Abstract
Study objective
To demonstrate the efficacy, safety, and appropriate mode of instillation of talc for sclerosis in treatment of malignant pleural effusions (MPEs).
Design
A prospective, randomized trial was designed to compare thoracoscopy with talc insufflation (TTI) to thoracostomy and talc slurry (TS) for patients with documented MPE.
Measurements
The primary end point was 30-day freedom from radiographic MPE recurrence among surviving patients whose lungs initially re-expanded > 90%. Morbidity, mortality, and quality of life were also assessed.
Results
Of 501 patients registered, those eligible were randomized to TTI (n = 242) or TS (n = 240). Patient demographics and primary malignancies were similar between study arms. Overall, there was no difference between study arms in the percentage of patients with successful 30-day outcomes (TTI, 78%; TS, 71%). However, the subgroup of patients with primary lung or breast cancer had higher success with TTI than with TS (82% vs 67%). Common morbidity included fever, dyspnea, and pain. Treatment-related mortality occurred in nine TTI patients and seven TS patients. Respiratory complications were more common following TTI than TS (14% vs 6%). Respiratory failure was observed in 4% of TS patients and 8% of TTI patients, accounting for five toxic deaths and six toxic deaths, respectively. Quality-of-life measurement demonstrated less fatigue with TTI than TS. Patient ratings of comfort and safety were also higher for TTI, but there were no differences on perceived value or convenience of the procedures.
Conclusions
Both methods of talc delivery are similar in efficacy; TTI may be better for patients with either a lung or breast primary. The etiology and incidence of respiratory complications from talc need further exploration.
Keywords: insufflation, malignant pleural effusion, slurry, talc
Malignant pleural effusion (MPE) is a common, debilitating complication of advanced cancer. Fluid accumulation resulting from tumor involvement of the pleura and lymphatics typically gives rise to dyspnea and chest pain. Although the optimal form of intrapleural therapy remains controversial, effective palliation can usually be achieved with tube thoracostomy and subsequent pleurodesis. Clinical studies1–6 of various sclerosing agents support the superior clinical effectiveness of intrapleural talc, but its safety and appropriate mode of administration are still debated.
Talc has been commonly used for treatment of MPE due to its well-known effectiveness for producing pleural symphysis. Thoracoscopic talc poudrage was introduced by Bethune7 in 1934 as an effective method of inducing adhesions to facilitate lobectomy. Use of talc slurry in animal models of sterile pleuritis was reported in 1940 as a more convenient variant of Bethune’s method.8 Chambers9 first reported the successful use of talc slurry to treat MPE in 1958. Since that time, a significant number of single institution reports have been published, primarily using thorascopically insufflated talc. However, a growing number of authors have advocated talc slurry via a percutaneously placed chest tube as a simpler and equally effective method for control of MPE with minimal short-term morbidity. However, several reports10,11 of serious respiratory complications with talc have also been published.
The objectives of the current trial Cooperative Groups Cancer and Leukemia Group B (CALGB) 9334 compare tube thoracostomy with talc slurry (TS) to surgical thoracoscopy with talc insufflation (TTI), and assesses their efficacy at 30 days, in addition to the safety and associated quality of life in a randomized multicenter trial. Portions of this work have been presented in abstract form.12
Materials and Methods
This was an intergroup cooperative trial led by the CALGB and monitored semiannually by its Data and Safety Monitoring Board, with participation by the Radiation Therapy Oncology Group, the Eastern Cooperative Oncology Group (ECOG), and the North Central Cooperative Oncology Group, encompassing both private and teaching hospitals. Credentialing of participating surgeons was required. Institutional review board approval and written informed patient consent were obtained.
Patients were identified either by the surgical or medical staff with eligibility criteria that included a history of a malignancy, pleural effusion requiring sclerosis, ECOG/Zubrod status 0–2, life expectancy > 2 months, and ability to undergo general anesthesia. Exclusion criteria included pregnancy, previous intrapleural therapy or radiation therapy encompassing the entire hemithorax, changes in systemic therapy within 2 weeks prior to randomization or subsequent to sclerosis (except addition of tamoxifen), and chylous or bilateral effusions requiring therapy.
