Abstract
There are several common causes of acute pancreatitis, principally excessive alcohol intake and gallstones, and there are many rare causes. However, cases of pancreatitis still occur in the absence of any recognizable factors, and these cases of idiopathic pancreatitis suggest the presence of unrecognized etiologies. Five cases of acute pancreatitis in four patients came to attention due to a strong temporal association with exposure to nerve stimulators and energy drinks. Given that these cases of pancreatitis were otherwise unexplained, and given that these exposures were not clearly known to be associated with pancreatitis, we performed a search for precedent cases and for mechanistic bases. No clear precedent cases were found in PubMed and only scant, weak precedent cases were found in public-health databases. However, there was a coherent body of intriguing literature in support of a mechanistic basis for these exposures playing a role in the etiology of pancreatitis.
Keywords: Pancreatitis, Energy drinks, Transcutaneous electric nerve stimulation, Etiology, Chronic pain
Core tip: This may be the first report of nerve stimulators or energy drinks playing an etiologic role in the development of pancreatitis. Five recent cases of otherwise unexplained pancreatitis recently came to attention due to a strong temporal association between pancreatitis and exposure to nerve stimulators (3 cases in 3 patients) and energy drinks (2 cases in 1 patient). Although causality is not shown, the temporal association is striking.
INTRODUCTION
Acute pancreatitis is a common and potentially life-threatening disease, whose incidence is increasing[1]. While the etiology of the vast majority of cases is identified as either alcohol or biliary stones, many cases (10%-34%)[1,2] are labeled idiopathic or cryptogenic, due to unknown etiology, and this proportion too is increasing[2,3]. Other, less common causes of pancreatitis that are identifiable include hypertriglyceridemia, tumors and stones, autoimmune diseases, and medications.
Use of energy drinks and nerve stimulators is also on the rise. Use of energy drinks such as RedBull and RockStar is increasing due in part to aggressive marketing campaigns and other psychosocial factors. The range and relative concentrations of their various ingredients vary widely, and per capita consumption has recently doubled in the United States[4-6]. Electrical nerve stimulation, such as transcutaneous electrical nerve stimulation (TENS) are employed at various intensities and frequencies for pain suppression, a controversial use[7].
Hypothesis
Very few data on idiopathic or cryptogenic causes of pancreatitis are available. Some cases of so-called idiopathic pancreatitis are likely due to unrecognized, or poorly understood, genetic defects[3,8-13] and pancreaticobiliary malformations[14,15]. Yet, there still remains a group of patients with so-called true idiopathic pancreatitis (TIP), whose etiology cannot be identified[16]. Three such patients with seemingly TIP, with no identified cause, were found to have a clear temporal association with the use of nerve-stimulation devices, and a fourth such patient was found to have a clear temporal association between 2 separate episodes of pancreatitis and consumption of the popular energy drink, Rockstar™ (Table 1). Other causes of pancreatitis, including gallstones, alcohol, autoimmune pancreatitis (elevated IgG4 levels), and hypertriglyceridemia, were ruled out, leading to the hypothesis that exposures to nerve stimulation or to energy drinks could play a role in the etiology of acute pancreatitis.
Table 1.
Patient characteristics
| Pt | Age | Gender | Type of exposure | Time gap between exposure and pancreatitis | Duration of exposure | Severity of pancreatits | TG | IgG4 | Confounding factors |
| 1 | 40s | F | TENS | Narrow | Short | Mild | Nl | Nl3 | CCB and PPI |
| 2 | 30s | F | IENS | Narrow | Unk | Mild | Unk | Unk | Unk2 |
| 3 | 50s | F | TENS | Narrow | Long | Severe | Nl | Nl3 | None1 |
| 4 | 40s | M | Energy drink | Narrow | Consumed Rockstar™ prior to 2 unexplained episodes | Both mild | Nl | Nl | Initial episode due to alcohol, but abstinent for 1 yr prior to these 2 episodes |
No medications, no alcohol, no gallstones (by transabdominal and intraoperative, but not endoscopic, ultrasound), there was no evidence for or against genetic causes and pancreas divisum;
No alcohol, and no gallstones (by transabdominal but not endoscopic ultrasound), but the patient could not recall her medication history;
Total IgG, not IgG4, was sent. Pt: Patient; Nl: Normal; Unk: Unknown; TG: Triglyceride level; TENS: Transcutaneous electrical nerve stimulation; F: Female; M: Male; CCB: Calcium-channel blocker; PPI: Proton-pump inhibitor; IENS: Implanted electrical nerve stimulator.
