Abstract
Immunosuppressive agents, such as thiopurines, methotrexate, and biologics, have revolutionized the treatment of inflammatory bowel disease (IBD). However, a number of case reports, case control studies and retrospective studies over the last decade have identified a concerning link between immunosuppression and lymphoproliferative disorders (LPDs), the oncological phenomenon whereby lymphocytes divide uncontrollably. These LPDs have been associated with Epstein-Barr virus (EBV) infection in which the virus provides the impetus for malignant transformation while immunosuppression hampers the immune system’s ability to detect and clear these malignant cells. As such, the use of immunosuppressive agents may come at the cost of increased risk of developing LPD. While little is known about the LPD risk in IBD, more is known about immunosuppression in the post-transplantation setting and the development of EBV associated post-transplantation lymphoproliferative disorders (PTLD). In review of the PTLD literature, evidence is available to demonstrate that certain immune suppressants such as cyclosporine and T-lymphocyte modulators in particular are associated with an increased risk of PTLD development. As well, high doses of immunosuppressive agents and multiple immunosuppressive agent use are also linked to increased PTLD development. Here, we discuss these findings in context of IBD and what future studies can be taken to understand and reduce the risk of EBV-associated LPD development from immunosuppression use in IBD.
Keywords: Epstein-Barr virus, Immunosuppression, Post-transplantation lymphoproliferative disorders, Lymphoproliferative disorders, Inflammatory bowel disease
Core tip: Immunosuppressive agents, such as thiopurines, methotrexate, and biologics, have revolutionized the treatment and maintenance therapy of inflammatory bowel disease (IBD). However, their use may come at the cost of increased risk of developing lymphoproliferative disorders (LPD). While little is known about this risk in IBD, more is known about immunosuppression risk in the fields of rheumatoid arthritis and post-transplantation with regards to the development of Epstein-Barr virus (EBV) associated LPD. Here, we attempt to review lymphoma risk in the setting of immunosuppression use in various medical conditions, discuss what lessons may be translatable to the IBD field and what future directions can be taken to reduce the risk of EBV-associated LPD from immunosuppression use in IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a term that describes a collection of autoimmune gastrointestinal conditions, most notably Crohn’s disease (CD) and ulcerative colitis (UC). While UC is confined to the colon, CD can involve the entire digestive tract from mouth to anus. The pathogenesis of IBD is currently thought to be the result of a combination of host/genetic, environmental and microbial factors that perpetuate chronic and inappropriate inflammation of the gastrointestinal tract[1]. IBD has a bimodal age distribution with first diagnoses occurring between 15 to 40 years of age or 50 to 80 years of age[2]. In addition to age, a range of other risk factors have been linked to the development of IBD including gender, ethnicity, smoking, gut microbiome and medications[3]. One concerning consequence of IBD, and its treatment, is the increased incidence of lymphoproliferative diseases (LPD). LPDs include B- and T-cell lymphoma, the development of which can be the result of Epstein-Barr virus (EBV) mediated malignant transformation of normal B- and T- lymphocytes to divide uncontrollably. Other pathogens, such as other human T-cell lymphotropic virus-1, human herpesvirus-8, hepatitis B and C, human papilloma virus, Kaposi’s sarcoma-associated herpesvirus, Merkel cell polyomavirus and Helicobacter pylori have also been implicated in malignant transformation of the infected host[4,5]. LPD encompasses a diverse group of hematological malignancies that can either be acute or chronic in nature; either leukemic or lymphoid in morphology. One unique group of LPD includes the post-transplantation lymphoproliferative disorders (PTLD), which can develop due to both primary and secondary immunosuppression[6]. IBD itself, even independent of immunosuppressive treatment, is thought to be associated with either no or a slight increase in the risk of LPD development[7-10]. However, an increase in rates of LPD development in those with IBD who are on immunosuppressive therapy has been noted by different groups worldwide, documented in a variety of population-based, retrospective and case control studies[8,11,12]. Collectively, these studies point to the possibility that increased malignancy rates may be due the use of particular immunosuppressive therapies that inhibit normal host immunity and exposure to EBV, which in an immunosuppressed host, can infect host cells and result in malignant transformation. While limited data is available in the IBD population, there is a wealth of studies conducted on PTLD and rheumatoid arthritis patients. The development of PTLD primarily involves either reactivation of latent EBV infection or new EBV infection and as such, the development of PTLD is screened for in the most high-risk population (EBV negative recipient matched with EBV positive donor) by monitoring EBV viral load. In the rheumatoid arthritis population, the use of methotrexate is well described to confer a significant risk of lymphoma development. In this review, we first describe the well-established causal relationship between EBV infection and LPD development. Second, we explore the effect of immunosuppression, including biologics, in the post-transplantation and rheumatoid arthritis populations on EBV-associated LPD development. Third, we examine what is known currently about the risk of EBV-associated LPD development in patients treated with immune suppressants in IBD. Lastly, we discuss what can be translated from the post-transplantation literature to IBD to manage risks of EBV-associated LPD while on immune suppressants.
EBV CAUSES LPD
EBV is a double-stranded DNA virus belonging to the herpesvirus family that is ubiquitously found worldwide in roughly 90%-95% of adults[13]. The peak incidence by age is bimodal as roughly half of children under five years of age in developed countries acquire this relatively benign infection, often passing as a constellation of unremarkable upper respiratory tract infection symptom while the second peak of infections occurs in the 15 to 24 years old group[13]. EBV spreads via oral secretions and blood, capable of triggering B-lymphocyte and epithelial cell uptake[13]. Once intracellular, EBV initiates the lytic phase of infection, resulting in the lysis of the cellular host and subsequent release of viral progenies. In an immunocompetent host, cell-mediated immunity is activated as cytotoxic T lymphocytes (CTLs) target viral infected cells for apoptosis[13]. A proportion of EBV infected B-lymphocytes escape CTLs detection and continues on to become long-lived infected memory B-lymphocytes where the virus persists in the latent phase of its life cycle[13].
In latent phase, viral proteins are capable of initiating host malignant transformation in a subset of individuals, resulting in uncontrolled memory B-lymphocytes proliferation, or a LPD. A number of prospective and case-control studies worldwide have identified EBV infection as a risk factor to the development of LPDs such as Hodgkin lymphoma, Burkitt’s lymphoma, and a subset of aggressive non-Hodgkin lymphomas[14,15]. Hodgkin lymphoma has been the best studied and remains the lymphoma with the strongest association between EBV[16]. A causal relationship has been established in in vitro studies where EBV infection of human B-lymphocytes results in the uncontrolled proliferation of infected cells[17]. One study suggests that the rate of malignant transformation in EBV infected individuals occurs at a rate of 1:1000 over the span of four years from infection to Hodgkin lymphoma detection[14].
Certain risk factors have been associated with higher rates of LPDs. A case-control study from England revealed that the age of first infection is associated with a higher odds-ratio of developing Hodgkin’s lymphoma with the highest odds-ratio in the 16-24 years of age group[18]. Immune deficient patients have increased susceptibility to LPD development in part due to an inability to mount an EBV-specific immune response. Those with compromised immunity[19] or those receiving immunosuppressive therapies[20,21] have been found to have an altered humoral immunity against EBV. As such, increased rates of EBV-associated LPD have been documented in patients with human immune-deficiency virus[22] those with inherited immune-deficiencies[23] and in post-transplanted patients receiving immunosuppressive therapy[24].
