Abstract
IκB kinase (IKKε) is a serine/threonine protein kinase that belongs to the IKK kinase family. Recent studies have shown that IKKε functions as a breast and ovarian cancer oncogene. We demonstrated frequent overexpression of IKKε in pancreatic ductal adenocarcinoma (PDA). We immunohistochemically evaluated 78 PDAs using the avidin-biotin-peroxidase method and the anti-IKKε rabbit polyclonal antibody. Elevated IKKε reactivity (immunohistochemical score, 4–9) was observed in 64% of PDAs (50/78), but in 0.0% of nonneoplastic pancreatic ductal epithelium (0/113; P < .001). Kaplan-Meier analysis of overall survival revealed that patients with high IKKε-immunohistochemical scores (4–9) had significantly shorter survival than did patients with low IKKε immunohistochemical scores (0–3; P = .023; log-rank test) independent of tumor stage or grade. These data indicate that deregulation of IKKε is a common event in PDA and might have an important role in the pathogenesis of this deadly disease. In addition, IKKε could serve as a prognostic marker and potential therapeutic target for PDA intervention.
Keywords: IκB kinase ε, IKKε, Kinase, Oncogene, Pancreatic cancer, Tumor progression
Pancreatic ductal adenocarcinoma (PDA) is the most common pancreatic epithelial tumor, and it ranks fourth as the overall leading cause of death in men and women.1 The annual incidence of pancreatic cancer has risen during the last decade, and more than 42,000 new cases were expected in 2010.1 Furthermore, the 5-year survival of patients with ductal pancreatic cancer is less than 5%, and it has shown no improvement during the past 3 decades despite molecular advances and the application of alternative therapeutic modalities.1,2
During the last decade, new treatments for cancer have been developed using agents targeting specific regulatory molecules of signal transduction pathways controlling malignant transformation, tumor invasion, and metastasis. The biochemical and molecular alterations underlying the progression from normal to cancerous pancreas are being elucidated and have included oncogenes, tumor suppressor genes, stromal components, and apoptotic molecules.3–18
Recently, Boehm and colleagues19 identified IκB kinase (IKKε) as an oncogene capable of replacing PI3K/AKT signaling and downstream Ras signaling, to promote cellular transformation in immortalized human mammary epithelial cells. IKKε belongs to 5 distinct but closely related proteins, the IκB kinase (IKK) family, consisting of IKKα, IKKβ, IKKγ, IKKε, and TBK1. IKKε activates NFκB through phosphorylation and degradation of IκB.19 Frequent overexpression of IKKε has been reported in breast and ovarian cancers.19–21 In human pancreatic cancer, IKKα and IKKβ were shown to be important regulators of NFκB activation, and their suppression was associated with inhibition of cell proliferation and induction of apoptosis.22–29 However, IKKε expression in PDAs has not been studied.
In the present study, we evaluated the expression of IKKε in PDAs using immunohistochemical studies and tissue microarray (TMA) technology. The TMA included formalin-fixed, paraffin-embedded samples of PDAs from patients who underwent surgical resection of their tumors.
Materials and Methods
Selection of Cases
The present study focused on 78 patients with pancreatic cancer who had a pancreatoduodenectomy at the Moffitt Cancer Center, Tampa, FL. The histologic diagnosis in each case was confirmed by pathologic review of H&E-stained sections cut from fixed surgical specimens (by A.C. and D.C.). Data from the Moffitt Cancer Registry, the patient’s medical record, the anatomic pathology database (CoPath, Cerner, North Kansas City, MO), and the Social Security Death Index were reviewed to determine the patient’s clinicopathologic characteristics, the American Joint Committee on Cancer anatomic staging, and the survival of each patient after surgery, whether the patient had died as of most recent knowledge, and whether there was evidence of disease at that time. The study was approved by the University of South Florida Institutional Review Board (Tampa).
