Abstract
Purpose
In 2004, the American Heart Association (AHA) had published an algorithm for the diagnosis of incomplete Kawasaki disease (KD). The aim of the present study was to investigate characteristics of supplemental laboratory criteria in this algorithm.
Methods
We retrospectively examined the medical records of 355 patients with KD who were treated with intravenous immunoglobulin (IVIG) during the acute phase of the disease. Laboratory data were obtained before the initial IVIG administration and up to 10 days after fever onset. In 106 patients, laboratory testing was performed more than twice.
Results
The AHA supplemental laboratory criteria were fulfilled in 90 patients (25.4%), and the frequency of laboratory examination (odds ratio [OR], 1.981; 95% confidence interval [CI], 1.391-2.821; P<0.001) was a significant predictor of it. The fulfillment of AHA supplemental laboratory criteria was significantly associated with refractoriness to the initial IVIG administration (OR, 2.388; 95% CI, 1.182-4.826; P=0.013) and dilatation of coronary arteries (OR, 2.776; 95% CI, 1.519-5.074; P=0.001).
Conclusion
Repeated laboratory testing increased the rate of fulfillment of the AHA supplemental laboratory criteria in children with KD.
Keywords: Kawasaki disease, Incomplete Kawasaki disease, Laboratory examinations, Diagnosis of Kawasaki disease
Introduction
Kawasaki disease (KD) is an acute self-limiting vasculitis of unknown etiology that predominantly occurs in young children and can cause coronary artery abnormalities in up to 25.0% of untreated patients1). KD cannot be diagnosed according to any specific tests; rather, the clinician should maintain a high index of suspicion for KD when a febrile patient presents with several compatible clinical findings. In the clinical practice, KD is typically diagnosed by applying recognized diagnostic criteria2). However, clinician's occasionally meet febrile children showing less than four of the principal clinical features of KD and without any other evident cause of fever; some of these children have been diagnosed with incomplete KD3). Incomplete KD is thought to represent approximately 15.0%-36.2% of KD cases3,4,5,6,7,8,9,10) and the risk of coronary artery abnormalities appears to be no lower in patients with incomplete KD than in patients with a complete presentation5). Also, it is possible that coronary artery abnormalities may become worse in incomplete KD patients due to delayed diagnosis and treatment3,11,12).
In 2004, the American Heart Association (AHA) published an algorithm for diagnosing incomplete KD and these criteria have been clinically applied ever since2). Laboratory criteria within that algorithm are as follows: (1) serum albumin ≤3.0 g/dL, (2) anemia for age, (3) increased alanine aminotransferase (ALT) levels, (4) platelet count ≥450,000/mm3 after the 7th day of fever, (5) white blood cell (WBC) count ≥15,000/mm3, and (6) urine WBC ≥10/high power field. More than three laboratory criteria showing increased levels of acute phase reactants support the diagnosis of an incomplete KD2). However, the performance of these laboratory criteria has been partially assessed in a single study only. In this study were all subjects children with coronary artery aneurysms13).
The aim of the present study was to investigate characteristics of the AHA supplemental laboratory criteria by conducting a retrospective analysis of the medical records of patients with KD.
Materials and methods
1. Subjects
From January 2006 to December 2012, 511 children with KD were admitted to Asan Medical Center. The study subjects were comprised of 355 children among them. All subjects presented with persisting fever and had at least 4 principal clinical features2): changes in extremities, polymorphous exanthem, bilateral bulbar conjunctival injection without exudate, changes in lips and oral cavity, and cervical lymphadenopathy. They were treated with initial intravenous immunoglobulin (IVIG) within 10 days after fever onset. Following patients were excluded from the study: 19 patients who were transferred from other institutes after initial IVIG treatment, 64 patients showing incomplete presentation, 10 patients that were admitted after fever lasting for more than 10 days and 63 patients in whom fever spontaneously subsided before initial IVIG administration.