Patients were randomized to receive 4 to 5 g of talc, either administered as a slurry in 100-mL saline solution through a chest tube at the bedside (TS group) or insufflated during thoracoscopy in the operating room (TTI group). After drainage of the pleural fluid, lung re-expansion by > 90% as estimated by the surgeon was required to proceed on protocol. Patients randomized to TS underwent sclerosis within 24 to 36 h of chest tube placement. The chest tube was subsequently clamped for 2 h and then reattached to suction drainage. There was no requirement for the patient to rotate positions. The chest tube (possibly more than one for TTI patients) was removed once the 24-h drainage was < 150 mL, and a baseline chest radiograph (CXR) was obtained. Additional radiographs were obtained after 30 days and then monthly until death or for 6 months.
The primary objective was to determine the percentage of patients whose lung initially re-expanded > 90% and who had a successful pleurodesis at 30 days after treatment. Surgeons were to make the visual estimation during surgery following drainage of the effusion of the > 90% re-expansion prior to sclerosis. For the talc slurry study arm, the surgeon will estimate radiographically the > 90% re-expansion prior to instillation of the talc via the chest tube. Success was defined as no pleural fluid re-accumulation greater than that seen on the baseline CXR as evaluated by the surgeon. Success rates were compared using Fisher exact test. Radiologic review (E.S.) was performed by comparing 30-day CXRs with baseline CXRs. Secondary end points included time to recurrence of effusion (compared using log-rank test), frequency of complications and toxicities (Fisher exact test), and the ability to re-expand the lung as assessed by CXR (Fisher exact test). A Wilcoxon two-sample test was used to compare treatment groups with respect to the following: (1) intensity of pain as measured by a visual analog scale, and (2) satisfaction with treatment as measured by 4-point Likert scales that assessed convenience, medical safety, comfort, and cost. Change in quality of life at 30 days relative to baseline was measured using the European Organization for Research and Treatment of Cancer QLQ-C3013 and analyzed using analysis of covariance.
Results
Between January 1995 and September 1999, 501 patients were randomized (TS, n = 250; TTI, n = 251). Nineteen patients were excluded: 13 were ineligible and 6 withdrew consent. Thus, 240 TS and 242 TTI patients remained for outcome analysis. Patient demographics (Table 1) and distribution of underlying malignancies (Table 2) were similar between study arms.
Table 1.
Characteristics of Eligible Patients*
| Characteristics | TS | TTI |
|---|---|---|
| Patients, No. | 240 | 242 |
| Female gender | 140 (58) | 129 (53) |
| Age, yr | ||
| Mean (SD) | 61.5 (12.9) | 62.5 (11.7) |
| Median (range) | 65 (23–87) | 64 (34–85) |
| Race | ||
| White | 198 (82) | 192 (79) |
| Hispanic | 5 (2) | 7 (3) |
| African American | 30 (13) | 36 (15) |
| Other | 7 (3) | 7 (3) |
| Performance status | ||
| 0 | 28 (12) | 30 (12) |
| 1 | 111 (46) | 88 (36) |
| 2 | 95 (40) | 113 (47) |
| 0–2 | 6 (2) | 11 (5) |
Data are presented as No. (%) unless otherwise indicated.
Table 2.
Distribution of Primary Malignancies*
| Primary Site | TS | TTI |
|---|---|---|
| Lung | 93 (39) | 89 (37) |
| Breast | 56 (23) | 59 (24) |
| GI | 25 (10) | 20 (8) |
| Gynecologic | 8 (3) | 12 (5) |
| Genitourinary | 8 (3) | 12 (5) |
| Sarcoma | 6 (2) | 6 (2) |
| Head and neck | 3 (1) | 9 (4) |
| Melanoma | 1 (< 1) | 4 (2) |
| Lymphoma | 1 (< 1) | 2 (1) |
| Mesothelioma | 4 (2) | 0 |
| Other or unknown | 35 (14) | 29 (11) |
Data are presented as No. (%).
