Abstract
Purpose
Incomplete Kawasaki disease (KD) is frequently associated with delayed diagnosis and treatment. Delayed diagnosis leads to increasing risk of coronary artery aneurysm. Anterior uveitis is an important ocular sign of KD. The purpose of this study was to assess differences in laboratory findings, including echocardiographic measurements, clinical characteristics such as fever duration and treatment responses between KD patients with and those without uveitis.
Methods
We conducted a prospective study with 110 KD patients from January 2008 to June 2013. The study group (n=32, KD with uveitis) was compared with the control group (n=78, KD without uveitis). Laboratory data were obtained from each patient including complete blood count (CBC), erythrocyte sedimentation rate (ESR), platelet count, and level of alanine aminotransferase, aspartate aminotransferase, serum total protein, albumin, C-reactive protein (CRP), and N-terminal probrain natriuretic peptide (NT-pro BNP). Echocardiographic measurements and intravenous immunoglobulin responses were compared between the two groups.
Results
The incidence of uveitis was 29.0%. Neutrophil counts and patient age were higher in the uveitis group than in the control group. ESR and CRP level were slightly increased in the uveitis group compared with the control group, but the difference between the two groups was not significant. No significant differences in coronary arterial complication and treatment responses were observed between the two groups.
Conclusion
Uveitis is an important ocular sign in the diagnosis of incomplete KD. It is significantly associated with patient age and neutrophil count.
Keywords: Kawasaki disease, Uveitis, Brain natriuretic peptide
Introduction
Kawasaki disease (KD) is an acute, self-limited vasculitis which is a cause of secondary heart disease in children1,2). Coronary artery aneurysm could be a cause of death in some cases of KD, so it is important to diagnose coronary artery complication2,3).
KD could be diagnosed by following 5 criteria: prolonged fever longer than 5 days and 4 of the 5 following main clinical features: changes in the peripheral extremities, polymorphous rash, oropharyngeal changes, acute nonpurulent conjunctivitis, cervical lymphadenopathy accompanied with lymph node greater than 1.5 cm4,5). Recently patients with incomplete KD less than 4 criteria, known as incomplete KD have been increasing4,6). Incomplete KD can be diagnosed by American Heart Association (AHA)5). The risk of delayed diagnosis could lead to coronary artery complication7,8).
Uveitis is the most common ophthalmic finding in KD. Bilateral conjunctival injection is a common diagnostic criterion for KD. Iridocyclitis, superficial punctuate keratitis, vitreous opacities and papiledema are also found in KD9). Burns et al.10) reported that 83% of children diagnosed with KD manifest uveitis within a week. Manifestation of uveitis is mild. It is bilateral and is sometimes associated with keratic precipitates. In general, it occurs a week after fever onset and recovers within 2-8 weeks after disease onset without any sequelae10). According to increasing incomplete KD, uveitis has become a more important factor in early diagnosis of incomplete KD.
In this study, we analyzed differences between KD patients with and without uveitis in clinical characteristics, laboratorial findings including echocardiographic measurements and responses to treatment.
Materials and methods
1. Patients and materials
This study was conducted at Ewha Womans University Mock-dong Hospital, Department of Pediatrics, from January 2008 to June 2013. A total of 110 KD patients were included for this study. The study was divided into 2 groups: patients with KD with uveitis (study group), patients with KD without uveitis (control group). Patients had diagnostic criteria established by the AHA: fever lasting longer than 5 days and 4 of the 5 following main clinical features: changes in the peripheral extremities, polymorphous rash, oropharyngeal changes, acute nonpurulent conjunctivitis, cervical lymphadenopathy with lymph node diameter greater than 1.5 cm4,5). Incomplete KD was also decided by AHA guideline; prolonged fever over 5 days and 2-3 of standard clinical features of KD.
The study was carried out with the approval of the ethics committee of Ewha Womans University Mokdong Hospital Institutional Review Board (IRB No. 11-62-04) and written informed consents were got from the parents of all the subjects.
2. Laboratory data
Laboratory data were obtained from each patient including complete blood count (CBC), erythrocyte sedimentation rate (ESR), platelet count, alanine aminotransferase, aspartate aminotransferase, serum total protein, albumin, C-reactive protein (CRP), N-terminal probrain natriuretic peptide (NT-pro BNP).