The study was approved by the Institutional Review Board of Asan Medical Center (2014-0283) and the need for a patient consent was waived.
2. Data collection
Clinical, laboratory, and echocardiographic data were collected from a review of medical records. Only data of C-reactive protein and 6 other laboratory variables as factors constituting the AHA supplemental laboratory criteria were collected that were obtained before initial IVIG administration and up to 10 days postfever onset.
The body surface area was calculated using DuBois's formula14). Laboratory tests were performed more than twice prior to IVIG administration in 106 patients (29.9%). As a result there were 493 laboratory data sets. The highest values were selected if C-reactive protein levels, WBC counts, ALT levels and urine WBC counts were repeatedly performed; the lowest values were selected in case of hemoglobin levels and albumin levels. The highest value of platelet counts was selected in 46 patients in whom complete blood counts were performed from day 8 to day 10 postfever onset. The response to initial IVIG treatment was deemed "refractory" if persistent or recrudescent fever was noted at least 36 hours after IVIG administration. Z scores for coronary artery diameter were calculated according to the formula of Dallaire and Dahdah15). Coronary arterial dilatation was defined as a z scores for any coronary artery of at least 2.52,16).
Data of individual laboratory variable were categorized into negative or positive, according to whether or not the value of individual laboratory variable corresponded to the AHA supplemental laboratory criteria. Age-adjusted normal ranges of hemoglobin and serum ALT17) were used on this categorization. The fulfillment of the AHA supplemental laboratory criteria in a subject was determined if serum level of C-reactive protein at least 3.0 mg/dL and there were at least 3 positive results of laboratory variables.
In addition, 493 laboratory data sets were examined to assess the relationship between the fulfillment of the AHA supplemental laboratory criteria in each data set determined through the same method to a subject and the day on which the corresponding laboratory test was performed.
3. Statistical analysis
All numerical data are presented as median and range. All statistical analyses were performed using SPSS ver. 21.0 (SPSS Korea Data Solutions, Seoul, Korea). Statistical significance was defined as a two-sided P-value of <0.05. Univariate logistic regression analyses of clinical variables were performed to find a predictor of the fulfillment of AHA supplemental laboratory criteria. Multivariate analysis of clinical variables which were significant predictors in univariate analyses was additionally performed. Chi-square test was used in the analysis of the association of the fulfillment of the AHA supplemental laboratory criteria with a repetition of laboratory testing, a refractory to initial IVIG and the dilatation of coronary arteries. The likelihood ratio test for trends was used to analyze the relationship between the fulfillment of the AHA supplemental laboratory criteria and the day on which corresponding laboratory tests were performed.
Results
1. Analyses of data from 355 patients with complete presentation of KD
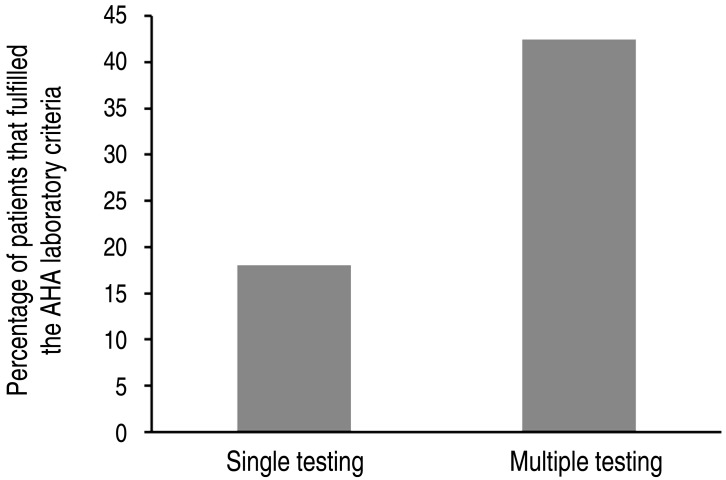
The patients' clinical and laboratory data are shown in Tables 1, 2. The AHA laboratory criteria were fulfilled in 90 patients (25.4 %). Clinical variables found to be associated with the fulfillment of the AHA supplemental laboratory criteria were as follows: the day of initial IVIG (P=0.029) and the frequency of laboratory examination (P<0.001). The frequency of laboratory examination (odds ratio [OR], 1.981; 95% confidence interval [CI], 1.391-2.821; P<0.001) also remained as a significant predictor for the fulfillment of the AHA supplemental laboratory criteria in multivariate analysis. One hundred six patients who underwent multiple laboratory testing fulfilled the AHA supplemental laboratory criteria more often than 249 patients who underwent single testing (43.0 % vs. 18.1%, P<0.001) (Fig. 1).