The criterion of > 90% lung re-expansion was met by 163 of 240 TS patients (68%) and 177 of 242 TTI patients (73%) [p = 0.231]. Among these, 33 of 163 TS patients (20%) and 25 of 177 TTI patients (14%) died within 30 days of the procedure. Table 3 summarizes 30-day recurrence-free survival among all eligible (both groups with either > 90% or < 90% re-expansion) patients, eligible patients treated with talc, all treated patients with > 90% lung re-expansion, and the subset of the patients with > 90% expansion who were alive at 30 days. There is no difference between the two treatment approaches in the rate of successful pleurodesis at 30 days among all patients treated with talc. However, the same comparison among all treated patients with > 90% lung re-expansion significantly favored TTI (67%) vs TS (56%) [p = 0.045], but successful pleurodesis among those alive at 30 days did not differ between study arms. Subgroup analyses revealed that the 30-day outcome among patients with breast or lung cancer differed between treatment arms (favoring TTI) independent of denominator (Table 3). Among TTI patients with > 90% re-expansion alive at 30 days, 82% had successful pleurodesis vs 67% of comparable TS patients (p = 0.022).
Table 3.
Efficacy of Talc Slurry and Talc Insufflation*
| TS | TTI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Denominator | No. | Alive Without Recurrence, No. |
% | CI | No. | Alive Without Recurrence, No. |
% | CI | p Value |
| All malignancies | |||||||||
| All eligible patients | 240 | 126 | 53 | 46–59 | 242 | 145 | 60 | 53–66 | 0.119 |
| Eligible, treated | 221 | 126 | 57 | 50–64 | 228 | 145 | 64 | 57–70 | 0.177 |
| > 90%, treated | 163 | 92 | 56 | 50–64 | 177 | 119 | 67 | 65–77 | 0.045 |
| > 90%, alive | 130 | 92 | 71 | 62–78 | 152 | 119 | 78 | 71–85 | 0.169 |
| Lung or breast cancer | |||||||||
| All eligible patients | 149 | 75 | 50 | 148 | 96 | 65 | 0.014 | ||
| Eligible, treated | 136 | 75 | 55 | 140 | 96 | 69 | 0.026 | ||
| > 90%, treated | 98 | 52 | 53 | 108 | 78 | 72 | 0.006 | ||
| > 90%, alive | 78 | 52 | 67 | 95 | 78 | 82 | 0.022 | ||
| Other cancers | |||||||||
| All eligible patients | 91 | 51 | 56 | 94 | 49 | 51 | 0.659 | ||
| Eligible, treated | 85 | 51 | 60 | 88 | 49 | 56 | 0.645 | ||
| > 90%, treated | 65 | 40 | 62 | 69 | 41 | 59 | 0.861 | ||
| > 90%, alive | 52 | 40 | 77 | 57 | 41 | 72 | 0.662 | ||
CI = confidence interval.
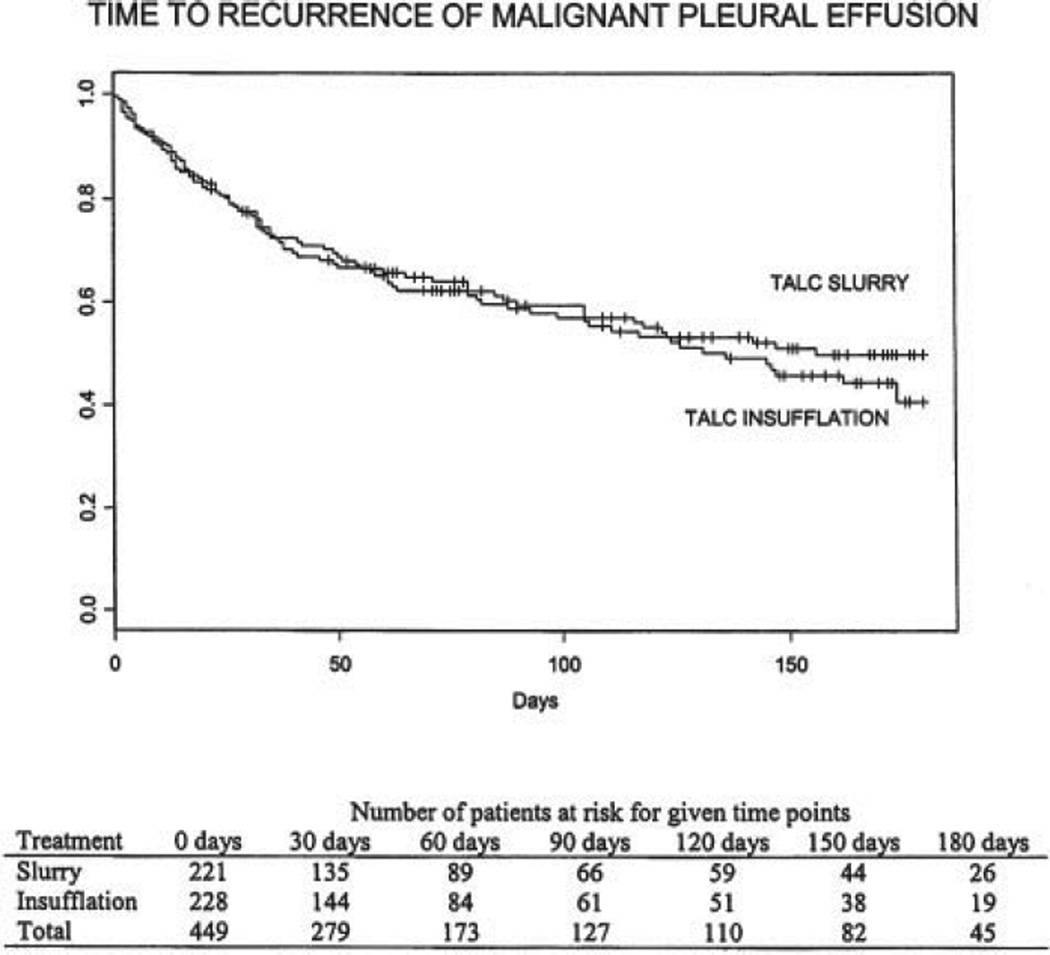
