Abstract
Objectives
Cytoplasmic clusterin (Clusterin), a ubiquitous multifunctional secretory sulfated glycoprotein, plays a role in apoptosis and is reportedly overexpressed in a variety of tumors. The role of Clusterin in pancreatic neuroendocrine tumors (PNETs) has not been investigated. In this study, Clusterin expression was evaluated in a subset of PNETs, and the results were correlated with the clinical-pathological features of the tumors.
Methods
Fifty-nine surgical cases were used to evaluate the immunohistochemical expression of Clusterin in PNETs. Using the avidinbiotin complex method, tissue sections from each case were stained with a rabbit anticlusterin antibody (Abcam, Cambridge, Mass). The immunohistochemical reactions were qualitatively and semiquantitatively evaluated by 2 pathologists.
Results
Strong Clusterin reactivity was identified in 36 (61%) of 59 PNETs. In 23 (39%) of 59 cases, the Clusterin score was 3 or less. Clusterin expression scores significantly associated with tumor size (P = 0.03) and with tumor stage (P = 0.02). The immunohistochemical score index did not correlate with tumor grade (P = 0.15).
Conclusions
We report the expression of Clusterin in PNETs. The correlation of Clusterin with tumor size and stage suggests involvement of this molecule in pancreatic neuroendocrine tumor progression. Clusterin may represent a new target of therapy for PNETs.
Keywords: clusterin, pancreatic neuroendocrine tumor, immunohistochemistry
Neuroendocrine neoplasms of the pancreas are rare, accounting for 1% to 2% of all pancreatic neoplasms.1 They all have malignant potential, although the rate of progression may be slow. Currently, the extent of tumor spread and the grade of the tumor, based on proliferative rate and presence of necrosis, are features that reportedly correlate best with prognosis.2–7 Frequently, the disease course is variable, and additional prognostic criteria are needed.
Clusterin gene (CLU) is a single-copy gene, organized into 9 exons (8 introns) and a 5′-untranslated region, located on chromosome 8 (8p21).8 In humans, CLU gene encodes for a nuclear (nCLU) and secreted (Clusterin) protein isoform that reportedly may have a role in proapoptotic and antiapoptotic functions, respectively.9 Clusterin is a multifunctional, stress-induced, ATP-independent molecular chaperone, previously known as testosterone-repressed prostate message 2, apolipoprotein J, sulfated glycoprotein 2, and complement lysis inhibitor.8,10 Recent data have demonstrated a significant role for Clusterin in carcinogenesis and progression of several human malignancies. The overexpression of Clusterin has been reported in prostate, breast, kidney, ovarian, and colorectal cancer as well as hematopoietic neoplasms such as anaplastic large cell lymphoma.11–16 Clusterin expression also positively correlates with increasing malignancy and pathological grading of tumors in breast and prostate cancer.17,18
Only 1 study, to our knowledge, has reported clusterin expression in pancreatic neuroendocrine tumors (PNETs) and solid pseudopapillary tumor of the pancreas but only included 30 PNETs.19
Based on the accumulated reported data, we sought to investigate the expression pattern of Clusterin in a larger set of pancreatic neuroendocrine neoplasms by immunohistochemistry (IHC) and to assess its role as a prognostic biomarker of pancreatic neuroendocrine neoplasm behavior.
MATERIALS AND METHODS
Selection of Cases
Fifty-nine surgical cases of PNETs were identified from the Moffitt Cancer Center Anatomic Pathology Department database, CoPath between 1990 and 2007, following institutional review board approval. All of the specimens were preserved in 10% neutral-buffered formalin before embedding in paraffin. The selected cases included tumors of different histologic grade and pathological stage. A custom-designed tissue microarray, including three 1-mm diameter cores of tumor tissue per block, was constructed using the representative paraffin blocks from the selected cases.