Table 1. Clinical characteristics of 355 patients with Kawasaki disease.
| Characteristic | Value |
|---|---|
| Age (mo) | 25.2 (1.6-186.0) |
| Female sex | 142 (40.0) |
| Body weight (kg) | 12.5 (5.2-58.1) |
| Height (cm) | 90.0 (52.9-176.4) |
| Body surface area (m2) | 0.54 (0.27-1.72) |
| Family history | 2 (0.6) |
| Recurrent illness | 11 (3.1) |
| Principal clinical features | |
| Conjunctival injection | 347 (97.7) |
| Changes in lips/oral cavity | 337 (94.9) |
| Changes in extremities | 330 (93.0) |
| Polymorphous exanthem | 325 (91.5) |
| Cervical lymphadenopathy | 235 (66.2) |
| Frequency of laboratory examination | 1 (1-5) |
| Day of IVIG | 6 (3-10) |
Values are presented as median (range) or number (%).
IVIG, intravenous immunoglobulin.
Table 2. Laboratory characteristics and number of patients with laboratory values corresponding with AHA supplemental laboratory criteria.
| Characteristic | Median (range) | Cutoff | No. (%) |
|---|---|---|---|
| CRP (mg/dL) | 7.95 (0.26-44.51) | ≥3.0 | 331 (93.2) |
| Supplemental laboratory criteria | 90 (25.4) | ||
| WBC count (/mm3) | 13,450 (3,000-31,300) | ≥15,000 | 140 (39.4) |
| Hemoglobin (g/dL) | 11.5 (8.4-16.8) | Anemia | 100 (28.2) |
| Platelet (×103/mm3) | 312 (51-864) | ≥450 | 12 (27.9*) |
| Albumin (g/dL) | 3.4 (2.0-4.5) | ≤3.0 | 70 (19.7) |
| ALT (IU/L) | 38 (5-1,028) | >45 | 170 (47.9) |
| Urine WBC (/HPF) | - | ≥10 | 114 (32.1) |
*Percentage was calculated in 46 patients who underwent complete blood count examination on 8th-10th day after fever onset.
AHA, American Heart Association; CRP, C-reactive protein; WBC, white blood cell; ALT, alanine aminotransferase; HPF, high power field.
Fig. 1. Percentage of patients fulfilling American Heart Association (AHA) supplemental laboratory criteria for the diagnosis of incomplete Kawasaki disease. Comparison between the results of patients who underwent a single laboratory test and those of patients who underwent multiple tests.
A refractory to initial IVIG was shown in 38 patients (10.7%). The fulfillment of the AHA supplemental laboratory criteria was significantly associated with the refractory to initial IVIG (OR, 2.388; 95% CI, 1.182-4.826; P=0.013). A coronary arterial dilatation was observed in 56 patients (15.8%). The fulfillment of AHA supplemental laboratory criteria was also significantly associated with the dilatation of coronary arteries (OR, 2.776; 95% CI, 1.519-5.074; P=0.001).