Of the 271 eligible patients who were alive at 30 days without recurrent MPE, 214 patients (79%; TS, n = 103; TTI, n = 111) had one or more subsequent follow-up assessments, and 60 late recurrences were documented (23 of 103 TS patients [22%], and 37 of 111 TTI patients [33%]). Kaplan-Meier product limit estimator was used to evaluate the distribution of time to recurrence of MPE (Fig 1). A log-rank test revealed no significant difference in time to recurrence between study arms (p = 0.622).
Figure 1.
Time to recurrence of MPE.
Independent radiologic review (TS, n = 102; TTI, n = 102) of patients alive at 30 days corroborated the results of the clinical evaluation. Seventy percent of patients with TS had stable or improved radiologic status at 30 days compared to 76% of TTI patients.
Table 4 summarizes surgical complications experienced by at least two patients. The most common adverse event following either procedure was fever (> 38.5°C). Respiratory complications (atelectasis, pneumonia, or respiratory failure) were experienced by significantly more TTI patients (13.5%) than TS patients (5.6%) [p = 0.007; χ2 with 80% power for a two-tailed test conducted at the 0.05 level of significance].
Table 4.
Surgical Complications Among Treated Patients*
| Toxicity | TS (n = 196) |
TTI (n = 223) |
|---|---|---|
| RBC transfusion | 5 (2.6) | 10 (4.5) |
| Postprocedure fever | 68 (34.7) | 68 (30.0) |
| Wound infection | 2 (1.0) | 1 (0.4) |
| Empyema | 2 (1.0) | 1 (0.4) |
| Bronchopleural fistula | 4 (2.0) | 6 (2.7) |
| Atelectasis (requiring more than two bronchoscopies) | 0 (0) | 3 (1.3) |
| Pneumonia (requiring antibiotics) | 7 (3.6) | 21 (9.3) |
| Respiratory failure | 8 (4.0) | 18 (8.1) |
| Dysrhythmia (requiring treatment) | 9 (4.6) | 12 (5.4) |
| Myocardial infarction | 1 (0.5) | 1 (0.4) |
| Deep vein thrombosis | 0 (0) | 7 (3.1) |
| Pulmonary embolism | 0 (0) | 4 (1.8) |
| Postoperative death | 12 (6.1) | 19 (8.4) |
Data are presented as No. (%).
Adverse events were also graded according to National Cancer Institute common toxicity criteria.14 Toxicity of at least grade 3 was experienced by 26% of patients in the TS study arm and 32% in the TTI study arm. Dyspnea (TS, 16%; TTI, 16%) and pain (TS, 10%; TTI, 5%) were the most common toxicities. Seven treatment-related deaths were reported for TS (respiratory failure [n = 5], cardiac [n = 2]), and nine treatment-related deaths were reported for TTI (respiratory [n = 6], cardiac [n = 1], infection [n = 2]).