The clinical charts, the pathology reports, and all available slides on each case were reviewed by 2 pathologists with expertise in enteropancreatic neuroendocrine tumors (A.N., D.C.), and the tumors were graded following the World Health Organization 2010 criteria. The tumors were staged according to the American Joint Committee on Cancer TNM staging system.17
Immunohistochemistry
Serial 4-μm-thick paraffin sections of the tissue microarray block were subjected to immunohistochemical study. The immunohistochemical staining was performed manually at room temperature, using the avidin-biotin-peroxidase complex method (Vectastatin Elite ABC kit; Vector Lab, Burlingame, Calif). Briefly, pretreatment for antigen retrieval with a pressure cooker involved heating the slides with a microwave oven in 250 mL of unmasking solution (Vector Lab) for 10 minutes at a high-power level, followed by 20 minutes of cooling. Endogenous peroxidase and non-specific background staining were blocked by incubating slides with 50:50 solution of 3% hydrogen peroxide and methanol for 20 minutes. After washing with phosphate-buffered saline (PBS) for 5 minutes, slides were blocked with fetal calf serum for 20 minutes, followed by incubation with the primary rabbit polyclonal antibody for clusterin (anti–Yapolipoprotein J antibody [ab69644]; Abcam, Cambridge, Mass) at a dilution of 1:100, for 2 hours at room temperature. After rinsing with PBS for 5 minutes, slides were incubated with a biotinylated secondary antibody for 30 minutes and washed again. After washing with PBS for 5 minutes, slides were incubated with avidin-biotin complex for 30 minutes and washed again. The slides were developed with 3,3-diaminobenzidine (DAB substrate kit for peroxidase); Vector Lab). The specificity of the antibody was proven with 3 different experiments (immunofluorescence, IHC, and immunoblotting). The results of these experiments are provided by the manufacturer and can be found at http://www.abcam.com/Apolipoprotein-J-antibody-ab69644.pdf. All of the slides were lightly counterstained with hematoxylin for 10 seconds before dehydration and mounting. Immunostaining was scored with a Leitz Orthoplan 2 microscope, and representative images were captured by a compact digital camera with the Smart Capture Program (Vysis, Downers Grove, Ill). Negative control was included by using nonimmune rabbit sera and omitting the primary antibodies during the primary antibody incubation step.
Immunohistochemical Data Analysis
The clusterin-stained tissue sections were examined by 2 independent observers (A.N., D.C.) simultaneously, and a consensus score was reached for each specimen. Clusterin immunoreactivity was scored into 4 categories according to the intensity of staining: 0, 1+, 2+, and 3+. The percentages of positive tumor cells were scored semiquantitatively on a 4-tiered scale: 0 (0%), 1 (1%–33%), 2 (34%–66%), and 3 (67%–100%). The product of the intensity by percentage score determined the final score. The final scores were classified as 0 = negative, 1 to 3 = weak, 4 to 6 = moderate, and 7 to 9 = strong. The staining of normal pancreatic islet cells in comparison to tumor was evaluated.
Statistical Analysis
Spearman correlation coefficient was used to assess the correlation between Clusterin expression and tumor size. Kruskal-Wallis test was used to test association between Clusterin and categorical clinicopathological variables (eg, stage and grade). The Tukey test was used for post hoc pairwise comparison. All analyses were performed with SAS software (Cary, NC).
RESULTS
Clinicopathological Features
The series consisted of 28 males and 31 females with an average age of 55.2 years (range, 17–79 years). The tumors measured between 1.4 and 14.5 cm with an average size of 4.2 cm. Following the World Health Organization 2010 classification, 38 tumors were grade 1 (well differentiated) (G1), 15 were grade 2 (well differentiated) (G2), and 6 were grade 3 (poorly differentiated) (G3). The stage of the tumors was as follows: 18 patients had stage I, 8 had stage II, 8 had stage III, and 25 had stage IV disease.
