scholar]
- 36.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 37.Pillai A, et al. Decreased BDNF Levels in CSF of Drug-Naive First-Episode Psychotic Subjects: Correlation with Plasma BDNF and Psychopathology. Int J Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- 38.Terracciano A, et al. Genetics of serum BDNF: Meta-analysis of the Val66Met and genome-wide association study. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2013;14 doi: 10.3109/15622975.2011.616533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalish H, Phillips TM. Analysis of neurotrophins in human serum by immunoaffinity capillary electrophoresis (ICE) following traumatic head injury. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:194–200. doi: 10.1016/j.jchromb.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur J Phys Rehabil Med. 2010;46:549–556. [PubMed] [Google Scholar]
- 41.Wagner AK, Zitelli KTA. Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiol Off J Int Soc Pathophysiol ISP. 2012 doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Baker SP, O'Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 43.Social Security Death Index. GenealogyBank.com at < http://www.genealogybank.com/gbnk/ssdi/>.
- 44.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 45.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedman ML, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 47.Nettiksimmons J, et al. The Associations between Serum Brain-Derived Neurotrophic Factor, Potential Confounders, and Cognitive Decline: A Longitudinal Study. PLoS ONE. 2014;9:e91339. doi: 10.1371/journal.pone.0091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972:187–220. [Google Scholar]
- 49.Chiaretti A, et al. Correlation between neurotrophic factor expression and outcome of children with severe traumatic brain injury. Intensive Care Med. 2003;29:1329–1338. doi: 10.1007/s00134-003-1852-6. [DOI] [PubMed] [Google Scholar]
- 50.Hicks RR, Numan S, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res. 1997;48:401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 51.Almeida RD, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 52.Lindvall O, Kokaia Z, Bengzon J, Elmér E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 53.Baguley IJ, et al. Dysautonomia after traumatic brain injury: a forgotten syndrome? J Neurol Neurosurg Psychiatry. 1999;67:39–43. doi: 10.1136/jnnp.67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes JR, et al. Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci Off J Soc Neurosci. 2012;32:4610–4622. doi: 10.1523/JNEUROSCI.0374-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vidaurre OG, et al. Imbalance of neurotrophin receptor isoforms TrkB-FL/TrkB-T1 induces neuronal death in excitotoxicity. Cell Death Dis. 2012;3:e256. doi: 10.1038/cddis.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasmussen P, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 57.Dawood T, et al. Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Mol Psychiatry. 2007;12:981–983. doi: 10.1038/sj.mp.4002059. [DOI] [PubMed] [Google Scholar]
- 58.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89:279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakahashi T, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 60.Fujimura H, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- 61.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lommatzsch M, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Erickson KI, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci Off J Soc Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 65.Cernak I, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2010;32:442–453. doi: 10.1159/000320085. [DOI] [PubMed] [Google Scholar]
- 66.Oakley R, Tharakan B. Vascular hyperpermeability and aging. Aging Dis. 2014;5:114–125. doi: 10.14336/AD.2014.0500114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuccato C, et al. Brain-derived neurotrophic factor in patients with Huntington's disease. PloS One. 2011;6:e22966. doi: 10.1371/journal.pone.0022966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuchimine S, Sugawara N, Ishioka M, Yasui-Furukori N. Preanalysis storage conditions influence the measurement of brain-derived neurotrophic factor levels in peripheral blood. Neuropsychobiology. 2014;69:83–88. doi: 10.1159/000358061. [DOI] [PubMed] [Google Scholar]
- 69.Trajkovska V, et al. Measurements of brain-derived neurotrophic factor: Methodological aspects and demographical data. Brain Res Bull. 2007;73:143–149. doi: 10.1016/j.brainresbull.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Bus BAA, et al. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2012;13:39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piccinni A, et al. Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: an analysis of sex differences. Chronobiol Int. 2008;25:819–826. doi: 10.1080/07420520802387773. [DOI] [PubMed] [Google Scholar]
- 72.Wise EK, Hoffman JM, Powell JM, Bombardier CH, Bell KR. Benefits of exercise maintenance after traumatic brain injury. Arch Phys Med Rehabil. 2012;93:1319–1323. doi: 10.1016/j.apmr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


