Abstract
Objective
To compare single-session radiofrequency ablation (RFA) and ethanol ablation (EA) for treating predominantly cystic thyroid nodules (PCTNs).
Materials and Methods
This single-blind, randomized trial was approved by the Institutional Review Board of two centers and informed consent was obtained from all patients before enrollment. Fifty patients with a single PCTN (cystic portion less than 90% and greater than 50%) were randomly assigned to be treated by either RFA (25 patients) or EA (25 patients) at two hospitals. The primary outcome was the tumor volume reduction ratio (%) at the six-month follow-up and the superiority margin was set at 13% (RFA minus EA). Analysis was performed primarily in an intention-to-treat manner. The secondary outcomes were the therapeutic success rate, improvement of symptomatic and cosmetic problems, and the number of major complications.
Results
The mean volume reduction was 87.5 ± 11.5% for RFA (n = 22) and 82.4 ± 28.6% for EA (n = 24) (p = 0.710; mean difference [95% confidence interval], 5.1% [-8.0 to 18.2]), indicating no significant difference. Regarding the secondary outcomes, therapeutic success (p = 0.490), mean symptom (p = 0.205) and cosmetic scores (p = 0.710) showed no difference. There were no major complications in either group (p > 0.99).
Conclusion
The therapeutic efficacy of RFA is not superior to that of EA; therefore, EA might be preferable as the first-line treatment for PCTNs.
Keywords: Ultrasound, Radiofrequency, Ethanol, Predominantly cystic thyroid nodule, Thyroid
INTRODUCTION
The majority of studies on ultrasound (US)-guided ethanol ablation (EA) and radiofrequency ablation (RFA) for thyroid nodules have reported their efficacy according to the proportion of the solid component of these nodules (i.e., either cystic [cystic portion > 90%], predominantly cystic [cystic portion, > 50% and < 90%], or solid [cystic portion < 50%]) (1,2,3). For cystic thyroid nodules, EA has been suggested as a first-line treatment option in review articles as well as in prospective and retrospective studies (4,5,6,7). A retrospective comparison study of EA with RFA demonstrated that the two modalities showed similar efficacy, but the number of treatment sessions was fewer for EA (1.2 vs. 1.7; p < 0.03) (8). A recent randomized clinical trial comparing EA and RFA for cystic nodules confirmed the results of previous retrospective studies (9). Therefore, EA rather than RFA can be a treatment of choice for cystic nodules.
With predominantly cystic thyroid nodules (PCTNs), RFA showed better volume reduction than EA (2,10,11). The volume reduction by RFA is 10-14% greater than by EA (i.e., an estimated mean volume reduction ratio of 80.0% for RFA, and 65.8-69.8% for EA) and EA shows a high recurrence rate (range from 26-33%) (12,13). Recurrence after EA is mainly due to re-growing or bleeding from the vascular solid component in PCTNs (12). In the recurrent cases, RFA achieved excellent volume reduction (91-92%) (12,13). In addition, Lim et al. (2) used RFA as a first-line modality to treat PCTNs, resulting in volume reductions of approximately 80%. Although RFA appears to be superior to EA for treating PCTNs in existing retrospective studies, the best treatment modality for PCTNs is still unclear because there does not appear to have been any study comparing the capabilities of the two modalities. To establish the best treatment modality for treating PCTNs, clinical trial is necessary.
Therefore, the purpose of this study was to compare single-session RFA and EA for treating PCTNs, primarily focusing on tumor volume reduction.
MATERIALS AND METHODS
Study Design
This study was a single-blind (i.e., outcome assessor blinded) randomized trial. The Institutional Review Board of Asan Medical Center and Daerim St. Mary's Hospital approved this study. From February 2013 to May 2013, patients who met the eligibility criteria and provided written informed consent were randomly assigned to undergo either EA or RFA at a 1:1 ratio using simple randomization.
The eligibility criteria for inclusion in the study were: 1) patients with PCTN (proportion of cystic component, less than 90% and greater than 50% of the nodule); 2) reports of pressure symptoms or cosmetic problems caused by thyroid nodules; 3) benign cytological confirmation in at least two separate US-guided, fine-needle aspiration or core needle biopsies (14); and 4) normal serum levels of thyroid hormone, thyrotropin, and calcitonin. The exclusion criteria were: 1) nodules showing malignant features (i.e., taller than wide, spiculated margin, markedly hypoechoic, micro- or macrocalcification) on US (15,16); 2) lack of informed consent; 3) younger than 20 years of age; and 4) pregnant woman (9).