For the immunohistochemical study, we used a human pancreatic cancer TMA (previously prepared by the Histology Laboratory, Moffitt Cancer Center Tissue Core Facility) containing tissue tumor samples of the selected 78 pancreatic cancer cases. The cases were selected to include tumors of low (I and II) and high (III and IV) stage. The TMA also contained 113 samples of nonneoplastic pancreatic ductal epithelium (NMP) derived from areas adjacent to PDAs (as far as possible from the tumor). Of the 113 NMP samples, 75 were derived from the same PDA specimens represented on the TMA. Areas of pancreatic intraepithelial neoplasia were not present in this TMA constructed to represent NMPs and PDAs of different stages. All tissues were derived from pancreatic surgical resection specimens obtained between 1987 and 2006. Two separate tissue cores of PDAs and NMPs were collected for the construction of the TMAs. All of the specimens were preserved in 10% buffered formalin before embedding in paraffin.
Immunohistochemical Staining
The tissues were immunostained using the avidin-biotin-peroxidase method and the anti-IKKε rabbit polyclonal antibody (catalog No. 14907, dilution 1:250; Sigma-Aldrich, St Louis, MO). The TMA slides were dewaxed by heating at 55°C for 30 minutes and by 3 washes, 5 minutes each, with xylene. Tissues were rehydrated by a series of 5-minute washes in 100%, 95%, and 80% ethanol and distilled water. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 minutes. After blocking with universal blocking serum (OmniMap, Ventana Medical Systems, Tucson, AZ) for 30 minutes, the samples were incubated with anti-IKKε rabbit polyclonal antibody (Sigma-Aldrich) at 4°C overnight. This antibody shows no cross-reactivity with IKKα, IKKβ, or IKKγ. The samples were then incubated with biotin-labeled secondary antibody and streptavidin-horseradish peroxidase for 30 minutes each (Ventana Medical Systems). The slides were developed with 3,3'-diaminobenzidine tetrahydrochloride substrate (Ventana Medical Systems) and counterstained with hematoxylin (Ventana Medical Systems). The tissue samples were dehydrated and coverslipped. Standard cell conditioning (following the Ventana proprietary recommendations) was used for antigen retrieval. The specificity of the anti-IKKε antibody was confirmed by immunoblot and immunohistochemical analysis of IKKε+ MCF7 cells Image 1K and Image 1L. Negative control was included by omitting the IKKε antibody during the primary antibody incubation step.
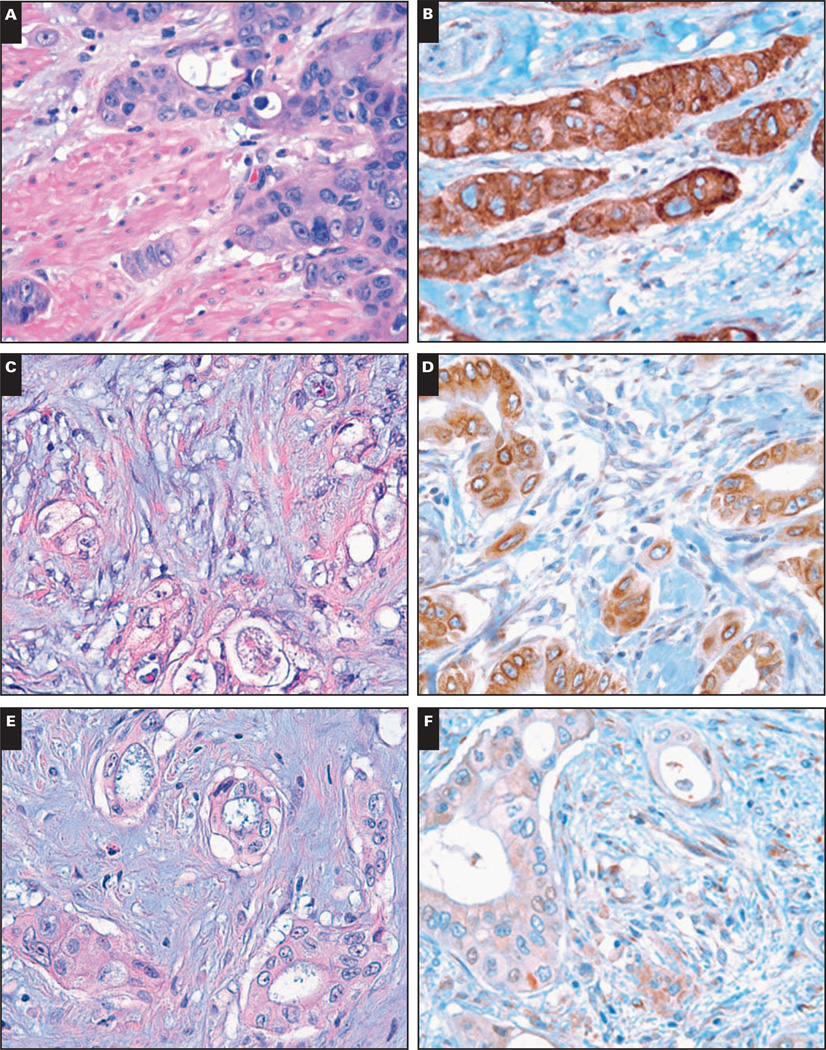
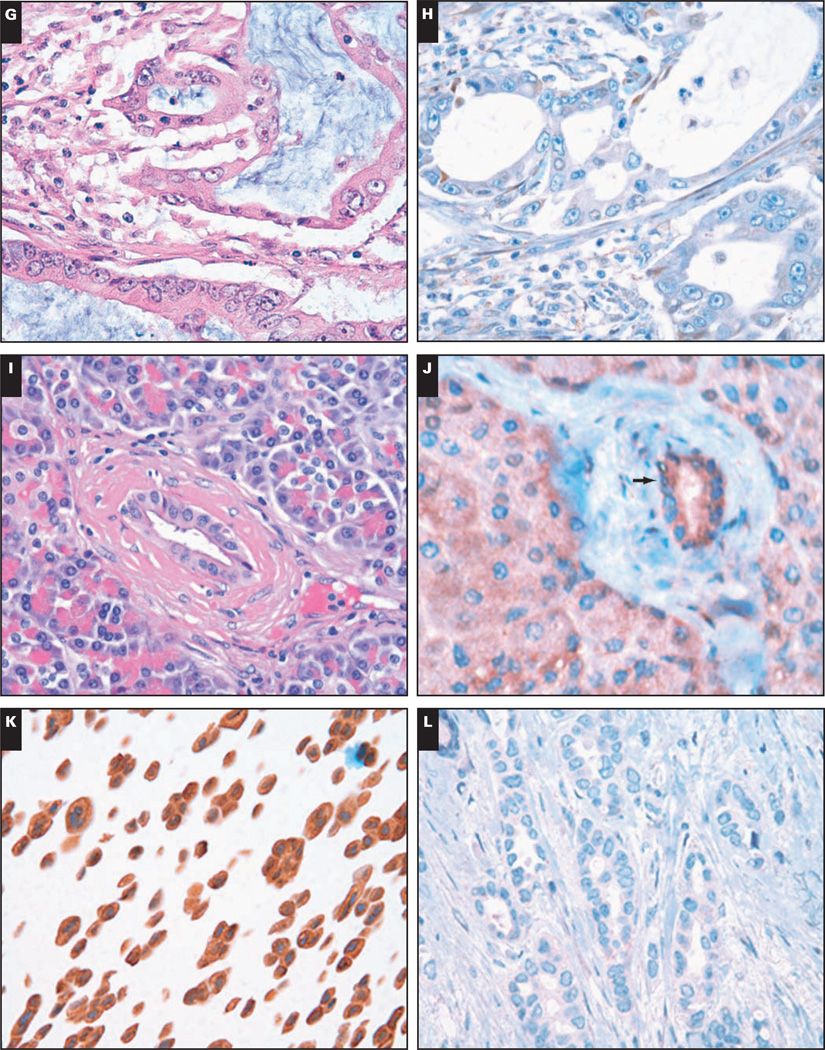
Image 1.
A pancreatic ductal adenocarcinoma (PDA) exhibiting strong and diffuse cytoplasmic positivity for I-κ-B kinase ε (IKKε) immunohistochemical (IHC) score, +9 (A, H&E ×400; B, IHC, ×400). A PDA with weak cytoplasmic IKKε expression, immunohistochemical score, +3 (C, H&E, ×400; D, IHC, ×400). E and F, A NMP showing weak IKKε staining of the benign ductal epithelium (immunohistochemical score, +3) (arrow) (E, H&E, ×400; F, IHC, ×400).