EFFECT OF POST-TRANSPLANTATION IMMUNOSUPPRESSION ON EBV-ASSOCIATED LPD DEVELOPMENT
EBV is thought to be responsible for the majority of cases of PTLD, defined as uncontrolled lymphoid or plasma cell proliferation post solid organ or hematologic transplantation in the setting of immunosuppressive agents[25]. PTLDs include a range of subtypes. Early lesions, which include plasmacytic hyperplasia and infectious mononucleosis, and polymorphic PTLD typically involve EBV and occur within the first year post transplant. On the other hand, monomorphic PTLDs, which are histologically identical to B- or T-cell derived non-transplant malignant lymphomas, tend to occur late post transplant, involve EBV less often, and are clinically more aggressive. Hodgkin lymphoma type PTLD is the least common subtype[26-30]. Similar to the risk factors for development of LPDs in immunocompetent patients infected with EBV, studies of post-transplant patients revealed the key risk factors for developing PTLD include the degree of T-lymphocyte immunosuppression and the EBV serostatus[31,32]. The risk of PTLD in renal transplant patients is thought to be 6-20 times higher than the general population while those receiving heart transplants have an estimated 200 times higher risk due to the relatively intensive immunosuppression that thoracic transplant recipients receive[29]. A number of multi-national retrospective database review studies revealed the greatest yearly incidence rate was seen in the first year post transplantation with the number of new cases steadily declining over the five years of study[32,33], suggesting that the degree of immunosuppression, which typically is highest during the first year post-transplantation, may increase the risk for PTLD development[29]. Studies with longer follow-up, however, show a second peak in incidence at around 8 years post-transplant, suggesting that prolonged high doses of immunosuppression are also associated with increased rates of PTLD development[30,34].
Different immune-suppressive induction agents have been hypothesized to confer different risk for developing PTLD[29,35,36]. In addition, combination therapy, while most successful at preventing rejection, is associated with greater risk of PTLD development in one pediatric population[37]. Agents that suppress CTLs, such as belatacept and efalizumab[38-40], and T-lymphocytes in general, such as OKT3 and thymoglobulin[36,38], were suggested to have a greater role in inducing PTLD than those that mediate general immunosuppression. Given that viral infected cells are cleared by activated CTLs, agents that hamper CTLs is thought to be permissive for viral infection and later malignant transformation of the infected host. The rates of PTLD were found to increase dramatically as well with the initial use of cyclosporine[41,42]. Fortunately, by implementing drug-level monitoring and dose reduction, rates of PTLD have dropped since the early days of cyclosporine use. Certain agents, such as mycophenolate mofetil, have not been associated with any increased risk of PTLD[38,43].
In addition to the degree and type of immunosuppression, EBV seronegativity is an independent risk factor for the development of PTLD. The risk of PTLD is greater in EBV seronegative patients who become infected while immune suppressed than in seropositive recipients reactivating latent EBV infection post-transplantation[24]. Numerous studies have identified EBV seroconversion after either solid organ or hematological transplantation as a risk factor for PTLD development[37,44-48]. EBV naïve patients receiving immune suppressants were found to be at a higher risk of developing PTLD compared to EBV positive patients in one landmark University of Alberta retrospective study[49]. Since then, others in different centers have likewise identified EBV seronegativity in the pre-transplantation individual as a significant risk factor for developing PTLD post transplantation[44,45,50]. EBV seronegativity has a stronger impact on the risk of PTLD that occurs early (i.e., within 1 year) as opposed to late post transplant[30,34]. As such, EBV seronegative patients are subjected to monitoring during the first year post-transplantation to detect PTLD development (discussed in the “PTLD Prevention” section).
PTLD prevention
To address the increased risk of malignant transformation of PTLD in context of immunosuppression and EBV infection, some have recommended that routine monitoring of EBV viral load be undertaken in the post-transplant settings. Rising viral load raises the suspicion of PTLD development since a high EBV viral load has been documented in some studies to precede the development of EBV-mediated PTLD[51-55]. As such, the absolute viral load has been proposed as prognostic of PTLD development[47,53]. However, in part due to a number of technical challenges of the EBV viral load assay, including a lack of standardized reference ranges for instrument calibration across multiple assay platforms, the positive predictive value of this assay remains low as an elevated viral load has high sensitivity but lacks specificity for PTLD development[56-61]. Thus, the utility of EBV viral load monitoring in a seropositive patient remains highly controversial[62-64]. On the other hand, serial EBV viral load monitoring in the seronegative recipient is an effective tool to identify those at clear risk of developing PTLD[65,66]. By routine monitoring of EBV viral load in the seronegative recipient, pre-emptive interventions, such as anti-viral treatment and rituximab therapy, may be undertaken to prevent PTLD development when rising EBV viral load is detected[55,65,67-71].
EBV-ASSOCIATED LPD DEVELOPMENT IN RHEUMATOID ARTHRITIS PATIENTS TREATED WITH IMMUNE SUPPRESSANTS
Several large-scale population studies have demonstrated a mildly elevated risk of LPD and EBV-associated LPD in those with rheumatoid arthritis (RA) and an even higher risk in patients being actively immunosuppressed compared to the healthy population[72-74]. Mechanistically, patients with RA have been shown to have defective EBV-specific T cell function, resulting in a greater number of infected lymphocytes and as such are at a higher risk than the general population for development of LPD[75]. The addition of an immunosuppressive agent further elevates the risk of EBV-associated LPD, increasing the relative risk for LPD development from 2.5 (RA without immunosuppression) to 10 (RA with immunosuppression)[76]. The highest incidence of LPD development typically occurs within the first year post treatment[77,78]. Various immune suppressive agents have been linked to an increased risk of malignancies. The best-studied immune suppressant in context of RA and LPD development is methotrexate (MTX). This immunosuppressive agent has such a strong association with LPD development that the 2008 World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues recognized MTX-associated LPD as in independent entity[79]. MTX-associated LPD is commonly characterized by the presence of EBV virus in the lymphoma tissue and that discontinuation of MTX in results in regression of LPD in many but not all patients[80,81]. Furthermore, this risk of MTX-associated LPD increases with higher treatment doses[82]. Thus, it has been proposed that should MTX treated RA patients develop LPD, they should then receive EBV serologic screening to determine if MTX should be discontinued[83] as the likelihood of regression post MTX discontinuation appear to be linked to EBV status[80].
Anti-tumor necrosis factor (TNF)α antibody therapy has also been shown to increase rates of LPD in RA patients compared to healthy controls in a systematic meta-analysis[84]. In one head to head comparison of anti-TNFα (infliximab) against MTX treatment, anti-TNFα agents were associated with higher rates of LPD than MTX[85]. The risk of LPD development is likewise correlated with higher doses of anti-TNFα (either infliximab or adalimumab)[84]. One recent report has even linked adalimumab to EBV-associated lymphoproliferative disorder development after two years of treatment[86].
