Abstract
Tularemia is a potentially severe zoonotic disease caused by Francisella tularensis. A lack of awareness about tularemia can be embarrassing and could result in delayed treatment because of improper diagnosis. The diagnosis of tularemia is difficult, because the infections are rare and the clinical spectrum is broad. As only 1 adult case has been reported in Korea thus far, pediatricians in Korea may be unfamiliar with tularemia. We report our experience with a 14-year-old male adolescent with tularemia who presented with atypical pneumonia and possible infective endocarditis. Although the infectivity and mortality rates for tularemia are very high if left untreated, we did not suspect tularemia in this case until the incidental isolation of F. tularensis. The present case suggests that clinicians in Korea should be more aware of tularemia. This case also suggests that tularemia should be considered in undetermined cases of atypical pneumonia or acute febrile illness without local signs.
Keywords: Francisella tularensis, Pneumonia, Endocarditis, Pediatrics, Korea
Introduction
Tularemia is a potentially severe zoonotic disease caused by the gram-negative coccobacillus Francisella tularensis1). Early suspicions are important because delayed treatment may result in prolonged morbidity, increase the risk of complications, or even end in mortality. However, the diagnosis of tularemia is difficult because infections are rare, exposures are diverse, and the clinical spectrum is exceedingly broad. Although tularemia occurs endemically in most countries in the Northern hemisphere, the nationwide incidence is only 0.4 to 1.3 human cases per million population in the United States2) and less than 10 human cases per year in Japan3). Therefore, tularemia is frequently overlooked by clinicians, even in highly endemic areas such as Missouri in the United States4). Tularemia tends to not be suspected until after incidental isolations of F. tularensis in blood4). In Korea, only one case was reported in 1999, which was an adult case of ulceroglandular tularemia5). Therefore, tularemia is extremely unfamiliar to clinicians in Korea.