Pre-Enrollment Procedures and Assessment
All US and US-guided biopsies in addition to the laboratory and clinical results were evaluated prior to ablation. Two radiologists (with 17 and 12 years of thyroid US experience, respectively) performed US and US-guided fine needle aspitation using a 10-MHz linear probe and a real-time US system (Aplio SSA-770A; Toshiba Medical Systems, Otawara, Japan). Core needle biopsy was performed using the solid component of the PCTNs (17). Benign cytological results were determined according to the Bethesda classification system (18).
Before treatment, patients were asked to rate their symptoms on a 10-cm visual analog scale (scale, 0-10), and the physician recorded the cosmetic score as follows (1, no palpable mass; 2, no cosmetic problem but a palpable mass; 3, cosmetic problem on swallowing only; and 4, readily detected cosmetic problem at all times) (1,19). Vascularity was graded into four categories (grade 0, no intranodular vascularity; grade 1, perinodular vascularity only; grade 2, intranodular vascularity < 50%; grade 3, intranodular vascularity > 50%) (1).
Ablation Procedures
Both RFA and EA were performed in an outpatient setting. Before the treatment, the three orthogonal diameters of each nodule that were measured were the largest diameter and two mutually perpendicular diameters. The volume of each nodule was calculated using the equation volume = π abc / 6, where a is the largest diameter, and b and c are the other mutually perpendicular diameters (19). We carefully evaluated the vessels located along the approach route and injected 2% lidocaine at the site of skin puncture. After puncturing the skin, target nodules were approached using the trans-isthmic approach method that involves insertion of the electrode or needle into the short axis of the nodule from the isthmus to the targeting nodules (19,20).
Radiofrequency Ablation
Radiofrequency ablation was performed by two experienced thyroid radiologists. An 18-gauge monopolar modified internally cooled electrode (VIVA, STARmed, Goyang, Korea) with a 1-cm active tip was used together with a radiofrequency generator (VIVA RF generator, STARmed, Goyang, Korea) and a peristaltic pump (VIVA pump, STARmed, Goyang, Korea). The pump continuously infused 0℃ saline solution into the lumen of the electrode to maintain the temperature of the electrode at less than 20℃ (20). As much of the internal fluid as possible was aspirated before starting the RFA (9).
Previous studies have proposed the moving shot technique for thyroid RFA (9,21,22). Before RFA, we removed as much cystic fluid in the nodule as possible. We then divided the thyroid nodules into multiple conceptual ablation units and performed RFA sequentially on each unit by moving the electrode tip. These conceptual ablation units were smaller in the periphery of the nodule and larger in the central safe portion. Initially, the electrode tip was positioned in the deepest, most remote conceptual unit of the nodule to enable easy monitoring of the electrode tip without disturbance from the transient hyperechoic zone. The electrode was moved within the thyroid mass by tilting it upward or downward and carefully monitoring critical structures around the thyroid gland (23). When ablation in the peripheral unit was finished, the electrode was moved backward and in the superficial direction. Ablation was begun with 40 watts of RF power. If a transient hyperechoic zone did not form at the electrode tip within several seconds, the RF power was increased in 10-watt increments up to 80-watts. If the patient did not tolerate pain during the ablation, the RF power was reduced or local anesthesia was injected around the thyroid gland. Ablation was terminated when all units had changed to transient hyperechoic zones (14).
To assess safety, any possible complications both during and immediately after the procedure were evaluated (12). Procedure-related pain was graded into four categories (grade 0, RF power did not have to be turned off because the patient experienced no pain; grade 1, RF power was turned off once or twice to reduce pain levels; grade 2, RF power was turned off more than three times; and grade 3, RF procedure was incompletely terminated due to the patient's severe pain) (9). After RFA, each patient was observed for 1-2 hours while still in the hospital.