EBV-ASSOCIATED LPD DEVELOPMENT IN IBD PATIENTS TREATED WITH IMMUNE SUPPRESSANTS
EBV and IBD
Given the extensive data from the post-transplantation and RA literature that shows the risk of EBV-associated LPD increases with immunosuppression, one logical question to ask is whether similar immunosuppression in other disease states, such as IBD, is also associated with increased rates of LPD. Furthermore, given that IBD patients are often diagnosed and initiate treatment younger than 30 years of age (an age demographic with many unexposed to EBV), concern for LPD risk in EBV seronegative patients may be raised.
Even without treatment, IBD patients have higher rates of infections such as non-antibiotic associated Clostridium difficile colitis[87], cytomegalovirus[87,88], and infectious colitis[88]. It is unclear why IBD is associated with higher rates of these specific infections. Regardless, it is clear is that with the addition of immunosuppressive agents, this infectious risk increases substantially[88-91]. However, with respect to EBV, Reijasse et al[92] did not find a relationship between EBV viral load and severity of CD activity or the type of immunosuppression (infliximab infusion, corticosteroids, azathioprine and cyclosporine) used. Similarly, Fernandez Salazar et al[93] found that EBV seropositive CD patients in remission maintained on either no immunosuppression, azathioprine and/or infliximab did not have any significant changes to the viral load. However, interestingly patients with the most severe uncontrolled CD activity were found to have transient dramatic spikes in EBV viral load[92,94] with EBV DNA detected in colonic mucosal B-lymphocytes[94].
EBV-associated LPD and IBD
The link between EBV-associated LPD and IBD remains somewhat controversial as there have been a number of conflicting major studies done to date. The majority of large population-based studies have failed to find a significant association between IBD and LPD[95-100]. On the other hand, studies based in tertiary referral centers, which may have an inherent a referral bias towards those with more severe disease, showed that after factoring in the type and dose of immune suppressants used, IBD by itself does confer a slightly elevated risk for LPD[7,101]. In addition, when subgroup analysis was undertaken in one population-based study from the University of Manitoba, an increased risk of LPD in male patients with CD was found[102]. The difficulty in large population studies is that a number of factors, such as IBD disease severity and immune suppressant usage, are often not accounted for. Thus, it remains unclear how much IBD by itself, without the influence of immune suppressants, contributes to LPD development.
While the influence of IBD on EBV-associated LPD development has not been independently determined, analysis of IBD patients on immunosuppressive therapy demonstrates a clear risk for the development of EBV-associated LPD. An estimated 50% of IBD patients on immunosuppressive therapy with LPD were EBV seropositive[8,103] with a number of case reports identifying EBV DNA present in LPDs that developed post immunosuppression in IBD patients[104-107]. In reviewing the post-transplantation literature, a major risk for the development of PTLD is EBV seroconversion or EBV naivety while on immune suppressants and those who were EBV seropositive prior to transplantation habouring a latent infection represents a minor risk factor[44,45,49,50]. Currently, only a handful of case reports have documented the link between EBV seroconversion and LPD development in context of IBD and immunosuppression. Van Biervliet et al[108] reported the case of a young EBV seronegative CD patient who developed LPD shortly after treatment with azathioprine. Similarly, a 16-year-old CD patient who became seropositive while on therapy of mesalamine, azathioprine and infliximab infusion consequently developed EBV-associated LPD[109]. Lastly, a 25-year-old CD patient develops LPD after undergoing EBV seroconversion while on azathioprine[110]. Taken together, these reports may indicate a risk of LPD development from EBV seroconversion while on immune suppressants in IBD patients on immune suppressants. Perhaps an argument can thus be made for EBV serological monitoring in the EBV naïve IBD population. However, more research is needed to determine the effectiveness and utility of such an approach.
Role of immune suppressants on EBV-associated LPD development in IBD
Medical therapy for IBD is often individualized and there are nuanced differences between the management of CD and UC[111]. Regardless, typical immunosuppressive regimens may include prednisone, mesalazine, cyclosporine, thiopurines, such as azathioprine (AZA) and 6-mercaptopurine (6-MP), and infliximab[111]. (MTX is also used in IBD treatment, though much less frequently than in RA management and thus scant safety data is available in the IBD population). These therapies have been examined for a correlation with EBV-associated LPD development. Mechanistically, it is theorized that increased cancer risk may be conferred with a disturbed mucosal barrier and increased inflammation resulting in an accumulation of genetic mutations provides the opportunity for EBV-mediated malignant transformation. Immunosuppressive agents hamper the innate and adaptive responses for tumor surveillance and clearance[95].
The best studied of all IBD treatment agents, AZA and 6-MP, were associated with an increased risk of LPD when standard dosing (AZA 2.5 mg/kg per day; 6-MP 1.5 mg/kg per day) were used[8,10-12]. Dayharsh et al[112] found in a retrospective study that thiopurine use dramatically increased the rates of EBV-associated LPD in their IBD population (17% increase to 50%). Similarly, thiopurine treatment in a French nationwide prospective observational cohort study (CESAME) was associated with increased EBV-associated lymphomas[113]. A recent review of the Kaiser Permanente Cancer Registry of 16023 IBD patients revealed an increased incidence of lymphomas in thiopurine treated patients[9]. Finally, in a recent meta-analysis[114] and a retrospective cohort study of the United States Veteran Affairs database[11], both publications demonstrated a 4-fold increased risk of lymphoma in AZA or 6-MP treated IBD patients compared with the general population[11,114]. The meta-analysis found the lymphoma development risk increased with duration of immunosuppression and decrease with discontinuation of therapy[114]. In fact, one case report described lymphoma regression upon withdrawal of thiopurine[115]. Thus, given the higher risk of EBV-associated LPD development in young male IBD patients, some groups have proposed the avoidance of thiopurine use in this particular population altogether[116,117].
In addition to thiopurine, other IBD treatment agents have been studied, albeit to a lesser extent. MTX is one such agent. There has been scant data on MTX and EBV-associated LPD development in the IBD population. Kandiel et al[11] found that 2 of the 4 cases of lymphoma development in IBD patients involved treatment with MTX (31 patients of the 782 person study received MTX in total). While studies in IBD are lacking, studies involving patients with rheumatoid arthritis found MTX treatment to be associated with increased risk of lymphoma development[118,119]. One case report documented the development of EBV-associated LPD in a patient with rheumatoid arthritis receiving MTX with lymphoma regression upon discontinuation of MTX use[120].