3. Ophthalmologic examination
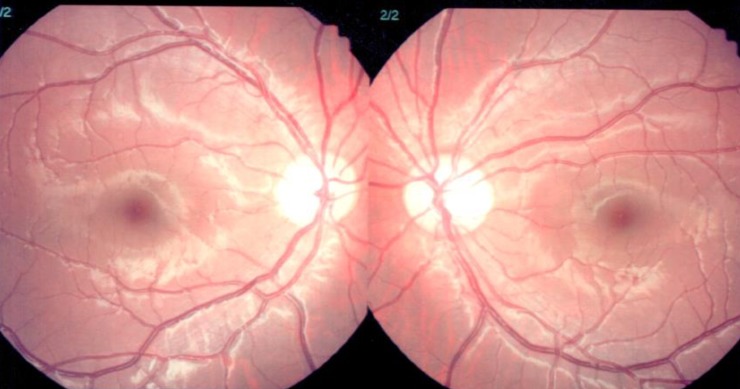
Patients who had not been examined by an ophthalmologist were excluded; to minimize errors between the two groups. Uveitis was diagnosed by slit lamp examination (Fig. 1). The severity of uveitis was decided by deep cell count and degree of flare. The grading of flare (0+ to 4+) was determined by slit lamp examination. Grade 0 is the absence of flare, grade 1 is faint, grade 2 is moderate (iris and lens details clear), grade 3 is marked (iris and lens details hazy), grade 4 is intense (fibrin or plastic aqueous)11). Aqueous cells are mostly lymphocytes and located in a deep anterior chamber. The grade 0 is no cell, grade 1 corresponds to 6 to 15 cells, and grade 2 has 16 to 25 cells, grade 3 has 26 to 50 cells. Grade 4 has more 50 cells per field (Table 1)12).
Fig. 1. Slit lamp biomicroscopic examination result in a Kawasaki disease patient with uveitis. Both eyes showed conjunctival injection (+/+), with deep cells (++/++), as a sign of uveitis.
Table 1. Ophthalmologic finding in the Kawasaki disease patients with uveitis.
| Grade | No. of deep cells | No. of patients | Stage |
|---|---|---|---|
| 0 | <1 | 0 | Acute |
| 0.5+ | 1-5 | 4 | Acute |
| 1+ | 6-15 | 11 | Acute |
| 2+ | 16-25 | 12 | Acute |
| 3+ | 26-50 | 3 | Acute |
| 4+ | >50 | 2 | Acute |
4. Echocardiography findings
Echocardiography was performed using an IE33 machine (Philips Medical System, Andover, MA, USA) with a S8 transducer. Standard parasternal and apical views were acquired. Two dimensional Echocardiography and M-mode echocardiogram, pulsed, color-flow Doppler and tissue Doppler imaging were obtained.
Coronary arteries were defined as abnormal if the internal lumen diameter was >3 mm in children younger than 5 years old; if the internal diameter of a segment measured ≥1.5 times that of an adjacent segment; or if the coronary lumen was clearly irregular13).
5. Response to treatment
Upon diagnosis, all patients were treated with IVIG (2 g/kg/day) and aspirin 30 mg/kg until defervescence, followed by aspirin 3-5 mg/kg/day as a single daily dose thereafter. In IVIG-resistant patients, a second dose of IVIG 2 g/kg was administrated if fever was over 36 hours or recrudescent (temperature ≥38.0℃). They were classified as IVIG-resistant5).
Data was analyzed according to patient's clinical characteristics, laboratory findings, ophthalmologic finding and responses to treatment of IVIG.
6. Statistical analysis
IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. A P<0.05 was considered statistically significant. Wilcoxon Rank Sum test and Mann-Whitney test were used to compare the laboratory results between KD patients with uveitis and without uveitis. Clinical characteristics and laboratory data were also analyzed by independent t test.
Univariate analysis was performed to investigate the correlations between grade of deep cell and serum NT-pro BNP level.
Results
1. Clinical characteristics
The mean age in the uveitis group was older than the control group (40.5±21.4 months vs. 33.4±29.3 months, P=0.043). Age range of the patients varied 3-147 months. Duration of fever (>5 days, 62.5% vs. 60.3%, P=0.959) had no significant difference in this study (Table 2).