Ethanol Ablation
Ethanol ablation was also performed by two experienced thyroid radiologists. EA was performed as has been described previously (1,8). Briefly, a 16-gauge aspiration needle was inserted into the nodule through an isthmic area. After the needle tip was placed into the cystic portion, the internal fluid was aspirated to the maximum extent possible (24), followed by slow injection of 99% ethanol into the cystic space. If the cyst contents were viscous, the viscous fluid was aspirated followed by normal saline irrigation to remove any viscous material, after which ethanol was slowly injected into the cystic cavity (with care taken to avoid the solid portion). The volume of ethanol injected usually corresponded to about 50% of the aspirated volume (9). After two minutes of ethanol retention with the needle in place (1,9), the injected ethanol was completely removed and the needle was withdrawn. Ethanol was not injected into the solid component.
In order to assess the safety of this method, we checked for any complications during and immediately after the procedure. Procedure-related pain was graded into four categories (grade 0, no pain or mild pain similar to pain experienced during the lidocaine injection; grade 1, pain greater than that of the lidocaine injection, but not requiring medication; grade 2, pain requiring medication; and grade 3, procedure terminated prior to completion owing to severe pain) (1,9). Following the procedure, each patient was observed for 30 minutes while still in the hospital (9).
Follow-Up and Outcome Assessment
Outcomes were assessed by two experienced radiologists (thyroid US experience of 6 years and 15 years) other than the operators who were blind to the treatment group allocation and study design. US examination was performed in all patients at the time of the 1- and 6-month follow-up examinations. Upon US examination, the changes in the volume of the nodules were evaluated. The volume, therapeutic success rate and the improvement of symptomatic and cosmetic problems were checked. Any adverse events that occurred during the 6-month follow-up period were checked.
Statistical Analysis
Sample size was calculated as follows. The clinically relevant difference in the primary outcome for this study was set at 13% in accordance with previous reports (2,10,11,25). This corresponds to an estimated mean volume reduction ratio of 80.0% and 67.0% (65.8-69.8%) for the RFA and EA groups, respectively. The US measurementre-measurement variability according to the results of previous studies was 5.1-6.6% (26,27,28) and the relevant difference in the primary outcome was therefore set at 13%, double the value of 6.6%. The estimated standard deviation was set as 15% in both groups, also for consistency with previous studies (2,25). Given a type 1 error of 0.05 (two-sided), power of 80%, 1:1 allocation for each group, and an expected study dropout rate of 10%, a total of 50 patients (25 for each group) were deemed to be required. Statistical comparison between the two groups was performed using the chi-square test for gender, the Wilcoxon rank sum test for continuous outcomes and Fisher's exact test for binary outcomes. A two-sided p value less than 0.05 was considered to indicate a statistically significant difference. Data analysis was performed primarily in an intention-to-treat manner, and per-protocol analysis is provided supplementarily. Statistical analysis was performed using SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA).
Study Outcomes
The primary outcome of this study was the quantitative volume reduction ratio of a thyroid nodule at six months. The secondary outcomes for the clinical outcomes included a binary therapeutic success rate (the proportion of patients who showed volume reduction > 50%) (1), improvement of symptomatic and cosmetic scores, and the number of major complications (1,9,12). Recurrence was defined as treatment of a lesion that failed to achieve therapeutic success after EA. Major complications were defined according to the definitions of the Society of Interventional Radiology (29).