CASE REPORT
Three nerve-stimulation cases
A healthy female in her 40s underwent cholecystectomy for suspected biliary pancreatitis based on a suspicion of gallbladder sludge, but then re-presented several months later with recurrent pancreatitis. Upon further questioning it became clear that she occasionally used TENS to treat pain, including prior to her current episode of interstitial edematous pancreatitis. She did not recall if she used it prior to her pre-cholecystectomy pancreatitis. She responded to standard nonoperative therapy and recovered well.
A healthy female in her 30s, with an implanted electrical nerve stimulator device for chronic back pain, developed interstitial edematous pancreatitis. She responded to standard nonoperative therapy and recovered well.
A healthy female in her 50s wore a TENS device for back pain during a 10-h car trip. Shortly after arrival she developed severe, extensive, necrotizing pancreatitis and disconnected-duct syndrome, requiring necrosectomy, after which she recovered well. Although following her stay in the intensive-care unit, and her extensive operation and requisite recovery, she could not recall the exact settings she used for TENS, but thought that it was a moderate setting and that the pads were applied to her back for the vast majority of her 10-h car trip. Pathology following operation revealed extensive necrotic pancreatic and peripancreatic tissue.
Two energy-drink cases
A healthy male in his 40s with a remote history of severe acute alcoholic pancreatitis with pseudocyst formation, all now resolved. He was thought certainly to be free of recent alcohol use, but developed 2 episodes of interstitial edematous pancreatitis, both following Rockstar™ consumption. He responded well to nonoperative therapy.
Literature search
To inquire whether these could be two heretofore unappreciated etiologies of acute pancreatitis, a literature search was performed in PubMed looking for a mechanistic basis, using the title and abstract terms “pancreatitis” with either “idiopathic” or “etiology” and combined this with either “energy drink” or “nerve stimulation”. In addition, a search was performed of www.fda.gov for pancreatitis, nerve stimulation, energy drink, and several ingredients in common energy beverages (e.g., milk thistle, guarana, ginkgo, ginseng)[17].
The PubMed search revealed no case reports associating pancreatitis with either energy drinks or with nerve stimulation. Although energy drinks are associated with a variety of adverse signs and symptoms, such as nausea, vomiting, diarrhea, abdominal pain, hyperhidrosis, tachycardia, irritability, insomnia, stroke, and psychotic and bipolar disorders, the Food and Drug Administration (FDA) search on www.fda.gov revealed only one report of pancreatitis (which required hospitalization) associated with the energy drink Redbull™[18] and two reports of pancreatic disorders associated with 5-Hour Energy Booster™ and Monster Energy™[19]. Four reports of pancreatitis associated with ginkgo were found in www.fda-reports.com.
DISCUSSION
The observation of five cases of pancreatitis in 4 patients without the usual risk factors for pancreatitis, but with other exposures, which themselves were notable, either because the exposure was unusually prolonged (patient #3, with exposure to nerve stimulation during the entirety of a 10 h car ride), or unusually recurrent (patient #4, with pancreatitis episodes occurring following each consumption of the energy drink Rockstar™) led us to hypothesize that these could conceivably be unrecognized etiologies of pancreatitis. Although some of the patients in Table 1 has confounding factors, such as medications loosely associated with AP, and pancreas divisum and genetic causes were not possible to rule out in each and every case, nevertheless it is striking that the patient with the most severe pancreatitis had the purest form of seemingly TIP (lacking any identifiable confounders) had the greatest exposure to TENS, and had the most severe pancreatitis, necrotizing pancreatitis requiring multidisciplinary management[20]. Our search of the literature and FDA records revealed no published case reports and only scant FDA evidence, but did reveal supporting evidence of this hypothesis worthy of discussion.