2. Relationship between the fulfillment of AHA supplemental laboratory criteria, its individual factors and the day of laboratory testing in 493 laboratory data sets
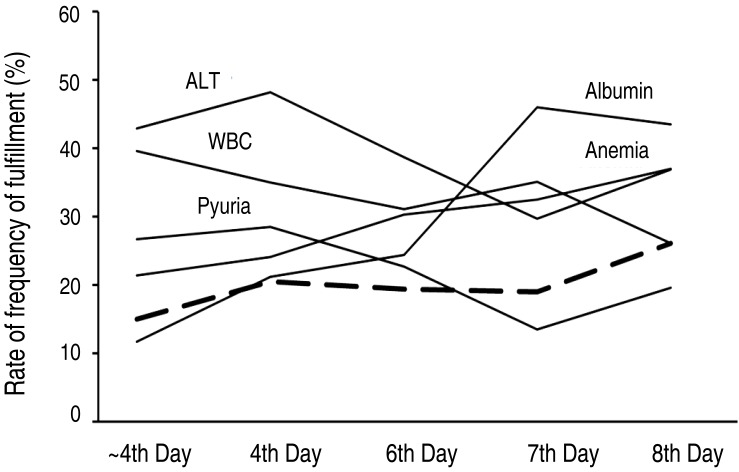
The observed increase in the tendency to fulfill the AHA supplemental laboratory criteria during a sustained bout of fever was not significant (P=0.075). Both the incidence of anemia (P=0.012) and hypoalbuminemia (P<0.001) tended to increase following a bout of sustained fever; however, neither the incidence of leukocytosis (P=0.077), the increase of ALT (P=0.126) nor the urine WBC ≥10/high power field (P=0.156) showed a significant tendency (Table 3, Fig. 2).
Table 3. Relationship between the fulfillment of AHA supplemental laboratory criteria and individual factors (laboratory variables) and the day at which laboratory examination was performed.
| Variable | ≤4th Day (n=154) | 5th Day (n=137) | 6th Day (n=119) | 7th Day (n=37) | 8th-10th Day (n=46) |
|---|---|---|---|---|---|
| CRP≥3.0 mg/dL | 133 (86.4) | 128 (93.4) | 107 (89.9) | 32 (86.5) | 41 (89.1) |
| Supplemental laboratory criteria | 21 (13.6) | 27 (19.7) | 22 (18.5) | 7 (18.9) | 12 (26.1) |
| WBC count ≥15,000/mm3 | 21 (13.6) | 27 (19.7) | 22 (18.5) | 7 (18.9) | 12 (26.1) |
| Anemia* | 33 (21.4) | 33 (24.1) | 36 (30.3) | 12 (32.4) | 17 (37.0) |
| Platelet ≥450×103/mm3 | - | - | - | - | 14 (30.4) |
| Albumin ≤3.0 g/dL* | 18 (11.7) | 29 (21.2) | 29 (24.4) | 17 (46.0) | 20 (43.5) |
| ALT >45 IU/L | 66 (42.9) | 66 (48.2) | 46 (38.7) | 11 (29.7) | 17 (37.0) |
| Urine WBC ≥10/HPF | 38 (24.7) | 36 (26.3) | 25 (21.0) | 5 (13.5) | 9 (19.6) |
Values are presented as number (%).
AHA, American Heart Association; CRP, C-reactive protein; WBC, white blood cell; ALT, alanine aminotransferase; HPF, high power field.
*P<0.05, showing significant tendency during a sustained bout of fever.
Fig. 2. Relationship between the day of laboratory testing and the frequency at which individual laboratory variables met American Heart Association (AHA) supplemental laboratory criteria. The thick dash denotes the rate of fulfillment of AHA supplemental laboratory criteria according to the day of laboratory testing. ALT, alanine aminotransferase; WBC, white blood cell.
Discussion
This study showed that repeated laboratory testing could increase the rate of fulfillment of the AHA supplemental laboratory criteria and that the incidences of anemia and hypoalbuminemia tend to increase following a sustained fever. In addition, the fulfillment of the AHA supplemental laboratory criteria was significantly associated with the refractory to initial IVIG and the dilatation of coronary arteries. These results will help a clinician to manage children suspected of having KD.
