Abstract
Rice is a staple and most important security food crop consumed by almost half of the world’s population. More rice production is needed due to the rapid population growth in the world. Rice blast caused by the fungus, Magnaporthe oryzae is one of the most destructive diseases of this crop in different part of the world. Breakdown of blast resistance is the major cause of yield instability in several rice growing areas. There is a need to develop strategies providing long-lasting disease resistance against a broad spectrum of pathogens, giving protection for a long time over a broad geographic area, promising for sustainable rice production in the future. So far, molecular breeding approaches involving DNA markers, such as QTL mapping, marker-aided selection, gene pyramiding, allele mining and genetic transformation have been used to develop new resistant rice cultivars. Such techniques now are used as a low-cost, high-throughput alternative to conventional methods allowing rapid introgression of disease resistance genes into susceptible varieties as well as the incorporation of multiple genes into individual lines for more durable blast resistance. The paper briefly reviewed the progress of studies on this aspect to provide the interest information for rice disease resistance breeding. This review includes examples of how advanced molecular method have been used in breeding programs for improving blast resistance. New information and knowledge gained from previous research on the recent strategy and challenges towards improvement of blast disease such as pyramiding disease resistance gene for creating new rice varieties with high resistance against multiple diseases will undoubtedly provide new insights into the rice disease control.
Keywords: rice blast disease, molecular breeding, DNA markers, QTL mapping, marker-aided selection, gene pyramiding
Introduction
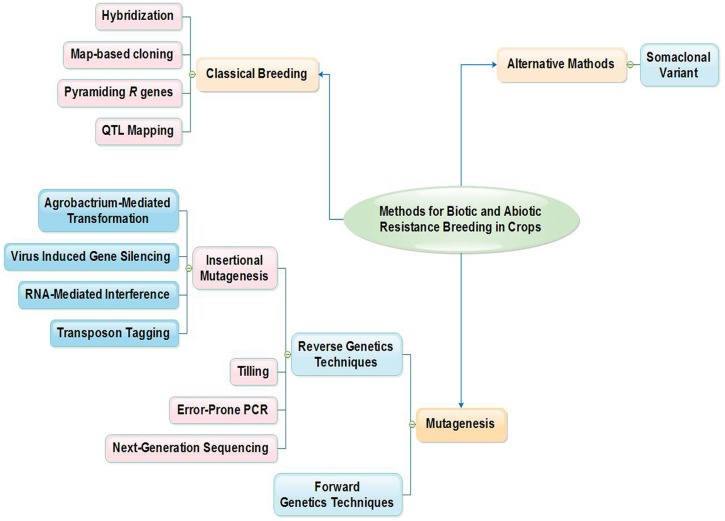
Biotic and abiotic stresses cause significant yield losses in food crop production and Improvement in stress tolerance of plant is a major breeding goal. Nowadays, different methods are being used to improve stress tolerance in plants (Figure 1). Diseases are among the most important limiting factors that affect rice production. More than 70 diseases caused by fungi, bacteria, viruses or nematodes have been reported on rice (Zhang et al., 2009). Rice blast (Magnaporthe oryzae) is the most devastating disease of rice because of its wide distribution and its destructiveness under conductive conditions (Skamnioti and Gurr, 2009; Helliwell and Yang, 2013; Helliwell et al., 2013). Among the biotic stresses blast disease is most important. Since there have been many blast disease outbreaks in rice, efforts have been made to develop new cultivars resistant to the blast disease. Earlier studies on the variability of this fungus relied mainly on the phenotypic characters and virulence test using a set of host differentials. These studies were only focused on screening and selection of rice varieties or advanced lines toward selected local blast pathotypes. Most of these phenotypic traits are highly variable as this pathogen is genetically unstable. Such kinds of studies are labor-intensive and time-consuming, require large greenhouse space and often lead to ambiguous results. Furthermore, they are influenced by environmental conditions, inoculation techniques and human errors during scoring (Shivayogi et al., 2002). Now strategic research concentrates on filling the gaps in the existing knowledge of biotic stresses on rice, especially improving molecular genetics of blast disease, with a view to develop an integrated management program for blast resistance. Over the past decades, we have seen the successful use of advanced molecular and genomic tools such as molecular markers, expressed sequence tags (ESTs), microarrays, and genetic transformations to explore the genetic basis of stress tolerance and eventually to develop crop cultivars improved for stress tolerance. The recent developments in DNA marker technology have helped to develop the concepts of QTLs mapping, marker-aided selection (MAS) and genetic transformation to produce plants of superior quality. In addition, molecular markers can be used for assessing genetic diversity, fingerprinting genotypes, separating hybrids from selfed progeny, and other uses. The actual identification of candidate DNA markers linked to resistance genes using fine mapping may well allow rice breeders to efficiently transfer these genes from donor cultivars into new, elite rice cultivars using marker-assisted selection (MAS). There is also a need to connect knowledge about genes and gene function to create new productive varieties that are a necessary element of a sustainable food supply for the future (Ashkani et al., 2015). Here we highlight a set of molecular tools that are currently being used to study the rice blast fungus. The information generated on recent methodology will help breeders to expedite breeding research in rice crops and explore a promising new concept which utilizes such molecular data to breed for durable resistance to rice blast.
FIGURE 1.
Method for biotic and abiotic resistance breeding in crops.
Rice And Rice Blast Importance
Rice (Oryza sativa), is the principal food for over half of the population of the world and supplies the main energy resource for almost 50% of the world’s population (Yu et al., 2002). In Asia, where 60% of the earths’ people live 90% of the world’s rice is grown and more than 3 billion Asians obtain 35–75% of their calories from rice and its products (Khush and Jena, 2009). Even though the world’s rice production increased from 257 million tons in 1966 to 600 million tons in 2000, the increase has not kept up with the demand for rice because of the corresponding increase in the human population during this time. It is estimated that rice production must increase by at least 40% in 2030 to meet ever-increasing demands (Khush, 2005). Hence, population increasing at an alarming rate, making the food security the major challenge in future.
Rice serves as an economically important crop and advances in molecular biology have made it a model monocot species among the cereal for genetics studies in breeding programs. Rice in comparison to other grass species has several attributes such as: small genome; extensive genetic resources; genetic transformation potential; synteny with other cereal genomes; comprehensive genetic and physical map of the genome; high density molecular map for gene mapping and map-based gene cloning; complete sequencing of the genome in indica and japonica rice cultivars; development of bacterial artificial chromosome (bac) and yeast artificial chromosome (YAC) libraries and development of the Oryza map alignment project (OMAP); and development of the genetic maps of chloroplast and mitochondrial genomes.
The study of homologies and diversities of markers and genes within and between species, genus or other taxonomic divisions is mentioned to comparative mapping (Paterson et al., 1991). This comparison involves analyzing the conserved area between maps of the order wherein markers occur; the conserved marker order is named ‘synteny.’ Comparative mapping may help in the construction of new linkage maps and the locations predictions of QTLs in varius mapping populations (Young, 1994). Infact, previous linkage maps may show an insight which markers are polymorphic and show an insight of linkage groups and the order of markers in the linkage groups. In the last few years, high-density molecular linkage maps of rice containing approximately 3000 markers have been developed making the marker density in the rice genome, on average, one marker per cM (Causse et al., 1994; Harushima et al., 1998; Lopez-Gerena, 2006).
Rice blast is by far the most important disease that attack rice. The fungus M. oryzae = M. grisae (Cooke) Sacc [anamorph: Pyricularia oryzae], is the causal agent of blast disease (Couch and Kohn, 2002). The fungus colonizes leaves (leaf blast), panicles (panicle blast) and other parts of the rice plants, and causes huge crop loss in rice growing areas. Its most infections occur on the leaves and first symptoms of the disease appear as small brown or grayish dots on the leaves. After 2–3 days the dots develop to almost 1.5 cm long and 0.3–0.5 cm wide diamond-shaped lesions with a gray or white center (Figure 2), causing the infected leaves to die. The yield losses due to blast were reported to be between 30 and 50% in large rice producing areas under favorable environmental conditions (Correa-Victoria José and Zeigler, 1993; Skamnioti and Gurr, 2009). Efforts are underway to develop rice varieties with durable blast resistance. Therefore, continuous studies on blast disease are important in order to overcome this disease problem sustaining rice production in the future.
FIGURE 2.
Leaf blast disease symptoms. Lesions are typically spindle-shaped; wide in the center and pointed toward either end. Large lesions usually develop a diamond shape with a grayish center and brown margin.
Management Of Rice Blast And Efficient Ways For Crop Protection
Management of rice blast through the breeding of blast-resistant varieties is the most desirable means of managing blast, especially in developing countries. Rice cultivars with durable blast resistance have been recognized in several production systems. The deployment of rice cultivars with broad-spectrum resistance is practical means of controlling the fungal pathogen (Bonman, 1992; Bonman et al., 1992; Fukuoka et al., 2015). Rice blast control with resistant cultivars is much desired for farmers and consumers, because it can decrease fungicide application, subsequently reducing agrochemical pollution in the rice fields, thus reducing the cost of production. Local and wild varieties are normally used as sources for introgression of a new resistance gene into cultivated rice. Genetic resistance has been, and will continue to be, the major method of disease control of blast. The resistance in newly released rice cultivars to rice blast caused by M. oryzae can be lost due to the high level of instability in the genome of this fungus or due to the frequent breakdown of resistance under field conditions (Bonman, 1992; Bonman et al., 1992; Zeigler et al., 1994). One way to overcome this problem is through pyramiding of multiple R genes, each recognizing a unique set of M. oryzae isolates, into a single cultivar. Molecular markers techniques can be utilize in gene pyramiding in rice breeding programs to produce resistant cultivars and overcome to breakdown of disiease in early stage thereby sustaining rice yields and eventually to map based cloning of the gene. Such techniques as simpler method will save time and minimize costs especially for traits with laborious screening and it is more efficient to use of resources for plant breeding.