K and L, The positive control sample (MCF7 cells constitutively expressing IKKε) is shown in K (×400), and in L is the negative control sample (in which the IKKε antibody was omitted during the primary antibody incubation step; ×400).
(G, H&E, ×400; H, IHC, ×400; I, H&E, ×400; J, IHC, ×400).
Immunohistochemical Data Analysis
The IKKε-stained tissue cores were examined by 2 independent observers (A.C. and D.C.) experienced in immunohistochemical methods, and a consensus score was reached for each specimen. They scored the stained tissue sections for intensity of IKKε immunostain and percentage of cells stained. The intensity of staining was subjectively ranked on a scale of 0 to 3, in which 0 represents no staining and 3 is maximal staining. The percentage of cells stained was estimated on a scale of 0 to 3, in which 0 represents no cells stained and the values 1 to 3 reflect the lowest to highest tertiles, respectively. The IKKε expression score was calculated as the product of immunostain intensity and the percentage of cells stained. Thus, the IKKε final score can take on only the values 0, 1, 2, 3, 4, 6, and 9. The specimens were also classified by the types of tissue staining positively: NMP or PDA.
Analytic and Statistical Methods
Correlations of IKKε overexpression with the tumor grade and stage were analyzed by χ2 testing. Multivariate analysis was used to evaluate the prognostic value of IKKε expression in PDA. Overall survival (OS) was calculated only for patients who achieved complete remission, from the date of the first documentation of diagnostic biopsy until the date of progression or death. The OS curve was estimated by using the Kaplan-Meier product-limit method and was compared by using the log-rank test. All statistical analyses were 2-sided, and comparisons in which the P value was less than .05 were considered statistically significant.
Results
Clinicopathologic Features
A total of 78 patients who underwent resection of ductal pancreatic cancer had fixed surgical specimens immunostained for IKKε. Samples of 78 PDAs and 113 NMPs were used. The ages of patients ranged between 42 and 82 years (mean ± SD, 64 ± 9.8 years). Of the patients, 28 were men and 50 were women. The tumor size ranged between 0.7 and 12 cm (mean, 3 cm). Of the tumors, 67 were located in the head of the pancreas, 9 were in the body, and 2 were in the tail; 16 tumors were well differentiated, 47 were moderately differentiated, and 15 were poorly differentiated. Of the patients, 25 (32%) had stage I or II cancer and 53 (68%) had stage III or IV disease.
Immunohistochemical Results
The immunohistochemical results are summarized in Table 1, Image 1A, Image 1B, Image 1C, Image 1D, Image 1E, Image 1F, Image 1G, Image 1H, Image 1I, and Image 1J (Images 1K and 1L are the relative controls). Elevated IKKε reactivity (immunohistochemical score, 4–9) was observed in 64% of the PDAs (50/78) but in 0.0% of NMPs (0/113), a difference that was statistically significant (P < .001). Low IKKε positivity (immunohistochemical score, 1–3) was seen in 35% of PDAs (27/78) and in 80 (70.8%) of 113 NMPs. This difference was also statistically significant (P < .001). Only 1 (1%) of 78 PDAs but 33 (29.2%) of 113 NMPs were IKKε− (P = .002). These data indicate that the increased expression of IKKε is a frequent event in PDAs and suggest that IKKε could have an important role in pancreatic carcinogenesis.
Table 1.
Expression of IKKε in PDA
| IKKε Expression | PDA | NMP | P |
|---|---|---|---|
| High (4–9) | 50 | 0 | .001 |
| Low (0–3) | 28 | 113 | |
| Total | 78 | 113 | |
| Positive (1–9) | 77 | 80 | .002 |
| Negative (0) | 1 | 33 | |
| Total | 78 | 113 |
IKKε, I-κ-B kinase ε; NMP, nonneoplastic pancreatic ductal epithelium; PDA, pancreatic ductal adenocarcinoma.