However, the rate of fulfillment of AHA supplemental laboratory criteria that is identical to its diagnostic sensitivity was only 25.4 % in overall subjects and 43.0% in subjects with repeated laboratory testing. Those diagnostic sensitivities were disappointingly low. Yellen et al.13) found that all 53 patients with incomplete KD in their study fulfilled the AHA supplemental laboratory criteria. The discrepancy between our data and that of Yellen et al.13) might not be caused by the presentation type of illness. No remarkable differences of laboratory findings have been reported between two presentation types4,18), although a low level of serum ALT4,13) and a low frequency of pyuria13) were exceptionally reported in children with incomplete presentations of KD. One possible explanation for this discrepancy may be differences in the study subjects. All the subjects recruited by Yellen et al.13) had coronary abnormalities and all laboratory tests were performed up to 21 days postfever onset, which is later than the 10 days in the present study. We believe that the ideal diagnostic criteria for incomplete KD should not be based on coronary artery abnormalities only and that they should be based on clinical/laboratory findings obtained within 10 days post fever onset. The possibility of future cardiovascular risk has not been excluded even in patients without definite coronary artery abnormalities during the acute phase of the disease. Thus, a long-term observation of those patients has been recommended19,20). We think it is needed to consider the future cardiovascular risk in the management of children with incomplete presentations of KD also. In addition, the fever duration is an important predictor of coronary artery abnormalities21,22). Indeed, the AHA recommends the administration of IVIG within the first 10 days of illness2). Therefore, ideal diagnostic criteria for an incomplete presentation of KD could support the diagnosis within 10 days postfever onset.
For the future, we recommend brain natriuretic peptide (BNP) as a factor of upgraded criteria if an upgrade of supplemental laboratory criteria for the diagnosis of an incomplete KD is considered. It has been reported that the value of BNP is above 50 pg/mL in nearly half of patients with KD23,24).
One limitation of this study is its retrospective design. Firstly, none of the laboratory variables were evaluated on a daily basis, which may at least partly be the reason for the low diagnostic sensitivity of the AHA supplemental laboratory criteria. Secondly, there were no age/gender-matched controls presenting with fever due to other causes. Therefore, we were unable to examine the diagnostic specificity of the AHA supplemental laboratory criteria as another important performance issue.
In conclusion, repeated laboratory testing could increase the rate of fulfillment of the AHA supplemental laboratory criteria in children with KD.
Footnotes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease: a 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 3.Sudo D, Monobe Y, Yashiro M, Mieno MN, Uehara R, Tsuchiya K, et al. Coronary artery lesions of incomplete Kawasaki disease: a nationwide survey in Japan. Eur J Pediatr. 2012;171:651–656. doi: 10.1007/s00431-011-1630-3. [DOI] [PubMed] [Google Scholar]
- 4.Manlhiot C, Christie E, McCrindle BW, Rosenberg H, Chahal N, Yeung RS. Complete and incomplete Kawasaki disease: two sides of the same coin. Eur J Pediatr. 2012;171:657–662. doi: 10.1007/s00431-011-1631-2. [DOI] [PubMed] [Google Scholar]
- 5.Sonobe T, Kiyosawa N, Tsuchiya K, Aso S, Imada Y, Imai Y, et al. Prevalence of coronary artery abnormality in incomplete Kawasaki disease. Pediatr Int. 2007;49:421–426. doi: 10.1111/j.1442-200X.2007.02396.x. [DOI] [PubMed] [Google Scholar]