Molecular Breeding Research To Improve Disease-Resistant Varieties
New rice varieties that combine higher yield potential with excellent grain quality, resistance to biotic and abiotic stresses, and input use efficiency are desperately needed. Diverse strategies for breeding durable resistance have been offered for rice blast. Some of these strategies, such as pyramiding (Bonman, 1992; Bonman et al., 1992), lineage exclusion (Zeigler et al., 1994), multilines (Abe, 2004) and mixtures (Zhu et al., 2000), are based on the use of complete and specific resistance genes. In general, in current agriculture three major strategies are used to improve disease resistance in crops. The first strategy involves improvement of cultural practices. The second approach comprises the improvement of crops through conventional or molecular marker-assisted breeding of disease resistant cultivars. The third strategy is the direct transformation of resistant genes into elite cultivars (Baulcombe, 2004). The use of molecular techniques for example the agrobacterium-mediated transformation allows the stable transfer of a transgene in a different variety or species, as well as to use a different promoter on a resistance-related gene to alter the intensity of gene expression (Baulcombe, 2004). To increase rice production and resistance, conventional rice breeding carried out during the last 50 years, resulted in the release of modern resistant varieties with high quality and yield. Despite, this method has played an important role in rice cultivar development over the past decades there are drawbacks as well. Conventional breeding progress is slow owing to several obstacles, such as: time-consuming and laborious selection process, difficulties in appropriate genotype selection due to the quantitative nature of most agronomic traits, several generations of crossing, selfing, and testing plants for resistance. In addition, traditional breeding is often negatively affected by linkage drag, which resulted in the transferring of loci conferring potentially undesired agronomic traits due to its close linkage with resistance loci. Recent advances in molecular genetics of rice have provided new tools for breeders to develop the rice varieties of the future which is known as molecular breeding. Only few years ago, the status of rice genetics was considered far behind that of other food crops such as maize, wheat, barley, and tomato. However, the last decade has seen a knowledge explosion in this area and rice is now considered a model plant for such research on cereal crops. Rice has been performed as a successful crop in biotechnology approaches leading to crop improvements. A vast reservoir of germplasm (>200,000 accessions) of both domestic and wild rice is available for genetic and breeding research. With the completion of rice genome sequence, many rice research now focused on functional characterization of rice genes, elucidation of the underlying mechanisms involved in major agronomic traits (e.g., high yield, grain quality, abiotic stress tolerance, and disease resistance), and subsequent translation of genomic knowledge into agricultural productivity via molecular breeding and improved cultural practice (Helliwell and Yang, 2013; Helliwell et al., 2013). Genetic studies of blast resistance in rice were established in Japan as early as 1917 (Ballini et al., 2008). To date, there are various molecular and biotechnological approaches to genetically improve rice crop for effective, durable and/or broad-spectrum resistance to major diseases. Currently, many resistance genes (R-genes) and QTLs in rice for blast have been identified and sequenced (Ashkani et al., 2014). These genes contribute to the understanding of the interaction between the disease and the host for breeding proposes. In addition, a wide variety of genes and mechanisms involved in rice defense response (e.g., pathogenesis-related proteins and other defense genes) have been identified and elucidated. Also, many molecular approaches including use of specialized promoters, modification of target protein structures have been studied and proposed to improve the effectiveness of transgenes (Baulcombe, 2004). During the past two decades, some rice research institute has been involved in the rice mutation breeding program to generate new varieties, in support of the crop breeding program. The main purposes of mutation breeding of rice have been improvement of agronomic traits, inducing resistance against diseases and pests, and enhancing the grain quality and grain taste. In the breeding program for rice in collaboration with IRRI (the Philippines) and JIRCAS (Japan) many modern varieties have also been released for commercial use. Traditional rice varieties have been widely used as genetic resources for biotic and abiotic traits of hybridization program. To complement conventional breeding method, molecular and transgenic method represents an increasingly important approach for genetic improvement of disease resistance and reduction of pesticide usage and various molecular strategies including use of specialized promoters, modification of target protein structures have been studied and proposed to improve the effectiveness of transgenes (Helliwell and Yang, 2013; Helliwell et al., 2013). MAS for quick indirect selection of the target gene by using molecular markers closely linked to a target gene as a molecular tag, quantitative trait locus (QTL) analysis and genetic transformation techniques are the most useful tools for rice molecular breeding especially to improve disease-resistant varieties. These techniques have been used to identify new germplasms and elite rice cultivars.
Blast Disease Improvement Due To Molecular Markers Techniques
Molecular markers have played an increasing role in rice breeding for cultivar improvement, screening, selection and germplasm collections (Wang et al., 2007). The new sequencing tools provide valuable informations for the discovery, validation and assessment of genetic markers in populations (Sahebi et al., 2015). For instance, the analysis of next generation sequencing (NGS) data by means of bioinformatics developments allows discovering new genes and regulatory sequences and their positions, and makes available large collections of molecular markers (Perez-de-Castro et al., 2012). The whole genome sequence data substantially enhanced the efficiency of polymorphic marker development for QTL fine mapping and the identification of possible candidate genes (Wan et al., 2006). These performances can be useful as genetic resources for breeding of rice cultivars. The use of molecular markers for rice has been recently reviewed (Temnykh et al., 2001; Xu, 2002; Semagn et al., 2006; Lang et al., 2008; Kumar et al., 2009; Benali et al., 2011). In the case of rice blast (M. oryzae) a large number of the major genes had been identified and was targeted for mapping investigations using a variety of marker systems and approaches (Ashkani et al., 2014). DNA markers including : simple sequence repeats (SSRs), Single-nucleotide polymorphisms (SNPs) and small insertions/deletions (InDels), amplified fragment length polymorphisms (AFLPs), random amplified polymorphic DNAs (RAPDs), cleaved amplified polymorphic sequences (CAPS), and restriction fragment length polymorphisms (RFLPs) have been identified to be linked with blast resistance genes in rice (Ashkani et al., 2014; Tanweer et al., 2015). In recent year, scientists have used these markers for genetic mapping to identify candidate genes and QTLs in many plant species. Several genes of agronomic importance such as those that confer resistance to blast, bacterial leaf blight, brown planthopper, tungro and grassy stunt virus have been transferred from the wild species into the elite breeding lines of rice, including the quantitative trait loci (QTLs) for biotic and abiotic stress resistance (Amante-Bordeos et al., 1992; Brar and Khush, 1997).
DNA markers techniques provide us and rapid tool to select for the existence of multiple blast resistance genes without the need to test the progeny or inexact phenotypic disease screening (Fjellstrom et al., 2004). Through molecular markers tools many useful markers linked to the race-specific blast resistance genes (Pi-genes), has been identified and screened in segregating populations in rice (Fjellstrom et al., 2004; Sharma et al., 2005; Ashkani et al., 2011). PCR-based markers as example SSRs are precise, reliable and cost effective; this marker has been applied for the selection of plants containing blast resistance genes in rice at an early stage (Hittalmani et al., 2002; Ashkani et al., 2012). Microsatellites are SSR markers, and have been used extensively to identify genes and QTLs associated blast resistance in rice. Microsatellites are abundant in plants (McCouch et al., 2001), causing more polymorphism and better repetition over other marker systems. The genetic map covering all 12 rice chromosomes with at least one microsatellite at the distance of 0.5 cM has been developed by International Rice Microsatellite Initiative (IRMI; McCouch et al., 2002). Currently, breeders are focusing on MAS instead of using conventional breeding. Application of MAS reduces the time for phenotypic selection and saves the costs to select a desired trait (Koide et al., 2009). This method is helpful tool, and more accurate approach in introducing novel cultivars and it also help breeders to expedite breeding research in crops by enabling selection based on the genotype rather than on the phenotype. After the discovery of molecular markers linked with gene of interest, selection of specified traits to develop new cultivar could be made at an early level (Zhu et al., 2012). Pyramiding of linked genes into a single line or cultivar is one of the common applications of MAS.
Marker-assisted backcross breeding (MABC) as another technique recently has been given attention in rice breeding for the introgression of blast resistance genes (one or a few genes) into the susceptible or in an adapted or elite varieties. MABC is the process of using markers to select for target loci, minimize the length of the donor segment containing a target locus and accelerate the recovery of the recurrent parent (RP) genome during backcrossing (Charcosset, 1997; Hospital, 2001; Hasan et al., 2015). The main purpose of MABC is to transfer the desired character/or targeted gene along with recovering the recurrent parent characters/or genes. MABC is now playing an important role for the development of blast-resistant cultivars (Sundaram et al., 2009) and is superior to conventional backcrossing in precision and efficiency and time saving. Molecular markers which are tightly linked with important traits are used in MABC. Therefore, molecular markers are the tools that can be used to detect the presence of desire character in backcrossing and greatly increases the efficiency of selection. The methods and potential application of MAS and MABC for the Improvement of rice have been recently reviewed and described (Collard and Mackill, 2008; Hasan et al., 2015). Recently through application of MABC many blast resistance genes have been successfully introgressed into the genetic background and improved the blast resistance. Some successful examples for application of MAS and MABC in rice breeding programs aimed at improving blast resistance in this species are presented in Table 1.
Table 1.