Nerve stimulation and neurogenic inflammation
Ironically, nerve stimulation, such as TENS, has been used to treat pain of pancreatitis, as well as pain of many other sources[21-23] but the number of patients is too small, and the follow-up too inconsistent, for there to be any observed causal relationship between pancreatitis and nerve stimulation. Certainly it is well known that the pancreas is richly innervated, and mounting evidence suggests that pathologic activation of pancreatic neurons and the inflammatory sequelae of that activation (known as neurogenic inflammation) play a role in the development of pancreatitis (Table 2). The concept of neurogenic inflammation is additionally supported by the observation that most of the neurotransmitters of C and Aδ fibers of the pancreas have proinflammatory actions[24].
Table 2.
List of observations associating electrical nerve stimulations and the development of acute pancreatitis
| Ref. | |
| Observation | |
| All cases reported here were considered idiopathic | N/A |
| There was a strong temporal association of exposure and AP | N/A |
| There was a directly proportional relationship between duration of exposure and severity of subsequent AP | N/A |
| Neurogenic inflammation is increasingly recognized to play a role in development of AP, with sensory nerves in particular being considered a final common pathway in AP | [24,30,39,40] |
| TRPV1 and TRPA1 expression and function in pancreatic afferent neurons increases and blocking this pathway attenuates pancreatitis in a mouse model of AP | [25,26] |
| Over-stimulation of nerves associated with pancreatic disease (decrease in pancreatic blood flow and DNA synthesis) in rats | [34] |
| Neural cross talk between the duodenum and pancreas (duodeno-pancreatic reflex at T6-T13) can promote AP in a rat model | [41] |
| Possibly contradictory observations | |
| Stimulation by electroacupuncture of dorsal segmental points corresponding to levels that innervate pancreas (by splanchnic nerves; T9-T11) causes decrease in fasting blood glucose | [33] |
| Electroacupuncture protects against CCK-induced AP in rats | [31] |
| Electroacupuncture to paraumbilical point ST25 (dermatome T10) down-regulates pro-inflammatory cytokines (TNFα, IL-6) and attenuates the morphological damage to pancreas in a rat model of AP | [32] |
AP: Acute pancreatitis; N/A: Not available; TNF: Tumor necrosis factor; IL- 6: Interleukin- 6; TRPV1: Transient receptor potential vanilloid 1; TRPA1: Transient receptor potential cation channel, subfamily A, member 1; CCK: Cholecystokinin.
Further intriguing experimental evidence exists linking neurogenic inflammation and pancreatitis. For example, sensory neurons of the pancreas express channels whose activation induces pancreatic inflammation, and whose blockade attenuates experimental pancreatitis[25-27]. Similarly, in a study of the modulatory role of bradykinin in neurogenic inflammation, a potent inhibitor of bradykinin was administered in an animal model of pancreatitis and this administration attenuated the hypotension, edema, and hypovolemia associated with the pancreatitis, suggesting modulation of nerve stimulation modulates severity of pancreatitis[28].
Studying the neuropeptide substance P (SP), a common neurotransmitter mediating pain and other nerve signals, Figini et al[29] found that administration of SP to mice stimulated plasma extravasation from postcapillary venules in the pancreas, and that this effect was blocked by the administration of antagonists to the SP receptor. More recently, in a neonatal model of pancreatitis, administration of the neuron-denervating agent capsaicin significantly reduced histological severity scores and abolished plasma extravasation associated with pancreatitis[30], findings which support the notion that primary sensory neurons constitute a common final pathway for pancreatitis.
Figure 1 illustrates in a schematic model some of these possible processes contributing to a neurogenic etiology of pancreatitis. Various stimuli may excite vanilloid receptor TRPV1 on primary sensory neurons (SN), which play the pivotal role in initiation of neurogenic inflammation. Subsequent depolarization release various neuropeptides, such as SP, neurokinin A, and calcitonin gene related peptide. The result is edema of pancreatic tissue due to massive vasodilation and white blood cells infiltration. Remarkably, those changes in pancreas may be caused by irritation of SN in duodenal mucosa, because of axonal dichotomy and central convergence at spinal or higher levels (Table 2). Damage to pancreatic acinar cells may also be mediated by non-specific excitation of ryanodine or inositol-3-phosphate receptors with subsequent calcium release from endoplasmic reticulum and activation of enzymes in zymogen granules inside the cell.