TTI was perceived to provide more comfort (p = 0.019) and medical safety (p = 0.013) than TS, and patient perceptions of pain control tended in the same direction (p = 0.07). Similar differences were observed in the subgroup of patients whose lungs expanded > 90% (p = 0.006, p = 0.007, and p = 0.028, respectively), but not in the < 90% expansion subgroup. There was no difference between the TS and TTI study arms in patients’ perception of convenience or cost of the procedure, or the degree to which it was worthwhile to their overall care. There was no difference between study arms in the patients’ daily indication of pain using a visual analog scale.
Fourteen subscales of the quality-of-life questionnaire were performed at baseline and at each subsequent follow-up for 133 TS patients (55%) and 131 TTI patients (54%). Only fatigue was significantly different between the two study arms, with patients in the TTI arm demonstrating a decrease in fatigue, compared to increased fatigue for TS patients (p = 0.016).
Discussion
The current finding of no difference in the ability of talc, whether insufflated or placed as slurry, to prevent recurrence of MPE at 30 days is not surprising. An equivalence design was used because there was no a priori reason to expect that efficacy would differ between the study arms. Although there is considerable variability in reported “success” rates among published studies, a review15 of MPE studies published through 1994 reveals identical overall success rates of 91% for slurry (n = 166) and poudrage (n = 461). A small, randomized trial16 published during the current study also demonstrated no difference between the two modes of application. Further, an animal study17 demonstrate a similar density and distribution of adhesions following slurry vs poudrage delivery of intrapleural talc.
The observed efficacy rates in both arms of this study are, however, lower than those reported in previous trials and case series, most of which were single institution studies with small enrollment. Reports in the literature vary between 68% and 97% “success” for poudrage4,18 and from 72 to 94% for slurry.19,20 Varying definitions of recurrence (radiologic, symptomatic, requiring treatment) and choice of denominator may account for some of the discrepancy among studies.
A significant 30-day mortality rate was observed for MPE patients in both study arms. Reported 30-day mortality in previous studies varies considerably, from 0 to 32% for TS19,21 and from 3 to 24% for TTI.22,23 In some studies, lower mortality corresponds to a high percentage of breast cancer and higher mortality to a preponderance of lung cancer in the case mix. However, Kennedy and colleagues24 reported 24% (14 of 58 patients) 30-day mortality despite a large percentage of patients with breast cancer. The results of a multivariate analysis of 85 patients with MPE25 suggest that performance status may be the only clinical variable related to survival. The present finding of 20% mortality among TS patients and 14% among TTI patients (despite entry criteria of good performance status and life expectancy of 2 months) underscores the difficulty in predicting life expectancy in this patient population.
Morbidity predominantly included postprocedure fever, dyspnea, and pain. Incidence of these complications did not differ between the two study arms. Pain—either from the chest tube or the sclerosing agent—and fever are well-recognized adverse effects of talc pleurodesis. Although self-reports of daily pain were similar between the study arms, reported satisfaction with pain control favored the TTI study arm. However, TTI patients had pain management by anesthesiologists, whereas no pain management protocol was specified for TS patients. It has been reported that postsclerosis pain may be lessened for TS patients by adding local anesthetic to the slurry.20 Increased respiratory complications experienced by TTI patients may have been related to atelectasis from the general anesthetic and/or single-lung ventilation.