Examples for application of marker-assisted selection (MAS) and marker-assisted backcrossing (MABB) in rice.
| Trait | Gene(s)/QTL(s) | Marker(s) used | Technique used | Application | Reference |
|---|---|---|---|---|---|
| Blast resistance | Pi1, Piz-5, Pita | RFLP | MAS | Pyramiding of three near isogenic lines (C101LAC, C101A51 and C101PKT) for blast resistance in into a single cultivar Co-39, each carrying the major genes Pi1, Piz-5 and Pita, respectively | Hittalmani et al., 2000 |
| Blast resistance | Pi1 | SSR and ISSR | MAS | Applied for backcross breeding of variety (Zhenshan 97A) | Liu et al., 2002b |
| Bacterial blight Resistance + Blast resistance | Xa21, Piz | SSR | MAS | Functional for pyramiding of target traits | Narayanan et al., 2002 |
| Blast resistance | Pid1, Pib, Pita and Pi2 | SSR | MAS | Pid1, Pib and Pita genes were introduced into G46B cultivar, while Pi2 Zhenshan97B cultivars of rice | Chen et al., 2004 |
| Blast resistance | Pi-z | SSR | MAS | Closely linked with Pi-z locus has been successfully used for selection of blast resistance in a wide array of rice germplasm | Fjellstrom et al., 2006 |
| Blast resistance + Bacterial blight resistance + Sheath blight resistance | Xa13, Xa21, Pi54, qSBR11 | SSR [for blast resistance (Xa13 and Xa21), for bacterial blight resistance (Pi54), and Sheath blight resistance (qSBR11)] | MAS | MAS-assisted transfer of genes conferring the resistance toward three different diseases in rice | Singh et al., 2012a |
| Blast resistance + Bacterial blight resistance | Pi-genes, Xa5 | SSR | MAS | Near-isogenic lines (NILs) derived from two blast resistant crosses (RD6 × P0489 and RD6 × Jao Hom Nin) were pyramided with IR62266 (xa5), to transfer bacterial leaf blight resistance to RD6 lines | Pinta et al., 2013 |
| Blast resistance | Pi-ta | Gene specific marker | MAS | Existence of the Pi-ta gene in 141 rice germplasm has been successfully determined, but the results were more articulated when Pi-ta gene was introduced through advanced breeding lines | Wang et al., 2007 |
| Submergence tolerance + Brown planthopper resistance + Blast resistance + Bacterial blight resistance | chr9 QTL, Xa21, Bph and QTLs blast, and quality loci | SSR and STS | MABB | MABB confirmed the transfer of gene and QTL for into cultivar KDML105 | Toojinda et al., 2005 |
| Blast resistance | Pi1, Pi2, Pi33 | SSR | MABB | Introgressed into Jin23B cultivar through MABB | Chen et al., 2008 |
| Blast resistance + Bacterial blight | Pi1, Pi2, Xa23 | SSR [For blast resistance (Pi1, Pi2), for bacterial blight resistance (Xa23) | MABB | Sucessfully applied for breeding the variety (Rongfeng B) | Fu et al., 2012 |
| Blast resistance | Piz-5, Pi54 | SSR | MABB | Combination of blast resistance gene from donor lines (C101A51 and Tetep) into cultivar PRR78 to develop Pusa1602 (PRR78 + Piz5) and Pusa1603 (PRR78 + Pi54), respectively | Singh et al., 2012b |
| Blast resistance | Pi-9(t) | pB8 | MABB | MABB applied to introgress the cultivar Luhui17 | Wen and Gao, 2011 |
| Blast resistance | Pi-1, Pi-z | SSR | MABB | Pyramiding of Pi-1 and Piz-5 genes into introduced PRR78 cultivars | Gouda et al., 2013 |
Resistant Genes And Qtls For Blast Disease
Resistance to blast was classified into complete and partial resistance (Wang et al., 1994). Complete resistance is a qualitative character and race specific controlled by a major gene (R genes). Meanwhile, partial resistance is a quantitative character and non-race specific, which is controlled by many genes known as quantitative resistance loci (QRL; Young, 1996). However, if the resistance is highly partial, it can also be controlled by a major gene and is race specific. Qualitative and quantitative blast resistances have been reported in rice germplasm (Ou, 1985). Many qualitative resistance major genes (∼100 genes) for blast resistance have been identified and mapped in the rice genome (Sharma et al., 2012; Ashkani et al., 2014). About 22 R-genes have been successfully cloned and molecularly characterized. In the practice of resistance breeding, using a single R gene which has a broad resistance spectrum is more effective. There have been many reports on introgression of Pi genes related to blast disease into commercial and elite varieties. For example, the Pi-9 gene that exists in the indica rice line 75-1-127 (Liu et al., 2002a), was introgressed from the wild species O. minuta (Amante-Bordeos et al., 1992). The Pi-ta allele was identified in O. rufipogon and O. nivara, or in their hybrids with O. sativa (Jena and Khush, 2000). These R genes function in a gene-for-gene fashion, so the pathogen can adapt by mutating or deleting the corresponding a-virulence gene. Therefore varieties those carrying R genes which confer high levels of resistance typically lose their resistance after a few years (Chen et al., 2003). Quantitative resistance donated by quantitative trait loci (QTL) are long-lasting disease resistance against a wide-range of pathogens, promising for sustainable rice production in the future (Song and Goodman, 2001). QTL mapping is a modern type of study to locate genes controlling a quantitative trait. Since the first publication of a QTL analysis of rice resistant to blast (Wang et al., 1994), several QTLs related to blast resistance have been detected using different type of markers, population and environment and have been published. We have summerized all these events in tabular form (Table 2).
Table 2.
Quantitative trait loci (QTL), identified for rice blast resistance.
| Mapping population | Parents used in crossing | Total No. of QTLs detected | Used markers | Reference |
|---|---|---|---|---|
| Recombinant Inbred Lines (RILs) | CT9993-5-10-1-m × KDML105 (F8); Zhenshan 97 × Minghui 63 (RILs); Moroberekan × Co39 (F7); Lemont × Teqing (F8); Lemont × Teqing (F14); Bala × Azucena (F6); Zhong 156 × Gumei 2 (F8); Oryzica Llanos 5 × Fanny (F5 and F6); SHZ-2 × Lijiangxin-tuan-heigu (LTH) (RILs); KDML105 × JHN (F6); Suweon365 × Chucheong (RILs) | 186 | RFLPs, SSR, RAPD, Isozymes, AFLPs, DR gene markers | Sirithunya et al., 2002; Chen et al., 2003; Wang et al., 1994; Tabien et al., 2002; Loan et al., 2003; Liu et al., 2004; Talukder et al., 2005; Wu et al., 2005; Lopez-Gerena, 2006; Noenplab et al., 2006; Cho et al., 2008; |
| Doubled Haploid (DH) | IR64 × Azucena; IR64 × Azucena; ZYQ8 × JX17 | 146 | RFLPs, RAPD, Isozymes | Xu et al., 2004; Sallaud et al., 2003; Bagali et al., 2000 |
| Single-segment substitution lines (SSSLs) | Developed by the use of HXJ74 as recipient and 24 accessions as donors | 11 | SSR | Zhang et al., 2012 |
| Back cross population | Way Rarem × Oryzica Llanos 5 (IRGC 117017); Oryza sativa cv MR219 × O. rufipogon IRGC 105491; SHZ-2 × TXZ-13; Oryzarufipogon × cultivated rice IR64 | 45 | SSR, SNP | Lestari et al., 2011; Rahim et al., 2012; Utani et al., 2008; Liu et al., 2011 |
| F2, F3, and F4 | Nipponbare × Owarihatamochi (F4 lines); Kahei × Koshihikari (F2:3); Tainung 69 × Koshihikari (F2); URN12 × Koshihikari (F2); Norin29 × Chubu32 (F3); Pongsu Seribu 2 × Mahsuri (F2:3); TAM × KHZ (F2:3); Junambyeo × O. minuta introgression line IR71033-121-15 (F2:3); Danghang-Shali × Hokkai 188 (F2:3) | 60 | RFLPs, SSR STS | Fukuoka and Okuno, 2001; Miyamoto et al., 2001; Sato et al., 2006; Zenbayashi et al., 2002; Ashkani et al., 2013a,b Nguyen et al., 2006; Rahman et al., 2011; Sabouri et al., 2011 |
Quantitative trait loci detection approach has been employed to map major or minor genes involved in the resistance (Wang et al., 1994; Fukuoka and Okuno, 2001; Miyamoto et al., 2001; Tabien et al., 2002; Zenbayashi et al., 2002; Chen et al., 2003; Sallaud et al., 2003; Talukder et al., 2004; Wu et al., 2005; Ashkani et al., 2013a,b). Identification of QTLs, associated with blast resistance has been delivered the effective genetics evidences for the molecular marker assisted breeding and cloning of the major genes. In the other word, QTL mapping is useful in identifying multiple loci controlling complete resistance in a highly resistant cultivar as well as in estimating the number, location and effect of genomic region involved in partial blast resistance (Sallaud et al., 2003). Many rice improvement programs now aim to incorporate quantitative or polygenic resistance into rice varieties. Previous studies have verified that genetic linkage maps constructed with various DNA markers are very useful for the analysis and detection of qualitative trait loci (Bao et al., 2000; Price et al., 2000). Molecular linkage maps have helped resolve the effects of minor and major QTLs and estimate the amount phenotypic variation explained at each locus. Molecular linkage maps have led to better understanding of genetic phenomena, such as interloci (epistasis) and intralocus (dominant) interactions (Grandillo and Tanksley, 1996), heterosis (Stuber et al., 1992) and identifying transgressive segregants (Tanksley, 1988, 1993).