Figure 1.
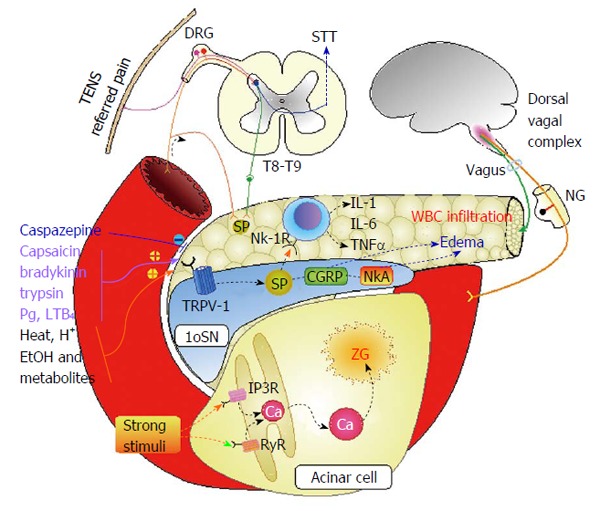
Schematic model illustrating possible mechanisms of neurogenic pancreatitis. AP: Acute pancreatitis; STT: Spinothalamic tract; TENS: Transcutaneous electrical nerve stimulation; DRG: Dorsal root ganglion; IL: Interleukin; TNF: Tumor necrosis factor; NK-1R: Neurokinin receptor 1; 1oSN: Primary sensory neuron; NG: Nodose ganglion; Pg: Prostaglandins; SP: Substance P; TRPV-1: Transient receptor potential vanilloid 1; CGRP: Calcitonin gene related protein; NkA: Neurokinin A; IP3R: Inositol-3-phosphate receptor; RyR: Ryanodine receptor.
Interestingly, there is also evidence that some forms of nerve stimulation, such as electroacupunture, seem to be protective against pancreatitis (Table 2)[31-33]. However, the concept of dose may well explain why a small amount of stimulation can have the opposite effect as a large amount, as is commonly observed in medicine, when a small amount of an exposure is safe and effective, such as acetaminophen (paracetamol) in therapeutic doses working as a safe antipyretic and analgesic, but a higher dose is toxic and can cause liver failure and death. Perhaps a very high dose of TENS, as in patient #3, can cause severe acute necrotizing pancreatitis, known to require prolonged multidisciplinary management[20], where as a small dose may be protective, and a moderate dose may cause only a minor episode of pancreatitis.
Indeed, experimental neurochemical precedent exists for the notion that over-stimulation of nerves may cause pancreatic disease. In conscious rats, for example, stimulation of sensory nerves with low-dose capsaicin reduces basal pancreatic secretory function, while moderate doses increase this function, and large (neurotoxic) doses cause a 27% decrease in pancreatic blood flow accompanied by a decrease in DNA synthesis in pancreatic tissue[34].
Energy drinks
Several aspects of the increasingly popular energy drinks are concerning. For example, because many energy drinks contain “natural” ingredients, such as ginseng, ginkgo, milk thistle, guarna-seed extract[17], these drinks are regulated as dietary supplements and not as medications, freeing manufacturers from the usual transparency associated with FDA-regulated products[6]. Similarly, several of the ingredients have been linked to health problems, but these links are either weak or inconsistent, and indeed, several of these same ingredients are commonly taken as remedies or preventive supplements to combat various common ailments.
For example, while there is some weak evidence that ginkgo is protective against pancreatitis[35,36], there are four low-quality “FDA reports” of pancreatitis in patients taking ginkgo[37]. Search of the presumably more reliable www.fda.gov site reveals no reports of this association.
While the health benefits and risks of caffeine are well known, none of these risks appear to be related to pancreatitis, and at least weak data suggest that caffeinated coffee may protect against alcoholic pancreatitis[38]. Similarly, no convincing association could be found between pancreatitis and other energy-drink ingredients, such as milk thistle and guarana. However, the FDA’s Center for Food Safety and Applied Nutrition Adverse Event Reporting System has reported several associations between pancreatitis and the cocktails of various energy drinks, such as RedBull™, 5-h Energy Booster™, and Monster Energy™[18,19]. Nevertheless, the possibility remains that what we have observed in patient #4 is merely a coincidence between exacerbations of autonomous alcoholic pancreatitis and the consumption of energy drinks. Still the fact that the otherwise unexplained pancreatitis occurred in this single patient twice, both times immediately following consumption of the energy drinks, is striking.
