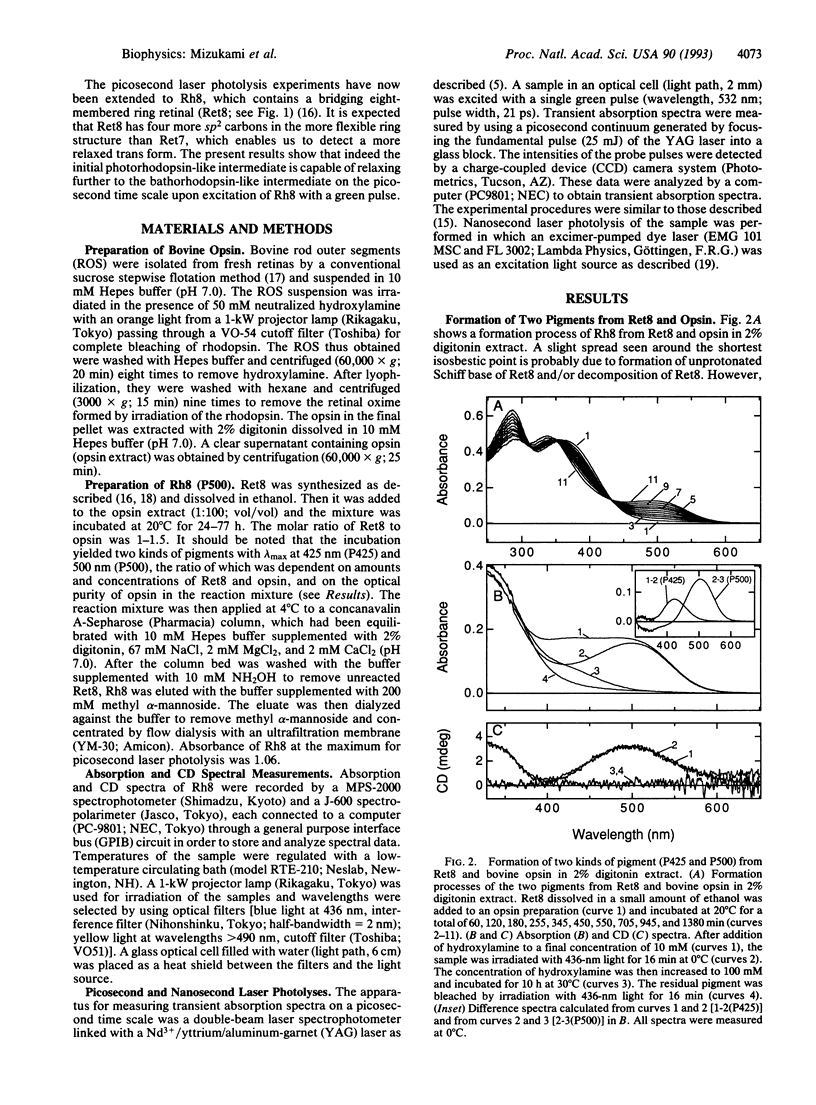
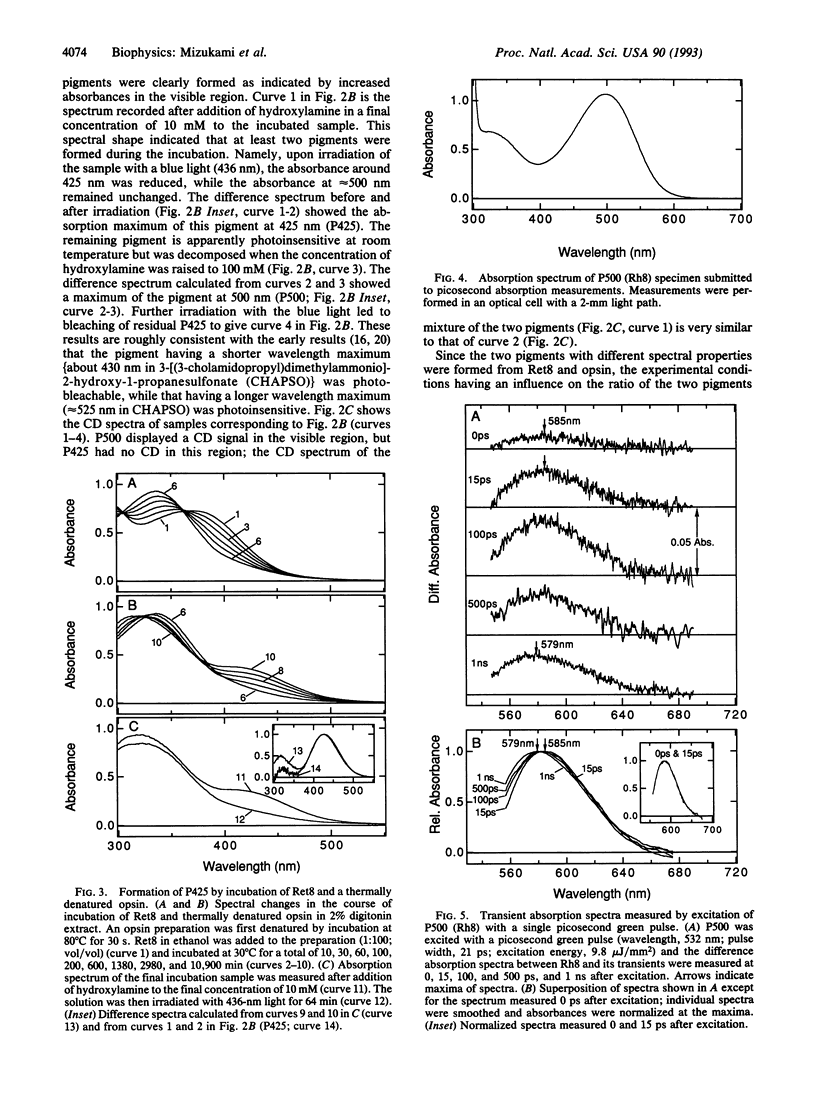
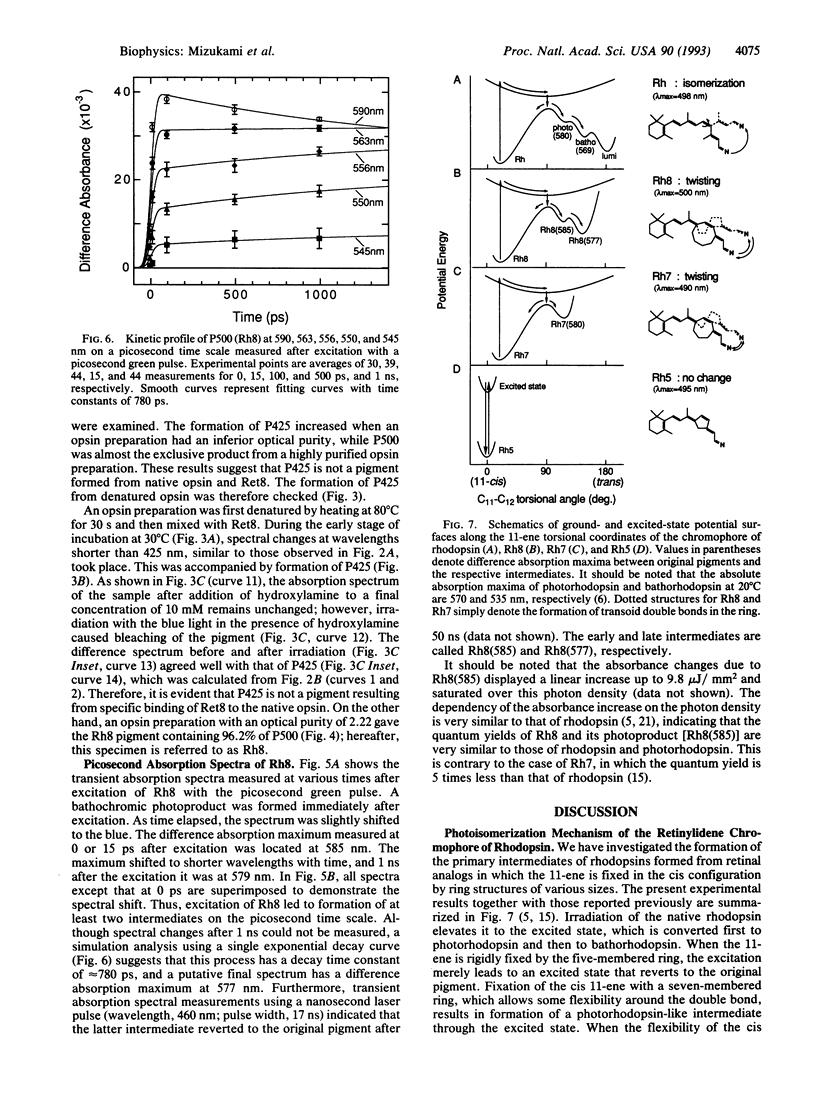
Abstract
The primary photochemical event in rhodopsin is an 11-cis to 11-trans photoisomerization of its retinylidene chromophore to form the primary intermediate photorhodopsin. Earlier picosecond studies have shown that no intermediate is formed when the retinal 11-ene is fixed through a bridging five-membered ring, whereas a photorhodopsin-like intermediate is formed when it is fixed through a flexible seven-membered ring. Results from a rhodopsin analog formed from a retinal with locked 11-ene structure through the more flexible eight-membered ring (Ret8) are described. Incubation of bovine opsin with Ret8 formed two pigments absorbing at 425 nm (P425) and 500 nm (P500). P425, however, is an artifact because it formed from thermally denatured opsin or other proteins and Ret8. Excitation of P500 with a picosecond green pulse led to formation of two intermediates corresponding to photo- and bathorhodopsins. These results demonstrate that an appearance of early intermediates is dependent on the flexibility of the 11-ene and that the photoisomerization of P500 proceeds by stepwise changes of chromophore-protein interaction, which in turn leads to a relaxation of the highly twisted all-trans-retinylidene chromophore in photorhodopsin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K. A., Balogh-Nair V., Croteau A. A., Dollinger G., Ebrey T. G., Eisenstein L., Hong M. K., Nakanishi K., Vittitow J. Fourier-transform infrared difference spectroscopy of rhodopsin and its photoproducts at low temperature. Biochemistry. 