RESULTS
Patients
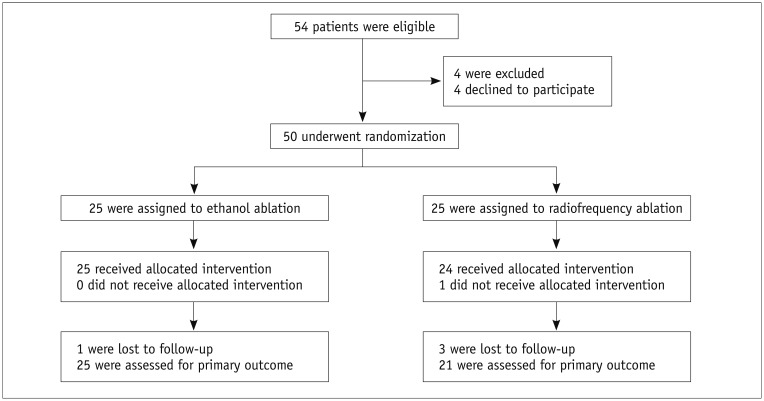
The flowchart in Figure 1 indicates the processes used for enrollment, randomization, and follow-up in this study. From February 2013 to May 2013, 54 patients with a single PCTN were identified as eligible for inclusion. Of these 54 patients, 4 patients declined to participate and the remaining 50 patients were randomly assigned to undergo either RFA (25 patients; mean age, 47.6 years; M:F = 4:21; mean age for women, 45.1 years; and for men, 50.0 years) or EA (25 patients; mean age, 50.8 years; M:F = 7:18; mean age for women, 53.3 years; and for men, 44.1 years). Only one patient in the RFA group did not meet the requirements of the study protocol. This patient was enrolled as a member of the EA group, and was included in the intention-to-treat analysis but excluded from the per-protocol analysis. One patient in the EA group and three patients in the RFA group were lost to follow-up after receiving the allocated procedures. Consequently, while 24 patients in the EA group and 22 patients in the RFA group were included in the intention-to-treat analysis, 25 patients in the EA group and 21 patients in the RFA group were included in the per-protocol analysis.
Fig. 1. Flowchart of patient enrollment process.
Regarding the treatment characteristics, the mean time of RFA was 218.4 ± 71.8 seconds (range, 120-450 seconds) and the mean RF power used was 57.4 ± 11.0 watts (range, 40-80 watts). The mean energy applied was 12482.2 ± 4308.4 joules (range, 6000-22500 Joule) and the mean energy per mL of nodule volume was 2302.7 ± 1358.7 joule/mL (range, 225-6125 joule/mL). The retention time of ethanol was 2 minutes in all cases.
Table 1 lists the demographic characteristics of the two study groups. The demographic data showed no differences between these groups but the average tumor diameter and volume were both significantly larger in the EA group. The symptom score was also greater for members of the EA group. Intra-nodular vascularity (vascularity grade 3) showed no significant difference in the RFA and EA groups (4/24 vs. 3/22, p > 0.99).
Table 1. Demographic Characteristics of Study.
| Characteristic | Radiofrequency Ablation (n = 22) | Ethanol Ablation (n = 24) | P |
|---|---|---|---|
| Gender (male:female) | 3:19 | 6:18 | 0.464 |
| Age (yr) | 49.8 ± 13.5 (24-76) | 50.8 ± 15.2 (23-82) | 0.819 |
| Nodule diameter (cm) | 3.0 ± 1.0 (2.0-5.6) | 3.6 ± 1.1 (1.7-6.2) | 0.032 |
| Nodule volume (mL) | 8.6 ± 9.4 (1.7-48.1) | 14.7 ± 13.7 (1.8-60.4) | 0.037 |
| Symptom score | 2.9 ± 1.8 (0-8) | 4.0 ± 1.7 (1-8) | 0.02 |
| Cosmetic score | 3.8 ± 0.4 (3-4) | 3.8 ± 0.4 (3-4) | 0.823 |
| Intra-nodular vascularity* | 3/22 | 4/24 | > 0.99 |
| Cost (USD)† | 1590 | 450 |
Except for numbers of men and women, values are means ± standard deviations, with ranges indicated in parentheses. *Intra-nodular vascularity: vascularity grade 3, †Average cost of enrolled two institutions
Outcomes
The clinical outcomes of the two groups at 6 months were analyzed in an intention-to-treat manner (Table 2). Regarding the primary outcome, the mean volume reduction of the RFA group was 87.5 ± 11.5%, and that of the EA group was 82.4 ± 28.6%, for a mean difference of 5.1% (95% confidence interval, -8.0 to 18.1) (p = 0.710).