- 6.Witt MT, Minich LL, Bohnsack JF, Young PC. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics. 1999;104:e10. doi: 10.1542/peds.104.1.e10. [DOI] [PubMed] [Google Scholar]
- 7.Barone SR, Pontrelli LR, Krilov LR. The differentiation of classic Kawasaki disease, atypical Kawasaki disease, and acute adenoviral infection: use of clinical features and a rapid direct fluorescent antigen test. Arch Pediatr Adolesc Med. 2000;154:453–456. doi: 10.1001/archpedi.154.5.453. [DOI] [PubMed] [Google Scholar]
- 8.Falcini F, Cimaz R, Calabri GB, Picco P, Martini G, Marazzi MG, et al. Kawasaki's disease in northern Italy: a multicenter retrospective study of 250 patients. Clin Exp Rheumatol. 2002;20:421–426. [PubMed] [Google Scholar]
- 9.Hsieh YC, Wu MH, Wang JK, Lee PI, Lee CY, Huang LM. Clinical features of atypical Kawasaki disease. J Microbiol Immunol Infect. 2002;35:57–60. [PubMed] [Google Scholar]
- 10.Perrin L, Letierce A, Guitton C, Tran TA, Lambert V, Kone-Paut I. Comparative study of complete versus incomplete Kawasaki disease in 59 pediatric patients. Joint Bone Spine. 2009;76:481–485. doi: 10.1016/j.jbspin.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Minich LL, Sleeper LA, Atz AM, McCrindle BW, Lu M, Colan SD, et al. Delayed diagnosis of Kawasaki disease: what are the risk factors? Pediatrics. 2007;120:e1434–e1440. doi: 10.1542/peds.2007-0815. [DOI] [PubMed] [Google Scholar]
- 12.Chang FY, Hwang B, Chen SJ, Lee PC, Meng CC, Lu JH. Characteristics of Kawasaki disease in infants younger than six months of age. Pediatr Infect Dis J. 2006;25:241–244. doi: 10.1097/01.inf.0000202067.50975.90. [DOI] [PubMed] [Google Scholar]
- 13.Yellen ES, Gauvreau K, Takahashi M, Burns JC, Shulman S, Baker AL, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010;125:e234–e241. doi: 10.1542/peds.2009-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311. [PubMed] [Google Scholar]
- 15.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Burns JC. Kawasaki disease update. Indian J Pediatr. 2009;76:71–76. doi: 10.1007/s12098-009-0031-3. [DOI] [PubMed] [Google Scholar]
- 17.Hong CE. Textbook of pediatrics. 10th ed. Seoul: Korea Textbook Publishing Co.; 2012. [Google Scholar]
- 18.Fukushige J, Takahashi N, Ueda Y, Ueda K. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994;83:1057–1060. doi: 10.1111/j.1651-2227.1994.tb12985.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukazawa R. Long-term prognosis of Kawasaki disease: increased cardiovascular risk? Curr Opin Pediatr. 2010;22:587–592. doi: 10.1097/MOP.0b013e32833e12f7. [DOI] [PubMed] [Google Scholar]
- 20.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 21.Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr. 1986;108:388–392. doi: 10.1016/s0022-3476(86)80878-2. [DOI] [PubMed] [Google Scholar]
- 22.Daniels SR, Specker B, Capannari TE, Schwartz DC, Burke MJ, Kaplan S. Correlates of coronary artery aneurysm formation in patients with Kawasaki disease. Am J Dis Child. 1987;141:205–207. doi: 10.1001/archpedi.1987.04460020095035. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, Wago M, Kawaguchi H, Tahara M, Yuge M. Plasma brain natriuretic peptide concentrations in patients with Kawasaki disease. Pediatr Int. 2000;42:241–248. doi: 10.1046/j.1442-200x.2000.01225.x. [DOI] [PubMed] [Google Scholar]
- 24.Bang S, Yu JJ, Han MK, Ko HK, Chun S, Choi HS, et al. Log-transformed plasma level of brain natriuretic peptide during the acute phase of Kawasaki disease is quantitatively associated with myocardial dysfunction. Korean J Pediatr. 2011;54:340–344. doi: 10.3345/kjp.2011.54.8.340. [DOI] [PMC free article] [PubMed] [Google Scholar]