In conclusion, there is insufficient direct evidence to support causality between pancreatitis and exposures to nerve stimulators and to energy drinks. However, the observations presented here, coupled with the rising use of the offending products, are cause for concern and warrant further study. Possibilities for such study include either cellular or animal models of pancreatitis using these potentially offending agents, analyses of large databases, and the establishment of an international registry.
COMMENTS
Case characteristics
Five cases of pancreatitis in four patients occurring after exposure to nerve stimulators or energy drinks.
Clinical diagnosis
Elevated lipase and/or imaging evidence of pancreatic inflammation.
Differential diagnosis
Pancreatitis could have been due to another cause not tested for, or missed, such as genetic polymorphisms or mutations.
Laboratory diagnosis
Elevated lipase, but normal IgG4 and triglycerides.
Imaging diagnosis
Computed tomography or magnetic resonance imaging showed evidence of pancreatic inflammation. It was not possible to rule out pancreas divisum in each and every case.
Pathological diagnosis
Pancreatic debridement in patient #3 yielded only necrotic and saponified tissue, as expected.
Treatment
The patients were treated with standard supportive care during their episodes of pancreatitis. Patient #3 required open pancreatic debridement and recovered well.
Related reports
The causes of idiopathic pancreatitis remain, of course, unknown, by definition. The authors know no other cases associating nerve stimulators and pancreatitis, and only very weak evidence exists associating some ingredients in energy drinks with pancreatitis.
Experiences and lessons
This case report presents several cases that suggest the possibility of new etiologies of pancreatitis. However, this observation should be interpreted with caution, as it is impossible to rule out every possible systemic, structural, and genetic cause of pancreatitis. In no way does this report prove any cause.
Peer-review
This is a very interesting manuscript from the clinical point of view. Both hypotheses are interesting.
Footnotes
P- Reviewer: BoettoR, Shen HN, Vujasinovic M S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
Institutional review board statement: Study of the acute pancreatitis patients was approved by SAH-IRB 2011-030.
Informed consent statement: Need for consent was waived by SAH-IRB 2011-030. There are no details that might identify the patients in this manuscript.
Conflict-of-interest statement: None of the authors have any conflict of interest regarding this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 28, 2015
First decision: August 20, 2015
Article in press: October 27, 2015
References
- 1.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 2.Lankisch PG, Schirren CA, Schmidt H, Schönfelder G, Creutzfeldt W. Etiology and incidence of acute pancreatitis: a 20-year study in a single institution. Digestion. 1989;44:20–25. doi: 10.1159/000199888. [DOI] [PubMed] [Google Scholar]
- 3.Joergensen M, Brusgaard K, Crüger DG, Gerdes AM, de Muckadell OB. Incidence, prevalence, etiology, and prognosis of first-time chronic pancreatitis in young patients: a nationwide cohort study. Dig Dis Sci. 2010;55:2988–2998. doi: 10.1007/s10620-009-1118-4. [DOI] [PubMed] [Google Scholar]
- 4.Wolk BJ, Ganetsky M, Babu KM. Toxicity of energy drinks. Curr Opin Pediatr. 2012;24:243–251. doi: 10.1097/MOP.0b013e3283506827. [DOI] [PubMed] [Google Scholar]
- 5.Sepkowitz KA. Energy drinks and caffeine-related adverse effects. JAMA. 2013;309:243–244. doi: 10.1001/jama.2012.173526. [DOI] [PubMed] [Google Scholar]