1985 Oct 22;24(22):6055–6071. doi: 10.1021/bi00343a006. [DOI] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Broek A., Lugtenburg J., Mathies R. Assignment and interpretation of hydrogen out-of-plane vibrations in the resonance Raman spectra of rhodopsin and bathorhodopsin. Biochemistry. 1982 Jan 19;21(2):384–393. doi: 10.1021/bi00531a028. [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Tokunaga F., Yoshizawa T. Circular dichroism of cattle rhodopsin and bathorhodopsin at liquid nitrogen temperatures. Biochim Biophys Acta. 1980 Jul 8;591(2):445–457. doi: 10.1016/0005-2728(80)90175-9. [DOI] [PubMed] [Google Scholar]
- Kandori H., Matuoka S., Shichida Y., Yoshizawa T. Dependency of photon density on primary process of cattle rhodopsin. Photochem Photobiol. 1989 Feb;49(2):181–184. doi: 10.1111/j.1751-1097.1989.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Kandori H., Matuoka S., Shichida Y., Yoshizawa T., Ito M., Tsukida K., Balogh-Nair V., Nakanishi K. Mechanism of isomerization of rhodopsin studied by use of 11-cis-locked rhodopsin analogues excited with a picosecond laser pulse. Biochemistry. 1989 Jul 25;28(15):6460–6467. doi: 10.1021/bi00441a045. [DOI] [PubMed] [Google Scholar]
- Kandori H., Shichida Y., Yoshizawa T. Absolute absorption spectra of batho- and photorhodopsins at room temperature. Picosecond laser photolysis of rhodopsin in polyacrylamide. Biophys J. 1989 Sep;56(3):453–457. doi: 10.1016/S0006-3495(89)82692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Yoshizawa T. Existence of a beta-ionone ring-binding site in the rhodopsin molecule. Nature. 