Gene Pyramiding For Blast Resistance
Pyramiding is the accumulation of genes into a single line or cultivar. In a gene pyramiding, strategy is to cumulate genes identified in multiple parents into a single genotype (Figure 3). The end product of a gene-pyramiding program is a genotype with all of the target genes. Pyramiding multiple resistance genes provides durable stress resistance expression in crops. Gene Pyramiding technique broadly is used for combining multiple disease or pest resistance genes for specific races of a pathogen or insect to develop durable resistance. It helps in crop improvement program and reduces breeding duration. Different R-genes often confer resistance to different isolates, races or biotypes. Combining their resistance broadens the number of races or isolates that a more than one character in a variety at the same time. Developing elite breeding lines and varieties often requires plant breeders to combine desirable traits from multiple parental lines, particularly in the case of disease resistance. Gene pyramiding can be accelerated by using molecular markers to identify and select plants that contain the desired allele combination in very early stage, resulting in obvious savings of resources including greenhouse or field space, water, and fertilizer. Therefore, marker technology can help existing plant breeding programs and allows researchers to access, transfer and combine genes at a rate and with a precision not previously possible. MAS based gene pyramiding could facilitate in pyramiding of genes effectively into a single genetic background (Joshi and Nayak, 2010). Factors such as the number of genes to be transferred, the distance between the target genes and flanking markers calculated in genetic mapping studies, the number of genotype selected in each breeding generation and the nature of germplasm is critical for successful gene pyramiding program. Gene pyramiding is considered one of the most effective strategies for achieving durable resistance against blast disease in rice (Shinoda et al., 1971; Hittalmani et al., 2000; Koide et al., 2010) and have successfully used for accumulating different blast resistance genes in elite rice cultivars (Table 3).
FIGURE 3.
Gene pyramiding scheme for cumulating six desired genes (G1–G6) which are present in 6 different parents or lines (P1–P6). The gene pyramiding consists of two steps, pedigree, which aims at cumulating of all target genes in a single genotype (Root genotype) by crossing and selection; the second step is fixation which aims at fixing the target genes into a homozygous state (Ideal/target genotype).
Table 3.
Example of gene pyramiding for blast resistance trait in rice.
| Traits | Parental lines | Pyramided genes | DNA marker(s) used | Reference |
|---|---|---|---|---|
| Blast resistance | C101LAC, C101A51 | Pi1, Pi2 and Pi33 | SSR | Chen et al., 2008 |
| Blast resistance | IR5, IR8, IR20, IR22, IR24, IR26, IR28, IR29, IR30, IR32, IR34, IR36, IR38, IR40, IR42, IR43, IR44, IR45, IR46, IR48, IR50 IR52, IR54, IR56, IR58, IR60, IR62, IR64, IR65, IR66, IR68, IR70, IR72, IR74 | Pib and Pita | SSR | Fujita et al., 2009 |
| Blast resistance | CO39 | Pish and Pib | SSR | Koide et al., 2010 |
| Blast resistance | IR64, JHN | Multiple resistance QTLs | SSR | Sreewongchai et al., 2010 |
| Blast resistance | Rongfeng B | Pi1, Pi2 Xa23 | SSR | Fu et al., 2012 |
| Blast resistance | Jin 23B | Pi1, Pi2, and D12 | SSR | Jiang et al., 2012 |
| Blast resistance | C101LAC, C101A51 | Pi-1 and Pi-2 | RG64 and C481 | Mahdian and Shahsavari, 2013 |
| Blast and bacterial leaf blight resistance | RD6 × P0489; RD6 × JHN | Four QTLs for blast resistance and one gene for bacterial leaf blight (xa5) | SSR | Pinta et al., 2013 |
| Blast resistance | C101A51, Tetep | Piz5 and Pi54 | SSR | Singh et al., 2013 |
| Blast resistance | Carnaroli, Baldo, Arborio | Piz and Pi5 | SSR | Urso et al., 2013 |
| Leaf blast resistance | Koshihikari | Pi21, Pi34, and Pi35 | SSR | Yasuda et al., 2014 |
| Blast resistance | GZ63-4S | Pi2 and Xa23 | SSR (M-Xa23) | Jiang et al., 2015 |
Allele Mining And Blast Resistance Genes
Allele mining is the commonly used approach to identify novel alleles or allelic variants of a gene/or candidate genes of interest, based on the available information about the genes, from a wide range of germplasm. This technique possesses good potential to be used in molecular plant breeding of crop improvement programs. The success of allele mining mainly depends on the type of genetic materials used for screening and should be as diverse as possible and availability of genome and gene sequence information of a particular crop species. For efficient allele mining, wild relatives and local landraces are used because they are reservoirs of useful alleles hidden in their phenotype (Tanksley et al., 1996). The current availability of complete rice genome sequences in addition to several bioinformatic tools have made it possible to mine allelic diversity throughout rice germplasm. EcoTilling and sequence based allele mining are the two widely used approaches in allele mining. Compared to EcoTilling, sequence based allele mining strategy is reported to be simpler and cost effective approach (Ramkumar et al., 2010; Ashkani et al., 2015). Allele mining possesses wide range of applications within crop improvement among them are, allele identification, allelic variation characterization, haplotypes identification, analysis of haplotypes diversity among different haplotypes of the same gene or among the related haplotypes, evolutionary relationship, similarity analysis and development of molecular markers to differentiate a particular allele from other alleles. To date allele mining strategy has been well demonstrated by many researchers. Allele mining of genes from wild and cultivated rice species aims to detect superior alleles for blast resistance (Kumari et al., 2013). So far, mining approaches have been used to identify novel and superior alleles of many major blast resistance genes from different cultivated rice varieties and wild species (Table 4). Through allele mining techniques functional marker to differentiate the resistance and susceptible alleles of Pi54 has been developed (Ramkumar et al., 2011). Costanzo and Jia (2010) analyzed the sequence level similarity for Pikm alleles, derived from 15 different rice cultivars. M. oryza has also been differentiated from M. grisea by using allele mining (Couch and Kohn, 2002).
Table 4.
Summary of allele mining report for blast resistance genes.
| R-Genes/Locus | Chromosome | Rice germplasm | Reference |
|---|---|---|---|
| Pi-ta | 12 | From wild rice species [O. rufipogon (Griff) and from O. rufipogon (ETOR)] | Yang et al., 2007; Geng et al., 2008 |
| Pi-ta | 12 | From O. rufipogon | Huang et al., 2008 |
| Pi-ta | 12 | From cultivated (AA) and wild species and invasive weedy rice | Lee et al., 2009, 2011 |
| Pi-ta | 12 | In 26 accessions, consisting of wild rice (O. rufipogon), cultivated rice (O. sativa) and related wild rice species (O. meridionalis and O. officinalis) collected from ten different countries of the world | Yoshida and Miyashita, 2009 |
| Pi-ta | 12 | From landraces and wild Oryza species | Ramkumar et al., 2010 |
| Pi-ta | 12 | In Indian land races of rice | Sharma et al., 2010 |
| Pi-ta | 12 | From Indian landraces of rice collected from different ecogeographical regions including the northwestern Himalayan region of India | Thakur et al., 2013a |
| Pi-kh (Pi54) | 11 | From wild and cultivated species of rice | Rai et al., 2011 |
| Pi-kh (Pi54) | 11 | From the blast-resistant wild species of rice, O. rhizomatis | Das et al., 2012 |
| Pi-kh (Pi54) | 11 | From six cultivated rice lines and eight wild rice species | Kumari et al., 2013 |
| Pi-kh (Pi54) | 11 | In Indian land races of rice | Sharma et al., 2010 |
| Pi-z(t) | 06 | In Indian land races of rice | Sharma et al., 2010 |
| Piz(t) | 06 | In 529 land races of rice collected at three different geographical locations of India | Thakur et al., 2013b |
| Pid3 | 06 | From 36 accessions of wild rice O. rufipogon | Shang et al., 2009; Xu et al., 2014 |
| Pid3-A4 | 06 | From wild rice A4 (O. rufipogon) | Lv et al., 2013 |
| Pi9 | In different rice species, five AA genome Oryza species including two cultivated rice species (O. sativa and O. glaberrima) and three wild rice species (O. nivara, O. rufipogon, and O. barthii). | Liu et al., 2011 | |
| AC134922 | 11 | Rice lines from various sources | Wang et al., 2014 |
Conclusion
Disease management extremely needed to sustain the world for food consumption. Rice blast caused by M. oryzae is the most severe fungal disease, which limits the rice production and causing the yield loss of 157 million tons of rice per annum in the worldwide (Kaundal et al., 2006). Development of resistant varieties with durable resistance by incorporating new genes into the improved germplasm has been proved to be economical, environmentally friendly and effective to control the rice blast disease (Skamnioti and Gurr, 2009). The availability of different molecular tools allows characterization of genes of interest and identification of plants carrying the target genes and might well serves to improve the efficiency of conventional breeding. Due to molecular dissection it is now possible to identify blast resistance genes and QTLs or combined effects of multiple loci with major and minor effects. The marker developed from these genes or QTLs can be used in marker assisted selection for selection of resistance without confounding the effects of environmental factors. DNA markers that co-segregate with the gene are a powerful method for use in crop protection and can be routinely employed in various aspects of plant genome analysis such as genetics and plant breeding. Information provided on genetics of blast resistance of local traditional variety is very useful for rice resistance breeding program in every country. Recent molecular breeding strategy such as gene pyramiding and allele mining holds greater prospects to attain durable resistance against biotic and abiotic stresses in crops. Identification of novel and superior resistance alleles of the blast resistance genes is an important task in the rice breeding program. The novel alleles are very useful in breeding programs and can be utilized gainfully to develop productive and superior plants.