We recently experienced the first pediatric tularemia case in Korea. The patient was presented with pneumonia and possible infective endocarditis, uncommon presentations of tularemia. However, we did not suspect tularemia in this case until the incidental isolation of F. tularensis. In addition, we were not able to find the exposure route. We are concerned that this infection can become a serious public health problem in Korea, considering its high infectivity and fatality without treatment.
Case report
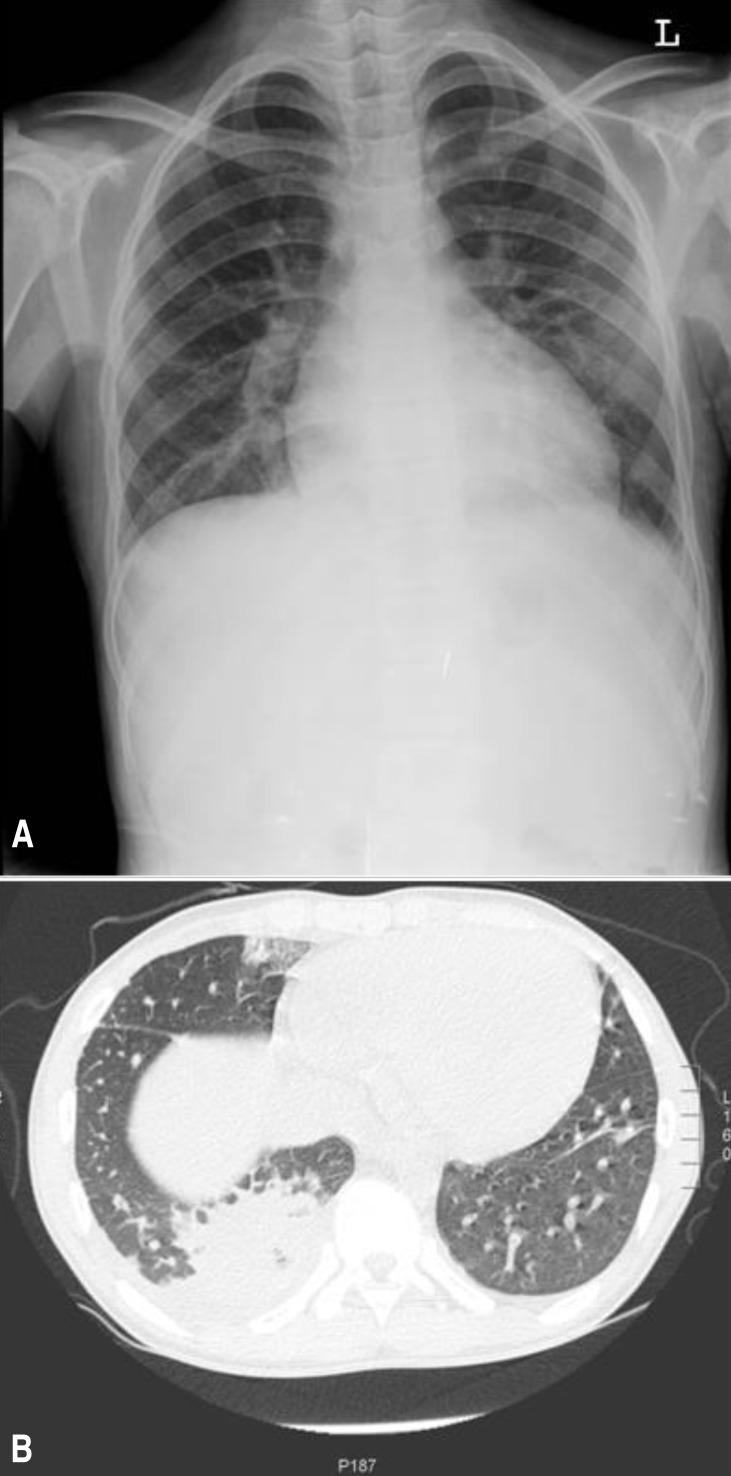
A 14-year-old male adolescent patient was admitted to the Gyeongsang National University Hospital on 3 December 2012 because of a 3-day history of high fever, general myalgia, and dry cough. He complained of a 1-day history of pain on the right lower side of the chest. The patient appeared pale and ill. His temperature was 38.5℃, pulse was 100 beats/min, and blood pressure was 100/60 mmHg. Physical examination revealed slightly decreased breath sounds at the right lung base and a continuous murmur with a thrill over the left subclavicular and left upper sternal border. He has been heard about the heart murmur and he complained of exertional dyspnea over the past 2-3 months. However, these signs of cardiac problems have not been evaluated. He had no cutaneous symptoms and no lymphadenopathy. However, the liver and spleen edges were palpable to be 5 and 3 cm below the costal margin, respectively. The patient had no pharyngotonsillitis or oral ulceration. There was no evidence of insect bites. A tuberculin skin test was negative. Increased pulmonary vascular markings were apparent, but obvious consolidations were not found on chest x-ray on the admission day (Fig. 1A). Echocardiography showed a large patent ductus arteriosus (7 mm with a left-to-right shunt), mitral valvular regurgitation (Δp=48 mmHg), dilated left ventricular internal dimension, and pericardial effusion. However, valvular anomaly or vegetation was not observed. The leukocyte count was 14.3×109/L (85% polymorphonuclear neutrophils with left-shift maturation), the hemoglobin level was 8.4 g/dL, the platelet count was 240×109/L, and the C-reactive protein level was 162 mg/L. The antistreptolysin O titer was 260 IU/mL. An antibody test for mycoplasma was negative. Polymerase chain reaction tests with respiratory specimens for respiratory viruses and Mycobacterium tuberculosis were negative. Bacterial culture of a throat swab and urine and stool samples were negative. Three consecutive blood cultures (blood was drawn >12 hours apart from different sites) were performed.