Table 2. Outcomes of RFA and EA Groups at 6 Months after Treatment According to Intention-to-Treat Analysis.
| Outcome | Radiofrequency Ablation (n = 22) | Ethanol Ablation (n = 24) | P |
|---|---|---|---|
| Primary outcome | |||
| Volume reduction (%) | 87.5 ± 11.5 (63.1-99.5) | 82.4 ± 28.6 (-35.7-99.2) | 0.710 |
| Secondary outcomes | |||
| Symptom score | 0.2 ± 0.4 (0-1) | 0.7 ± 1.3 (0-6) | 0.205 |
| Cosmetic score | 1.5 ± 0.5 (1-2) | 1.7 ± 1.0 (1-4) | 0.710 |
| Therapeutic success (%) | 100 (22/22) | 91.7 (22/24) | 0.490 |
| Major complications | 0/22 | 1/24 | > 0.99 |
Values are means ± standard deviations (with ranges), except for major complications. EA = ethanol ablation, RFA = radiofrequency ablation
Regarding the secondary outcomes, the therapeutic success rate was higher for the RFA group than for the EA group; however, this was not statistically significant (100% vs. 91.7%, p > 0.99). Although the difference in the levels of therapeutic success was not statistically significant, two patients in the EA group failed to achieve therapeutic success. For both patients, the treated nodules grew during the follow-up period owing to internal bleeding from intra-nodular vessels. Both patients showed evidence of intra-nodular vascularity before treatment. One of these cases who showed increased nodule volume during follow-up was managed effectively using RFA. There was no significant difference between the EA and RFA groups in either mean symptoms (p = 0.280) or cosmetic scores (p = 0.901).
The outcomes of the two groups according to the protocol employed are summarized in Table 3. The mean volume reduction was 87.1 ± 11.6% in the RFA group and 83.1 ± 28.7% in the EA group, for a mean difference of 4.0% (95% CI, -9.1 to 17.4) (p = 0.904). The mean symptom and cosmetic scores, therapeutic success rate and major complications were not significantly different between the two groups.
Table 3. Outcomes of RFA and EA Patient Groups at 6 Months after Treatments According to Per-Protocol Analysis.
| Outcome | Radiofrequency Ablation (n = 21) | Ethanol Ablation (n = 25) | P |
|---|---|---|---|
| Primary outcome | |||
| Volume reduction (%) | 87.1 ± 11.6 (63.1-99.5) | 83.1 ± 28.7 (-35.7-99.2) | 0.904 |
| Secondary outcomes | |||
| Symptom score | 0.2 ± 0.4 (0-1) | 0.7 ± 1.3 (0-6) | 0.280 |
| Cosmetic score | 1.5 ± 0.5 (1-2) | 1.6 ± 0.9 (1-4) | 0.901 |
| Therapeutic success (%) | 100 (21/21) | 92 (23/25) | 0.493 |
| Major complications | 0/21 | 1/25 | > 0.99 |
Values are means ± standard deviations (with ranges), except for major complications. EA = ethanol ablation, RFA = radiofrequency ablation
One patient complained of a voice change immediately after EA but the issue was completely resolved by the 2-month follow-up without treatment. During and after the procedure, the degree of pain was grade 0 in all patients in the EA group and grade 0 (n = 9), grade 1 (n = 8), or grade 2 (n = 5) in members of the RFA group. Although there were no significant differences in major complications (p > 0.99), members of the RFA group revealed a greater tendency to experience pain.
DISCUSSION
Our current study is the first study comparing the efficacy and safety of RFA and EA for PCTNs. This study demonstrated that both single-session RFA and EA ensure the effective and safe treatment of PCTNs. Regarding the primary outcome, the mean volume reduction achieved using RFA was not superior to that using EA. Although previous retrospective studies showed superior volume reduction with RFA than with EA (2,10,11), the results of the current study could change the best treatment modality for treating PCTNs from RFA to EA. In addition, there were no significant differences between these two ablation approaches in terms of the secondary outcomes tested. Hence, EA might be used as a first-line treatment modality for PCTNs, given that it is a simpler and less expensive procedure. The results of this randomized controlled trial will be helpful for the development of future guidelines.