Immunohistochemistry
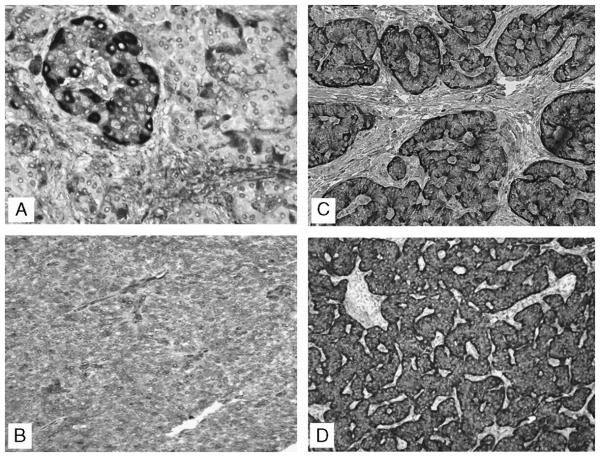
Immunoreactivity of Clusterin was cytoplasmic. No case demonstrated nuclear staining. Strong Clusterin immunoreactivity was identified in 36 (61%) of 59 tumors. In 23 (39%) of 59 cases, the Clusterin IHC score was 3 or less. Of these 30.5% (18/59) tumors had low Clusterin IHC scores between 3 and 1, and 5 of them (17%) were Clusterin negative (Figs. 1B–D). In the normal islets, 2 cellular populations could be identified. The first, exhibiting strong Clusterin cytoplasmic stain, was composed of cells distributed at the periphery of the islets and along the capillaries in the islet’s interior (Fig. 1A). These cells recapitulate the distribution of the glucagon-producing cells. The second population is characterized by cells with a weaker Clusterin cytoplasmic stain and localized within the inner portion of the islet. These cells are known to be mostly composed of insulin and PP-producing cells. In the Clusterin-positive PNETs, the stain was localized in the basal area of each cell, a pattern of stain previously described as “secretory.”19 The remaining part of the cytoplasm stained weakly. The remaining PNETs either were negative or exhibited a diffuse and homogeneously weak Clusterin positivity throughout the tumor.
FIGURE 1.
Clusterin expression in nonneoplastic islets and in PNETs. A, Nonneoplastic pancreatic islet: clusterin expression localized mainly at the periphery. B, Neuroendocrine carcinoma: weak intensity with 100% of cells staining. C, Neuroendocrine carcinoma: strong clusterin stain in the basal area of each cell (″secretory″ pattern) and weak stain of the remaining part of the cytoplasm.D, Neuroendocrine carcinoma: strong intensity with 100% of cells staining.
Statistical Analysis
There was significant association between clusterin score and disease stage (P = 0.02). In particular, Tukey test for multiple comparison showed that the average Clusterin score was statistically significant when comparing stage I versus stage II (P = 0.02), and stage I versus stages III and IV (P = 0.03). A moderate statistical correlation was found between Clusterin scores and tumor size (P = 0.03). The immunohistochemical score index did not correlate significantly with tumor grade (P = 0.15), lymph node involvement (P = 0.31), or vascular invasion (P = 0.12).
DISCUSSION
In this analysis of 59 surgically resected PNETs, we observed a correlation of Clusterin immunoreactivity with tumor size and pathological stage. Although the expression of Clusterin in PNETs was recently reported by Mourra et al,19 these authors did not observe any correlation of Clusterin with tumor size or invasiveness (perineural invasion, vascular permeation, or lymph node metastasis). Based on the results of our analysis, we suggest that this molecule is involved in PNET progression.
Clusterin was initially discovered in rat testis fluid as a protein capable of inducing the clustering of a variety of cell types in culture.8 This protein seems to regulate a variety of biological processes, sometimes with opposite functions. For example, it shows proapoptotic function when expressed in the nucleolus and antiapoptotic effect if secreted in the cytoplasm.9,21 Secreted clusterin (Clusterin) is a 75- to 80-kd glycosylated α-β heterodimer present in almost all physiological fluids, and it consists of 2 chains of about 40 kd each and linked by 5 disulfide bonds.22 Clusterin, initially shown to have a role in tissue remodeling, lipid transport, complement regulation, and apoptosis,23 has recently been linked to cancer progression, prognosis, and response to therapy.12,24 There is accumulating evidence demonstrating that changes in clusterin expression in a variety of human cancers may reflect its role in cancer development. Although clusterin is not detected in normal ovarian or breast tissue, its expression is up-regulated during progression from cystadenoma to carcinoma in the ovary and from ductal carcinoma in situ to invasive mammary carcinoma and metastatic breast cancer.12,24 In contrast, clusterin is down-regulated in prostate cancer in respect to normal/benign tissue, and it is inversely proportional to the Gleason grade and advanced tumor stage.25
In our study, we demonstrated that a large percentage of PNETs (61%) express Clusterin. Importantly, Clusterin expression correlated significantly with tumor stage and tumor size. These findings differ from those reported by Mourra et al,19 who in their study of 30 PNET could not find any correlation between clusterin immunoreactivity and neither tumor size nor stage.19 It is possible that these differences may be related to the fact that different antibodies were used in the 2 studies (a clusterin α/β rabbit polyclonal antibody H330 from Santa Cruz Biotechnology in the study of Mourra et al and a clusterin rabbit polyclonal antibody from Abcam in our study). In our study, the specificity of the primary antibody was determined by appropriate blocking experiments reported by the manufacturer (http://www.abcam.com/Apolipoprotein-J-antibody-ab69644.html). In addition, we used antigen retrieval and a scoring system different from the one used in the study of Mourra et al. Finally, in both studies, the normal pancreatic islets, when present, adjacent to the tumor were Clusterin positive.