1975 Dec 11;258(5535):523–526. doi: 10.1038/258523a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Yoshizawa T. Recognition of opsin to the longitudinal length of retinal isomers in the formation of rhodopsin. Vision Res. 1978;18(5):607–609. doi: 10.1016/0042-6989(78)90212-2. [DOI] [PubMed] [Google Scholar]
- Okada T., Kandori H., Shichida Y., Yoshizawa T., Denny M., Zhang B. W., Asato A. E., Liu R. S. Spectroscopic study of the batho-to-lumi transition during the photobleaching of rhodopsin using ring-modified retinal analogues. Biochemistry. 1991 May 14;30(19):4796–4802. doi: 10.1021/bi00233a022. [DOI] [PubMed] [Google Scholar]
- Schoenlein R. W., Peteanu L. A., Mathies R. A., Shank C. V. The first step in vision: femtosecond isomerization of rhodopsin. Science. 1991 Oct 18;254(5030):412–415. doi: 10.1126/science.1925597. [DOI] [PubMed] [Google Scholar]
- Shichida Y., Ono T., Yoshizawa T., Matsumoto H., Asato A. E., Zingoni J. P., Liu R. S. Electrostatic interaction between retinylidene chromophore and opsin in rhodopsin studied by fluorinated rhodopsin analogues. Biochemistry. 1987 Jul 14;26(14):4422–4428. doi: 10.1021/bi00388a035. [DOI] [PubMed] [Google Scholar]
- Shichida Y., Tokunaga F., Yoshizawa T. Circular dichroism of squid rhodopsin and its intermediates. Biochim Biophys Acta. 1978 Dec 7;504(3):413–430. doi: 10.1016/0005-2728(78)90064-6. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., KITO Y. Chemistry of the rhodopsin cycle. Nature. 1958 Dec 6;182(4649):1604–1605. doi: 10.1038/1821604a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]