- 6.Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks--a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database Syst Rev. 2008;(4):CD003008. doi: 10.1002/14651858.CD003008.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Sun XT, Weng XL, Zhou DZ, Sun C, Xia T, Hu LH, Lai XW, Ye B, Liu MY, et al. Comprehensive screening for PRSS1, SPINK1, CFTR, CTRC and CLDN2 gene mutations in Chinese paediatric patients with idiopathic chronic pancreatitis: a cohort study. BMJ Open. 2013;3:e003150. doi: 10.1136/bmjopen-2013-003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XT, Xia T, Hu LH, Zhou DZ, Li ZS, Liao Z. Identification of a novel deletion of CFTR in a 13-year-old: patient with idiopathic chronic pancreatitis. Pancreas. 2014;43:659–660. doi: 10.1097/MPA.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 10.Rai P, Sharma A, Gupta A, Aggarwal R. Frequency of SPINK1 N34S mutation in acute and recurrent acute pancreatitis. J Hepatobiliary Pancreat Sci. 2014;21:663–668. doi: 10.1002/jhbp.111. [DOI] [PubMed] [Google Scholar]
- 11.Masson E, Chen JM, Audrézet MP, Cooper DN, Férec C. A conservative assessment of the major genetic causes of idiopathic chronic pancreatitis: data from a comprehensive analysis of PRSS1, SPINK1, CTRC and CFTR genes in 253 young French patients. PLoS One. 2013;8:e73522. doi: 10.1371/journal.pone.0073522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaRusch J, Barmada MM, Solomon S, Whitcomb DC. Whole exome sequencing identifies multiple, complex etiologies in an idiopathic hereditary pancreatitis kindred. JOP. 2012;13:258–262. [PMC free article] [PubMed] [Google Scholar]
- 13.Audrézet MP, Chen JM, Le Maréchal C, Ruszniewski P, Robaszkiewicz M, Raguénès O, Quéré I, Scotet V, Férec C. Determination of the relative contribution of three genes-the cystic fibrosis transmembrane conductance regulator gene, the cationic trypsinogen gene, and the pancreatic secretory trypsin inhibitor gene-to the etiology of idiopathic chronic pancreatitis. Eur J Hum Genet. 2002;10:100–106. doi: 10.1038/sj.ejhg.5200786. [DOI] [PubMed] [Google Scholar]
- 14.Takuma K, Kamisawa T, Hara S, Tabata T, Kuruma S, Chiba K, Kuwata G, Fujiwara T, Egashira H, Koizumi K, et al. Etiology of recurrent acute pancreatitis, with special emphasis on pancreaticobiliary malformation. Adv Med Sci. 2012;57:244–250. doi: 10.2478/v10039-012-0041-7. [DOI] [PubMed] [Google Scholar]
- 15.Gonoi W, Akai H, Hagiwara K, Akahane M, Hayashi N, Maeda E, Yoshikawa T, Tada M, Uno K, Ohtsu H, et al. Pancreas divisum as a predisposing factor for chronic and recurrent idiopathic pancreatitis: initial in vivo survey. Gut. 2011;60:1103–1108. doi: 10.1136/gut.2010.230011. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, Enns R. Review of idiopathic pancreatitis. World J Gastroenterol. 2007;13:6296–6313. doi: 10.3748/wjg.v13.i47.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Tuttle TD, Higgins CL. Energy beverages: content and safety. Mayo Clin Proc. 2010;85:1033–1041. doi: 10.4065/mcp.2010.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.USDHHS/FDA/CFSAN. Voluntary Reports on Red Bull Energy Drink. 2012. [Google Scholar]
- 19.USDHHS/FDA/CFSAN. Voluntary and Mandatory Reports on 5-Hour Energy, Monster Energy and Rockstar Energy Drink. 2012. [Google Scholar]
- 20.Sabo A, Goussous N, Sardana N, Patel S, Cunningham SC. Necrotizing pancreatitis: a review of multidisciplinary management. JOP. 2015;16:125–135. doi: 10.6092/1590-8577/2947. [DOI] [PubMed] [Google Scholar]
- 21.Nielson KD, Adams JE, Hosobuchi Y. Experience with dorsal column stimulation for relief of chronic intractable pain: 1968-1973. Surg Neurol. 1975;4:148–152. [PubMed] [Google Scholar]
- 22.Roberts HJ. Transcutaneous electrical nerve stimulation in the management of pancreatitis pain. South Med J. 1978;71:396–398. doi: 10.1097/00007611-197804000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Ballegaard S, Christophersen SJ, Dawids SG, Hesse J, Olsen NV. Acupuncture and transcutaneous electric nerve stimulation in the treatment of pain associated with chronic pancreatitis. A randomized study. Scand J Gastroenterol. 1985;20:1249–1254. doi: 10.3109/00365528509089285. [DOI] [PubMed] [Google Scholar]