Table 2. Clinical characteristics of the KD patients in the uveitis and control groups.
| Characteristic | Uveitis group (n=32) | Control group (n=78) | P value* |
|---|---|---|---|
| Sex | |||
| Male:female | 18:14 | 45:33 | 0.959 |
| Age (yr) | 40.5±21.4* | 33.4±29.3 | 0.043 |
| Duration of fever (>5 days) | 20 (62.5) | 47 (60.3) | 0.966 |
| Conjunctival injection | 30 (93.7) | 60 (76.9) | 0.064 |
| Cervical lymphadenopathy | 10 (31.2) | 23 (29.5) | 0.792 |
| Polymorphous skin rash | 24 (75.0) | 55 (70.5) | 0.651 |
| Abnormalities of lip or oral mucosa | 27 (84.3) | 55 (70.5) | 0.113 |
| Abnormalities of extremities | 22 (68.7) | 47 (60.3) | 0.476 |
| BCGitis | 6 (18.7) | 28 (35.9) | 0.077 |
| Complete type KD | 18 (56.2)* | 26 (33.3) | 0.027 |
Values are presented as mean±standard deviation or number (%).
KD, Kawasaki disease; BCGitis, the inflammation at the bacille Calmette-Guérin inoculation site.
*P<0.05 significantly different from control group.
The number of male and female was 18:14 in the uveitis group and 45:33 in the control group. Fever duration more than 5 days was 62.5% in the uveitis group and 60.3% in the control group. Conjunctival injection is higher in the uveitis group (93.7%) than in the control group (76.9%), but there was no statistical significance (P=0.064). Cervical lymphadenopathy (31.2% vs. 29.5%, P=0.792), polymorphous skin rash (75.0% vs. 70.5%, P=0.651), mucosa change (84.3% vs. 70.5%, P=0.113) and abnormality of extremities (68.7% vs. 60.3%, P=0.476) were slightly increased in the uveitis group but there was no statistical importance. Only BCGitis (the inflammation at the bacille Calmette-Guérin inoculation site) was increased in the control group rather than the uveitis group although there was no statistical significance (18.7% vs. 35.9%, P=0.077).
Although clinical characteristics were similar, the uveitis group showed a higher incidence of complete type KD in this study (56.2%).
2. Ophthalmologic finding by slit lamp examination
Table 1 showed that uveitis in KD was acute state and limited in anterior chamber. Anterior uveitis included iritis, iridocyclitis, and anterior cyclitis. Most KD patients had 1-2 grades of deep cells.
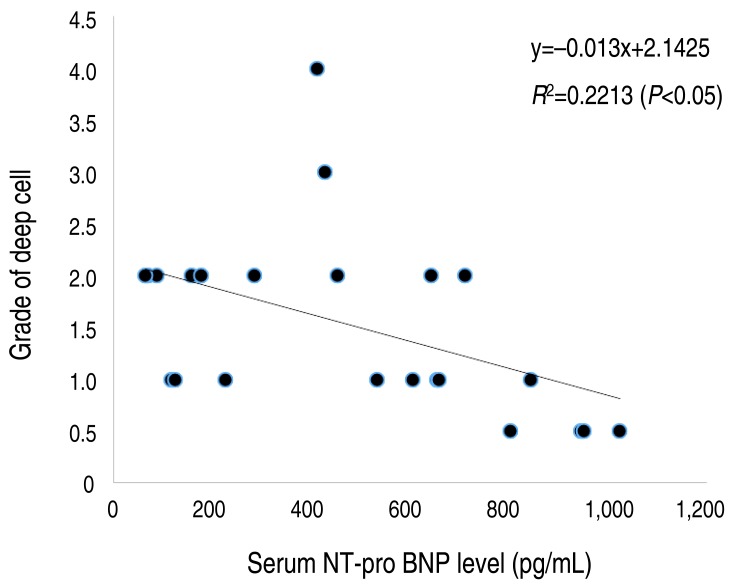
Fig. 2 demonstrated that there was weak negative linear correlation between grade of deep cell and serum NT-pro BNP level. Cell grading was not corresponding to the severity of disease.
Fig. 2. Linear regression analysis between grade of deep cell and serum NT-pro BNP level. The linear regression model shows a negative correlation between grade of deep cells and serum NT-pro BNP level. NT-pro BNP, N-terminal probrain natriuretic peptide.
Within one week, uveitis was subsided after steroid eye drop treatment. No cell was detected after steroid eye drop treatment. Photophobia is one of the most common symptoms in uveitis but in our study, no patient showed it as a sign of uveitis. Most patients were so young that it was difficult to complain photophobia. Conjunctival injection was the only symptom of uveitis in some patients.