Respiratory failure was observed in 4% of TS cases and 8% of TTI cases, accounting for five toxic deaths and six toxic deaths, respectively. These observations are consistent with some previous reports (although others report no acute respiratory failure in large series26,27). Using 10 g of talc in a slurry, Rinaldo et al10 reported three cases of ARDS with bilateral infiltrates apparently precipitated by talc. Kennedy et al24 reported that 5 of 58 patients (9%) treated with 10 g of slurry had respiratory failure, with 3 patients requiring intubation. The high incidence of respiratory complications in these series may be related to the large dose.27 However, Marom et al19 found no difference in complication rates among 60 patients treated with 5 g vs 10 g of TS. Rehse and colleagues11 described the development of ARDS in 7 of 78 patients (9%) after 5 g of talc by poudrage (n = 3) and slurry (n = 4). Also, several reports28,29 describe acute pulmonary distress of patients who received only 2 g of talc as a poudrage. Inconsistent incidence among reported series suggests a possible relationship to the size of talc particles30 or to specific contaminants, both of which can vary among talc sources.31
The etiology of acute respiratory complications is unclear. Intrapleural talc (slurry or powder) can clearly migrate or be transported into the lungs and other organs29,32 and may directly trigger adverse consequences. Indeed, long-term talc exposure in people who mine or process the substance has been reported to reduce measures of pulmonary function.33 However, the nature of any acute response and the factors that render specific patients susceptible are unknown. In the current study, the requirement for a performance status of 0–2 was intended to eliminate patients with inadequate respiratory reserve. However, patients with MPE may have substantial interstitial pulmonary disease with low oxygen saturation, which may not be clinically evident. Unfortunately, data were not gathered in the current study to test this hypothesis. Rapid evacuation of large pleural effusions is associated with risk of re-expansion pulmonary edema. However, in only a few of the respiratory deaths were the pleural effusions large enough for this to be of concern. Despite a thorough review of the treatment-related deaths, no consistent characteristic was evident.
Thus, it is often difficult to predict which patients will have successful and uncomplicated pleurodesis with talc. Patients with lung cancer, the largest subgroup of MPE patients, have been reported to have lower rates of success, although this trend was not substantiated in the current study. Danby et al34 noted that all failures in their series were lung cancer patients. In some patients, the lung may become “trapped” by tumor or fibrin, a condition that commonly leads to pleurodesis failure. Effusions characterized by low pH or glucose level have been reported to correlate with treatment failure and poor survival.35,36 However, the predictive utility of pleural fluid pH is questioned by other authors.37 Heffner and colleagues reported that pH has only “modest” value as a predictor of pleurodesis failure38 or survival,39 based on a meta-analysis that included 231 patients treated with thoracoscopic talc. Clinicians should acknowledge a small but currently unpredictable risk of severe respiratory complications including death. Future studies should evaluate the predictive value of oxygen saturation or diffusion capacity for such complications.
Given the equivalent efficacy of TS and TTI, the appropriate mode of delivery for a given patient will depend on several considerations. Thoracoscopy affords an opportunity to directly inspect the pleura and to address adhesions and loculations. This may be indicated for patients who have had prior ipsilateral surgery or attempted pleurodesis, or for whom there is a significant possibility of a trapped lung. Subset analysis in the current study suggests that TTI may be more advantageous for patients with lung or breast cancer. Other authors have expressed the opinion that thoracoscopy should be used to assess all patients with lung cancer and ipsilateral MPE.18 TTI is perceived by patients to afford greater com fort and medical safety, as well as less fatigue relative to TS. These factors may significantly impact treatment preferences for patients who rank quality of life as a principal goal of care.
Bedside TS, however, is a simpler, less invasive procedure that in this study was associated with a lower risk of respiratory complications and possibly with lower cost. Further work is still required to better define the etiology and risk of respiratory failure due to talc instillation. Other factors being equal, one should opt for the least intrusive method of palliation in this population of end-stage cancer patients. In this regard, a number of authors have reported successful control of MPE using smallbore, indwelling catheters without pleurodesis. As a follow-up to the current study, this intergroup consortium has recently undertaken a randomized trial comparing TS as the least invasive arm of the current study to small-bore catheter outpatient drainage.
ACKNOWLEDGMENT
We thank the cooperative oncology groups (Radiation Therapy Oncology Group, ECOG, North Central Cooperative Oncology Group, CALGB) and all of the surgeons who participated in placing patients onto this trial.
This work was supported by the National Cancer Institute (2 U01 CA65170).