Clusterin expression is believed to affect cellular differentiation, especially during development.26 However, in prior studies, the correlation of clusterin with tumor grade has been variable. For example, 1 study has reported the correlation of clusterin expression with low-grade breast cancers,17 but another has shown the correlation of clusterin with high-grade prostate adenocarcinomas.18 In our study, we found no correlation between clusterin expression and grade of PNET. Taken together, these findings suggest that the correlation between clusterin and tumor grade may be tumor type dependent or that such a correlation is affected by other factors.
Xie et al,27 who also reported on clusterin expression in pancreatic cancer, focused their analysis on pancreatic ductal adenocarcinomas and observed that 49% of 33 ductal adenocarcinomas as well as 50% of 26 cases of chronic pancreatitis stained for clusterin. These authors concluded that clusterin expression was induced in pancreatic cancer and that it was inversely correlated with grade and stage, as tumors of lower stage and grade had higher clusterin expression. Based on this observation, they postulated that clusterin may be involved in the progression of pancreatic ductal adenocarcinomas.
In our study, we observed that 39% of the PNETs studied had Clusterin down-regulation, and when all data were evaluated, we did not find a statistical correlation with tumor grade. However, we found an increased expression of Clusterin in the PNETs that were larger and of higher stage, suggesting that Clusterin may play a significant role in PNET progression. In addition, it is intriguing that 30.5% (18/59) of the PNETs we studied had low Clusterin IHC scores (3 to 1) and that 5 of them were Clusterin negative. It is possible that the biology of these tumors may be different from those that have higher Clusterin expression. For example, recently Chen et al10 have reported that Clusterin may confer gemcitabine resistance in pancreatic cancer cells. The reported data show that in vivo systemic administration of clusterin antisense oligonucleotides and gemcitabine significantly decreased the subcutaneous tumor volume of a pancreatic cancer cell line.10 This observation led to the development of an antisense inhibitor targeting the translation initiation site of human exon II CLU (OGX-011) and of Custirsen, a second-generation clusterin antisense oligonucleotide with a long half-life of ~7 days. The use of OGX-011 in the clinical setting has shown improved efficacy of adjuvant therapy (chemotherapy, radiation, and hormonal withdrawal) by inhibiting Clusterin expression and favoring apoptosis of tumors in the preclinical setting (xenograft models of prostate, lung, breast, and other cancers).28–30 Our cases of negative or low Clusterin PNETs were mostly of small size and did not receive adjuvant therapy; therefore, we could not correlate the Clusterin IHC score with tumor response to adjuvant therapy.