3. Laboratory data
Thirty-two patients (29.0%) had uveitis out of the 110 KD patients. Laboratory data was collected in acute phase. Neutrophil counts were higher in the uveitis group compared with the control group (64.3±15.8 [×103/mm3] vs. 54.4±19.3 [×103/mm3], P=0.018). CRP and ESR were slightly increased in uveitis group but there was no statistical significance.
There was no significant difference in serum NT-pro BNP level (558.4±465.1 pg/mL vs. 784.1±878.2 pg/mL, P=0.659) and coronary artery diameter (3.0±1.0 mm vs. 2.8±1.0 mm, P=0.139) between two groups. Other laboratory results did not show the significant differences between the two groups (Table 3).
Table 3. Laboratory data of the KD patients in the uveitis and control groups.
| Laboratory findings | Uveitis group (n=32) | Control group (n=78) | P value* |
|---|---|---|---|
| Hb (g/dL) | 11.4±0.8 | 11.6±0.9 | 0.479 |
| WBC (×109/L) | 12.5±4.3 | 11.2±5.9 | 0.148 |
| Neutrophil (%) | 64.3±15.8* | 54.4±19.3 | 0.018 |
| Platelet (×109/L) | 311.7±88.3 | 304.4±77.3 | 0.937 |
| ESR (mm/hr) | 43.3±27.2 | 30.8±24.6 | 0.050 |
| CRP (mg/dL) | 8.1±6.1 | 7.9±10.7 | 0.306 |
| AST (IU/L) | 52.1±57.7 | 71.2±44.2 | 0.608 |
| ALT (IU/L) | 62.4±85.8 | 62.8±66.8 | 0.903 |
| Total protein (g/dL) | 6.5±0.7 | 6.5±0.6 | 1.000 |
| Albumin (g/dL) | 3.8±0.3 | 4.2±3.8 | 0.688 |
| NT-pro BNP (pg/mL) | 558.4±465.1 | 784.1±878.2 | 0.659 |
| Coronary artery diameter (mm) | 3.0±1.0 | 2.8±1.0 | 0.139 |
Values are presented as mean±standard deviation.
KD, Kawasaki disease; Hb, hemoglobin; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NT-pro BNP, N-terminal probrain natriuretic peptide.
*P<0.05 significantly different from control group.
4. Response to treatment
Table 4 demonstrated the response to treatment in KD patients. Response to first IVIG was higher in the control group (94.9%) compared with the uveitis group (90.6%). Retreatment of IVIG was 5.1% in the control group and 9.3% in the uveitis group. The recurrence rate was 2.6% in the control group and 6.2% in the uveitis group. Family history was found only in 2.6% of the control group. However, there was no statistical significance between the two groups. Uveitis was not related with response to treatment.
Table 4. Treatment response of the KD patients in the uveitis and control groups.
| Treatment response | Uveitis group (n=32) | Control group (n=78) | P value |
|---|---|---|---|
| IVIG responder | 29 (90.6) | 74 (94.9) | 0.307 |
| Retreatment IVIG | 3 (9.3) | 4 (5.1) | 0.307 |
| Family history | 0 (0) | 2 (2.6) | 0.392 |
| History of recurrence | 2 (6.2) | 2 (2.6) | 0.275 |
Values are presented as number (%).
KD, Kawasaki disease; IVIG, intravenous immunoglobulin.
Discussion
KD is self-limited systemic vasculitis that occurs in children of all age groups. The incidence rate of KD is increasing, but the cause of KD remains unknown1). Incidence rates of KD are different among countries, and Asian countries show a higher incidence rates than other countries1,3). Because we do not know why the incidence rate is higher in Asian countries, there is much interest in genetic studies2,3,14). Many physicians have been trying to find out the specific genes causing KD.
In recent years, patients who do not fulfill the diagnostic criteria of KD have increased4,6). They have a febrile condition and have some features compatible with KD1,3,4,5,6,7,15). So it is not easy to decide whether starting treatment KD or not. The one thing to consider before starting treatment is that delayed treatment is related to complication of coronary artery6,8). Therefore, early diagnosis and treatment is becoming more important for reducing coronary artery complications.
Incomplete KD and the severity of coronary artery complication are higher in younger age group especially under 6 months old3,6,7,8). Younger than 6 month or older than 6 year patients show a higher incidence of incomplete KD, but the reason has not been found yet3).