IKKε Correlation With Clinicopathologic Features
We examined the relationships between overexpression of IKKε and tumor grade and stage. Statistical analysis showed no significant differences of IKKε immunohistochemical scores between tumor stage and grade (P = .562 and P = .865, respectively) Table 2. Actually, the frequency of IKKε overexpression seems to be higher in low-grade and early-stage than high-grade and late-stage tumor, suggesting that IKKε might involve tumor initiation rather than progression.
Table 2.
IKKε Expression in PDA: Correlation With Clinicopathologic Features
| IKKε Expression | |||
|---|---|---|---|
| Variable | No. of Low/No |
No. (%) of High/Moderate |
P |
| Stage | |||
| I/II (n = 25) | 12 | 13 (52) | .562 |
| III/IV (n = 53) | 26 | 27 (51) | |
| Grade | |||
| 1 (n = 16) | 8 | 8 (50) | .865 |
| 2 (n = 47) | 21 | 26 (55) | |
| 3 (n = 15) | 8 | 7 (47) | |
IKKε, I-κ-B kinase ε; PDA, pancreatic ductal adenocarcinoma.
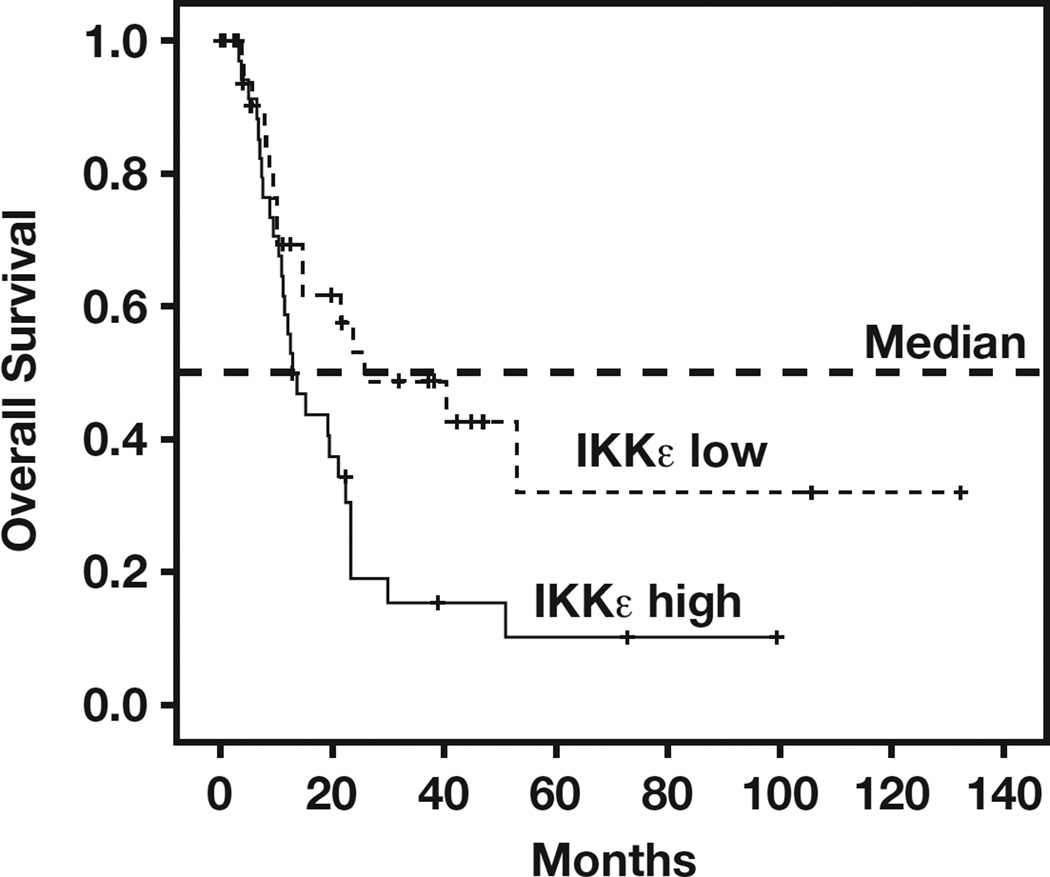
To study whether IKKε is a prognostic factor for pancreatic cancer, we correlated IKKε expression in tumors with a median follow-up of 12.7 months after pancreatic cancer surgery. In our patient cohort (n = 78), all had complete follow-up information. We found a strong association between IKKε and survival. A multivariate Cox proportional hazards model with the following variables was used to analyze their simultaneous association with OS: age, sex, grade, and IKKε expression. As shown in Table 3, IKKε expression is an independent predictor with a hazard ratio of 2.030 (95% confidence interval, 1.089–3.782) and a P value of .026. Furthermore, Kaplan-Meier analysis of OS revealed that patients with high IKKε immunohistochemical scores (4–9) had a significantly shorter survival than did patients with low IKKε immunohistochemical scores (0–3) Figure 1. Median survival time was also significantly different between IKKε+ (12.70 months) and IKKε− (25.77 months) cases (P = .023; log-rank test), suggesting that IKKε could be a strong prognostic marker in PDA.