Future Perspectives And Consideration
The major difficulty in controlling rice blast is the durability of genetic resistance. Enhancing the host plant resistance is being considered as the best approach to handle the rice blast disease. Rice cultivars containing only a single R gene to a specific pathogen race often become susceptible over time due to the emergence of new virulent races. Understanding of genetic identity of contemporary M. oryzae is important for accurate deployment of rice cultivars with different R genes. Stacking R genes with overlapped resistance spectra can lead to long lasting resistance. For combinations of different blast resistance genes in host plant in the rice blast breeding programs, superior alleles of the targeted genes should be considered. During the evolution and artificial selection processes, a significant portion of beneficial alleles have been left behind in the landraces and wild species (McCouch et al., 2007), which can be used for the development of better rice varieties. Effective blast management also requires international cooperation. The knowledge gained by collaborative effort ought to lead to more effective methods to reduce crop loss due to blast disease worldwide. Although considerable progress has been made toward understanding the nature of disease resistance genes, defense responses, and the signal transduction leading to activation of defense responses in rice, the whole story is still far from clear. Studies of the molecular biology of disease resistance will be helpful in improving rice varieties for high production for increasing population. The completion of the rice genome project and availability of structural genomic data for the public can undoubtedly accelerate research on the molecular biology of rice disease resistance. Development of new molecular techniques, methodologies such as functional geonomics and DNA microarrays for global analysis of gene expression should urge rice breeders to integrate these techniques to conventional breeding. Rice research should more focus on identifying more durably resistant genes, tagging of these genes with molecular markers and pyramiding these genes or QTLs through molecular MAS. Monogenic resistance to blast is less stable but varieties with pyramided monogenes or QTLs are durably resistant. Molecular breeding strategy can help in the introduction of durably blast-resistant rice cultivars thereby sustaining rice yields. Candidate gene identification through rice functional genomics has great potential for developing more durably resistant varieties.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our university colleagues for their helpful suggestions, insightful comments and discussions on this valuable review. This work was supported by the Long-term Research Grants Scheme (LRGS), Rice Food Security Project from the Ministry of Education, Malaysia.
References
- Abe S. (2004). Breeding of a blast resistant multiline variety of rice. Sasanishiki BL. Japan Agric. Res. Q. 38 149–154. 10.6090/jarq.38.149 [DOI] [Google Scholar]
- Amante-Bordeos A., Sitch L., Nelson R., Dalmacio R., Oliva N., Aswidinnoor H., et al. (1992). Transfer of bacterial blight and blast resistance from the tetraploid wild rice Oryza minuta to cultivated rice. Oryza sativa. Theor. Appl. Genet. 84 345–354. 10.1007/BF00229493 [DOI] [PubMed] [Google Scholar]
- Ashkani S., Rafii M. Y., Rahim H. A., Latif M. A. (2013a). Genetic dissection of rice blast resistance by QTL mapping approach using an F3 population. Mol. Biol. Rep. 40 2503–2515. 10.1007/s11033-012-2331-3 [DOI] [PubMed] [Google Scholar]
- Ashkani S., Rafii M. Y., Rahim H. A., Latif M. A. (2013b). Mapping of the quantitative trait locus (QTL) conferring partial resistance to rice leaf blast disease. Biotechnol. Lett. 35 799–810. 10.1007/s10529-012-1130-1 [DOI] [PubMed] [Google Scholar]
- Ashkani S., Rafii M. Y., Rusli I., Sariah M., Abdullah S. N. A., Rahim H. A., et al. (2012). SSRs for marker-assisted selection for blast resistance in rice (Oryza sativa L.). Plant Mol. Biol. Rep. 30 79–86. 10.1007/s11105-011-0315-4 [DOI] [Google Scholar]
- Ashkani S., Rafii M. Y., Sariah M., Siti N. A. A., Rusli I., Rahim H. A., et al. (2011). Analysis of simple sequence repeat markers linked with blast disease resistance genes in a segregating population of rice (Oryza sativa). Genet. Mol. Res. 10 1345–1355. 10.4238/vol10-3gmr1331 [DOI] [PubMed] [Google Scholar]
- Ashkani S., Rafii M. Y., Shabanimofrad M., Ghasemzadeh A., Ravanfar S., Latif M. A. (2014). Molecular progress on the mapping and cloning of functional genes for blast disease in rice (Oryza sativa L.): current status and future considerations. Crit. Rev. Biotechnol. 14 1–15. [DOI] [PubMed] [Google Scholar]
- Ashkani S., Rafii M. Y., Shabanimofrad M., Azadi A., Ghasemzadeh A., Azizi P., et al. (2015). Allele mining strategies: principles and utilisation for blast resistance genes in rice (Oryza sativa L.). Curr. Issues Mol. Biol. 17 57–74. [PubMed] [Google Scholar]
- Bagali P. G., Hittalmani S., Shashidhar S. Y., Shashidhar H. (2000). “Identification of DNA markers linked to partial resistance for blast disease in rice across four locations,” in Advances in Rice Blast Research, eds Tharreau D., Lebrun M. H., Talbot N. J., Notteghem J. L. (Berlin: Springer; ), 34–42. [Google Scholar]
- Ballini E., Morel J.-B., Droc G., Price A., Courtois B., Notteghem J.-L., et al. (2008). A genome-wide meta-analysis of rice blast resistance genes and quantitative trait loci provides new insights into partial and complete resistance. Mol. Plant Microbe Inter. 21 859–868. 10.1094/MPMI-21-7-0859 [DOI] [PubMed] [Google Scholar]
- Bao J., Zheng X., Xia Y., He P., Shu Q., Lu X., et al. (2000). QTL mapping for the paste viscosity characteristics in rice (Oryza sativa L.). Theor. Appl. Genet. 100 280–284. 10.1139/G10-070 [DOI] [Google Scholar]
- Baulcombe D. (2004). RNA silencing in plants. Nature 431 356–363. 10.1038/nature02874 [DOI] [PubMed] [Google Scholar]
- Benali S., Mohamed B., Eddine H. J., Neema C. (2011). Advances of molecular markers application in plant pathology research. Eur. J. Sci. Res. 50 110–123. [Google Scholar]
- Bonman J. (1992). Durable resistance to rice blast disease-environmental influences. Euphytica 63 115–123. 10.1007/BF00023917 [DOI] [Google Scholar]
- Bonman J., Khush G., Nelson R. (1992). Breeding rice for resistance to pests. Annu. Rev. Phytopathol. 30 507–528. 10.1146/annurev.py.30.090192.002451 [DOI] [Google Scholar]
- Brar D., Khush G. (1997). Alien introgression in rice. Plant Mol. Biol. 35 35–47. 10.1023/A:1005825519998 [DOI] [PubMed] [Google Scholar]
- Causse M. A., Fulton T. M., Cho Y. G., Ahn S. N., Chunwongse J., Wu K., et al. (1994). Saturated molecular map of the rice genome based on an interspecific backcross population. Genetics 138 1251–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcosset A. (1997). Marker-assisted introgression of quantitative trait loci. Genetics 147 1469–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chen Z., Ni S., Zuo S., Pan X., Zhu X. (2008). Pyramiding three genes with resistance to blast by marker assisted selection to improve rice blast resistance of Jin23B. Chinese J. Rice Sci. 22 23–27. [Google Scholar]
- Chen H., Wang S., Xing Y., Xu C., Hayes P. M., Zhang Q. (2003). Comparative analyses of genomic locations and race specificities of loci for quantitative resistance to Pyricularia grisea in rice and barley. Proc. Natl. Acad. Sci. U.S.A 100 2544–2549. 10.1073/pnas.0437898100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. W., Li S. G., Ma Y. Q., Li H. Y., Zhou K. D., Zhu L. H. (2004). Marker-assisted selection and pyramiding for three blast resistance genes, Pi-d (t) 1, Pi-b, Pi-ta2, in rice. Chinese J. Biotechnol. 20 708–714. [PubMed] [Google Scholar]
- Cho Y.-C., Kwon S.-W., Suh J.-P., Kim J.-J., Lee J.-H., Roh J.-H., et al. (2008). QTLs identification and confirmation of field resistance to leaf blast in temperate japonica rice (Oryza sativa L.). J. Crop Sci. Biotechnol. 11 269–276. [Google Scholar]
- Collard B. C., Mackill D. J. (2008). Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B 363 557–572. 10.1098/rstb.2007.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Victoria José F., Zeigler R. S. (1993). Pathogenic variability in Pyricularia grisea at a rice blast hot spot breeding site in eastern Colombia. Plant Dis. 77 1029–1035. 10.1094/PD-77-1029 [DOI] [Google Scholar]
- Costanzo S., Jia Y. (2010). Sequence variation at the rice blast resistance gene Pi-km locus: implications for the development of allele specific markers. Plant Sci. 178 523–530. 10.1016/j.plantsci.2010.02.014 [DOI] [Google Scholar]
- Couch B. C., Kohn L. M. (2002). A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94 683–693. 10.2307/3761719 [DOI] [PubMed] [Google Scholar]
- Das A., Soubam D., Singh P., Thakur S., Singh N., Sharma T. (2012). A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae. Funct. Integr. Genom. 12 215–228. 10.1007/s10142-012-0284-1 [DOI] [PubMed] [Google Scholar]