Fig. 1. (A) Chest radiography performed on the first day of hospital admission showed increased pulmonary vascular markings and hilar enlargement. (B) Chest computer tomography performed on the third day of hospital admission showed pneumonic consolidation and presence of pleural effusion.
Considering the possibility of infective endocarditis, the patient was treated with ampicillin/sulbactam, vancomycin, and gentamicin. After administration of antibiotics, the fever gradually subsided and the patient's general condition improved. However, on the third day of hospitalization, the patient complained of right lower chest pain aggravation. To rule out pulmonary embolism, chest computed tomography and lower-extremity venography computed tomography were performed. Pneumonia and pleural effusion were notified without evidence of embolism (Fig. 1B). Bloody and turbid, neutrophil-dominant exudate (red blood cell count>1,000/high power field, white blood cell count>1,000/high power field, polymorphonuclear neutrophils 69%, lymphocytes 14%, monocytes 12%, pH 7.5, glucose 102 mg/dL, protein 6.2 mg/dL, lactate dehydrogenase 1,100 U/L, and no bacteria) was removed by thoracostomy due to progressively increase in the amount of fluid. On the seventh day of hospitalization, F. tularensis was finally confirmed in all blood cultures by incubation for an extended period for the identification of fastidious bacteria; we did not suspect tularemia until the pathogen was identified. Another challenge we encountered was identification of the route of exposure to the pathogen. However, the patient had not been traveling, camping or hunting or exposed to arthropods or hay. He had not eaten any game or skinned any animals. The patient was treated with gentamicin for an additional 3 weeks. Echocardiography before discharge revealed disappearance of the pericardiac effusion and marked improvement in the mitral valvular regurgitation. He will be scheduled to undergo surgery for patent ductus arteriosus.
Discussion
Tularemia is entirely unfamiliar to clinicians in Korea. Although tularemia occurs endemically in most countries in the Northern hemisphere within the latitude range 30° to 71°1), only one adult case has been reported in Korea5). Even in highly endemic areas, clinicians tend to frequently overlook the diagnosis of tularemia because of the rarity of the infection and the diversity of the disease spectrum4). In addition, infections are particularly unusual in children6). Our patient presented pneumonia and possible infective endocarditis, in which both are uncommon presentations of tularemia. For these reasons, we did not suspect tularemia until the incidental isolation of F. tularensis.
Tularemia is a zoonotic disease caused by gram-negative coccobacillus F. tularensis. A large number of animals serve as a reservoir for F. tularensis, which can be transmitted through the bite of infected ticks or other biting insects, by contact with infected animals, by consumption of contaminated foods or water, or through inhalation7). Portal of entry for infection is critical in determining the clinical spectrums of tularemia; percutaneous inoculation typically causes the most two common forms of tularemia in children, lymphoadenopathy with (ulceroglandular tularemia) or without skin or mucosal ulceration (glandular tularemia)7). Consumption of contaminated foods results in oropharyngeal tularemia, which is presented by acute pharyngotonsillitis with ulcer7). In these cases, local signs of acquisition such as cutaneous papule, ulcer, or regional lymphadenopathy are important clues for diagnosis of tularemia. On the other hand, inhalation exposure can lead to respiratory tularemia, which has no local signs of acquisition4,7). In addition, the clinical features of tularemic pneumonia are the same as atypical pneumonia. That means that the development of a fever and a general illness without respiratory symptoms or crackles are frequently recognized in tularemic pneumonia7,8). In this case, we did not find any evidence of lower respiratory tract infection except for a dry cough and chest pain at the time of admission. Chest radiographic findings also vary widely and not specific8). Although hilar adenopathy (one of the most common abnormalities) was notified on the initial chest radiographs9), we did not suspect pneumonia at that time. Pleural effusions occur in 60% to 80% of patients as early as three days after the onset of symptoms8), however, we did not recognize it on initial chest radiograph. Our case suggested that recognition of pneumonia is likely to be delayed in tularemia.