Non-surgical treatments for thyroid nodules have been introduced over the past 20 years (6,7,30). Recent large-population studies on RFA, laser ablation and EA were reported for nonfunctioning benign nodules (2,7,31,32), hyperfunctioning nodules (33) and recurrent thyroid cancers (34,35). Regarding the treatment efficacy, the proportion of solid component has been suggested as an important factor in volume reduction (1,2,36). Therefore, in order to select the treatment modality, thyroid nodules were divided into three types according to their solid component (36). Regarding the treatment of cystic thyroid nodules, the findings of both a retrospective study (8) and a randomized clinical trial (9) suggested that EA might be the optimal treatment modality. The results of RFA and EA for PCTNs are different from those for cystic nodules. Previous retrospective studies reported that RFA resulted in better volume reduction than EA for treating PCTNs (2,10,11,25). Consequently, RFA was regarded as the first-line treatment option for PCTNs before the current study. However, the results of the current study did not indicate RFA superiority in the treatment of PCTNs and could therefore change the treatment strategy of PCTNs from RFA to EA.
It was shown in an earlier report that recurrence after EA is frequent in thyroid nodules with a vascular solid component (3). The findings in that study indicated that venous washout of injected ethanol was frequently observed during EA of solid thyroid nodules with high vascularity, suggesting that the vascular solid component might be one of the causes of recurrence after EA. In contrast, intra-nodular echo-staining is observed during EA when the nodule shows poor venous washout of the injected ethanol. Consequently, intra-nodular echo-staining is closely related to the success rate of EA. For these recurrent cases, RFA showed excellent results (37). Jang et al. (12) prospectively suggested that RFA is necessary for the treatment of PCTNs for which more than 20% of the solid component displays extensive intra-nodular vascularity. Lee et al. (13) also suggested that RFA is effective for managing patients with incompletely resolved cosmetic and/or symptomatic problems after EA. In our current study, EA achieved 50% (2/4) therapeutic success in PCTNs with intra-nodular vascularity. This indicates that while EA can completely collapse the cystic portion of a PCTN, it is less suitable for complete management of the vascular solid component, which can be a cause of recurrence. Although RFA failed to show superior efficacy compared to EA in our current study, the vascular solid component, which can be a source of recurrence after EA, was effectively managed by RFA. All three patients who had vascular solid components in the RFA group achieved therapeutic success. Further study is necessary to validate the efficacy of RFA and EA according to the solid component and vascularity.
Regarding procedural safety, although the EA and RFA showed no differences in our study in terms of major complications, one of our patients who underwent EA complained of a voice change immediately after the procedure. This issue was completely resolved at the 2-month follow-up without treatment. Although voice change is a possible complication after EA, voice change after this procedure is very rare (1,3,9,10,25,37). Leakage of ethanol outside the thyroid gland can cause voice changes owing to damage of the recurrent laryngeal nerve. In our current study, no patients complained of voice changes after RFA, although a previous large multicenter study reported that approximately 1% (15/1459) of patients had voice-related problems after RFA (38). Pain during and after EA and RFA was tolerable in all of our study patients. However, the degree of pain seemed to be somewhat greater for members of the RFA group than for those of the EA group. In addition, though we did not measure the procedure time, the procedure time for EA seemed shorter than for RFA.
Our study had several limitations worth noting. The first was the relatively short follow-up period. Although both treatment modalities produced more than 80% volume reduction within 6 months, the remaining undertreated solid component of a PCTN can grow during a longer-term follow-up period (20). The second limitation was that one patient refused RFA, and thus needed to be enrolled in the EA group. Although this necessitated per-protocol analysis, the results were consistent with those of the intention-to-treat analysis. The third limitation was the demographic differences between our two ablation groups. Although the EA group showed a larger initial diameter (and volume) and symptoms, the efficacy achieved was similar to that observed for the RFA group. The fourth limitation was that it was unfeasible for the patients to be kept unaware of the assigned treatment. However, outcomes were assessed by blinded assessors. The fifth limitation is the selection of the primary outcome from among volume reduction, remission rate of the cyst and patients' symptoms. We used volume reduction as the primary outcome, but did not measure cystic and solid areas. We did not use the remission rate of the cystic portion, but we used the therapeutic success rate as a second outcome. Regarding the primary outcome of non-surgical treatment of cystic or PCTNs, the volume reduction ratio after treatment (1,8,9,11) and cyst volume less than 1 mL (treatment success) (5) have been used. In a previous EA study (5), recurrence of the treated cyst was defined as cyst volume > 1 mL on US at the 1-month evaluation and treatment was repeated. In other studies (1,9,12), treatment efficacy was measured by volume reduction. Therefore, the best medical/radiological parameter to confirm the treatment efficacy for fluid-containing thyroid nodules is still debatable. Since nodule size is usually well-correlated with compressive symptoms (39), volume reduction is a reasonable primary outcome. Finally, we only compared RFA and EA. To verify the best non-surgical treatment modality for PCTNs, further studies should compare EA or RFA approaches with other thermal ablation modalities, such as laser ablation, microwave ablation, and high intensity focused US.