- Fjellstrom R., Conaway-Bormans C. A., Mcclung A. M., Marchetti M. A., Shank A. R., Park W. D. (2004). Development of DNA markers suitable for marker assisted selection of three genes conferring Resistance to multiple pathotypes. Crop Sci. 44 1790–1798. 10.2135/cropsci2004.1790 [DOI] [Google Scholar]
- Fjellstrom R., Mcclung A. M., Shank A. R. (2006). SSR markers closely linked to the Pi-z locus are useful for selection of blast resistance in a broad array of rice germplasm. Mol. Breed. 17 149–157. 10.1007/s11032-005-4735-4 [DOI] [Google Scholar]
- Fu C., Wu T., Liu W., Wang F., Li J., Zhu X., et al. (2012). Genetic improvement of resistance to blast and bacterial blight of the elite maintainer line Rongfeng B in hybrid rice (Oryza sativa L.) by using marker-assisted selection. Afr. J. Biotechnol. 11 13104–13114. 10.5897/AJB12.1465 [DOI] [Google Scholar]
- Fujita D., Ebron L. A., Kobayashi N., Fukuta Y. (2009). “DNA marker analysis of blast resistance genes Pib and Pita in IRRI-bred rice varieties comparision with gene estimation by a differential system,” in Advances in Genetics, Genomics and Control of Rice Blast Disease, eds Wang G. L., Valent B. (Berlin: Springer; ), 315–324. [Google Scholar]
- Fukuoka S., Okuno K. (2001). QTL analysis and mapping of pi21, a recessive gene for field resistance to rice blast in Japanese upland rice. Theor. Appl. Genet. 103 185–190. 10.1007/s001220100611 [DOI] [Google Scholar]
- Fukuoka S., Saka N., Mizukami Y., Koga H., Yamanouchi U., Yoshioka Y., et al. (2015). Gene pyramiding enhances durable blast disease resistance in rice. Sci. Rep. 5:7773 10.1038/srep07773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X. S., Yang M. Z., Huang X. Q., Cheng Z. Q., Fu J., Sun T., et al. (2008). Cloning and analyzing of rice blast resistance gene Pi-ta+ allele from Jinghong erect type of common wild rice (Oryza rufipogon Griff) in Yunnan. Yi Chuan 30 109–114. 10.3724/SP.J.1005.2008.00109 [DOI] [PubMed] [Google Scholar]
- Gouda P. K., Saikumar S., Varma C. M., Nagesh K., Thippeswamy S., Shenoy V., et al. (2013). Marker-assisted breeding of Pi-1 and Piz-5 genes imparting resistance to rice blast in PRR78, restorer line of Pusa RH-10 Basmati rice hybrid. Plant Breed. 132 61–69. 10.1111/pbr.12017 [DOI] [Google Scholar]
- Grandillo S., Tanksley S. (1996). QTL analysis of horticultural traits differentiating the cultivated tomato from the closely related species Lycopersicon pimpinellifolium. Theor. Appl. Genet. 92 935–951. 10.1007/BF00224033 [DOI] [PubMed] [Google Scholar]
- Harushima Y., Yano M., Shomura A., Sato M., Shimano T., Kuboki Y., et al. (1998). A high-density rice genetic linkage map with 2275 markers using a single F2 population. Genetics 148 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M. M., Rafii M. Y., Ismail M. R., Mahmood M., Rahim H. A., Alam M. A., et al. (2015). Marker-assisted backcrossing: a useful method for rice improvement. Biotechnol. Biotechnol. Equip. 29 237–254. 10.1080/13102818.2014.995920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell E. E., Wang Q., Yang Y. (2013). Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol. J. 11 33–42. 10.1111/pbi.12004 [DOI] [PubMed] [Google Scholar]
- Helliwell E. E., Yang Y. (2013). Molecular strategies to improve rice disease resistance. Methods Mol. Biol. 956 285–309. 10.1007/978-1-62703-194-3_21 [DOI] [PubMed] [Google Scholar]
- Hittalmani S., Parco A., Mew T., Zeigler R., Huang N. (2000). Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theor. Appl. Genet. 100 1121–1128. 10.1007/s001220051395 [DOI] [Google Scholar]
- Hittalmani S., Shashidhar H., Bagali P. G., Huang N., Sidhu J., Singh V., et al. (2002). Molecular mapping of quantitative trait loci for plant growth, yield and yield related traits across three diverse locations in a doubled haploid rice population. Euphytica 125 207–214. 10.1023/A:1015890125247 [DOI] [Google Scholar]
- Hospital F. (2001). Size of donor chromosome segments around introgressed loci and reduction of linkage drag in marker-assisted backcross programs. Genetics 158 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-L., Hwang S.-Y., Chiang Y.-C., Lin T.-P. (2008). Molecular evolution of the Pi-ta gene resistant to rice blast in wild rice (Oryza rufipogon). Genetics 179 1527–1538. 10.1534/genetics.108.089805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena K. K., Khush G. S. (2000). “Exploitation of species in rice improvement-opportunities, achievements and future challenges,” in Rice Breeding and Genetic Research Priorities and Challenges, ed. Nanda J. S. (Enfield: Science Publication; ), 269–284. [Google Scholar]
- Jiang H., Feng Y., Bao L., Li X., Gao G., Zhang Q., et al. (2012). Improving blast resistance of Jin 23B and its hybrid rice by marker-assisted gene pyramiding. Mol. Breed. 30 1679–1688. 10.1007/s11032-012-9751-6 [DOI] [Google Scholar]
- Jiang J., Yang D., Ali J., Mou T. (2015). Molecular marker-assisted pyramiding of broad-spectrum disease resistance genes, Pi2 and Xa23, into GZ63-4S, an elite thermo-sensitive genic male-sterile line in rice. Mol. Breed. 35 1–12. 10.1007/s11032-015-0282-9 [DOI] [Google Scholar]
- Joshi R. K., Nayak S. (2010). Gene pyramiding-A broad spectrum technique for developing durable stress resistance in crops. Biotechnol. Mol. Biol. Rev. 5 51–60. [Google Scholar]
- Kaundal R., Kapoor A. S., Raghava G. P. (2006). Machine learning techniques in disease forecasting: a case study on rice blast prediction. BMC Bioinformatics 7:485 10.1186/1471-2105-7-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush G. S. (2005). What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 59 1–6. 10.1007/s11103-005-2159-5 [DOI] [PubMed] [Google Scholar]
- Khush G. S., Jena K. K. (2009). “Current status and future prospects for research on blast resistance in rice (Oryza sativa L.),” in Advances in Genetics, Genomics and Control of Rice Blast Disease, eds Wang G. L., Valent B. (Berlin: Springer; ), 1–10. [Google Scholar]
- Koide Y., Kawasaki A., Telebancoâaaa-Yanoria M., Hairmansis A., Nguyet N., Bigirimana J., et al. (2010). Development of pyramided lines with two resistance genes, Pish and Pib, for blast disease (Magnaporthe oryzae B. Couch) in rice (Oryza sativa L.). Plant Breed. 129 670–675. 10.1111/j.1439-0523.2010.01781.x [DOI] [Google Scholar]
- Koide Y., Kobayashi N., Xu D., Fukuta Y. (2009). Resistance genes and selection DNA markers for blast disease in rice (Oryza sativa L.). Japan Agric. Res. Q. 43 255–280. 10.1016/j.crvi.2014.11.003 [DOI] [Google Scholar]
- Kumar P., Gupta V., Misra A., Modi D., Pandey B. (2009). Potential of molecular markers in plant biotechnology. Plant Omics J. 2 141–162. [Google Scholar]
- Kumari A., Das A., Devanna B., Thakur S., Singh P., Singh N., et al. (2013). Mining of rice blast resistance gene Pi54 shows effect of single nucleotide polymorphisms on phenotypic expression of the alleles. Eur. J. Plant Pathol. 137 55–65. 10.1007/s10658-013-0216-5 [DOI] [Google Scholar]
- Lang N., Buu B. C., Ismail A. (2008). Molecular mapping and marker-assisted selection for major-gene traits in rice (Oryza sativa L.). Omonrice 16 50–56. [Google Scholar]
- Lee S., Costanzo S., Jia Y., Olsen K. M., Caicedo A. L. (2009). Evolutionary dynamics of the genomic region around the blast resistance gene Pi-ta in AA genome Oryza species. Genetics 183 1315–1325. 10.1534/genetics.109.108266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Jia Y., Jia M., Gealy D. R., Olsen K. M., Caicedo A. L. (2011). Molecular evolution of the rice blast resistance gene Pi-ta in invasive weedy rice in the USA. PLoS ONE 6:e26260 10.1371/journal.pone.0026260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestari P., Trijatmiko K. R., Warsun A., Ona I., Cruz C. V., Bustamam M. (2011). Mapping quantitative trait loci conferring blast resistance in upland indica rice (Oryza sativa L.). J. Crop Sci. Biotechnol. 14 57–63. 10.1007/s12892-010-0030-y [DOI] [Google Scholar]
- Liu B., Zhang S., Zhu X., Yang Q., Wu S., Mei M., et al. (2004). Candidate defense genes as predictors of quantitative blast resistance in rice. Mol. Plant Microbe Inter. 17 1146–1152. 10.1094/MPMI.2004.17.10.1146 [DOI] [PubMed] [Google Scholar]
- Liu G., Lu G., Zeng L., Wang G.-L. (2002a). Two broad-spectrum blast resistance genes, Pi9 (t) and Pi2 (t), are physically linked on rice chromosome 6. Mol. Genet. Genomics 267 472–480. 10.1007/s00438-002-0677-2 [DOI] [PubMed] [Google Scholar]
- Liu S., Li X., Wang C., Li X., He Y. (2002b). Improvement of resistance to rice blast in Zhenshan 97 by molecular marker-aided selection. Acta Bot. Sinica 45 1346–1350. [Google Scholar]
- Liu Y., Zhu X. Y., Zhang S., Bernardo M., Edwards J., Galbraith D. W., et al. (2011). Dissecting quantitative resistance against blast disease using heterogeneous inbred family lines in rice. Theor. Appl. Genet. 122 341–353. 10.1007/s00122-010-1450-2 [DOI] [PubMed] [Google Scholar]