Abbreviations
- CALGB
Cooperative Groups Cancer and Leukemia Group B
- CXR
chest radiograph
- ECOG
Eastern Cooperative Oncology Group
- MPE
malignant pleural effusion
- TS
thoracostomy and talc slurry
- TTI
thoracoscopy with talc insufflation
Reference
- 1.Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med. 1994;120:56–64. doi: 10.7326/0003-4819-120-1-199401010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Fentiman IS, Rubens RD, Hayward JL. A comparison of intracavitary talc and tetracycline for the control of pleural effusions secondary to breast cancer. Eur J Cancer Clin Oncol. 1986;22:1079–1081. doi: 10.1016/0277-5379(86)90009-x. [DOI] [PubMed] [Google Scholar]
- 3.Hamed H, Fentiman IS, Chaudary MA, et al. Comparison of intracavitary bleomycin and talc for control of pleural effusions secondary to carcinoma of the breast. Br J Surg. 1989;76:1266–1267. doi: 10.1002/bjs.1800761214. [DOI] [PubMed] [Google Scholar]
- 4.Hartman DL, Gaither JM, Kesler KA, et al. Comparison of insufflated talc under thoracoscopic guidance with standard tetracycline and bleomycin pleurodesis for control of malignant pleural effusions. J Thorac Cardiovasc Surg. 1993;105:743–747. [PubMed] [Google Scholar]
- 5.Zimmer PW, Hill M, Casey K, et al. Prospective randomized trial of talc slurry vs bleomycin in pleurodesis for symptomatic malignant pleural effusions. Chest. 1997;112:430–434. doi: 10.1378/chest.112.2.430. [DOI] [PubMed] [Google Scholar]
- 6.Diacon AH, Wyser C, Bolliger CT, et al. Prospective randomized comparison of thoracoscopic talc poudrage under local anesthesia versus bleomycin instillation for pleurodesis in malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1445–1449. doi: 10.1164/ajrccm.162.4.2002030. [DOI] [PubMed] [Google Scholar]
- 7.Bethune N. Pleural poudrage: a new technic for the deliberate production of pleural adhesions as a preliminary to lobectomy. J Thorac Surg. 1935;4:251–261. [Google Scholar]
- 8.Hanrahan EM, Adams R, Klopstock R. The role of experimentally produced intrapleural adhesions in extrapleural pneumonolysis and in the prevention of surgical atelectasis in animals. J Thorac Surg. 1941;10:284–299. [Google Scholar]
- 9.Chambers JS. Palliative treatment of neoplastic pleural effusion with intercostal intubation and talc instillation. West J Surg Obstet Gynecol. 1958;66:26–28. [PubMed] [Google Scholar]
- 10.Rinaldo JE, Owens GR, Rogers RM. Adult respiratory distress syndrome following intrapleural instillation of talc. J Thorac Cardiovasc Surg. 1983;85:523–526. [PubMed] [Google Scholar]
- 11.Rehse DH, Aye RW, Florence MG. Respiratory failure following talc pleurodesis. Am J Surg. 1999;177:437–440. doi: 10.1016/s0002-9610(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Fleishman SB, Dresler C, Herndon JE, et al. Quality of life (QOL) advantage of sclerosis for malignant pleural effusion (MPE) via talc thoracoscopy over chest tube infusion of talc slurry: a Cancer and Leukemia Group B study [abstract] Proc Am Soc Clin Oncol. 2002;21:355a. [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQC30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Common toxicity criteria, version 2.0. Bethesda, MD: National Cancer Institute; 1999. [PubMed] [Google Scholar]
- 15.Kennedy L, Sahn SA. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215–1222. doi: 10.1378/chest.106.4.1215. [DOI] [PubMed] [Google Scholar]
- 16.Yim AP, Chan AT, Lee TW, et al. Thoracoscopic talc insufflation versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg. 1996;62:1655–1658. [PubMed] [Google Scholar]
- 17.Cohen RG, Shely WW, Thompson SE, et al. Talc pleurodesis: talc slurry versus thoracoscopic talc insufflation in a porcine model. Ann Thorac Surg. 1996;62:1000–1002. doi: 10.1016/0003-4975(96)00488-2. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez Panadero F. Lung cancer and ipsilateral pleural effusion. Ann Oncol. 1995;6(suppl):S25–S27. doi: 10.1093/annonc/6.suppl_3.s25. [DOI] [PubMed] [Google Scholar]