In conclusion, RFA and EA are not significantly different in terms of treatment efficacy and safety for treating PCTNs. Given that the therapeutic efficacy of RFA is not superior to that of EA, and that EA is simpler and less expensive than RFA, EA may be preferable as the first-line treatment option for PCTNs. The results of this randomized controlled trial will be helpful for the development of future guidelines.
References
- 1.Kim YJ, Baek JH, Ha EJ, Lim HK, Lee JH, Sung JY, et al. Cystic versus predominantly cystic thyroid nodules: efficacy of ethanol ablation and analysis of related factors. Eur Radiol. 2012;22:1573–1578. doi: 10.1007/s00330-012-2406-5. [DOI] [PubMed] [Google Scholar]
- 2.Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23:1044–1049. doi: 10.1007/s00330-012-2671-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim DW, Rho MH, Park HJ, Kwag HJ. Ultrasonography-guided ethanol ablation of predominantly solid thyroid nodules: a preliminary study for factors that predict the outcome. Br J Radiol. 2012;85:930–936. doi: 10.1259/bjr/81849588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zingrillo M, Torlontano M, Chiarella R, Ghiggi MR, Nirchio V, Bisceglia M, et al. Percutaneous ethanol injection may be a definitive treatment for symptomatic thyroid cystic nodules not treatable by surgery: five-year follow-up study. Thyroid. 1999;9:763–767. doi: 10.1089/thy.1999.9.763. [DOI] [PubMed] [Google Scholar]
- 5.Bennedbaek FN, Hegedüs L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–5777. doi: 10.1210/jc.2003-031000. [DOI] [PubMed] [Google Scholar]
- 6.Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98:3949–3957. doi: 10.1210/jc.2013-1806. [DOI] [PubMed] [Google Scholar]
- 7.Papini E, Pacella CM, Misischi I, Guglielmi R, Bizzarri G, Døssing H, et al. The advent of ultrasound-guided ablation techniques in nodular thyroid disease: towards a patient-tailored approach. Best Pract Res Clin Endocrinol Metab. 2014;28:601–618. doi: 10.1016/j.beem.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Sung JY, Kim YS, Choi H, Lee JH, Baek JH. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol. 2011;196:W210–W214. doi: 10.2214/AJR.10.5172. [DOI] [PubMed] [Google Scholar]
- 9.Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269:293–300. doi: 10.1148/radiol.13122134. [DOI] [PubMed] [Google Scholar]
- 10.Cho YS, Lee HK, Ahn IM, Lim SM, Kim DH, Choi CG, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. AJR Am J Roentgenol. 2000;174:213–216. doi: 10.2214/ajr.174.1.1740213. [DOI] [PubMed] [Google Scholar]
- 11.Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004;10:269–275. doi: 10.4158/EP.10.3.269. [DOI] [PubMed] [Google Scholar]
- 12.Jang SW, Baek JH, Kim JK, Sung JY, Choi H, Lim HK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81:905–910. doi: 10.1016/j.ejrad.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA) World J Surg. 2010;34:1488–1493. doi: 10.1007/s00268-010-0565-6. [DOI] [PubMed] [Google Scholar]
- 14.Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125. doi: 10.3348/kjr.2012.13.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak JY, Jung I, Baek JH, Baek SM, Choi N, Choi YJ, et al. Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean J Radiol. 2013;14:110–117. doi: 10.3348/kjr.2013.14.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the korean society of thyroid radiology. Korean J Radiol. 2015;16:391–401. doi: 10.3348/kjr.2015.16.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK, Song DE, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013;268:274–280. doi: 10.1148/radiol.13122247. [DOI] [PubMed] [Google Scholar]
- 18.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 19.Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250. doi: 10.1007/s00330-008-0880-6. [DOI] [PubMed] [Google Scholar]
- 20.Huh JY, Baek JH, Choi H, Kim JK, Lee JH. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session--prospective randomized study. Radiology. 2012;263:909–916. doi: 10.1148/radiol.12111300. [DOI] [PubMed] [Google Scholar]