Another commonly used class of IBD agents is the anti-TNFα antibody, including both adalimumab and infliximab[12]. Adalimumab has been linked to Hodgkin Lymphoma development[121] or recurrence[122]. However, the largest trial to date involving adalimumab use found no increased incidences of T-cell non Hodgkin Lymphoma development over control[123]. This study, however, did find increased risk of T-cell non Hodgkin Lymphoma development in those treated with anti-TNFα agents (either adalimumab or infliximab) in combination with a thiopurine[123]. The link between infliximab and LPD is likewise controversial. There are a number of trials that have identified a small but significant risk of lymphoma development in IBD patients on infliximab. In the ACCENT I maintenance infliximab infusion randomized placebo-controlled trial, two cases of EBV-associated non-Hodgkin lymphoma were found out of 573 patients (all participants had a score of at least 220 on the CD activity index)[124]. A second study based at the Mayo Clinic found one case of EBV-associated lymphoma out of 500 patients[11]. A third smaller randomized, double-blinded placebo controlled trial of 73 IBD patients who were either refractory to conventional treatments or responded sub-optimally to treatment were initiated on a course of four infliximab infusions every 8 wk[125]. One patient developed B-cell lymphoma 9.5 mo post initial infusion[125]. A large retrospective chart review of the Kaiser Permanente Cancer Registry revealed an increased standardized incidence rate ratio (5.5 for past use; 4.4 for current use) of lymphoma development over nearly 6-year span in the IBD population treated with infliximab over those without[9]. Finally, a recent meta-analysis of 26 publications found, in subgroup analysis, an increased risk of non-Hodgkin’s lymphoma development in anti-TNFα agent treated IBD male patients aged 20-54 years of age[12]. On the other hand, a number of studies have failed to find evidence of increased LPD risk from infliximab use. The large Crohn’s Therapy Resource, Evaluation, and Assessment Tool registry found no increased risk of lymphoma in IBD patients treated with infliximab over control population[126]. A selective small meta-analysis of randomized controlled trials failed to find an increased risk of lymphoma associated with infliximab over those that did not receive any anti-TNFα agents. Finally, a recent study of long-term safety of infliximab use found no increase LPD risk conferred by infliximab over control over the span of 14 years[127].
There are several inherent difficulties in establishing a role for infliximab in EBV-associated LPD development in IBD. First, most studies do not stratify the data based on disease severity. It may be reasonable to suspect that those requiring treatment with an anti-TNFα agent is associated with more refractory or severe disease as biologics are typically prescribed after other immune suppressants have failed. As such, more severe inflammatory disease may independently confer a higher LPD risk. Second, it maybe challenging to show the effect of anti-TNFα therapy alone in the development of LPD as the control group typically has received some form of immunosuppressive therapy. Third, very few patients will have received only anti-TNFα therapy without prior exposure to any other immunosuppressive agents. As such, there may be an accumulated risk from multiple agent use. This raises the hypothesis that it may not be any specific immunosuppressive agent that may be the culprit for LPD development, but rather the combination or addition of the third or the fourth agent that statistically increases LPD risk[128]. One observation that supports this theory is the increasing rates of hepatosplenic T-cell lymphoma (HSTCL) where the majority of reported cases involve young male patients (average age mid-twenties) receiving either prolonged thiopurine therapy (more than two years) or combination immunosuppression therapy of thiopurine and anti-TNF therapy[129,130]. As such, some have proposed that male patients under 35 years of age on prolonged thiopurine treatment or combination therapy should be monitored carefully for signs of HSTCL[129,130].
In summary, EBV-associated LPD may not be elevated in IBD from a population perspective but appears to occur more frequently in the younger male population, possibly due to the fact that significant EBV exposure occurs during this time. What might be behind the gender differences is currently unclear. In addition, regardless of patient demographics, thiopurines appear to confer the greatest risk of EBV-associated LPD development when compared to the methotrexate or biologics.
LESSONS FROM IMMUNE SUPPRESSION USE: FUTURE DIRECTIONS FOR IBD RESEARCH
Attempting to interpret findings from one field and apply them to another must be done with caution, as the dosing and treatment regimens of immune suppressants used in IBD are different than those used post-transplantation or in RA. Furthermore, the pathophysiology of these diseases, although incompletely elucidated, are likely quite different. However, given the sparse data available in the IBD field surrounding the risks of immune suppressants, complications from their use in the context of other inflammatory diseases should also not be overlooked. Currently, there is a trend amongst IBD physicians to move towards increased use of MTX for the purposes of both primary immunosuppression and also for suppression of anti-biologics antibody production. As the data linking lymphoma risk in MTX use in RA is mounting, the role of MTX in lymphoma development in IBD should be examined more closely. Furthermore, it remains largely unclear what effect the dose, the combination and the duration of IBD immunosuppressive therapy has on EBV-associated LPD development. In review of the available data, more questions remain than answers. Is there a role for EBV serological screening as in the post-transplantation field and if so, who should be screened and for how long? The younger male population appears to have a higher risk of LPD development while on immune suppressants and given the second peak of EBV seroconversion is within the same age range, should males between the ages of 18 to 30 be selected for routine EBV viral load screening while on therapy? Are there certain combinations of drugs or specific therapies that should be avoided or dose adjusted to minimize the risk of EBV-associated LPD? Should withdrawal of immunosuppressive therapy be initiated as soon as metrics of early remission is achieved to minimize LPD risk? How should this be balanced with the risk of disease flare or risk of subsequent surgery? The benefit of immunosuppressive therapies in IBD, much like in RA, is unequivocal but the risk of LPD development is a cost that while relatively small is one which not all patients are comfortable with. Many questions surrounding how best to utilize and discontinue these powerful immunosuppressive agents remain. As such, the development of an early screening tool to further minimize the risk of LPD may invaluable to all IBD patients on immunosuppressive treatment (Figure 1).
Figure 1.
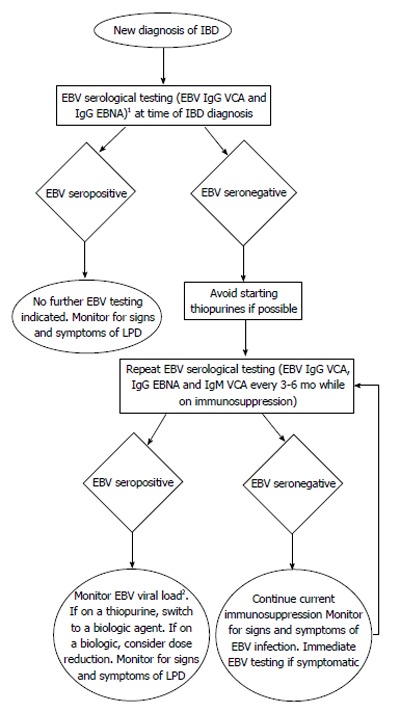
Proposed algorithm for treatment management of inflammatory bowel disease patients who are either Epstein-Barr virus seropositive or negative. 1EBV monospot or EBV IgM have not been shown to be helpful in in determining serostatus; 2EBV viral load should be done by polymerase chain reaction in whole blood in EDTA collection tube. EBV: Epstein-Barr virus; IBD: Inflammatory bowel disease; EBNA: Epstein-Barr virus-determined nuclear antigen; LPD: Lymphoproliferative disorders; VCA: Viral-capsid antigen; EDTA: Ethylenediaminetetraacetic acid.