Table 3.
Multivariate Analysis of Overall Survival of PDA Cases Studied
| Variable | Multivariate Analysis* | P |
|---|---|---|
| Sex, M vs F | 1.688 (0.874–3.259) | .119 |
| Age, >55 vs ≤55 y | 1.141 (0.524–2.481) | .741 |
| Grade, III/IV vs I/II | 1.604 (0.881–2.391) | .143 |
| IKKε, positive vs negative | 2.030 (1.089–3.782) | .026 |
IKKε, I-κ-B kinase ε; PDA, pancreatic ductal adenocarcinoma.
Data are given as hazard ratio (confidence interval).
Figure 1.
Poor overall survival in patients with elevated levels of I-κ-B kinase ε (IKKε) expression. Overall survival in patients with high levels of IKKε (n = 41) vs the remaining patients (n = 37) was plotted by the Kaplan-Meier method. Statistical comparison of survival between groups with the log-rank statistic analysis suggests that patients whose tumors express elevated levels of IKKε had poor survival compared with patients with low levels of IKKε (P = .023). The median survival times were as follows: low IKKε group, 25.77 months; high IKKε group, 12.70 months.
Discussion
In this study, we demonstrated the frequent overexpression of IKKε (~54%) in PDAs. IKKε has recently been shown to be an oncogene that is frequently overexpressed in carcinoma of breast and ovary.19–21 This is the first report of IKKε expression in human pancreatic cancer. Furthermore, we report a statistically significant correlation between an IKKε immunohistochemical score of more than 3 with worse survival, which is independent of tumor grade and stage.
Oncogenes, tumor suppressor genes, stromal components, and apoptotic molecules may be altered during the progression from normal to cancerous pancreas. Mutations and/or overexpression of p53 have been reported to occur in 54% to 73.5% of PDAs, depending on negative or positive smoking history, respectively.3,4 CD44 standard and variants 3, 4, and 6 have been found to be overexpressed in pancreatic tumor cells and predict decreased patient survival.5,6 Lysosomal cathepsins, laminin receptors, urokinase plasminogen activator, and its receptor have been linked to the metastatic potential of pancreatic cancer cells.7,8 Recently, it has been shown that the use of cathepsin protease and/or urokinase plasminogen activator receptor inhibitors negatively affects proliferation and migration of pancreatic adenocarcinoma cells.9,10 Proapoptotic and antiapoptotic proteins (Bax, Bcl-2, Bcl-XL, and c-FLIP) also have a role in pancreatic carcinogenesis.11,12 The tumor suppressor gene DPC4 has been reported to affect the behavior of primary carcinomas and the progression of pancreatic neoplasia.13 Furthermore, aberrant expression of growth factors and of cell receptors has been described in pancreatic cancer.14–17 Remarkably, K-ras oncogene mutations have been observed in 80% of PDAs.18
Herein we report the overexpression of IKKε as an additional alteration with a role in the progression from normal pancreatic ductal epithelium to pancreatic ductal carcinoma. IKKε is a member of the IKK family, consisting of IKKα, IKKβ, IKKγ, IKKε, and TBK1. Of these, only IKKα and IKKβ were found to be involved in pancreatic carcinogenesis by modulating the NF-κB pathway and apoptosis.22–29 Furthermore, decreased signaling via the IKKβ/NF-κB pathway was found to result in decreased expression of angiogenesis- and chemotaxis-related factors.30 However, alterations of IKKα and IKKβ at protein levels have not been reported. IKKε has recently attracted intense interest because it locates on chromosome 1q32.1, a region commonly undergoing amplification in human cancers.31 The 1q32.1 amplifications involving the IKKε gene occur in as many as 16.3% of breast cancers and 20% of ovarian cancers.20,21 But the frequency of elevated IKKε at the protein level is much higher than its DNA amplification in these 2 diseases, suggesting the deregulation of IKKε is at the transcriptional and/or at the translational level.20,21 Although amplification of chromosome 1q32.1 is not common in pancreatic cancer, a recent study shows one of the PDA susceptibility loci is on chromosome 1q32.1 that harbors NR5A2. Five highly significant single nucleotide polymorphisms were found in this locus.31 It is unknown whether these single nucleotide polymorphisms affect IKKε expression. Future study is required to determine the mechanism of overexpression of IKKε in PDAs.