Yu7) reported that the prevalence of incomplete KD was 15 to 36.2% among total KD patients. In our study, the incidence of incomplete KD was 43.8%. There is no definite sign to diagnose incomplete KD, but fever longer than 5 days could be a very important sign to diagnose KD. If physicians find other clues to diagnose KD earlier, it would be helpful to decrease KD complications.
Uveitis could be a useful sign to help different diagnose incomplete KD9). As mentioned before, uveitis is mild and bilateral. It sometimes is associated with keratic precipitates10). The cause of uveitis can be classified as infectious and noninfectious16,17). Noninfectious uveitis such as juvenile idiopathic arthritis, spondyloarthropathies is more common in children. Anatomical location is another clue to predict the diseases: anterior, posterior, panuveitis17). Anterior uveitis is common in KD18). It is necessary to investigate the cause of anterior uveitis. Clinical features, antinuclear antibody, human leukocyte antigen B-27, x-ray, echocardiography and respiratory virus polymerase chain reaction are important for differential diagnosis in uveitis16).
In our study, the incidence of uveitis was different from that of the result of Burn's10). Each disease has its own incidence rate according to the race. In KD, East Asia shows higher incidence than Europe and America, so incidence of uveitis in KD is also different according to the ethnic group. Kump et al.19) investigated the frequency of pediatric uveitis. That was not limited in KD but included all cases of pediatric uveitis, so only 2 of 169 patients were detected uveitis in KD.
In KD, uveitis resolves within 2-8 weeks after disease onset without any sequelae10). The grading of uveitis is decided according to flare and cell number, so slit lamp examination is necessary to diagnose uveitis. Ophthalmic evaluation includes visual activity, measurement of intraocular pressure, assessment of cell and flare by slit lamp examination16,17,20).
Uveitis in KD shows a mild clinical course. In most cases, topical corticosteroid is effective for treatment. KD patients who have anterior uveitis need to follow up evaluation depending on its severity between 1-7 days9,10).
In our study, 29% of patients with KD have uveitis. Although some patients do not have enough features to diagnose KD, we focus on the patients who have fever and uveitis. In uveitis group, 80% of patients showed conjunctival injection which is a characteristic of KD.
For detecting KD earlier, physicians should focus on fever and consider the other situations similar with KD. For example, adenovirus infection has similar clinical characteristics with KD such as fever, conjunctival injection and cervical lymphadenopathy. Adenoviral conjunctivitis is mild and subsided without any treatment. In this study, adenovirus was not detected in uveitis group. BenEzra et al.17) reported that KD was only 2.2% of the cause of uveitis in infectious patients. Usually, adenovirus is not the cause of uveitis but conjunctivitis.
In this case, slit lamp examination and echocardiography are very useful for differential diagnosis of KD. However, patients could have both uveitis and an adenoviral infection as well21). We found that some patients have both uveitis and an adenoviral infection. Uveitis is not the only clue to diagnose KD.
Although duration of fever was less than 5 days, some patients showed coronary artery complication in echocardiography. They had conjunctival injection before the fever showed up. That's the reason why they had the slit lamp examination. Uveitis was detected, and then, they underwent echocardiography.
The serum NT-pro BNP levels have been reported as an early diagnostic marker of myocardial involvement in KD22,23). In our study, there were no significant differences between KD without uveitis and KD with uveitis group, except age and neutrophil count. Absence of an exact indicator of KD is a challenge for pediatricians.
There is not any definite guideline to diagnose incomplete KD yet. Early diagnosis is needed to prevent coronary artery complications. Uveitis is not the only clue to diagnosing KD, but if a patient has conjunctivitis, ophthalmic examination is very helpful in detecting uveitis and it should be considered a sign of KD.
There are some limitations in our study. First, patients who have conjunctival injection were evaluated by silt lamp examination. It is difficult to decide the best timing for ophthalmic evaluation to diagnose uveitis. There is no specific guideline to determine the timing of slit lamp examination in KD. In our study, conjunctival injection was the only sign to perform slit lamp examination. Second, the study number was small. Third, in our study, we did not compare KD with other diseases that cause uveitis. Other chronic or immunologic diseases that lead to uveitis were excluded in this study.
In conclusion, uveitis is the one of the important ocular sings to help diagnose incomplete KD. As increasing incomplete type of KD, clinicians are responsible to diagnose and start treatment earlier to decrease coronary artery complications. Routine slit lamp examination will be helpful for early diagnosis of incomplete KD in patients who have conjunctival injection.