Footnotes
P- Reviewer: Grossi L, Maharshak N, Miheller P S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: Lam GY has no conflicts of interest to declare. Peters AC has been an advisory board member for Janssen, Roche, Lundbeck and Seattle Genetics. Halloran BP is a consultant and/or advisory board member for Abbvie and Janssen. Fedorak RN is a consultant or advisory board member of Abbvie, Ferring, Janssen, Shire, VSL#3, Celltrion. He is also the recipient of the following clinical/basic science research grants: Abbvie, Alba, Bristol Myers Squibb, Centocor, GSK, Genentec, Janssen, Merck, Millennium, Novartis, Pfizer, Proctor and Gamble, Roche, VSL#3, Celltrion. Finally, he is also the owner/Shareholder of Metablolomic Technologies Inc (www.metabolomictechnologies.ca).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 31, 2015
First decision: April 23, 2015
Article in press: September 28, 2015
References
- 1.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A. Epidemiology of inflammatory bowel disease. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;2:7–18; discussion 18-21. doi: 10.1159/000061792. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales-Sánchez A, Fuentes-Pananá EM. Human viruses and cancer. Viruses. 2014;6:4047–4079. doi: 10.3390/v6104047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis JD, Bilker WB, Brensinger C, Deren JJ, Vaughn DJ, Strom BL. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121:1080–1087. doi: 10.1053/gast.2001.28703. [DOI] [PubMed] [Google Scholar]
- 8.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 9.Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146–2153. doi: 10.1038/ajg.2011.283. [DOI] [PubMed] [Google Scholar]
- 10.Vos AC, Bakkal N, Minnee RC, Casparie MK, de Jong DJ, Dijkstra G, Stokkers P, van Bodegraven AA, Pierik M, van der Woude CJ, Oldenburg B, Hommes DW; WInitiative on Crohn's and Colitis (ICC) Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis. 2011;17:1837–1845. doi: 10.1002/ibd.21582. [DOI] [PubMed] [Google Scholar]
- 11.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 14.Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, Madsen M, Rosdahl N, Konradsen HB, Storm HH, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 15.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady J, et al. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Coghill AE, Hildesheim A. Epstein-Barr virus antibodies and the risk of associated malignancies: review of the literature. Am J Epidemiol. 2014;180:687–695. doi: 10.1093/aje/kwu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 18.Alexander FE, Jarrett RF, Lawrence D, Armstrong AA, Freeland J, Gokhale DA, Kane E, Taylor GM, Wright DH, Cartwright RA. Risk factors for Hodgkin’s disease by Epstein-Barr virus (EBV) status: prior infection by EBV and other agents. Br J Cancer. 2000;82:1117–1121. doi: 10.1054/bjoc.1999.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA, Marklund G, Rymo L, Wellinder C, Straus SE. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci USA. 1987;84:570–574. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grose C, Henle W, Horwitz MS. Primary Epstein-Barr virus infection in a renal transplant recipient. South Med J. 1977;70:1276–1278. doi: 10.1097/00007611-197711000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lange B, Arbeter A, Hewetson J, Henle W. Longitudinal study of Epstein-Barr virus antibody titers and excretion in pediatric patients with Hodgkin’s disease. Int J Cancer. 1978;22:521–527. doi: 10.1002/ijc.2910220503. [DOI] [PubMed] [Google Scholar]
- 22.Goedert JJ, Coté TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, Jaffe ES, Biggar RJ. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 23.Filipovich AH, Mathur A, Kamat D, Shapiro RS. Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer Res. 1992;52:5465s–5467s. [PubMed] [Google Scholar]
- 24.Green M, Michaels MG. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am J Transplant. 2013;13 Suppl 3:41–54; quiz 54. doi: 10.1111/ajt.12004. [DOI] [PubMed] [Google Scholar]
- 25.Hanto DW. Classification of Epstein-Barr virus-associated posttransplant lymphoproliferative diseases: implications for understanding their pathogenesis and developing rational treatment strategies. Annu Rev Med. 1995;46:381–394. doi: 10.1146/annurev.med.46.1.381. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematology Am Soc Hematol Educ Program. 2013;2013:95–102. doi: 10.1182/asheducation-2013.1.95. [DOI] [PubMed] [Google Scholar]
- 27.Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am J Transplant. 2013;13:1296–1304. doi: 10.1111/ajt.12192. [DOI] [PubMed] [Google Scholar]
- 28.Kasiske BL, Kukla A, Thomas D, Wood Ives J, Snyder JJ, Qiu Y, Peng Y, Dharnidharka VR, Israni AK. Lymphoproliferative disorders after adult kidney transplant: epidemiology and comparison of registry report with claims-based diagnoses. Am J Kidney Dis. 2011;58:971–980. doi: 10.1053/j.ajkd.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 30.Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, Velten M, Moulin B; French Transplant Centers. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682–693. doi: 10.1111/j.1600-6143.2011.03896.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith JM, Rudser K, Gillen D, Kestenbaum B, Seliger S, Weiss N, McDonald RA, Davis CL, Stehmen-Breen C. Risk of lymphoma after renal transplantation varies with time: an analysis of the United States Renal Data System. Transplantation. 2006;81:175–180. doi: 10.1097/01.tp.0000188687.18972.a8. [DOI] [PubMed] [Google Scholar]
- 32.Caillard S, Lelong C, Pessione F, Moulin B; French PTLD Working Group. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006;6:2735–2742. doi: 10.1111/j.1600-6143.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 33.Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342:1514–1516. doi: 10.1016/s0140-6736(05)80084-4. [DOI] [PubMed] [Google Scholar]
- 34.van Leeuwen MT, Grulich AE, Webster AC, McCredie MR, Stewart JH, McDonald SP, Amin J, Kaldor JM, Chapman JR, Vajdic CM. Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood. 2009;114:630–637. doi: 10.1182/blood-2009-02-202507. [DOI] [PubMed] [Google Scholar]
- 35.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4:87–93. doi: 10.1046/j.1600-6135.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 36.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, Dizikes GJ, Pifarre R, Fisher RI. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323:1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 37.McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, Wyatt R, Arar M, Liereman D, Bridges N, Harmon W; CCTPT Study Group. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. Am J Transplant. 2008;8:984–989. doi: 10.1111/j.1600-6143.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 38.Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, Potdar S, Shapiro R, Dharnidharka VR, Kauffman HM. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant. 2007;7:2619–2625. doi: 10.1111/j.1600-6143.2007.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B; Belatacept Study Group. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 40.Vincenti F, Mendez R, Pescovitz M, Rajagopalan PR, Wilkinson AH, Butt K, Laskow D, Slakey DP, Lorber MI, Garg JP, et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007;7:1770–1777. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 41.Beveridge T, Krupp P, McKibbin C. Lymphomas and lymphoproliferative lesions developing under cyclosporin therapy. Lancet. 1984;1:788. doi: 10.1016/s0140-6736(84)91293-5. [DOI] [PubMed] [Google Scholar]