In summary, our study evaluated the expression Clusterin in PNETs and its association with clinical-pathological parameters. We found a correlation of Clusterin expression with tumor size and stage, suggesting the role of clusterin in PNET progression. It is possible that the immunohistochemical assessment of Clusterin in human PNETs may be used to predict tumor response to targeted therapies. This latter aspect warrants further investigation.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Bosman F, Carneiro F, Hruban R, et al. WHO Classification of Tumours of the Digestive Stystem. 4th IARC Press; Lyon: 2010. [Google Scholar]
- 2.Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–7803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 3.Ferrone C, Tang L, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 4.Fischer L, Kleeff J, Esposito I, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 5.Hochwald S, Zee S, Conlon K, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 6.La Rosa S, Klersy C, ccella S, et al. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40:30–40. doi: 10.1016/j.humpath.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Pape U, Jann H, Muller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256–265. doi: 10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 8.Fritz IB, Burdzy K, Setchell B, et al. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28:1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- 9.Shannan B, Seifert M, Leskov K, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13:12–19. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Wang Z, Zhang K, et al. Clusterin confers gemcitabine resistance in pancreatic cancer. World J Surg Oncol. 2011;9:59–66. doi: 10.1186/1477-7819-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzi F, Bettuzzi S. The clusterin paradigm in prostate and breast carcinogenesis. Endocr Relat Cancer. 2010;17:R1–R17. doi: 10.1677/ERC-09-0140. [DOI] [PubMed] [Google Scholar]
- 12.Redondo M, Villar E, Torres-Munoz J, et al. Overexpression of clusterin in human breast carcinoma. Am J Pathol. 2000;157:393–399. doi: 10.1016/S0002-9440(10)64552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurahashi T, Muramaki M, Yamanaka K, et al. Expression of the secreted form of clusterin in renal cell carcinoma as a predictor of disease extension. BJU. 2005;99:895–899. doi: 10.1111/j.1464-410X.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 14.Hassan MK, Watari H, Han Y, et al. Clusterin is a potential molecular predictor for ovarian cancer patient’s survival: targeting clusterin improves response to paclitaxel. J Exp Clin Cancer Res. 2011;30:113. doi: 10.1186/1756-9966-30-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kevans D, Foley J, Tenniswood M, et al. High clusterin expression correlates with poor outcome in stage II colorectal cancers. Cancer Epidemiol Biomarkers Prev. 2009;18:393–399. doi: 10.1158/1055-9965.EPI-08-0302. [DOI] [PubMed] [Google Scholar]
- 16.Saffer H, Wahed A, Rassidakis GZ, et al. Clusterin expression in malignant lymphomas: a survey of 266 cases. Mod Pathol. 2002;15:1221–1226. doi: 10.1097/01.MP.0000036386.87517.AA. [DOI] [PubMed] [Google Scholar]
- 17.Yom CK, Woo HY, Min SY, et al. Clusterin overexpression and relapse-free survival in breast cancer. Anticancer Res. 2009;29:3909–3912. [PubMed] [Google Scholar]
- 18.Steinberg J, Oyasu R, Lang S. Intracellular levels of SGP-2 (clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res. 1997;3:1707–1711. [PubMed] [Google Scholar]
- 19.Mourra N, Couvelard A, Tiret E, et al. Clusterin is highly expressed in pancreatic endocrine tumours but not in solid pseudopapillary tumors. Histopathology. 2007;50:331–337. doi: 10.1111/j.1365-2559.2007.02608.x. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th Springer; New York: 2010. Exocrine and Endocrine Pancreas; pp. 241–249. [Google Scholar]
- 21.Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res. 2010;16:1088. doi: 10.1158/1078-0432.CCR-09-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Silva H, Stuart W, Gil C, et al. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29:5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg ME, Silkensen L. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27:633–645. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 24.Xie D, Lau S, Sham J, et al. Up-regulated expression of cytoplasmic clusterin in human ovarian carcinoma. Cancer. 2005;103:277–283. doi: 10.1002/cncr.20765. [DOI] [PubMed] [Google Scholar]
- 25.Vanaja D, Cheville J, Iturria S, et al. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Research. 2003;63:3877–3882. [PubMed] [Google Scholar]
- 26.Ahuja HS, Tenniswood M, Lockshin R, et al. Expression of clusterin in cell differentiation and cell death. Biochem Cell Biol. 1994;72(11–12):523–530. doi: 10.1139/o94-070. [DOI] [PubMed] [Google Scholar]
- 27.Xie MJ, Motoo Y, Su SB, et al. Expression of clusterin in human pancreatic cancer. Pancreas. 2002;25:234–238. doi: 10.1097/00006676-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Miyake H, Hara I, Gleave ME. Antisense oligodeoxynucleotide therapy targeting clusterin gene for prostate cancer: Vancouver experience from discovery to clinic. Int J Urol. 2005;12:785–794. doi: 10.1111/j.1442-2042.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 29.Sowery RD, Hadaschik BA, So AI, et al. Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxol-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int. 2008;102:389–397. doi: 10.1111/j.1464-410X.2008.07618.x. [DOI] [PubMed] [Google Scholar]
- 30.Gleave M, Miyake H. Use of antisense oligonucleotides targeting the cytoprotective gene, clusterin, to enhance androgen- and chemo-sensitivity in prostate cancer. World J Urol. 2005;23:38–46. doi: 10.1007/s00345-004-0474-0. [DOI] [PubMed] [Google Scholar]