- 24.Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559; discussion 559-560. doi: 10.1159/000082180. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, Gebhart GF. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140:1283–1291.e1-2. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liddle RA. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta. 2007;1772:869–878. doi: 10.1016/j.bbadis.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Félétou M, Lonchampt M, Robineau P, Jamonneau I, Thurieau C, Fauchère JL, Villa P, Ghezzi P, Prost JF, Canet E. Effects of the bradykinin B2 receptor antagonist S 16118 (p-guanidobenzoyl-[Hyp3,Thi5,D-Tic7,Oic8]bradykinin) in different in vivo animal models of inflammation. J Pharmacol Exp Ther. 1995;273:1078–1084. [PubMed] [Google Scholar]
- 29.Figini M, Emanueli C, Grady EF, Kirkwood K, Payan DG, Ansel J, Gerard C, Geppetti P, Bunnett N. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am J Physiol. 1997;272:G785–G793. doi: 10.1152/ajpgi.1997.272.4.G785. [DOI] [PubMed] [Google Scholar]
- 30.Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle RA. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–G946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 31.An HJ, Lee JH, Lee HJ, Yang WM, Park SK, Hong SH, Kim HM, Um JY. Electroacupuncture protects against CCK-induced acute pancreatitis in rats. Neuroimmunomodulation. 2007;14:112–118. doi: 10.1159/000107793. [DOI] [PubMed] [Google Scholar]
- 32.Xue QM, Huang L, Li N. [Effects of electroacupuncture at Tianshu (ST25) on pro- and anti-inflammatory cytokines in rats with severe acute pancreatitis] Zhongxiyi Jiehe Xuebao. 2011;9:658–664. doi: 10.3736/jcim20110613. [DOI] [PubMed] [Google Scholar]
- 33.Belivani M, Lundeberg T, Cummings M, Dimitroula C, Belivani N, Vasilakos D, Hatzitolios A. Immediate effect of three different electroacupuncture protocols on fasting blood glucose in obese patients: a pilot study. Acupunct Med. 2015;33:110–114. doi: 10.1136/acupmed-2014-010662. [DOI] [PubMed] [Google Scholar]
- 34.Warzecha Z, Dembiński A, Jaworek J, Ceranowicz P, Szlachcic A, Walocha J, Konturek SJ. Role of sensory nerves in pancreatic secretion and caerulein-induced pancreatitis. J Physiol Pharmacol. 1997;48:43–58. [PubMed] [Google Scholar]
- 35.Zeybek N, Gorgulu S, Yagci G, Serdar M, Simsek A, Kaymakcioglu N, Deveci S, Ozcelik H, Tufan T. The effects of gingko biloba extract (EGb 761) on experimental acute pancreatitis. J Surg Res. 2003;115:286–293. doi: 10.1016/s0022-4804(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 36.Xu XW, Yang XM, Bai YH, Zhao YR, Shi GS, Zhang JG, Zheng YH. Treatment with ginkgo biloba extract protects rats against acute pancreatitis-associated lung injury by modulating alveolar macrophage. Prz Gastroenterol. 2014;9:43–48. doi: 10.5114/pg.2014.40850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz PI, La A. FDA Reports. 2012. [Google Scholar]
- 38.Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. Am J Gastroenterol. 2004;99:731–738. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 39.Hegde A, Bhatia M. Neurogenic inflammation in acute pancreatitis. JOP. 2005;6:417–421. [PubMed] [Google Scholar]
- 40.Li Q, Peng J. Sensory nerves and pancreatitis. Gland Surg. 2014;3:284–292. doi: 10.3978/j.issn.2227-684X.2013.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Zhu Y, Shenoy M, Pai R, Liu L, Pasricha PJ. Anatomical and functional characterization of a duodeno-pancreatic neural reflex that can induce acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G490–G500. doi: 10.1152/ajpgi.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