- 42.Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, Rosenthal JT, Hakala TR, Shaw BW, Hardesty RL. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funch DP, Ko HH, Travasso J, Brady J, Kew CE, Nalesnik MA, Walker AM. Posttransplant lymphoproliferative disorder among renal transplant patients in relation to the use of mycophenolate mofetil. Transplantation. 2005;80:1174–1180. doi: 10.1097/01.tp.0000169035.10572.c6. [DOI] [PubMed] [Google Scholar]
- 44.Walker RC, Paya CV, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, Daly RC, McGregor CG. Pretransplantation seronegative Epstein-Barr virus status is the primary risk factor for posttransplantation lymphoproliferative disorder in adult heart, lung, and other solid organ transplantations. J Heart Lung Transplant. 1995;14:214–221. [PubMed] [Google Scholar]
- 45.Aris RM, Maia DM, Neuringer IP, Gott K, Kiley S, Gertis K, Handy J. Post-transplantation lymphoproliferative disorder in the Epstein-Barr virus-naïve lung transplant recipient. Am J Respir Crit Care Med. 1996;154:1712–1717. doi: 10.1164/ajrccm.154.6.8970360. [DOI] [PubMed] [Google Scholar]
- 46.Shahinian VB, Muirhead N, Jevnikar AM, Leckie SH, Khakhar AK, Luke PP, Rizkalla KS, Hollomby DJ, House AA. Epstein-Barr virus seronegativity is a risk factor for late-onset posttransplant lymphoroliferative disorder in adult renal allograft recipients. Transplantation. 2003;75:851–856. doi: 10.1097/01.TP.0000055098.96022.F7. [DOI] [PubMed] [Google Scholar]
- 47.Wheless SA, Gulley ML, Raab-Traub N, McNeillie P, Neuringer IP, Ford HJ, Aris RM. Post-transplantation lymphoproliferative disease: Epstein-Barr virus DNA levels, HLA-A3, and survival. Am J Respir Crit Care Med. 2008;178:1060–1065. doi: 10.1164/rccm.200804-531OC. [DOI] [PubMed] [Google Scholar]
- 48.Bakker NA, Verschuuren EA, Erasmus ME, Hepkema BG, Veeger NJ, Kallenberg CG, van der Bij W. Epstein-Barr virus-DNA load monitoring late after lung transplantation: a surrogate marker of the degree of immunosuppression and a safe guide to reduce immunosuppression. Transplantation. 2007;83:433–438. doi: 10.1097/01.tp.0000252784.60159.96. [DOI] [PubMed] [Google Scholar]
- 49.Cockfield SM, Preiksaitis JK, Jewell LD, Parfrey NA. Post-transplant lymphoproliferative disorder in renal allograft recipients. Clinical experience and risk factor analysis in a single center. Transplantation. 1993;56:88–96. doi: 10.1097/00007890-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Walker RC, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, McGregor CG, Paya CV. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20:1346–1353. doi: 10.1093/clinids/20.5.1346. [DOI] [PubMed] [Google Scholar]
- 51.Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, Porter DL, Vonderheide RH, Bagg A, Heitjan DF, et al. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am J Transplant. 2015;15:2665–2673. doi: 10.1111/ajt.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner HJ, Wessel M, Jabs W, Smets F, Fischer L, Offner G, Bucsky P. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001;72:1012–1019. doi: 10.1097/00007890-200109270-00006. [DOI] [PubMed] [Google Scholar]
- 53.Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, Meijer CJ, van Den Brule AJ, Middeldorp JM. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–1171. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 54.Tsai DE, Douglas L, Andreadis C, Vogl DT, Arnoldi S, Kotloff R, Svoboda J, Bloom RD, Olthoff KM, Brozena SC, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8:1016–1024. doi: 10.1111/j.1600-6143.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 55.Green M, Bueno J, Rowe D, Mazariegos G, Qu L, Abu-Almagd K, Reyes J. Predictive negative value of persistent low Epstein-Barr virus viral load after intestinal transplantation in children. Transplantation. 2000;70:593–596. doi: 10.1097/00007890-200008270-00010. [DOI] [PubMed] [Google Scholar]
- 56.Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA, Brenner MK, Rooney CM, Heslop HE. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood. 2004;103:3979–3981. doi: 10.1182/blood-2003-12-4287. [DOI] [PubMed] [Google Scholar]
- 57.Gärtner BC, Schäfer H, Marggraff K, Eisele G, Schäfer M, Dilloo D, Roemer K, Laws HJ, Sester M, Sester U, et al. Evaluation of use of Epstein-Barr viral load in patients after allogeneic stem cell transplantation to diagnose and monitor posttransplant lymphoproliferative disease. J Clin Microbiol. 2002;40:351–358. doi: 10.1128/JCM.40.2.351-358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loginov R, Aalto S, Piiparinen H, Halme L, Arola J, Hedman K, Höckerstedt K, Lautenschlager I. Monitoring of EBV-DNAemia by quantitative real-time PCR after adult liver transplantation. J Clin Virol. 2006;37:104–108. doi: 10.1016/j.jcv.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Doesch AO, Konstandin M, Celik S, Kristen A, Frankenstein L, Sack FU, Schnabel P, Schnitzler P, Katus HA, Dengler TJ. Epstein-Barr virus load in whole blood is associated with immunosuppression, but not with post-transplant lymphoproliferative disease in stable adult heart transplant patients. Transpl Int. 2008;21:963–971. doi: 10.1111/j.1432-2277.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 60.Hoshino Y, Kimura H, Tanaka N, Tsuge I, Kudo K, Horibe K, Kato K, Matsuyama T, Kikuta A, Kojima S, et al. Prospective monitoring of the Epstein-Barr virus DNA by a real-time quantitative polymerase chain reaction after allogenic stem cell transplantation. Br J Haematol. 2001;115:105–111. doi: 10.1046/j.1365-2141.2001.03087.x. [DOI] [PubMed] [Google Scholar]
- 61.van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F, Bacigalupo A, Schaefer UW, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood. 2001;98:972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- 62.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 63.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales-Stawinski G, Martinelli L, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 64.EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. Nephrol Dial Transplant. 2002;17 Suppl 4:1–67. [PubMed] [Google Scholar]
- 65.Lee TC, Savoldo B, Rooney CM, Heslop HE, Gee AP, Caldwell Y, Barshes NR, Scott JD, Bristow LJ, O’Mahony CA, et al. Quantitative EBV viral loads and immunosuppression alterations can decrease PTLD incidence in pediatric liver transplant recipients. Am J Transplant. 2005;5:2222–2228. doi: 10.1111/j.1600-6143.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 66.Green M, Cacciarelli TV, Mazariegos GV, Sigurdsson L, Qu L, Rowe DT, Reyes J. Serial measurement of Epstein-Barr viral load in peripheral blood in pediatric liver transplant recipients during treatment for posttransplant lymphoproliferative disease. Transplantation. 1998;66:1641–1644. doi: 10.1097/00007890-199812270-00012. [DOI] [PubMed] [Google Scholar]