The data obtained from breast cancer have implied that alterations of IKKε might be an early event in the development of some breast cancers because amplification of this locus was observed in 4 of 10 ductal carcinomas in situ.19,32 However, a recent study of ovarian cancer showed that an elevated level of IKKε could be associated with late-stage and high-grade tumor.20 Because the frequency of overexpression of IKKε is slightly higher in early-stage and low-grade PDAs (Table 2), IKKε may involve tumor initiation of PDA. However, an increase of IKKε is significantly associated with poor overall survival (Figure 1), suggesting that IKKε is an unfavorable prognostic marker in PDA.
In the present study, we demonstrated increased expression of IKKε in human primary pancreatic cancer. It is interesting that we found the aberrant expression of IKKε was not significantly different among tumor stages and grades. Furthermore, patients with pancreatic cancer with low levels of IKKε have a better outcome than do patients whose tumors express elevated levels of IKKε, regardless of tumor stage. Based on these findings, IKKε may exert an oncogene role in PDA.
Upon completion of this activity you will be able to:
define the function of IκB kinase (IKK)-ε in pancreatic cell proliferation.
summarize the role of IKK-ε in pancreatic cancer progression.
assess the role of IKK-ε as a marker of tumor response to therapy.
Acknowledgments
We thank the Histology Section of the Tissue Core at the Moffitt Cancer Center for the preparation of the pancreatic cancer TMA and for support in performing immunohistochemical stains. We also thank Andrea Dattilo for assistance during preparation and submission of the manuscript.
J.P.G. is a postdoctoral fellow award recipient (IKD04) from the James and Esther King Biomedical Research Program, Florida.
Footnotes
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009;69:3681–3688. doi: 10.1158/0008-5472.CAN-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsiambas E, Kravvaritis C, Tsounis D, et al. Correlation between different p53 expression patterns and chromosome 17 imbalances in pancreatic ductal adenocarcinoma based on tissue microarray analysis. J BUON. 2010;15:94–100. [PubMed] [Google Scholar]
- 5.Ringel J, Jesnowski R, Schmidt C, et al. CD44 in normal human pancreas and pancreatic carcinoma cell lines. Teratog Carcinog Mutagen. 2001;21:97–106. [PubMed] [Google Scholar]
- 6.Gotoda T, Matsumura Y, Kondo H, et al. Expression of CD44 variants and its association with survival in pancreatic cancer. Jpn J Cancer Res. 1998;89:1033–1040. doi: 10.1111/j.1349-7006.1998.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryschich E, Khamidjanov A, Kerkadze V, et al. Promotion of tumor cell migration by extracellular matrix proteins in human pancreatic cancer. Pancreas. 2009;38:804–810. doi: 10.1097/MPA.0b013e3181b9dfda. [DOI] [PubMed] [Google Scholar]
- 8.Hildenbrand R, Niedergethmann M, Marx A, et al. Amplification of the urokinase-type plasminogen activator receptor (uPAR) gene in ductal pancreatic carcinomas identifies a clinically high-risk group. Am J Pathol. 2009;174:2246–2253. doi: 10.2353/ajpath.2009.080785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elie BT, Gocheva V, Shree T, et al. Identification and pre-clinical testing of a reversible cathepsin protease inhibitor reveals anti-tumor efficacy in a pancreatic cancer model. Biochimie. 2010;92:1618–1624. doi: 10.1016/j.biochi.2010.04.023. [published online ahead of print May 4, 2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue A, Xue M, Jackson C, et al. Suppression of urokinase plasminogen activator receptor inhibits proliferation and migration of pancreatic adenocarcinoma cells via regulation of ERK/p38 signaling. Int J Biochem Cell Biol. 2009;41:1731–1738. doi: 10.1016/j.biocel.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto Y, Hosotani R, Wada M, et al. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 12.Haag C, Stadel D, Zhou S, et al. Identification of c-FLIPL and c-FLIPS as critical regulators of death receptor–induced apoptosis in pancreatic cancer cells. Gut. 2011;60:225–237. doi: 10.1136/gut.2009.202325. [published online ahead of print September 28, 2010]. [DOI] [PubMed] [Google Scholar]