Footnotes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Gerding R. Kawasaki disease: a review. J Pediatr Health Care. 2011;25:379–387. doi: 10.1016/j.pedhc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Kim JJ, Hong YM, Yun SW, Han MK, Lee KY, Song MS, et al. Assessment of risk factors for Korean children with Kawasaki disease. Pediatr Cardiol. 2012;33:513–520. doi: 10.1007/s00246-011-0143-1. [DOI] [PubMed] [Google Scholar]
- 3.Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child. 2014;99:74–83. doi: 10.1136/archdischild-2012-302841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DS. Kawasaki disease. Yonsei Med J. 2006;47:759–772. doi: 10.3349/ymj.2006.47.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 6.Freeman AF, Shulman ST. Kawasaki disease: summary of the American Heart Association guidelines. Am Fam Physician. 2006;74:1141–1148. [PubMed] [Google Scholar]
- 7.Yu JJ. Diagnosis of incomplete Kawasaki disease. Korean J Pediatr. 2012;55:83–87. doi: 10.3345/kjp.2012.55.3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonobe T, Kiyosawa N, Tsuchiya K, Aso S, Imada Y, Imai Y, et al. Prevalence of coronary artery abnormality in incomplete Kawasaki disease. Pediatr Int. 2007;49:421–426. doi: 10.1111/j.1442-200X.2007.02396.x. [DOI] [PubMed] [Google Scholar]
- 9.Ohno S, Miyajima T, Higuchi M, Yoshida A, Matsuda H, Saheki Y, et al. Ocular manifestations of Kawasaki's disease (mucocutaneous lymph node syndrome) Am J Ophthalmol. 1982;93:713–717. doi: 10.1016/0002-9394(82)90465-2. [DOI] [PubMed] [Google Scholar]
- 10.Burns JC, Joffe L, Sargent RA, Glode MP. Anterior uveitis associated with Kawasaki syndrome. Pediatr Infect Dis. 1985;4:258–261. doi: 10.1097/00006454-198505000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Guney E, Tugal-Tutkun I. Symptoms and signs of anterior uveitis. US Ophthalmic Rev. 2013;6:33–37. [Google Scholar]
- 12.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)--digest version. Circ J. 2010;74:1989–2020. doi: 10.1253/circj.cj-10-74-0903. [DOI] [PubMed] [Google Scholar]
- 14.Sundel RP. Kawasaki disease. Rheum Dis Clin North Am. 2015;41:63–73. doi: 10.1016/j.rdc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Bayers S, Shulman ST, Paller AS. Kawasaki disease: part I. Diagnosis, clinical features, and pathogenesis. J Am Acad Dermatol. 2013;69:501.e1–501.e11. doi: 10.1016/j.jaad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal RV, Murthy S, Sangwan V, Biswas J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010;58:11–19. doi: 10.4103/0301-4738.58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89:444–448. doi: 10.1136/bjo.2004.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klig JE. Ophthalmologic complications of systemic disease. Emerg Med Clin North Am. 2008;26:217–231. doi: 10.1016/j.emc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Kump LI, Cervantes-Castaneda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112:1287–1292. doi: 10.1016/j.ophtha.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Anesi SD, Foster CS. Anterior uveitis: etiology and treatment. Adv Ocul Care. 2011;2:32–34. [Google Scholar]
- 21.Barone SR, Pontrelli LR, Krilov LR. The differentiation of classic Kawasaki disease, atypical Kawasaki disease, and acute adenoviral infection: use of clinical features and a rapid direct fluorescent antigen test. Arch Pediatr Adolesc Med. 2000;154:453–456. doi: 10.1001/archpedi.154.5.453. [DOI] [PubMed] [Google Scholar]
- 22.Dahdah N, Siles A, Fournier A, Cousineau J, Delvin E, Saint-Cyr C, et al. Natriuretic peptide as an adjunctive diagnostic test in the acute phase of Kawasaki disease. Pediatr Cardiol. 2009;30:810–817. doi: 10.1007/s00246-009-9441-2. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, Wago M, Kawaguchi H, Tahara M, Yuge M. Plasma brain natriuretic peptide concentrations in patients with Kawasaki disease. Pediatr Int. 2000;42:241–248. doi: 10.1046/j.1442-200x.2000.01225.x. [DOI] [PubMed] [Google Scholar]