For these reasons, we initially focused on the signs of infective endocarditis. Using an empirical antibiotic regimen with gentamicin for treatment of infective endocarditis was fortunately effective for our patient. Gentamicin is the drug of choice for the treatment of tularemia in children9). Beta-lactam antibiotics have no effects on tularemia. However, our case was not met on definitive infective endocarditis due to presence of only 3 minor criteria; known cardiac lesion, high fever, and positive blood culture that does not meet a major criterion. Tularemic endocarditis is extremely rare; only one case in an adult has been reported10). Therefore, isolation of F. tularensis does not seem to meet a major criterion. However, considering the importance of blood culture for diagnosing infective endocarditis, tularemic endocarditis may be underestimated due to the exceedingly low sensitivity of routine blood culture and empirical therapy with gentamicin in infective endocarditis. Nevertheless, blood culture should not be emphasized for tularemia due to the danger to laboratory personnel. Instead, the microbiological diagnosis of tularemia relies mainly on serology such as serum agglutiniation tests and enzyme-linked immunosorbent assay.
Aerosols from farming activities (haying or threshing), lawn mowers, or dust from contaminated soil have been considered to cause of respiratory tularemia7). Although we could not identify any obvious exposure route, inhalation exposure is strongly suspected in this case. F. tularensis can survive in water and mud for months, and only 10 organisms can cause the disease if it is inhaled9). Large outbreaks of respiratory tularemia have been reported11). In line with recent boom of camping and outdoor activities, we are concerned about the possibility of increasing number of cases in Korea. A lack of awareness about the disease can cause embarrassing results from the delayed treatment following an improper diagnosis.
In conclusion, our case suggests clinicians in Korea should be aware for tularemia. Our case also suggests that diagnosis of tularemia should be considered in patients presenting with atypical pneumonia (nonresponsive to β lactam and macrolide) or infective endocarditis.
Footnotes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Hopla CE. The ecology of tularemia. Adv Vet Sci Comp Med. 1974;18:25–53. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Summary of notifiable diseases, United States 1994. MMWR Morb Mortal Wkly Rep. 1994;43:1–80. [PubMed] [Google Scholar]
- 3.Ohara Y, Sato T, Homma M. Epidemiological analysis of tularemia in Japan (yato-byo) FEMS Immunol Med Microbiol. 1996;13:185–189. doi: 10.1111/j.1574-695X.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 4.Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin Infect Dis. 2012;55:1283–1290. doi: 10.1093/cid/cis706. [DOI] [PubMed] [Google Scholar]
- 5.Ahn WS, Oh MG, Lee JH. A case of ulceroglandular tularemia. J Korean Surg Soc. 1999;57:304–310. [Google Scholar]
- 6.Gries DM, Fairchok MP. Typhoidal tularemia in a human immunodeficiency virus-infected adolescent. Pediatr Infect Dis J. 1996;15:838–840. doi: 10.1097/00006454-199609000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Tarnvik A, Berglund L. Tularaemia. Eur Respir J. 2003;21:361–373. doi: 10.1183/09031936.03.00088903. [DOI] [PubMed] [Google Scholar]
- 8.Gill V, Cunha BA. Tularemia pneumonia. Semin Respir Infect. 1997;12:61–67. [PubMed] [Google Scholar]
- 9.Schutze GE, Jacobs RF. Tularemia. In: Kliegman RM, Stanton B III, St. Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. pp. 978–980. [Google Scholar]
- 10.Tancik CA, Dillaha JA. Francisella tularensis endocarditis. Clin Infect Dis. 2000;30:399–400. doi: 10.1086/313678. [DOI] [PubMed] [Google Scholar]
- 11.Dahlstrand S, Ringertz O, Zetterberg B. Airborne tularemia in Sweden. Scand J Infect Dis. 1971;3:7–16. doi: 10.3109/inf.1971.3.issue-1.02. [DOI] [PubMed] [Google Scholar]