- 13.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton CM, Hall PA, Hughes CM, et al. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol. 1991;163:111–116. doi: 10.1002/path.1711630206. [DOI] [PubMed] [Google Scholar]
- 15.Sloss CM, Wang F, Palladino MA, et al. Activation of EGFR by proteasome inhibition requires HB-EGF in pancreatic cancer cells. Oncogene. 2010;29:3146–3152. doi: 10.1038/onc.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szepeshazi K, Halmos G, Schally AV, et al. Growth inhibition of experimental pancreatic cancers and sustained reduction in epidermal growth factor receptors during therapy with hormonal peptide analogs. J Cancer Res Clin Oncol. 1999;125:444–452. doi: 10.1007/s004320050301. [DOI] [PubMed] [Google Scholar]
- 17.Wagner M, Kleeff J, Friess H, et al. Enhanced expression of the type II transforming growth factor-beta receptor is associated with decreased survival in human pancreatic cancer. Pancreas. 1999;19:370–376. doi: 10.1097/00006676-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Alfa GK, Chapman PB, Feilchenfeldt J, et al. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. doi: 10.1097/COC.0b013e3181e84b1f. [published online ahead of print August 3, 2010]. [DOI] [PubMed] [Google Scholar]
- 19.Boehm JS, Zhao JJ, Yao J, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 20.Guo JP, Shu SK, He L, et al. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am J Pathol. 2009;175:324–333. doi: 10.2353/ajpath.2009.080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo JP, Shu SK, Esposito NN, et al. IKKε phosphorylation of estrogen receptor alpha Ser-167 and contribution to tamoxifen resistance in breast cancer. J Biol Chem. 2010;285:3676–3684. doi: 10.1074/jbc.M109.078212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 23.Hayden MS, Ghosh S. Shared principles in NFκB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 25.Tenoever BR, Ng SL, Chua MA, et al. Multiple functions of the IKK-related kinase IKKε in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 26.Breiman A, Grandvaux N, Lin R, et al. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKε. J Virol. 2005;79:3969–3978. doi: 10.1128/JVI.79.7.3969-3978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woronicz JD, Gao X, Cao Z, et al. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 28.Peters RT, Liao SM, Maniatis T. IKKε is part of a novel PMA-inducible IκB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 29.Shimada T, Kawai T, Takeda K, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IκB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 30.Hosoi F, Izumi H, Kamahara A, et al. N-myc downstream regulated gene 1/Cap43 suppresses tumor growth and angiogenesis of pancreatic cancer through attenuation of inhibitor of κB kinase β expression. Cancer Res. 2009;69:4983–4991. doi: 10.1158/0008-5472.CAN-08-4882. [DOI] [PubMed] [Google Scholar]
- 31.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eddy SF, Guo S, Demicco EG, et al. Inducible IκB kinase/IκB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-κB activation in breast cancer cells. Cancer Res. 2005;65:11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]