- 21.Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014;15:836–843. doi: 10.3348/kjr.2014.15.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesareo R, Pasqualini V, Simeoni C, Sacchi M, Saralli E, Campagna G, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100:460–466. doi: 10.1210/jc.2014-2186. [DOI] [PubMed] [Google Scholar]
- 23.Choi SH, Kim EK, Kim SJ, Kwak JY. Thyroid ultrasonography: pitfalls and techniques. Korean J Radiol. 2014;15:267–276. doi: 10.3348/kjr.2014.15.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Døssing H, Bennedbæk FN, Hegedüs L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules--a prospective randomized trial. J Clin Endocrinol Metab. 2013;98:E1213–E1217. doi: 10.1210/jc.2013-1503. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Ahn IM. Effectiveness of percutaneous ethanol injection therapy in benign nodular and cystic thyroid diseases: long-term follow-up experience. Endocr J. 2005;52:455–462. doi: 10.1507/endocrj.52.455. [DOI] [PubMed] [Google Scholar]
- 26.Bennedbaek FN, Nielsen LK, Hegedüs L. Effect of percutaneous ethanol injection therapy versus suppressive doses of L-thyroxine on benign solitary solid cold thyroid nodules: a randomized trial. J Clin Endocrinol Metab. 1998;83:830–835. doi: 10.1210/jcem.83.3.4673. [DOI] [PubMed] [Google Scholar]
- 27.Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules - a randomised study. Eur J Endocrinol. 2005;152:341–345. doi: 10.1530/eje.1.01865. [DOI] [PubMed] [Google Scholar]
- 28.Hegedüs L, Karstrup S, Rasmussen N. Evidence of cyclic alterations of thyroid size during the menstrual cycle in healthy women. Am J Obstet Gynecol. 1986;155:142–145. doi: 10.1016/0002-9378(86)90098-0. [DOI] [PubMed] [Google Scholar]
- 29.Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14(9 Pt 2):S243–S246. [PubMed] [Google Scholar]
- 30.Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH, Baek SH, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015;100:1903–1911. doi: 10.1210/jc.2014-4077. [DOI] [PubMed] [Google Scholar]
- 31.Suh CH, Baek JH, Ha EJ, Choi YJ, Lee JH, Kim JK, et al. Ethanol ablation of predominantly cystic thyroid nodules: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70:42–47. doi: 10.1016/j.crad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Ji Hong M, Baek JH, Choi YJ, Lee JH, Lim HK, Shong YK, et al. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. J Vasc Interv Radiol. 2015;26:55–61. doi: 10.1016/j.jvir.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Sung JY, Baek JH, Jung SL, Kim JH, Kim KS, Lee D, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25:112–117. doi: 10.1089/thy.2014.0100. [DOI] [PubMed] [Google Scholar]
- 34.Lim HK, Baek JH, Lee JH, Kim WB, Kim TY, Shong YK, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25:163–170. doi: 10.1007/s00330-014-3405-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Jung SL, Kim BS, Ahn KJ, Choi HS, Lim DJ, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol. 2014;15:817–826. doi: 10.3348/kjr.2014.15.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ha EJ, Baek JH. Advances in nonsurgical treatment of benign thyroid nodules. Future Oncol. 2014;10:1399–1405. doi: 10.2217/fon.14.59. [DOI] [PubMed] [Google Scholar]
- 37.Kim DW. Usefulness of two-stage ethanol ablation in the treatment of benign predominantly cystic thyroid nodules. Endocr Pract. 2014;20:548–555. doi: 10.4158/EP13458.OR. [DOI] [PubMed] [Google Scholar]
- 38.Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342. doi: 10.1148/radiol.11110416. [DOI] [PubMed] [Google Scholar]
- 39.Eng OS, Potdevin L, Davidov T, Lu SE, Chen C, Trooskin SZ. Does nodule size predict compressive symptoms in patients with thyroid nodules? Gland Surg. 2014;3:232–236. doi: 10.3978/j.issn.2227-684X.2014.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]