- Loan L., Du P., Li Z. (2003). Identification of genes conferring resistance to some Philippine and Vietnamese races of blast. Omonrice 11 49–62. [Google Scholar]
- Lopez-Gerena J. (2006). Mapping QTL Controlling Durable Resistance to Rice Blast in the Cultivar Oryzica llanos 5 Ph.D. thesis, Kansas State University, Manhattan, KS. [Google Scholar]
- Lv Q., Xu X., Shang J., Jiang G., Pang Z., Zhou Z., et al. (2013). Functional analysis of Pid3-A4, an ortholog of rice blast resistance gene Pid3 revealed by allele mining in common wild rice. Phytopathology 103 594–599. 10.1094/PHYTO-10-12-0260-R [DOI] [PubMed] [Google Scholar]
- Mahdian S., Shahsavari A. (2013). Pyramiding of blast resistance genes Pi-1 and Pi-2 in tarom mahalli rice cultivar. Seed Plant Improv. J. 29 391–395. [Google Scholar]
- McCouch S., Temnykh S., Lukashova A., Coburn J., Declerck G., Cartinhour S., et al. (2001). “Microsatellite markers in rice: abundance, diversity, and applications,” in Rice genetics IV, eds Khush G. S., Brar D. S., Hardy B. (Willingford: CAB International; ), 117–135. [Google Scholar]
- McCouch S. R., Teytelman L., Xu Y., Lobos K. B., Clare K., Walton M., et al. (2002). Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9 199–207. 10.1093/dnares/9.6.199 [DOI] [PubMed] [Google Scholar]
- McCouch S. R., Sweeney M., Li J., Jiang H., Thomson M., Septiningsih E., et al. (2007). Through the genetic bottleneck: O. rufipogon as a source of trait-enhancing alleles for O. sativa. Euphytica 154 317–339. 10.1007/s10681-006-9210-8 [DOI] [Google Scholar]
- Miyamoto M., Yano M., Hirasawa H. (2001). Mapping of quantitative trait loci conferring blast field resistance in the japanese upland rice variety kahei. Breed. Sci. 51 257–261. 10.1270/jsbbs.51.257 [DOI] [Google Scholar]
- Narayanan N., Baisakh N., Cruz V., Gnanamanickam S., Datta K., Datta S. (2002). Molecular breeding for the development of blast and bacterial blight resistance in rice cv. IR50. Crop Sci. 42 2072–2079. 10.2135/cropsci2002.2072 [DOI] [Google Scholar]
- Nguyen T., Koizumi S., La T., Zenbayashi K., Ashizawa T., Yasuda N., et al. (2006). Pi35 (t), a new gene conferring partial resistance to leaf blast in the rice cultivar Hokkai 188. Theor. Appl. Genet. 113 697–704. 10.1007/s00122-006-0337-8 [DOI] [PubMed] [Google Scholar]
- Noenplab A., Vanavichit A., Toojinda T., Sirithunya P., Tragoonrung S., Sriprakhon S., et al. (2006). QTL Mapping for leaf and neck blast resistance in Khao Dawk Mali105 and Jao Hom Nin recombinant inbred lines. Sci. Asia 32 133–142. 10.2306/scienceasia1513-1874.2006.32.133 [DOI] [Google Scholar]
- Ou S. H. (1985). Blast. Rice Diseases. Wallingford: CAB International. [Google Scholar]
- Paterson A. H., Tanksley S. D., Sorrells M. E. (1991). DNA markers in plant improvement. Adv. Agron. 44 39–90. 10.1016/S0065-2113(08)60578-7 [DOI] [Google Scholar]
- Perez-de-Castro A. M., Vilanova S., Canizares J., Pascual L., Blanca J. M., Diez M. J., et al. (2012). Application of genomic tools in plant breeding. Curr. Genomics 13 179 10.2174/138920212800543084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinta W., Toojinda T., Thummabenjapone P., Sanitchon J. (2013). Pyramiding of blast and bacterial leaf blight resistance genes into rice cultivar RD6 using marker assisted selection. Afr. J. Biotechnol. 12 4432–4438. 10.5897/AJB12.2028 [DOI] [Google Scholar]
- Price A., Steele K., Moore B., Barraclough P., Clark L. (2000). A combined RFLP and AFLP linkage map of upland rice (Oryza sativa L.) used to identify QTLs for root-penetration ability. Theor. Appl. Genet. 100 49–56. 10.1007/s001220050007 [DOI] [Google Scholar]
- Rahim H., Bhuiyan M., Lim L., Sabu K., Saad A., Azhar M., et al. (2012). Identification of quantitative trait loci for blast resistance in BC. Genet. Mol. Res. 11 3277–3289. 10.4238/2012.September.12.11 [DOI] [PubMed] [Google Scholar]
- Rahman L., Khanam S., Jaehwan R. (2011). Mapping of QTLs involved in resistence to rice blast (Magnaporthe grisea) using Oryza minuta introgression lines. Czech. J. Genet. Plant Breed. 47 85–94. [Google Scholar]
- Rai A. K., Kumar S. P., Gupta S. K., Gautam N., Singh N. K., Sharma T. R. (2011). Functional complementation of rice blast resistance gene Pi-kh (Pi54) conferring resistance to diverse strains of Magnaporthe oryzae. J. Plant Biochem. Biotechnol. 20 55–65. 10.1007/s13562-010-0026-1 [DOI] [Google Scholar]
- Ramkumar G., Biswal A. K., Mohan K. M., Sakthivel K., Sivaranjani A. K. P., Neeraja C. M., et al. (2010). Rani NS, Viraktamath, B.C., and, Madhav, M.S. (2010b). Identifying novel alleles of rice blast resistance genes Pikh and Pita through allele mining. Int. Rice Res. Notes 35 1–6. [Google Scholar]
- Ramkumar G., Srinivasarao K., Mohan K. M., Sudarshan I., Sivaranjani A., Gopalakrishna K., et al. (2011). Development and validation of functional marker targeting an InDel in the major rice blast disease resistance gene Pi54 (Pikh). Mol. Breed. 27 129–135. 10.1007/s11032-010-9538-6 [DOI] [Google Scholar]
- Sabouri H., Sabouri A., Jafarzadeh M. R., Mollashahi M. (2011). Detection of QTLs controlling field blast resistance in rice (Oryza sative L.). Plant Omics J. 4 1–5. [Google Scholar]
- Sahebi M., Hanafi M. M., Azizi P., Hakim A., Ashkani S., Abiri R. (2015). Suppression subtractive hybridization versus next-generation sequencing in plant genetic engineering: challenges and perspectives. Mol. Biotechnol. 57 880–903. 10.1007/s12033-015-9884-z [DOI] [PubMed] [Google Scholar]
- Sallaud C., Lorieux M., Roumen E., Tharreau D., Berruyer R., Svestasrani P., et al. (2003). Identification of five new blast resistance genes in the highly blast-resistant rice variety IR64 using a QTL mapping strategy. Theor. Appl. Genet. 106 794–803. [DOI] [PubMed] [Google Scholar]
- Sato H., Takeuchi Y., Hirabayashi H., Nemoto H., Hirayama M., Kato H., et al. (2006). Mapping QTLs for field resistance to rice blast in the Japanese upland rice variety Norin 12. Breed. Sci. 56 415–418. 10.1270/jsbbs.56.415 [DOI] [Google Scholar]
- Semagn K., Bjørnstad Å, Ndjiondjop M. (2006). An overview of molecular marker methods for plants. Afr. J. Biotechnol. 5 2540–2568. [Google Scholar]
- Shang J., Tao Y., Chen X., Zou Y., Lei C., Wang J., et al. (2009). Identification of a new rice blast resistance gene, Pid3, by genomewide comparison of paired nucleotide-binding site-leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 182 1303–1311. 10.1534/genetics.109.102871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T. R., Madhav M. S., Singh B. K., Shanker P., Jana T., Dalal V., et al. (2005). High-resolution mapping, cloning and molecular characterization of the Pi-k h gene of rice, which confers resistance to Magnaporthe grisea. Mol. Genet. Genom. 274 569–578. 10.1007/s00438-005-0035-2 [DOI] [PubMed] [Google Scholar]
- Sharma T., Rai A., Gupta S., Vijayan J., Devanna B., Ray S. (2012). Rice blast management through host-plant resistance: retrospect and prospects. Agric. Res. 1 37–52. 10.1007/s40003-011-0003-5 [DOI] [Google Scholar]
- Sharma T. R., Rai A. K., Gupta S. K., Singh N. K. (2010). Broad-spectrum blast resistance gene Pi-kh cloned from rice line Tetep designated Pi54. J. Plant Biochem. Biotechnol. 191 87–89. 10.1007/BF03323441 [DOI] [Google Scholar]
- Shinoda H., Toriyama K., Yunoki T., Ezuka A., Sakurai Y. (1971). Studies on the varietal resistance of rice to blast. 6. Linkage relationship of blast resistance genes. Bull. Chugoku Agric. Exp. Stn. Set. A 20 1–25. [Google Scholar]
- Shivayogi S., Vaishali M., Shashidhar H., Kumar K. G. (2002). Genetic analysis of rice blast fungus of southern Karnataka using DNA markers and reaction of popular rice genotypes. Curr. Sci. 82 732–735. [Google Scholar]
- Singh A., Singh V. K., Singh S., Pandian R., Ellur R. K., Singh D., et al. (2012a). Molecular breeding for the development of multiple disease resistance in Basmati rice. AoB Plants 2012:pls029 10.1093/aobpla/pls029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K., Singh A., Singh S., Ellur R. K., Choudhary V., Sarkel S., et al. (2012b). Incorporation of blast resistance into “PRR78”, an elite Basmati rice restorer line, through marker assisted backcross breeding. Field Crops Res. 128 8–16. 10.1016/j.fcr.2011.12.003 [DOI] [Google Scholar]
- Singh V. K., Singh A., Singh S., Ellur R. K., Singh D., Gopala Krishnan S., et al. (2013). Marker-assisted simultaneous but stepwise backcross breeding for pyramiding blast resistance genes Piz5 and Pi54 into an elite Basmati rice restorer line ‘PRR78’. Plant Breed. 132 486–495. [Google Scholar]
- Sirithunya P., Tragoonrung S., Vanavichit A., Pa-in N., Vongsaprom C., Toojinda T. (2002). Quantitative trait loci associated with leaf and neck blast resistance in recombinant inbred line population of rice (Oryza sativa). DNA Res. 9 79–88. 10.1093/dnares/9.3.79 [DOI] [PubMed] [Google Scholar]