- 67.Allen U, Hebert D, Petric M, Tellier R, Tran D, Superina R, Stephens D, West L, Wasfy S, Nelson S. Utility of semiquantitative polymerase chain reaction for Epstein-Barr virus to measure virus load in pediatric organ transplant recipients with and without posttransplant lymphoproliferative disease. Clin Infect Dis. 2001;33:145–150. doi: 10.1086/321806. [DOI] [PubMed] [Google Scholar]
- 68.McDiarmid SV, Jordan S, Kim GS, Toyoda M, Goss JA, Vargas JH, Martín MG, Bahar R, Maxfield AL, Ament ME, et al. Prevention and preemptive therapy of postransplant lymphoproliferative disease in pediatric liver recipients. Transplantation. 1998;66:1604–1611. doi: 10.1097/00007890-199812270-00006. [DOI] [PubMed] [Google Scholar]
- 69.Gridelli B, Spada M, Riva S, Colledan M, Segalin A, Lucianetti A, Sonzogni A, Furione M, Baldanti F, Torre G. Circulating Epstein-Barr virus DNA to monitor lymphoproliferative disease following pediatric liver transplantation. Transpl Int. 2000;13 Suppl 1:S399–S401. doi: 10.1007/s001470050370. [DOI] [PubMed] [Google Scholar]
- 70.Liu Q, Xuan L, Liu H, Huang F, Zhou H, Fan Z, Zhao K, Wu M, Xu L, Zhai X, et al. Molecular monitoring and stepwise preemptive therapy for Epstein-Barr virus viremia after allogeneic stem cell transplantation. Am J Hematol. 2013;88:550–555. doi: 10.1002/ajh.23452. [DOI] [PubMed] [Google Scholar]
- 71.van der Velden WJ, Mori T, Stevens WB, de Haan AF, Stelma FF, Blijlevens NM, Donnelly JP. Reduced PTLD-related mortality in patients experiencing EBV infection following allo-SCT after the introduction of a protocol incorporating pre-emptive rituximab. Bone Marrow Transplant. 2013;48:1465–1471. doi: 10.1038/bmt.2013.84. [DOI] [PubMed] [Google Scholar]
- 72.Askling J. Malignancy and rheumatoid arthritis. Curr Rheumatol Rep. 2007;9:421–426. doi: 10.1007/s11926-007-0067-1. [DOI] [PubMed] [Google Scholar]
- 73.Hemminki K, Liu X, Försti A, Ji J, Sundquist J, Sundquist K. Subsequent leukaemia in autoimmune disease patients. Br J Haematol. 2013;161:677–687. doi: 10.1111/bjh.12330. [DOI] [PubMed] [Google Scholar]
- 74.Baecklund E, Smedby KE, Sutton LA, Askling J, Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders--what are the driving forces? Semin Cancer Biol. 2014;24:61–70. doi: 10.1016/j.semcancer.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Tosato G, Steinberg AD, Blaese RM. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981;305:1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- 76.Kinlen LJ. Incidence of cancer in rheumatoid arthritis and other disorders after immunosuppressive treatment. Am J Med. 1985;78:44–49. doi: 10.1016/0002-9343(85)90245-1. [DOI] [PubMed] [Google Scholar]
- 77.Chen YJ, Chang YT, Wang CB, Wu CY. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63:352–358. doi: 10.1002/art.30134. [DOI] [PubMed] [Google Scholar]
- 78.Ekström K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, Askling J. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48:963–970. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 79.Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010;184:16–20. doi: 10.1016/j.cbi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, Schultz M, Murren J. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14:1943–1949. doi: 10.1200/JCO.1996.14.6.1943. [DOI] [PubMed] [Google Scholar]
- 81.Feng WH, Cohen JI, Fischer S, Li L, Sneller M, Goldbach-Mansky R, Raab-Traub N, Delecluse HJ, Kenney SC. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst. 2004;96:1691–1702. doi: 10.1093/jnci/djh313. [DOI] [PubMed] [Google Scholar]
- 82.Kameda T, Dobashi H, Miyatake N, Inoo M, Onishi I, Kurata N, Mitsunaka H, Kawakami K, Fukumoto T, Susaki K, et al. Association of higher methotrexate dose with lymphoproliferative disease onset in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2014;66:1302–1309. doi: 10.1002/acr.22306. [DOI] [PubMed] [Google Scholar]
- 83.Starkebaum G. Rheumatoid arthritis, methotrexate, and lymphoma: risk substitution, or cat and mouse with Epstein-Barr virus? J Rheumatol. 2001;28:2573–2575. [PubMed] [Google Scholar]
- 84.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 85.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50:1740–1751. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 86.Ikeda T, Toyama S, Ogasawara M, Amano H, Takasaki Y, Morita H, Ishizuka T. Rheumatoid arthritis complicated with immunodeficiency-associated lymphoproliferative disorders during treatment with adalimumab. Mod Rheumatol. 2012;22:458–462. doi: 10.1007/s10165-011-0501-0. [DOI] [PubMed] [Google Scholar]
- 87.Irving PM, Gibson PR. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol. 2008;5:18–27. doi: 10.1038/ncpgasthep1004. [DOI] [PubMed] [Google Scholar]
- 88.Epple HJ. Therapy- and non-therapy-dependent infectious complications in inflammatory bowel disease. Dig Dis. 2009;27:555–559. doi: 10.1159/000233297. [DOI] [PubMed] [Google Scholar]
- 89.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, Langholff W, Londhe A, Sandborn WJ. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524–2533. doi: 10.1038/ajg.2009.322. [DOI] [PubMed] [Google Scholar]
- 91.Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Reijasse D, Le Pendeven C, Cosnes J, Dehee A, Gendre JP, Nicolas JC, Beaugerie L. Epstein-Barr virus viral load in Crohn’s disease: effect of immunosuppressive therapy. Inflamm Bowel Dis. 2004;10:85–90. doi: 10.1097/00054725-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez Salazar L, Rojo S, De Lejarazu RO, Castro E, Higuera E, González JM. No increase in Epstein-Barr virus viral load in a group of 30 asymptomatic patients with Crohn’s disease. Am J Gastroenterol. 2013;108:1933–1935. doi: 10.1038/ajg.2013.250. [DOI] [PubMed] [Google Scholar]
- 94.Sankaran-Walters S, Ransibrahmanakul K, Grishina I, Hung J, Martinez E, Prindiville T, Dandekar S. Epstein-Barr virus replication linked to B cell proliferation in inflamed areas of colonic mucosa of patients with inflammatory bowel disease. J Clin Virol. 2011;50:31–36. doi: 10.1016/j.jcv.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aithal GP, Mansfield JC. Review article: the risk of lymphoma associated with inflammatory bowel disease and immunosuppressive treatment. Aliment Pharmacol Ther. 2001;15:1101–1108. doi: 10.1046/j.1365-2036.2001.01023.x. [DOI] [PubMed] [Google Scholar]
- 96.Bebb JR, Logan RP. Review article: does the use of immunosuppressive therapy in inflammatory bowel disease increase the risk of developing lymphoma? Aliment Pharmacol Ther. 2001;15:1843–1849. doi: 10.1046/j.1365-2036.2001.01125.x. [DOI] [PubMed] [Google Scholar]
- 97.Ekbom A, Helmick C, Zack M, Adami HO. Extracolonic malignancies in inflammatory bowel disease. Cancer. 1991;67:2015–2019. doi: 10.1002/1097-0142(19910401)67:7<2015::aid-cncr2820670731>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 98.Karlén P, Löfberg R, Broström O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 99.Palli D, Trallori G, Bagnoli S, Saieva C, Tarantino O, Ceroti M, d’Albasio G, Pacini F, Amorosi A, Masala G. Hodgkin’s disease risk is increased in patients with ulcerative colitis. Gastroenterology. 2000;119:647–653. doi: 10.1053/gast.2000.16487. [DOI] [PubMed] [Google Scholar]