- 19.Marom EM, Patz EF, Jr, Erasmus JJ, et al. Malignant pleural effusions: treatment with small-bore-catheter thoracostomy and talc pleurodesis. Radiology. 1999;210:277–281. doi: 10.1148/radiology.210.1.r99dc04277. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi CA, Wenger FA, Schmitz-Rixen T, et al. Talc pleurodesis in recurrent pleural effusions. Langenbecks Arch Surg. 1998;383:156–159. doi: 10.1007/s004230050108. [DOI] [PubMed] [Google Scholar]
- 21.Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8–15. doi: 10.1016/s0003-4975(10)63944-6. [DOI] [PubMed] [Google Scholar]
- 22.Schulze M, Boehle AS, Kurdow R, et al. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg. 2001;71:1809–1812. doi: 10.1016/s0003-4975(01)02586-3. [DOI] [PubMed] [Google Scholar]
- 23.Jones GR. Treatment of recurrent malignant pleural effusion by iodized talc pleurodesis. Thorax. 1969;24:69–73. doi: 10.1136/thx.24.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy L, Rusch VW, Strange C, et al. Pleurodesis using talc slurry. Chest. 1994;106:342–346. doi: 10.1378/chest.106.2.342. [DOI] [PubMed] [Google Scholar]
- 25.Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117:73–78. doi: 10.1378/chest.117.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Viallat JR, Rey F, Astoul P, et al. Thoracoscopic talc poudrage pleurodesis for malignant effusions: a review of 360 cases. Chest. 1996;110:1387–1393. doi: 10.1378/chest.110.6.1387. [DOI] [PubMed] [Google Scholar]
- 27.Weissberg D, Ben-Zeev I. Talc pleurodesis: experience with 360 patients. J Thorac Cardiovasc Surg. 1993;106:689–695. [PubMed] [Google Scholar]
- 28.Bouchama A, Chastre J, Gaudichet A, et al. Acute pneumonitis with bilateral pleural effusion after talc pleurodesis. Chest. 1984;86:795–797. doi: 10.1378/chest.86.5.795. [DOI] [PubMed] [Google Scholar]
- 29.de Campos JR, Vargas FS, de Campos Werebe E, et al. Thoracoscopy talc poudrage: a 15-year experience. Chest. 2001;119:801–806. doi: 10.1378/chest.119.3.801. [DOI] [PubMed] [Google Scholar]
- 30.Ferrer J, Montes JF, Villarino MA, et al. Influence of particle size on extrapleural talc dissemination after talc slurry pleurodesis. Chest. 2002;122:1018–1027. doi: 10.1378/chest.122.3.1018. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer J, Villarino MA, Tura JM, et al. Talc preparations used for pleurodesis vary markedly from one preparation to another. Chest. 2001;119:1901–1905. doi: 10.1378/chest.119.6.1901. [DOI] [PubMed] [Google Scholar]
- 32.Werebe EC, Pazetti R, Milanez de Campos JR, et al. Systemic distribution of talc after intrapleural administration in rats. Chest. 1999;115:190–193. doi: 10.1378/chest.115.1.190. [DOI] [PubMed] [Google Scholar]
- 33.Wegman DH, Peters JM, Boundy MG, et al. Evaluation of respiratory effects in miners and millers exposed to talc free of asbestos and silica. Br J Ind Med. 1982;39:233–238. doi: 10.1136/oem.39.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danby CA, Adebonojo SA, Moritz DM. Video-assisted talc pleurodesis for malignant pleural effusions utilizing local anesthesia and IV sedation. Chest. 1998;113:739–742. doi: 10.1378/chest.113.3.739. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Armengol A, Rodriguez-Panadero F. Survival and talc pleurodesis in metastatic pleural carcinoma, revisited. Report of 125 cases. Chest. 1993;104:1482–1485. doi: 10.1378/chest.104.5.1482. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Panadero F, Segado A, Martin Juan J, et al. Failure of talc pleurodesis is associated with increased pleural fibrinolysis. Am J Respir Crit Care Med. 1995;151:785–790. doi: 10.1164/ajrccm/151.3_Pt_1.785. [DOI] [PubMed] [Google Scholar]
- 37.Aelony Y, King RR, Boutin C. Thoracoscopic talc poudrage in malignant pleural effusions: effective pleurodesis despite low pleural pH. Chest. 1998;113:1007–1012. doi: 10.1378/chest.113.4.1007. [DOI] [PubMed] [Google Scholar]
- 38.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure. Chest. 2000;117:87–95. doi: 10.1378/chest.117.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest. 2000;117:79–86. doi: 10.1378/chest.117.1.79. [DOI] [PubMed] [Google Scholar]