- Skamnioti P., Gurr S. J. (2009). Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol. 27 141–150. 10.1016/j.tibtech.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Song F., Goodman R. M. (2001). Molecular biology of disease resistance in rice. Physiol. Mol. Plant Pathol. 59 1–11. 10.1006/pmpp.2001.0353 [DOI] [Google Scholar]
- Sreewongchai T., Toojinda T., Thanintorn N., Kosawang C., Vanavichit A., Tharreau D., et al. (2010). Development of elite indica rice lines with wide spectrum of resistance to Thai blast isolates by pyramiding multiple resistance QTLs. Plant Breed. 129 176–180. 10.1111/j.1439-0523.2009.01669.x [DOI] [Google Scholar]
- Stuber C. W., Lincoln S. E., Wolff D., Helentjaris T., Lander E. (1992). Identification of genetic factors contributing to heterosis in a hybrid from two elite maize inbred lines using molecular markers. Genetics 132 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram R. M., Vishnupriya M., Laha G. S., Rani N. S., Rao P. S., Balachandran S. M., et al. (2009). Introduction of bacterial blight resistance into Triguna, a high yielding, mid-early duration rice variety. Biotechnol. J. 4 400–407. 10.1002/biot.200800310 [DOI] [PubMed] [Google Scholar]
- Tabien R., Li Z., Paterson A., Marchetti M., Stansel J., Pinson S. (2002). Mapping QTLs for field resistance to the rice blast pathogen and evaluating their individual and combined utility in improved varieties. Theor. Appl. Genet. 105 313–324. 10.1007/s00122-002-0940-2 [DOI] [PubMed] [Google Scholar]
- Talukder Z. I., Mcdonald A. J. S., Price A. H. (2005). Loci controlling partial resistance to rice blast do not show marked QTL × environment interaction when plant nitrogen status alters disease severity. New Phytol. 168 455–464. 10.1111/j.1469-8137.2005.01507.x [DOI] [PubMed] [Google Scholar]
- Talukder Z. I., Tharreau D., Price A. H. (2004). Quantitative trait loci analysis suggests that partial resistance to rice blast is mostly determined by race-specific interactions. New Phytol. 162 197–209. 10.1111/j.1469-8137.2004.01010.x [DOI] [Google Scholar]
- Tanksley S. (1993). QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics 134 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley S. D. (1988). Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 335 721–726. 10.1038/335721a0 [DOI] [PubMed] [Google Scholar]
- Tanksley S., Grandillo S., Fulton T., Zamir D., Eshed Y., Petiard V., et al. (1996). Advanced backcross QTL analysis in a cross between an elite processing line of tomato and its wild relative L. pimpinellifolium. Theor. Appl. Genet. 92 213–224. 10.1007/BF00223378 [DOI] [PubMed] [Google Scholar]
- Tanweer F. A., Rafii M. Y., Sijam K., Rahim H. A., Ahmed F., Latif M. A. (2015). Current advance methods for the identification of blast resistance genes in rice. C. R. Biol. 338 321–334. 10.1016/j.crvi.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Temnykh S., Declerck G., Lukashova A., Lipovich L., Cartinhour S., Mccouch S. (2001). Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 11 1441–1452. 10.1101/gr.184001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S., Gupta Y., Singh P., Rathour R., Variar M., Prashanthi S., et al. (2013a). Molecular diversity in rice blast resistance gene Pi-ta makes it highly effective against dynamic population of Magnaporthe oryzae. Funct. Integr. Genom. 13 309–322. 10.1007/s10142-013-0325-4 [DOI] [PubMed] [Google Scholar]
- Thakur S., Singh P., Rathour R., Variar M., Prashanthi S., Singh A., et al. (2013b). Positive selection pressure on rice blast resistance allele Piz-t makes it divergent in Indian land races. J. Plant Inter. 8 34–44. [Google Scholar]
- Toojinda T., Tragoonrung S., Vanavichit A., Siangliw J. L., Pa-in N., Jantaboon J., et al. (2005). Molecular breeding for rainfed lowland rice in the Mekong region. Plant Prod. Sci. 8 330–333. 10.1626/pps.8.330 [DOI] [Google Scholar]
- Urso S., Orasen G., Perrini R., Tacconi G., Delfanti S., Biselli C., et al. (2013). “Pyramiding of Pi resistance genes to increase blast resistance in Italian rice varieties using marker-assisted selection approaches,” in Proceedings of the 57th Italian Society of Agricultural Genetics Annual Congress, 16th-19th September, Foggia. [Google Scholar]
- Utani D. W., Moeljopawiro S., Aswidinnoor H., Setiawan A., Hanarida I. (2008). Blast resistance genes in wild rice Oryza rufipogon and rice cultivar IR64 Indonesian. J. Agric. 1 71–76. [Google Scholar]
- Wan X. Y., Wan J. M., Jiang L., Wang J. K., Zhai H. Q., Weng J. F., et al. (2006). QTL analysis for rice grain length and fine mapping of an identified QTL with stable and major effects. Theor. Appl. Genet. 112 1258–1270. 10.1007/s00122-006-0227-0 [DOI] [PubMed] [Google Scholar]
- Wang D., Guo C., Huang J., Yang S., Tian D., Zhang X. (2014). Allele-mining of rice blast resistance genes at AC134922 locus. Biochem. Biophys. Res. Commun. 446 1085–1090. 10.1016/j.bbrc.2014.03.056 [DOI] [PubMed] [Google Scholar]
- Wang G.-L., Mackill D. J., Bonman J. M., Mccouch S. R., Champoux M. C., Nelson R. J. (1994). RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar. Genetics 136 1421–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Jia Y., Rutger J., Xia Y. (2007). Rapid survey for presence of a blast resistance gene Pi-ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-ta gene. Plant Breed. 126 36–42. 10.1111/j.1439-0523.2007.01304.x [DOI] [Google Scholar]
- Wen S., Gao B. (2011). Introgressing blast resistant gene Pi-9 (t) into elite rice restorer Luhui17 by marker-assisted selection. Rice Genom. Genet. 2 10.5376/rgg.2011.02.0004 [DOI] [Google Scholar]
- Wu J.-L., Fan Y.-Y., Li D.-B., Zheng K.-L., Leung H., Zhuang J.-Y. (2005). Genetic control of rice blast resistance in the durably resistant cultivar Gumei 2 against multiple isolates. Theor. Appl. Genet. 111 50–56. 10.1007/s00122-005-1971-2 [DOI] [PubMed] [Google Scholar]
- Xu J., Wang J., Ling Z., Zhu L. (2004). Analysis of rice blast resistance genes by QTL mapping. Chinese Sci. Bull. 49 337–342. 10.1007/BF02900315 [DOI] [Google Scholar]
- Xu X., Lv Q., Shang J., Pang Z., Zhou Z., Wang J., et al. (2014). Excavation of Pid3 orthologs with differential resistance spectra to Magnaporthe oryzae in rice resource. PLoS ONE 9:e93275 10.1371/journal.pone.0093275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. (2002). “Global View of QTL: rice as a model,” in Quantitative Genetics, Genomics, and Plant Breeding, ed. Kang M. S. (Willingford: CAB International; ), 109–134. [Google Scholar]
- Yang M.-Z., Cheng Z.-Q., Chen S.-N., Qian J., Xu L.-L., Huang X.-Q. (2007). A rice blast-resistance genetic resource from wild rice in Yunnan. China. J. Plant Physiol. Mol. Biol. 33 589–595. [PubMed] [Google Scholar]
- Yasuda N., Mitsunaga T., Hayashi K., Koizumi S., Fujita Y. (2014). Effects of pyramiding quantitative resistance genes pi21, Pi34, and Pi35 on rice leaf blast disease. Plant Dis. 99 904–909. 10.1094/PDIS-02-14-0214-RE [DOI] [PubMed] [Google Scholar]
- Yoshida K., Miyashita N. T. (2009). DNA polymorphism in the blast disease resistance gene Pita of the wild rice Oryza rufipogon and its related species. Genes Genet. Syst. 84 121–136. 10.1266/ggs.84.121 [DOI] [PubMed] [Google Scholar]
- Young N. D. (1994). “Constructing a plant genetic linkage map with DNA markers,” in DNA-Based Markers in Plants, eds Ronald I. K. V., Phillips L. (Dordrecht: Kluwer; ), 39–57. [Google Scholar]
- Young N. D. (1996). QTL mapping and quantitative disease resistance in plants. Annu. Rev. Phytopathol. 34 479–501. 10.1146/annurev.phyto.34.1.479 [DOI] [PubMed] [Google Scholar]
- Yu J., Hu S., Wang J., Wong G. K.-S., Li S., Liu B., et al. (2002). A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296 79–92. 10.1126/science.1068037 [DOI] [PubMed] [Google Scholar]
- Zeigler R. S., Leong S. A., Teng P. S. (1994). Rice Blast Disease. Wallingford: CAB International. [Google Scholar]
- Zenbayashi K., Ashizawa T., Tani T., Koizumi S. (2002). Mapping of the QTL (quantitative trait locus) conferring partial resistance to leaf blast in rice cultivar Chubu 32. Theor. Appl. Genet. 104 547–552. 10.1007/s00122-001-0779-y [DOI] [PubMed] [Google Scholar]
- Zhang H., Li G., Li W., Song F. (2009). Transgenic strategies for improving rice disease resistance. Afri. J. Biotechnol. 8 1750–1757. 10.1007/978-1-62703-194-3_21 [DOI] [Google Scholar]
- Zhang Y., Yang J., Shan Z., Chen S., Qiao W., Zhu X., et al. (2012). Substitution mapping of QTLs for blast resistance with SSSLs in rice (Oryza sativa L.). Euphytica 184 141–150. 10.1007/s10681-011-0601-0 [DOI] [Google Scholar]
- Zhu X., Chen S., Yang J., Zhou S., Zeng L., Han J., et al. (2012). The identification of Pi50 (t), a new member of the rice blast resistance Pi2/Pi9 multigene family. Theor. Appl. Genet. 124 1295–1304. 10.1007/s00122-012-1787-9 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Chen H., Fan J., Wang Y., Li Y., Chen J., et al. (2000). Genetic diversity and disease control in rice. Nature 406 718–722. 10.1038/35021046 [DOI] [PubMed] [Google Scholar]