- 100.Persson PG, Karlén P, Bernell O, Leijonmarck CE, Broström O, Ahlbom A, Hellers G. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology. 1994;107:1675–1679. doi: 10.1016/0016-5085(94)90807-9. [DOI] [PubMed] [Google Scholar]
- 101.Greenstein AJ, Gennuso R, Sachar DB, Heimann T, Smith H, Janowitz HD, Aufses AH. Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985;56:2914–2921. doi: 10.1002/1097-0142(19851215)56:12<2914::aid-cncr2820561232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 102.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 103.Sokol H, Beaugerie L. Inflammatory bowel disease and lymphoproliferative disorders: the dust is starting to settle. Gut. 2009;58:1427–1436. doi: 10.1136/gut.2009.181982. [DOI] [PubMed] [Google Scholar]
- 104.Allen PB, Laing G, Connolly A, O’Neill C. EBV-associated colonic B-cell lymphoma following treatment with infliximab for IBD: a new problem? BMJ Case Rep. 2013;2013:pii: bcr2013200423. doi: 10.1136/bcr-2013-200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bai M, Katsanos KH, Economou M, Kamina S, Balli C, Briasoulis E, Kappas AM, Agnantis N, Tsianos EV. Rectal Epstein-Barr virus-positive Hodgkin’s lymphoma in a patient with Crohn’s disease: case report and review of the literature. Scand J Gastroenterol. 2006;41:866–869. doi: 10.1080/00365520500529629. [DOI] [PubMed] [Google Scholar]
- 106.Loiudice TA, Nemer W, Rosenblum S, Murray B, Batizy S, Fleming L, Mubashir B, Demeter S. Crohn’s disease (regional enteritis) in association with Hodgkin’s disease. J Am Osteopath Assoc. 1989;89:519–523. [PubMed] [Google Scholar]
- 107.Castrellon A, Feldman PA, Suarez M, Spector S, Chua L, Byrnes J. Crohn’s disease complicated by primary gastrointestinal Hodgkin’s lymphoma presenting with small bowel perforation. J Gastrointestin Liver Dis. 2009;18:359–361. [PubMed] [Google Scholar]
- 108.Van Biervliet S, Velde SV, De Bruyne R, De Looze D, De Vos M, Van Winckel M. Epstein-Barr virus related lymphoma in inflammatory bowel disease. Acta Gastroenterol Belg. 2008;71:33–35. [PubMed] [Google Scholar]
- 109.Gidrewicz D, Lehman D, Rabizadeh S, Majlessipour F, Dubinsky M. Primary EBV infection resulting in lymphoproliferative disease in a teenager with Crohn disease. J Pediatr Gastroenterol Nutr. 2011;52:103–105. doi: 10.1097/MPG.0b013e3181e80410. [DOI] [PubMed] [Google Scholar]
- 110.N’guyen Y, Andreoletti L, Patey M, Lecoq-Lafon C, Cornillet P, Léon A, Jaussaud R, Fieschi C, Strady C. Fatal Epstein-Barr virus primo infection in a 25-year-old man treated with azathioprine for Crohn’s disease. J Clin Microbiol. 2009;47:1252–1254. doi: 10.1128/JCM.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 112.Dayharsh GA, Loftus EV, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, Macon WR, Burgart LJ. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- 113.Sokol H, Beaugerie L, Maynadié M, Laharie D, Dupas JL, Flourié B, Lerebours E, Peyrin-Biroulet L, Allez M, Simon T, Carrat F, Brousse N; CESAME Study Group. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2063–2071. doi: 10.1002/ibd.22889. [DOI] [PubMed] [Google Scholar]
- 114.Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145:1007–1015.e3. doi: 10.1053/j.gastro.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 115.Larvol L, Soule JC, Le Tourneau A. Reversible lymphoma in the setting of azathioprine therapy for Crohn’s disease. N Engl J Med. 1994;331:883–884. doi: 10.1056/NEJM199409293311321. [DOI] [PubMed] [Google Scholar]
- 116.Subramaniam K, D’Rozario J, Pavli P. Lymphoma and other lymphoproliferative disorders in inflammatory bowel disease: a review. J Gastroenterol Hepatol. 2013;28:24–30. doi: 10.1111/jgh.12015. [DOI] [PubMed] [Google Scholar]
- 117.Schwartz LK, Kim MK, Coleman M, Lichtiger S, Chadburn A, Scherl E. Case report: lymphoma arising in an ileal pouch anal anastomosis after immunomodulatory therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1030–1034. doi: 10.1016/j.cgh.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 118.Baecklund E, Askling J, Rosenquist R, Ekbom A, Klareskog L. Rheumatoid arthritis and malignant lymphomas. Curr Opin Rheumatol. 2004;16:254–261. doi: 10.1097/00002281-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 119.Buchbinder R, Barber M, Heuzenroeder L, Wluka AE, Giles G, Hall S, Harkness A, Lewis D, Littlejohn G, Miller MH, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008;59:794–799. doi: 10.1002/art.23716. [DOI] [PubMed] [Google Scholar]
- 120.Kamel OW, van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA, Montgomery PG, Warnke RA, Dorfman RF. Brief report: reversible lymphomas associated with Epstein-Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N Engl J Med. 1993;328:1317–1321. doi: 10.1056/NEJM199305063281806. [DOI] [PubMed] [Google Scholar]
- 121.Rodriguez AA, Kerner J, Luna-Fineman S, Berry GJ. Hodgkin lymphoma following adalimumab for the treatment of Crohn’s disease in an adolescent. Dig Dis Sci. 2014;59:2403–2405. doi: 10.1007/s10620-014-3191-6. [DOI] [PubMed] [Google Scholar]
- 122.Cassaday RD, Malik JT, Chang JE. Regression of Hodgkin lymphoma after discontinuation of a tumor necrosis factor inhibitor for Crohn’s disease: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2011;11:289–292. doi: 10.1016/j.clml.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 123.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-α) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2013;108:99–105. doi: 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 124.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 125.Rutgeerts P, D’Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Van Hogezand RA, Braakman T, DeWoody KL, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 126.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Langholff W, Londhe A, Sandborn WJ. Drug therapies and the risk of malignancy in Crohn’s disease: results from the TREAT™ Registry. Am J Gastroenterol. 2014;109:212–223. doi: 10.1038/ajg.2013.441. [DOI] [PubMed] [Google Scholar]
- 127.Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, Henckaerts L, Van Assche G, Vermeire S, Rutgeerts P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 128.Subramaniam K, Cherian M, Jain S, Latimer M, Corbett M, D’Rozario J, Pavli P. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339–1342. doi: 10.1111/imj.12287. [DOI] [PubMed] [Google Scholar]
- 129.Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-α inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol. 2011;23:1150–1156. doi: 10.1097/MEG.0b013e32834bb90a. [DOI] [PubMed] [Google Scholar]
- 130.Kotlyar DS, Osterman MT, Diamond RH, Porter D, Blonski WC, Wasik M, Sampat S, Mendizabal M, Lin MV, Lichtenstein GR. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36–41.e1. doi: 10.1016/j.cgh.2010.09.016. [DOI] [PubMed] [Google Scholar]


